Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer
Abstract
1. Introduction
2. Results and Discussion
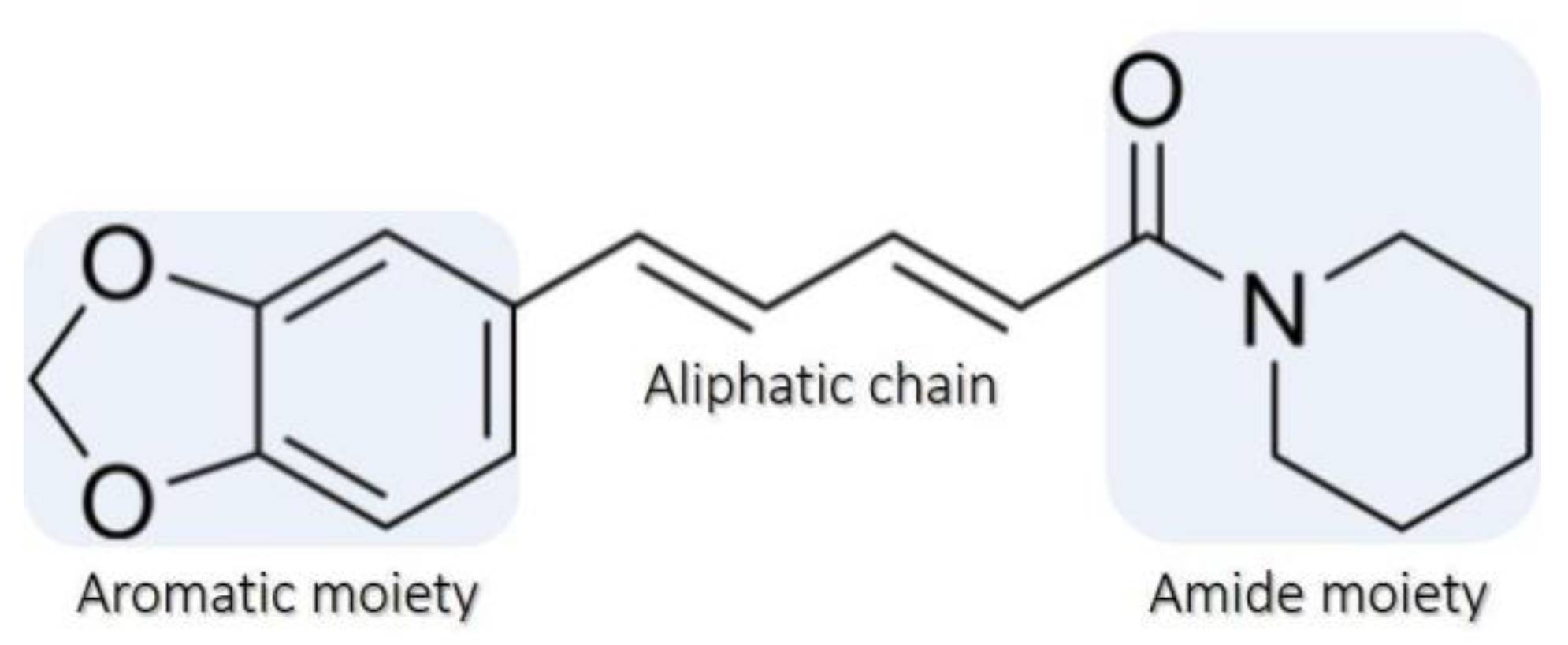
2.1. Isolation and Characterization of Piperine
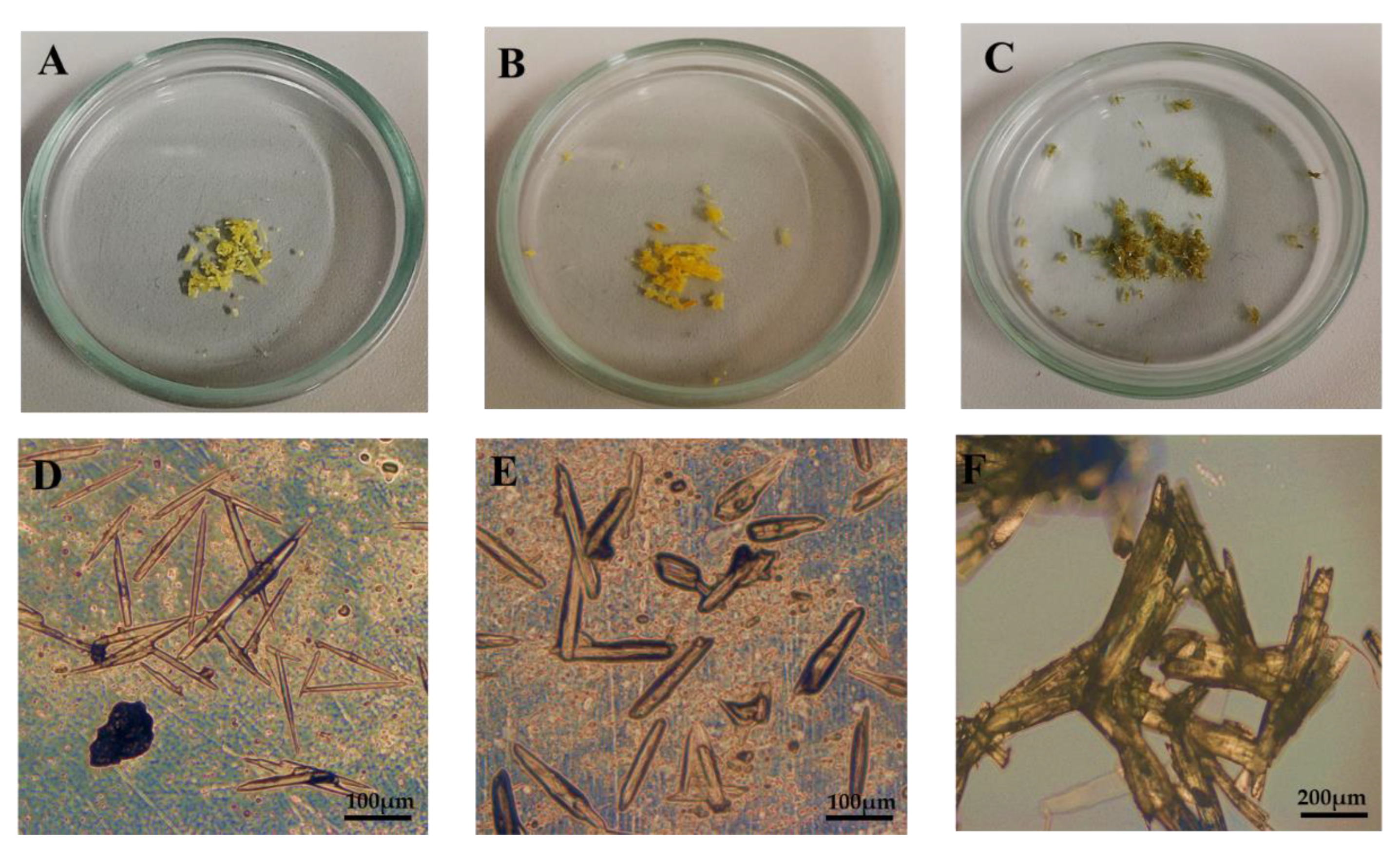
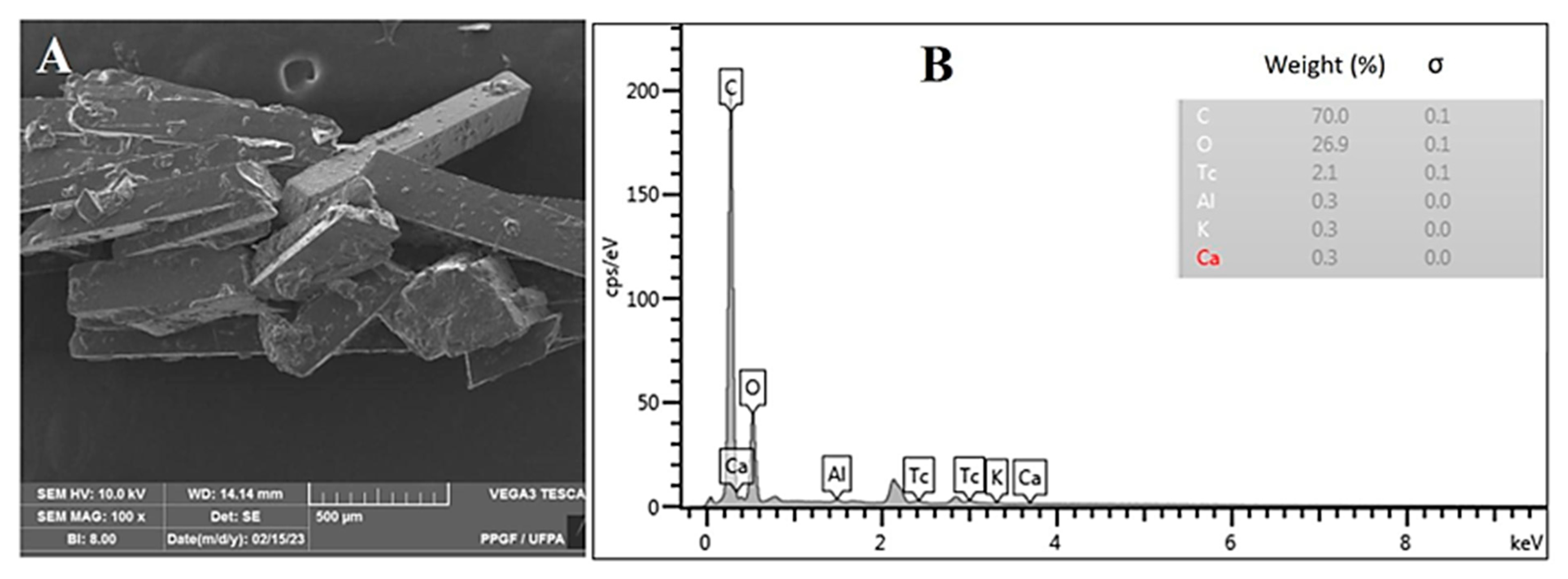
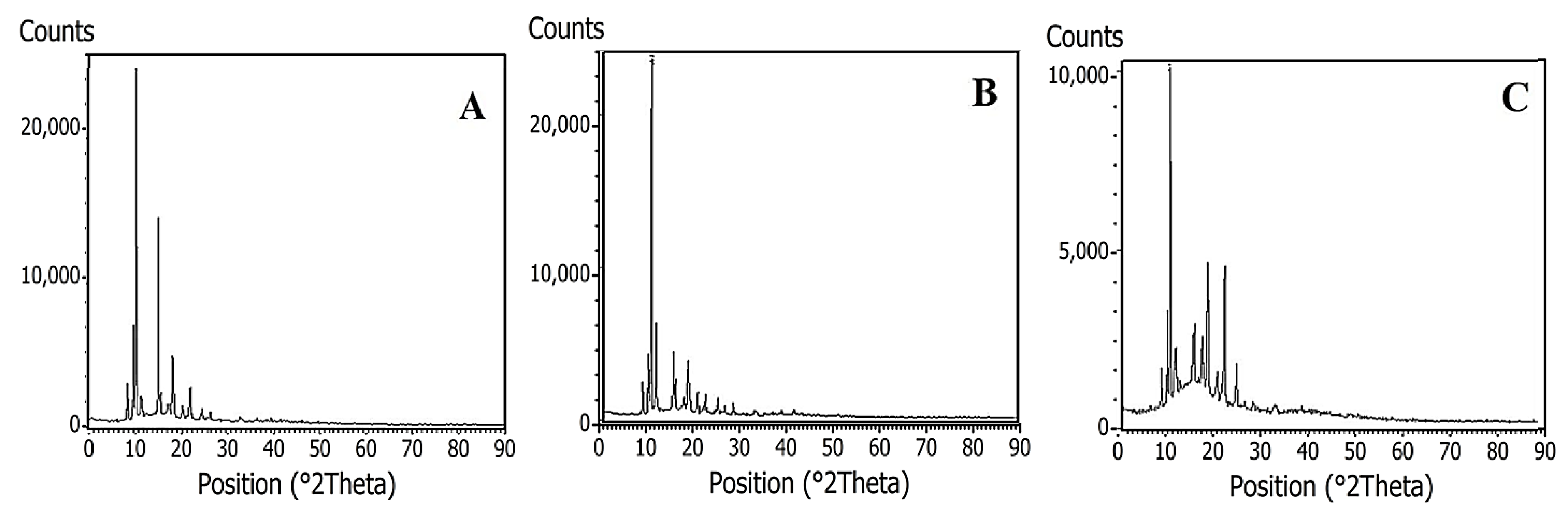
2.1.1. Microscopy and X-ray Diffraction Analysis
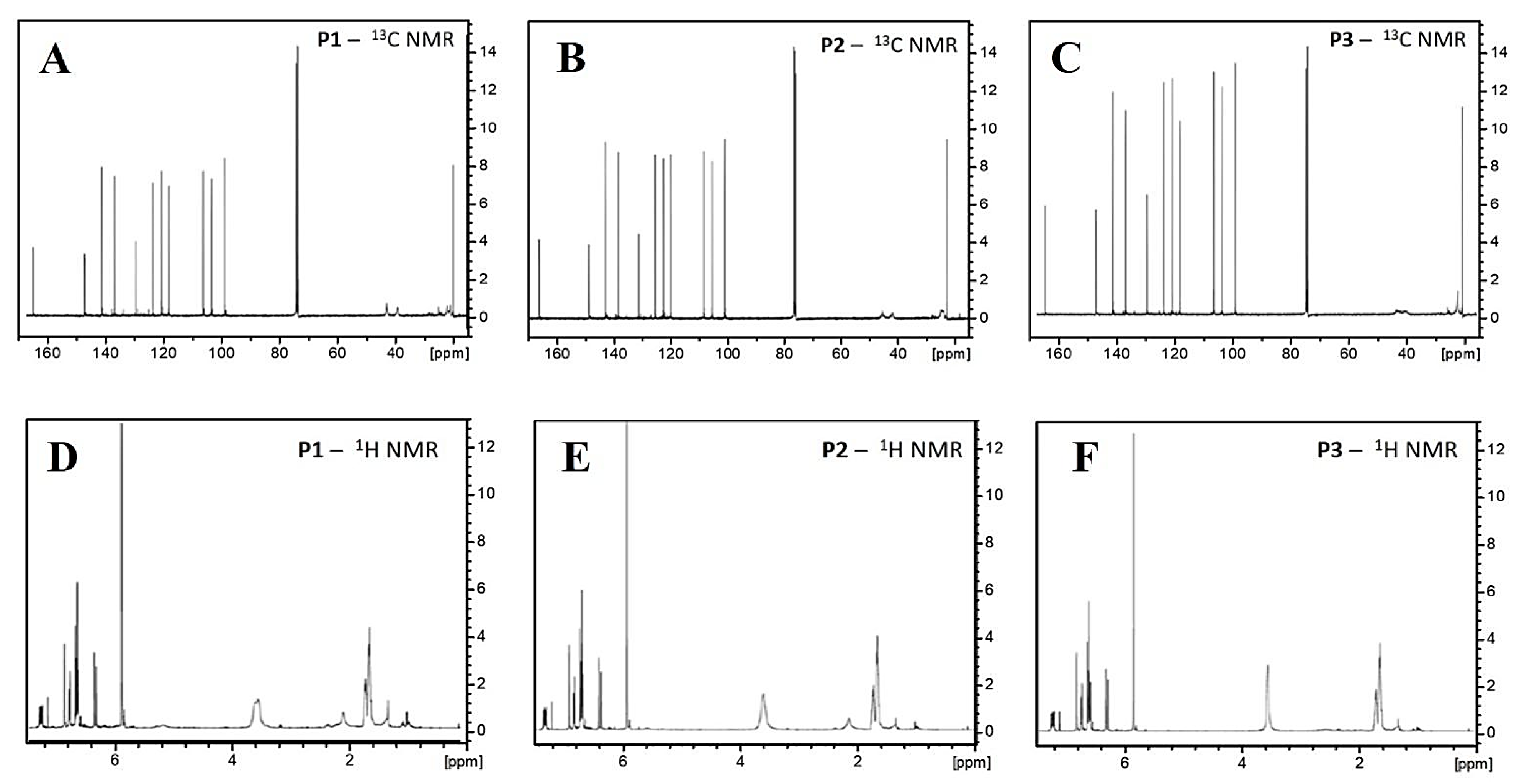
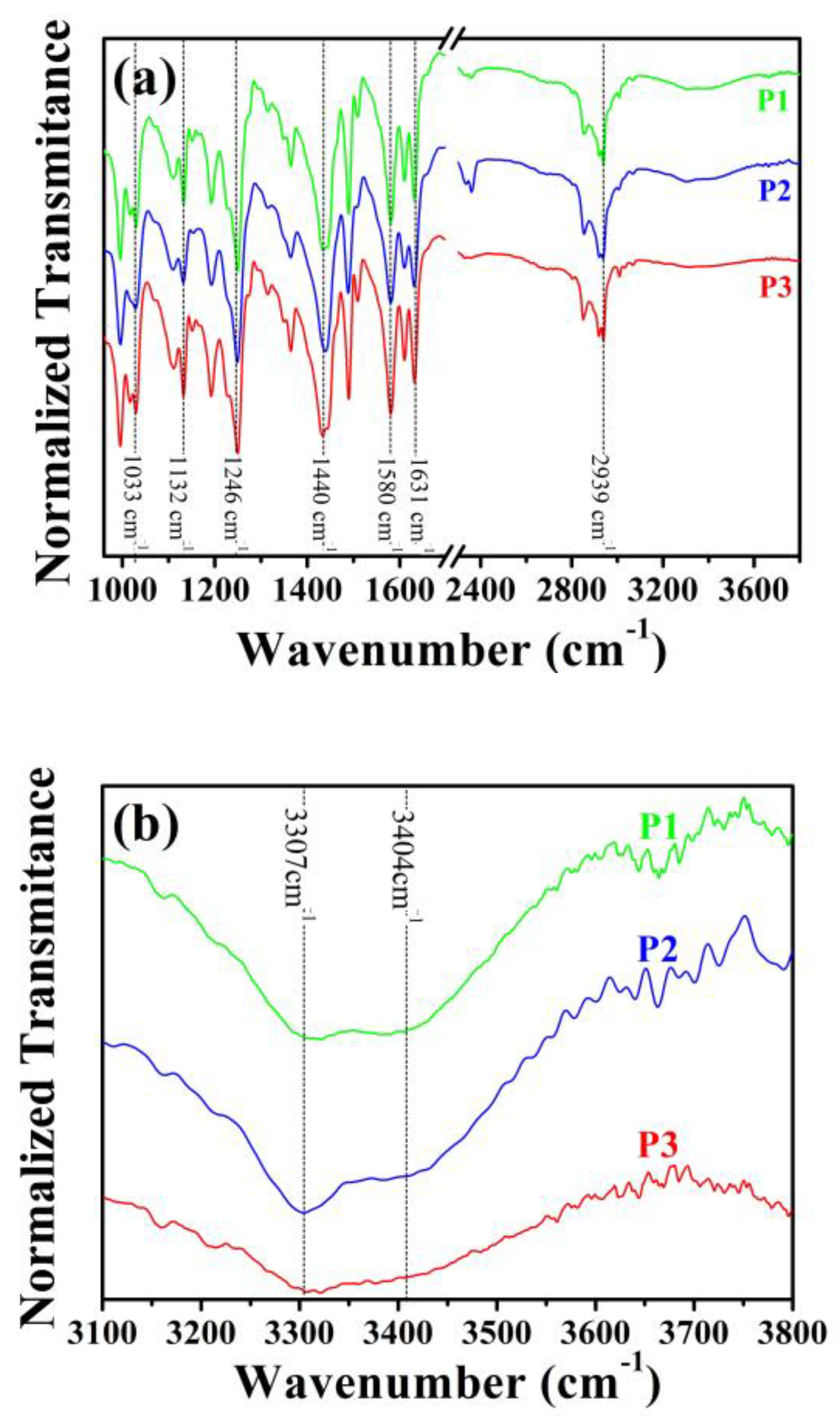
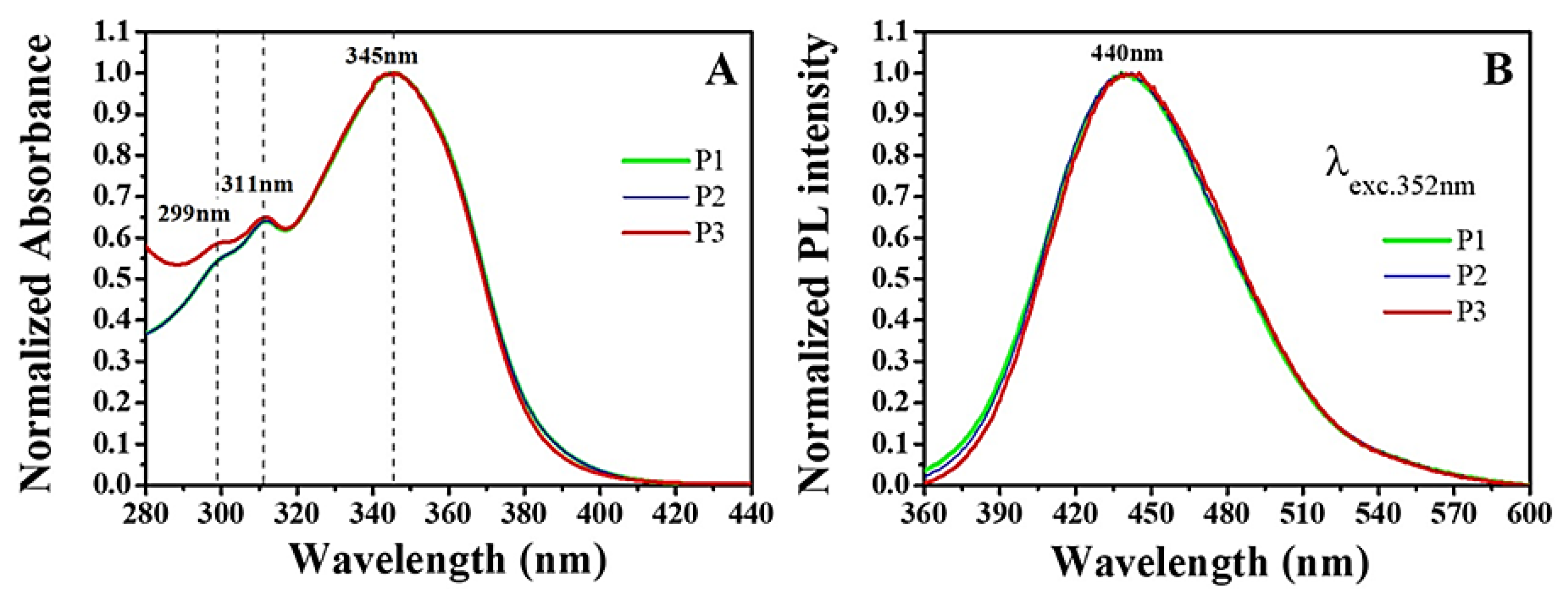
2.1.2. Spectroscopy Analysis
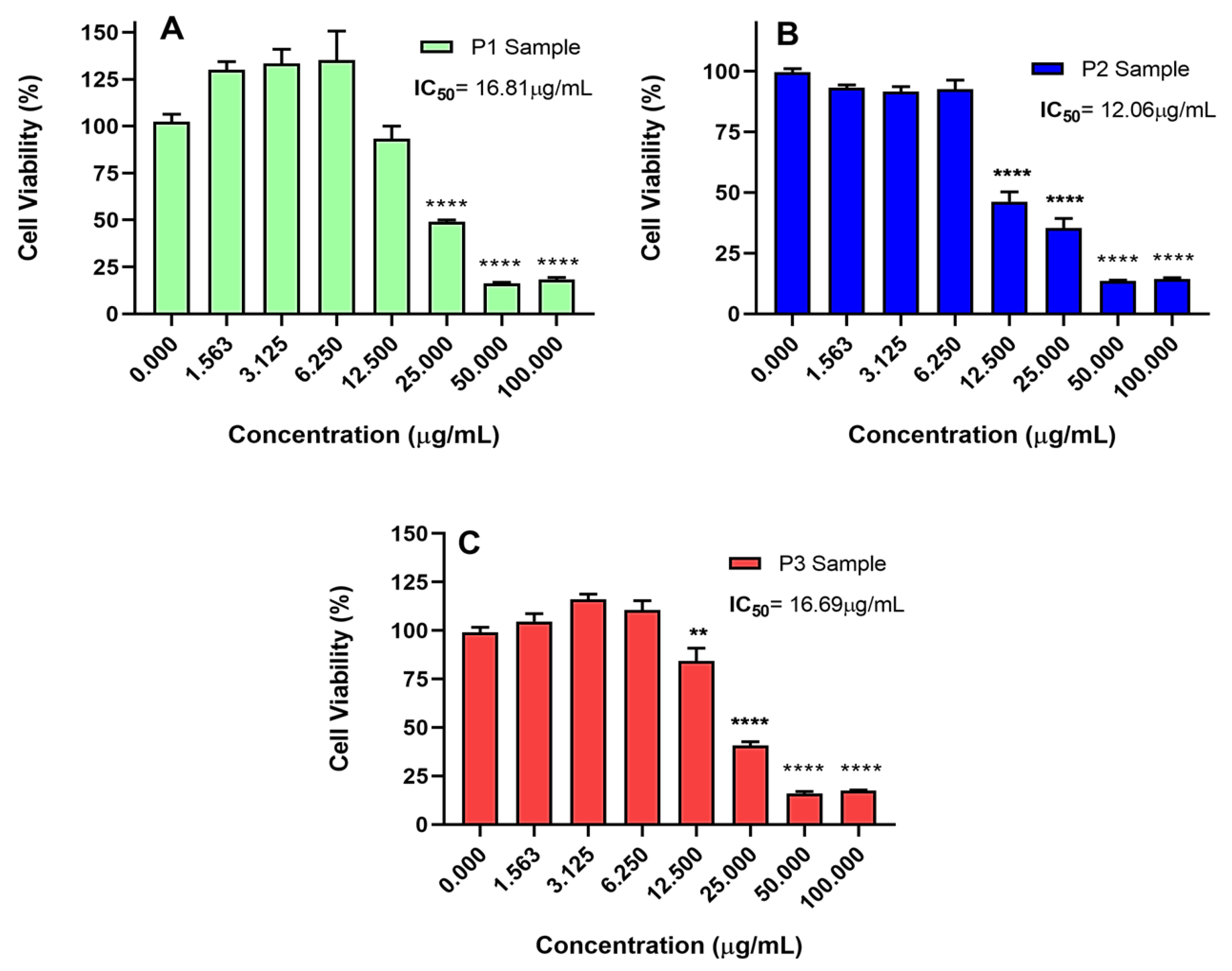
2.2. Piperine Cytotoxicity in Gastric Cancer Models
2.2.1. Isolated Piperine
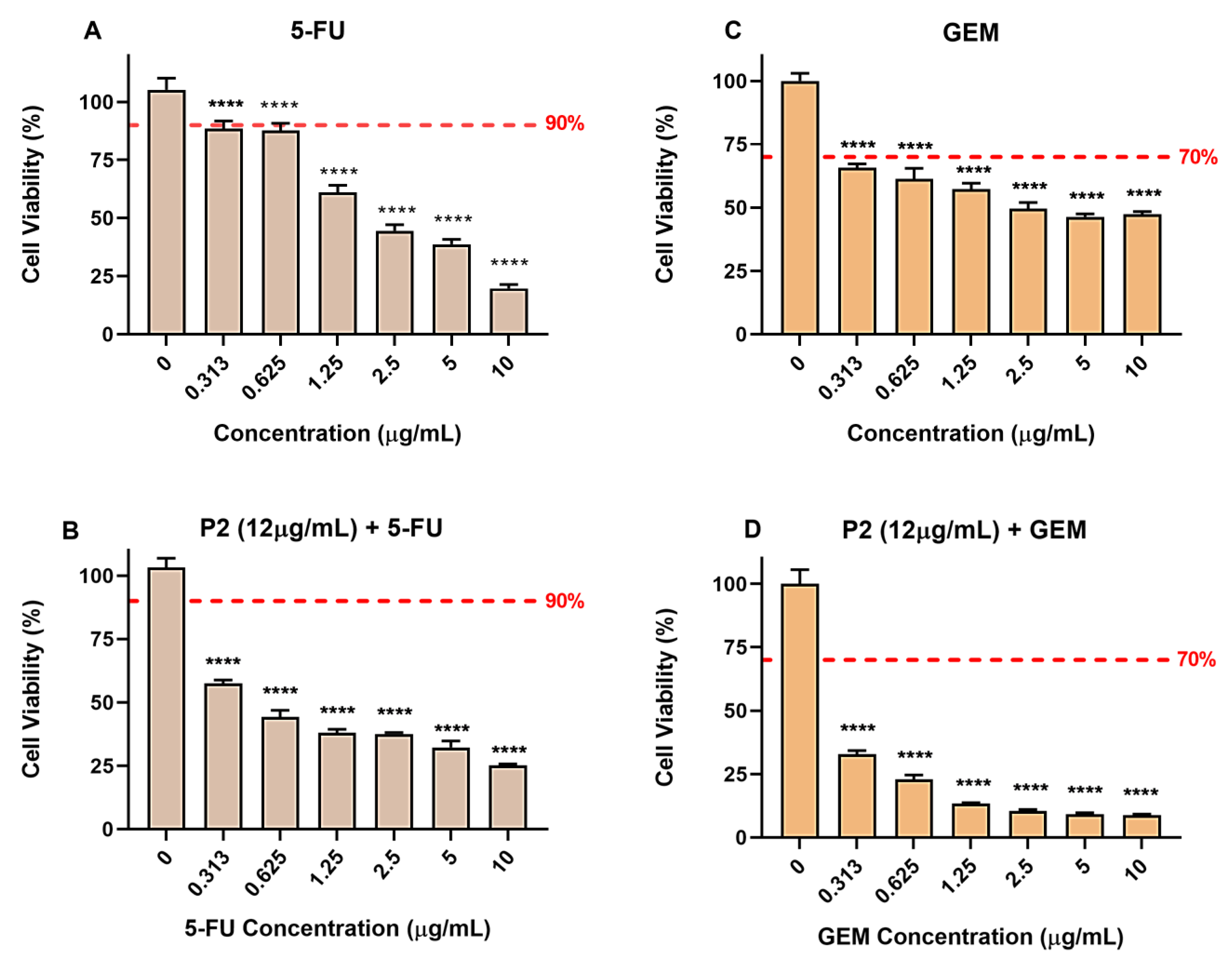
2.2.2. Piperine in Association with 5-Fluorouracil and Gemcitabine
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Plant Material and Obtention of Piperine Crystals
3.3. Characterization Methods
3.3.1. X-ray Diffraction (XRD)
3.3.2. Optical Microscopy and Scanning Electron Microscopy (SEM) Coupled with Energy-Dispersive X-ray Spectroscopy (EDS)
3.3.3. Nuclear Magnetic Resonance (NMR)
3.3.4. Infrared Spectroscopy
3.3.5. UV–Vis Absorption and Photoluminescence (PL) Spectroscopies
3.4. In Vitro Cytotoxicity Activity
3.4.1. Cell Culture
3.4.2. Cytotoxicity Assay
3.4.3. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Przegla̜d Gastroenterol. 2019, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The Current and Future Incidence and Mortality of Gastric Cancer in 185 Countries, 2020–2040: A Population-Based Modelling Study. eClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Duarte, H.O.; Gomes, J.; Machado, J.C.; Reis, C.A. Gastric Cancer: Basic Aspects. Helicobacter 2018, 23, e12523. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhu, J.; Li, Y.; Xu, Y.; Chen, K.; Lv, L.; Mao, W. Treatment Strategies for Metastatic Gastric Cancer: Chemotherapy, Palliative Surgery or Radiotherapy? Futur. Oncol. 2020, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.E.; Najeeb, M.; Hallak, A.; Diab, M.; Azmi, A.S. Gastric Cancer: A Comprehensive Review of Current and Future Treatment Strategies. Cancer Metastasis Rev. 2020, 39, 1179–1203. [Google Scholar] [CrossRef]
- Uno, Y.; Kanda, M.; Miwa, T.; Umeda, S.; Tanaka, H.; Tanaka, C.; Kobayashi, D.; Suenaga, M.; Hattori, N.; Hayashi, M.; et al. Increased Expression of DNAJC12 Is Associated with Aggressive Phenotype of Gastric Cancer. Ann. Surg. Oncol. 2019, 26, 836–844. [Google Scholar] [CrossRef]
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and Pharmacological Importance of Plant Secondary Metabolites in Modern Medicine. In Bioorganic Phase in Natural Food: An Overview; Springer: Cham, Switzerland, 2018; pp. 135–156. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Molecular Sciences Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci 2018, 19, 1578. [Google Scholar] [CrossRef]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Pejčić, M.; Dimitrijević, M.; Aleksić, A.; Anil Kumar, N.V.; Salehi, B.; Cho, W.C.; Sharifi-Rad, J. Piperine-A Major Principle of Black Pepper: A Review of Its Bioactivity and Studies. Appl. Sci. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A Review of Its Biological Effects. Phyther. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.; Subbramaniyan, P.; Noorul Ameen, J.; Raj, V. Double Bypasses Soxhlet Apparatus for Extraction of Piperine from Piper Nigrum. Arab. J. Chem. 2016, 9, S537–S540. [Google Scholar] [CrossRef]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A Comprehensive Review of Methods of Isolation, Purification, and Biological Properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Rathod, S.S.; Rathod, V.K. Extraction of Piperine from Piper Longum Using Ultrasound. Ind. Crops Prod. 2014, 58, 259–264. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential Microwave-Ultrasound-Assisted Extraction for Isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioprocess Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Olalere, O.A.; Abdurahman, N.H.; Yunus, R.b.M.; Alara, O.R.; Akbari, S. Evaluation of Optimization Parameters in Microwave Reflux Extraction of Piperine-Oleoresin from Black Pepper (Piper nigrum). Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 626–631. [Google Scholar] [CrossRef]
- Ongarora, B.C. Optimization of Piperine Extraction from Black Pepper (Piper nigrum) Using Different Solvents for Control of bedbugs. East African Agric. For. J. 2020, 84. [Google Scholar]
- Wu, Y.; Ma, H.; Han, Y. Solubility and Thermodynamic Properties of Piperine in (Acetone/Ethyl Acetate + Ethanol) at 278.15 K to 318.15 K and Its Correlation with the Jouyban-Acree and CNIBS/R-K Models. J. Chem. Thermodyn. 2021, 161, 106555. [Google Scholar] [CrossRef]
- Andrade, K.S.; Trivellin, G.; Ferreira, S.R.S. Piperine-Rich Extracts Obtained by High Pressure Methods. J. Supercrit. Fluids 2017, 128, 370–377. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Rev. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and Pharmacological Aspects of Piperine as a Potential Molecule for Disease Prevention and Management: Evidence from Clinical Trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Jaisin, Y.; Ratanachamnong, P.; Wongsawatkul, O.; Watthammawut, A.; Malaniyom, K.; Natewong, S. Antioxidant and Anti-Inflammatory Effects of Piperine on UV-B-Irradiated Human HaCaT Keratinocyte Cells. Life Sci. 2020, 263, 118607. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.S.; Cruz, J.N.; de Farias Ramos, I.N.; do Nascimento Brandão, D.L.; Queiroz, R.N.; da Silva, G.V.; da Silva, G.V.; Dolabela, M.F.; da Costa, M.L.; Khayat, A.S.; et al. Evaluation of Antimicrobial Activity and Cytotoxicity Effects of Extracts of Piper nigrum L. and Piperine. Separations 2023, 10, 21. [Google Scholar] [CrossRef]
- Corneliu Moraru, A.; Roşca, I.; Crăciun, B.; Nicolescu, A.; Chiriac, A.E.; Voicu, V. Insights of the Antimicrobial Activity of Piperine Extracted from Piper nigrum L. Farmacia 2019, 67, 6. [Google Scholar] [CrossRef]
- Imran, M.; Samal, M.; Qadir, A.; Ali, A.; Mir, S.R. A Critical Review on the Extraction and Pharmacotherapeutic Activity of Piperine. Polym. Med. 2022, 52, 31–36. [Google Scholar] [CrossRef]
- Priprem, A.; Chonpathompikunlert, P.; Sutthiparinyanont, S.; Wattanathorn, J.; Priprem, A.; Chonpathompikunlert, P.; Sutthiparinyanont, S.; Wattanathorn, J. Antidepressant and Cognitive Activities of Intranasal Piperine-Encapsulated Liposomes. Adv. Biosci. Biotechnol. 2011, 2, 108–116. [Google Scholar] [CrossRef]
- Souza, J.S.; Martins, E.P.S.; Souza, H.D.S.; De Oliveira, R.F.; Alves, F.S.; Lima, E.O.; Cordeiro, L.V.; Trindade, E.O.; Lira, B.F.; Rocha, G.B.; et al. Synthesis, Spectroscopic Characterization, DFT Calculations and Preliminary Antifungal Activity of New Piperine Derivatives. Artic. J. Braz. Chem. Soc 2021, 32, 490–502. [Google Scholar] [CrossRef]
- Moon, Y.S.; Choi, W.S.; Park, E.S.; Bae, I.K.; Choi, S.D.; Paek, O.; Kim, S.H.; Chun, H.S.; Lee, S.E. Antifungal and Antiaflatoxigenic Methylenedioxy-Containing Compounds and Piperine-Like Synthetic Compounds. Toxins 2016, 8, 240. [Google Scholar] [CrossRef]
- Zadorozhna, M.; Tataranni, T.; Mangieri, D. Piperine: Role in Prevention and Progression of Cancer. Mol. Biol. Rep. 2019, 46, 5617–5629. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Nabavi, S.M.; Setzer, W.N.; Jafari, S. Piperine as a Potential Anti-Cancer Agent: A Review on Preclinical Studies. Curr. Med. Chem. 2018, 25, 4918–4928. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.K. Piperine-Mediated Drug Interactions and Formulation Strategy for Piperine: Recent Advances and Future Perspectives. Expert Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Anand, U.; Jha, N.K.; Shekhawat, M.S.; Saha, S.C.; Nongdam, P.; Rengasamy, K.R.R.; Proćków, J.; Dey, A. Anticancer Applications and Pharmacological Properties of Piperidine and Piperine: A Comprehensive Review on Molecular Mechanisms and Therapeutic Perspectives. Front. Pharmacol. 2022, 12, 3549. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, D.B.; Sreedharan, S.; Mahadik, K.R. Role of Piperine as an Effective Bioenhancer in Drug Absorption. Pharm. Anal. Acta 2018, 9, 7. [Google Scholar] [CrossRef]
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib Synergistically Inhibit Colon Cancer Cell Proliferation via Modulating Wnt/β-Catenin Signaling Pathway. Phytomedicine 2021, 84, 153484. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Heimberg, K.; Lampen, A.; Ildico Hirsch-Ernst, K.; Lachenmeier, W. Safety Aspects of the Use of Isolated Piperine Ingested as a Bolus. Foods 2021, 10, 2121. [Google Scholar] [CrossRef]
- Bolat, Z.B.; Islek, Z.; Demir, B.N.; Yilmaz, E.N.; Sahin, F.; Ucisik, M.H. Curcumin- and Piperine-Loaded Emulsomes as Combinational Treatment Approach Enhance the Anticancer Activity of Curcumin on HCT116 Colorectal Cancer Model. Front. Bioeng. Biotechnol. 2020, 8, 50. [Google Scholar] [CrossRef]
- Fattah, A.; Morovati, A.; Niknam, Z.; Mashouri, L.; Asadi, A.; Rizi, S.T.; Abbasi, M.; Shakeri, F.; Abazari, O. The Synergistic Combination of Cisplatin and Piperine Induces Apoptosis in MCF-7 Cell Line. Iran. J. Public Health 2021, 50, 1037–1047. [Google Scholar] [CrossRef]
- Leal, M.F.; Martins do Nascimento, J.L.; da Silva, C.E.A.; Vita Lamarão, M.F.; Calcagno, D.Q.; Khayat, A.S.; Assumpção, P.P.; Cabral, I.R.; de Arruda Cardoso Smith, M.; Burbano, R.R. Establishment and Conventional Cytogenetic Characterization of Three Gastric Cancer Cell Lines. Cancer Genet. Cytogenet. 2009, 195, 85–91. [Google Scholar] [CrossRef]
- Alves, F.S.; Rodrigues Do Rego, J.d.A.; Da Costa, M.L.; Lobato Da Silva, L.F.; Da Costa, R.A.; Cruz, J.N.; Brasil, D.D.S.B. Spectroscopic Methods and in Silico Analyses Using Density Functional Theory to Characterize and Identify Piperine Alkaloid Crystals Isolated from Pepper (Piper nigrum L.). J. Biomol. Struct. Dyn. 2020, 38, 2792–2799. [Google Scholar] [CrossRef] [PubMed]
- Sulman, L. Isolation of Piperine from Black Pepper (Piper nigrum) in the Provision of Standard Compounds for Natural Chemical Practice and Research Activities. J. Pijar MIPA 2021, 16, 683–687. [Google Scholar] [CrossRef]
- Pfund, L.Y.; Chamberlin, B.L.; Matzger, A.J. The Bioenhancer Piperine Is at Least Trimorphic. Cryst. Growth Des. 2015, 15, 2047–2051. [Google Scholar] [CrossRef] [PubMed]
- Muthuraja, A.; Subramaniyan Raja, R.; Bharath, D. Growth and Characterization of Piperine (PPN) Single Crystal Grown by Slow Evaporation Solution Growth Technique. Mater. Res. Innov. 2019, 23, 228–232. [Google Scholar] [CrossRef]
- Kusumorini, N.; Nugroho, A.K.; Pramono, S.; Martien, R. Development of New Isolation and Quantification Method of Piperine from White Pepper Seeds (Piper nigrum L.) Using a Validated HPLC. Indones. J. Pharm. 2021, 32, 158–165. [Google Scholar] [CrossRef]
- Aziz, D.M.; Hama, J.R.; Alam, S.M. Synthesising a Novel Derivatives of Piperine from Black Pepper (Piper nigrum L.). J. Food Meas. Charact. 2015, 9, 324–331. [Google Scholar] [CrossRef]
- Tolkatchev, D.; Elnatan, D.; Nogara, L.; Ly, T.; Naber, N.; Haak, K.; Meech, R.; Cooke, R.; Kostyukova, A.S. Piperine, an Alkaloid Inhibiting the Super-Relaxed State of Myosin, Binds to the Myosin Regulatory Light Chain. Arch. Biochem. Biophys. 2018, 659, 75. [Google Scholar] [CrossRef]
- Reyes-Solís, L.M.; Restrepo, J.; Sánchez, R.A. Encapsulación de La Piperine Presente En La Especie Piper Tuberculatum Utilizando Vesículas Multilamelares y Determinación de Su Poder Antioxidante. Rev. Cienc. 2018, 21, 11–28. [Google Scholar] [CrossRef]
- Lim, E.T.; Lee, J.S.; Yong, S.; Wang, T.; Zhao, Y.; Setzler, B.P.; Bahri, S.; Ambarwati, Y.; Iqbal, M.; Baihaqy, A.A. Synthesis 4-Piperoilmorpholine from Piperine. J. Phys. Conf. Ser. 2019, 1338, 012010. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and Antimicrobial Activities of Various Solvent Extracts, Piperine and Piperic Acid from Piper Nigrum. LWT Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Perakis, F.; De Marco, L.; Shalit, A.; Tang, F.; Kann, Z.R.; Kühne, T.D.; Torre, R.; Bonn, M.; Nagata, Y. Vibrational Spectroscopy and Dynamics of Water. Chem. Rev. 2016, 116, 7590–7607. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.G.; Kumamoto, J.; Ibers, J.A. Vibrational Spectrum of Crystalline Potassium Hydroxide. J. Chem. Phys. 1960, 33, 1171–1177. [Google Scholar] [CrossRef]
- Zsila, F.; Hazai, E.; Sawyer, L. Binding of the Pepper Alkaloid Piperine to Bovine β-Lactoglobulin: Circular Dichroism Spectroscopy and Molecular Modeling Study. J. Agric. Food Chem. 2005, 53, 10179–10185. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, G.R.D.; Kiran, C.R.; Sundaresan, A.; Mony, R.S.; Venugopalan, V.V. Process Development Studies for Recovery of Bio Active Isolates from Spent Black Pepper Generated from Ayurvedic Industry. Ind. Crops Prod. 2015, 66, 144–149. [Google Scholar] [CrossRef]
- Murti, Y.B.; Hartini, Y.S.; Hinrichs, W.L.J.; Frijlink, H.W.; Setyaningsih, D. UV-Vis Spectroscopy to Enable Determination of the Dissolution Behavior of Solid Dispersions Containing Curcumin and Piperine. J. Young Pharm. 2018, 11, 26–30. [Google Scholar] [CrossRef]
- Debnath, S.; Mishra, J. Understanding the Intrinsic Fluorescence of Piperine in Microheterogeneous Media: Partitioning and Loading Studies. New J. Chem. 2020, 44, 8317–8324. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Chou, W.H.; Hsieh, M.C.; Chang, C.M.; Luo, W.T.; Tai, Y.T.; Chang, W.C. Genetic Alterations in Peritoneal Metastatic Tumors Predicted the Outcomes for Hyperthermic Intraperitoneal Chemotherapy. Front. Oncol. 2023, 13, 1471. [Google Scholar] [CrossRef]
- Norouzi, S.; Gorgi Valokala, M.; Mosaffa, F.; Zirak, M.R.; Zamani, P.; Behravan, J. Crosstalk in Cancer Resistance and Metastasis. Crit. Rev. Oncol. Hematol. 2018, 132, 145–153. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine Impairs Cell Cycle Progression and Causes Reactive Oxygen Species-Dependent Apoptosis in Rectal Cancer Cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef]
- Cardoso, L.P.; de Sousa, S.O.; Gusson-Zanetoni, J.P.; de Melo Moreira Silva, L.L.; Frigieri, B.M.; Henrique, T.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Piperine Reduces Neoplastic Progression in Cervical Cancer Cells by Downregulating the Cyclooxygenase 2 Pathway. Pharmaceuticals 2023, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, D.Y.; Zeng, L.H.; Pan, H.; Xu, L.H.; Wang, Y.; Liu, K.P.; He, X.H. Piperine Inhibits the Proliferation of Human Prostate Cancer Cells via Induction of Cell Cycle Arrest and Autophagy. Food Chem. Toxicol. 2013, 60, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of In-Vitro Bioassay Methods: Application in Herbal Drug Research, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 46, ISBN 9780128241271. [Google Scholar]
- Cushnie, T.P.T.; Cushnie, B.; Echeverría, J.; Fowsantear, W.; Thammawat, S.; Dodgson, J.L.A.; Law, S.; Clow, S.M. Bioprospecting for Antibacterial Drugs: A Multidisciplinary Perspective on Natural Product Source Material, Bioassay Selection and Avoidable Pitfalls. Pharm. Res. 2020, 37, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lee, D.Y.; Lim, J.H.; Oh, W.K.; Park, J.T.; Park, S.C.; Cho, K.A. Piperine: An Anticancer and Senostatic Drug. Front. Biosci. Landmark 2022, 27, 137. [Google Scholar] [CrossRef]
- Nakamura, H.; Maeda, H. Cancer Chemotherapy. In Fundamentals of Pharmaceutical Nanoscience; Springer: New York, NY, USA, 2023; pp. 401–427. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical Implications of Chemotherapeutic Agent Organ Toxicity on Perioperative Care. Biomed. Pharmacother. 2022, 146, 112503. [Google Scholar] [CrossRef] [PubMed]
- Khew, C.Y.; Harikrishna, J.A.; Wee, W.Y.; Lau, E.T.; Hwang, S.S. Transcriptional Sequencing and Gene Expression Analysis of Various Genes in Fruit Development of Three Different Black Pepper (Piper nigrum L.) Varieties. Int. J. Genom. 2020, 2020, 1540915. [Google Scholar] [CrossRef]
- Fofaria, N.M.; Kim, S.H.; Srivastava, S.K. Piperine Causes G1 Phase Cell Cycle Arrest and Apoptosis in Melanoma Cells through Checkpoint Kinase-1 Activation. PLoS ONE 2014, 9, e94298. [Google Scholar] [CrossRef]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Y.; Sheng, Y.J.; Wang, J.; Ruan, S.; Han, C. Mechanism of Piperine in Affecting Apoptosis and Proliferation of Gastric Cancer Cells via ROS-Mitochondria-Associated Signalling Pathway. J. Cell. Mol. Med. 2021, 25, 9513–9522. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahamad, M.S.; Jafri, A.; Afzal, M.; Arshad, M. Piperine Triggers Apoptosis of Human Oral Squamous Carcinoma Through Cell Cycle Arrest and Mitochondrial Oxidative Stress. Nutr. Cancer 2017, 69, 791–799. [Google Scholar] [CrossRef]
- Chen, H.; Sheng, H.; Zhao, Y.; Zhu, G. Piperine Inhibits Cell Proliferation and Induces Apoptosis of Human Gastric Cancer Cells by Downregulating Phosphatidylinositol 3-Kinase (PI3K)/Akt Pathway. Med. Sci. Monit. 2020, 26, e928403. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, Y.; Zhen, Y.; Li, D.; He, X.; Yang, H.; Zhang, H.; Liu, Q. Piperine Inhibits Colorectal Cancer Migration and Invasion by Regulating STAT3/Snail-Mediated Epithelial–Mesenchymal Transition. Biotechnol. Lett. 2020, 42, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, C.R.; Kuttan, G. Effect of Piperine on the Inhibition of Lung Metastasis Induced B16F-10 Melanoma Cells in Mice. Clin. Exp. Metastasis 2002, 19, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor Efficacy of Piperine in the Treatment of Human HER2-Overexpressing Breast Cancer Cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef] [PubMed]
- Quijia, C.R.; Chorilli, M. Piperine for Treating Breast Cancer: A Review of Molecular Mechanisms, Combination with Anticancer Drugs, and Nanosystems. Phytother. Res. 2022, 36, 147–163. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.; Cheng, J.; Zhang, Y.; Zhang, B. Study on the Effect and Mechanisms of Piperine against Cervical Cancer Based on Network Pharmacology and Experimental Validation. Biotechnol. Genet. Eng. Rev. 2023, 1–24. [Google Scholar] [CrossRef]
- Li, S.; Nguyen, T.T.; Ung, T.T.; Sah, D.K.; Park, S.Y.; Lakshmanan, V.K.; Do Jung, Y. Piperine Attenuates Lithocholic Acid-Stimulated Interleukin-8 by Suppressing Src/EGFR and Reactive Oxygen Species in Human Colorectal Cancer Cells. Antioxidants 2022, 11, 530. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rashid, S.; Arafah, A.; Qamar, W.; Alsaffar, R.M.; Ahmad, A.; Almatroudi, N.M.; Alqahtani, S.M.A.; Rashid, S.M.; Ahmad, S.B. Piperine Regulates Nrf-2/Keap-1 Signalling and Exhibits Anticancer Effect in Experimental Colon Carcinogenesis in Wistar Rats. Biology 2020, 9, 302. [Google Scholar] [CrossRef]
- Araújo, T.; Khayat, A.; Quintana, L.; Calcagno, D.; Mourão, R.; Modesto, A.; Paiva, J.; Lima, A.; Moreira, F.; Oliveira, E.; et al. Piwi like RNA-Mediated Gene Silencing 1 Gene as a Possible Major Player in Gastric Cancer. World J. Gastroenterol. 2018, 24, 5338–5350. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Konno, Y.; Ihira, K.; Xu, D.; Kobayashi, N.; Yue, J.; Watari, H. Critical Roles of PIWIL1 in Human Tumors: Expression, Functions, Mechanisms, and Potential Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 656993. [Google Scholar] [CrossRef]
- Buranrat, B.; Junking, M. Piperine Suppresses Growth and Migration of Human Breast Cancer Cells through Attenuation of Rac1 Expression. Asian Pac. J. Trop. Biomed. 2022, 12, 39. [Google Scholar] [CrossRef]
- Jeong, J.H.; Ryu, J.H.; Lee, H.J. In Vitro Inhibition of Piper Nigrum and Piperine on Growth, Migration, and Invasion of PANC-1 Human Pancreatic Cancer Cells. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Ge, S.; Xia, X.; Ding, C.; Zhen, B.; Zhou, Q.; Feng, J.; Yuan, J.; Chen, R.; Li, Y.; Ge, Z.; et al. A Proteomic Landscape of Diffuse-Type Gastric Cancer. Nat. Commun. 2018, 9, 1012. [Google Scholar] [CrossRef] [PubMed]
- Monster, J.L.; Kemp, L.J.S.; Gloerich, M.; van der Post, R.S. Diffuse Gastric Cancer: Emerging Mechanisms of Tumor Initiation and Progression. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188719. [Google Scholar] [CrossRef]
- Tanabe, S.; Quader, S.; Ono, R.; Cabral, H.; Aoyagi, K.; Hirose, A.; Yokozaki, H.; Sasaki, H. Molecular Network Profiling in Intestinal- and Diffuse-Type Gastric Cancer. Cancers 2020, 12, 3833. [Google Scholar] [CrossRef]
- Gunasekaran, V.; Elangovan, K.; Niranjali Devaraj, S. Targeting Hepatocellular Carcinoma with Piperine by Radical-Mediated Mitochondrial Pathway of Apoptosis: An in Vitro and in Vivo Study. Food Chem. Toxicol. 2017, 105, 106–118. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine Inhibits the Growth and Motility of Triple-Negative Breast Cancer Cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Afifi, N.; Barrero, C.A. Understanding Breast Cancer Aggressiveness and Its Implications in Diagnosis and Treatment. J. Clin. Med. 2023, 12, 1375. [Google Scholar] [CrossRef]
- Smith, B.R.; Stabile, B.E. Extreme Aggressiveness and Lethality of Gastric Adenocarcinoma in the Very Young. Arch. Surg. 2009, 144, 506–510. [Google Scholar] [CrossRef]
- Chen, D.; Xu, L.; Li, X.; Chu, Y.; Jiang, M.; Xu, B.; Zhao, M.; Wang, W.; Wang, H.; Kang, H.; et al. Enah Overexpression Is Correlated with Poor Survival and Aggressive Phenotype in Gastric Cancer. Cell Death Dis. 2018, 9, 998. [Google Scholar] [CrossRef]
- Takahashi, H.; Asaoka, M.; Yan, L.; Rashid, O.M.; Oshi, M.; Ishikawa, T.; Nagahashi, M.; Takabe, K. Biologically Aggressive Phenotype and Anti-Cancer Immunity Counterbalance in Breast Cancer with High Mutation Rate. Sci. Rep. 2020, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, A.; Nagel, A.; Szade, J.; Majewska, H.; Skokowski, J.; Seroczynska, B.; Stokowy, T.; Welnicka-Jaskiewicz, M.; Zaczek, A.J. Aggressive Phenotype of Cells Disseminated via Hematogenous and Lymphatic Route in Breast Cancer Patients. Transl. Oncol. 2018, 11, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Badgwell, B.D. Current Treatment and Recent Progress in Gastric Cancer. CA Cancer J. Clin. 2021, 71, 264–279. [Google Scholar] [CrossRef]
- Cainap, C.; Vlad, C.; Seicean, A.; Balacescu, O.; Seicean, R.; Constantin, M.; Balacescu, L.; Crisan, O.; Marta, M.M. Gastric Cancer: Adjuvant Chemotherapy versus Chemoradiation. A Clinical Point of View. J. BUON 2019, 24, 2209–2219. [Google Scholar] [PubMed]
- De Oliveira, I.; Maia, A.; Fernando, J. Factors Associated with Chemotherapy Toxicity in Outpatients: A Cohort Study. Braz. J. Oncol. 2020, 16, e-20200003. [Google Scholar]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, Q.; Lu, M.; Lo, Y.C.; Hsu, Y.H.; Wei, M.C.; Chen, Y.H.; Lo, S.C.; Wang, S.J.; Bain, D.J.; et al. A New Approach to Reduce Toxicities and to Improve Bioavailabilities of Platinum-Containing Anti-Cancer Nanodrugs. Sci. Rep. 2015, 5, 10881. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef]
- Hegeto, L.A.; Caleffi-Ferracioli, K.R.; Nakamura-Vasconcelos, S.S.; de Almeida, A.L.; Baldin, V.P.; Nakamura, C.V.; Siqueira, V.L.D.; Scodro, R.B.L.; Cardoso, R.F. In Vitro Combinatory Activity of Piperine and Anti-Tuberculosis Drugs in Mycobacterium Tuberculosis. Tuberculosis 2018, 111, 35–40. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bansal, P.; Bhardwaj, R.K.; Velpandian, T. Comparative Anti-Nociceptive, Anti-Inflammatory and Toxicity Profile of Nimesulide vs. Nimesulide and Piperine Combination. Pharmacol. Res. 2000, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, X.; Liu, Y. Effect of Piperine on the Bioavailability and Pharmacokinetics of Emodin in Rats. J. Pharm. Biomed. Anal. 2015, 115, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Singh, M.K.; Hussain, S.; Dwivedi, P.; Khattri, S.; Singh, K. Therapeutic Spectrum of Piperine for Clinical Practice: A Scoping Review. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, K.; Gupta, R. Bioavailability Enhancers of Herbal Origin: An Overview. Asian Pac. J. Trop. Biomed. 2013, 3, 253. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Wang, Q.; Ho, R.L.K.Y.; Huang, Y.; Chow, M.S.S.; Lam, C.W.K.; Zuo, Z. Enhanced Anti-Tumor Efficacy and Mechanisms Associated with Docetaxel-Piperine Combination- in Vitro and in Vivo Investigation Using a Taxane-Resistant Prostate Cancer Model. Oncotarget 2018, 9, 3338–3352. [Google Scholar] [CrossRef]
- Xie, Z.; Wei, Y.; Xu, J.; Lei, J.; Yu, J. Alkaloids from Piper nigrum Synergistically Enhanced the Effect of Paclitaxel against Paclitaxel-Resistant Cervical Cancer Cells through the Downregulation of Mcl-1. J. Agric. Food Chem. 2019, 67, 5159–5168. [Google Scholar] [CrossRef]
- Burande, A.S.; Viswanadh, M.K.; Jha, A.; Mehata, A.K.; Shaik, A.; Agrawal, N.; Poddar, S.; Mahto, S.K.; Muthu, M.S. EGFR Targeted Paclitaxel and Piperine Co-Loaded Liposomes for the Treatment of Triple Negative Breast Cancer. AAPS PharmSciTech 2020, 21, 151. [Google Scholar] [CrossRef]
- El-Shehawy, A.A.; Elmetwalli, A.; El-Far, A.H.; Mosallam, S.A.E.R.; Salama, A.F.; Babalghith, A.O.; Mahmoud, M.A.; Mohany, H.; Gaber, M.; El-Sewedy, T. Thymoquinone, Piperine, and Sorafenib Combinations Attenuate Liver and Breast Cancers Progression: Epigenetic and Molecular Docking Approaches. BMC Complement. Med. Ther. 2023, 23, 69. [Google Scholar] [CrossRef]
- Cisło, M.; Filip, A.A.; Offerhaus, G.J.A.; Ciseł, B.; Rawicz-Pruszyński, K.; Skierucha, M.; Polkowski, W.P.; Cisło, M.; Filip, A.A.; Arnold Offerhaus, G.J.; et al. Distinct Molecular Subtypes of Gastric Cancer: From Laurén to Molecular Pathology. Oncotarget 2018, 9, 19427–19442. [Google Scholar] [CrossRef]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General Cytotoxicity Assessment by Means of the MTT Assay. Methods Mol. Biol. 2015, 1250, 333–348. [Google Scholar] [PubMed]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell Proliferation and Cytotoxicity Assays. Curr. Pharm. Biotechnol. 2016, 17, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, 469–471. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, I.N.d.F.; da Silva, M.F.; Lopes, J.M.S.; Cruz, J.N.; Alves, F.S.; do Rego, J.d.A.R.; Costa, M.L.d.; Assumpção, P.P.d.; Barros Brasil, D.d.S.; Khayat, A.S. Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer. Molecules 2023, 28, 5587. https://doi.org/10.3390/molecules28145587
Ramos INdF, da Silva MF, Lopes JMS, Cruz JN, Alves FS, do Rego JdAR, Costa MLd, Assumpção PPd, Barros Brasil DdS, Khayat AS. Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer. Molecules. 2023; 28(14):5587. https://doi.org/10.3390/molecules28145587
Chicago/Turabian StyleRamos, Ingryd Nayara de Farias, Monique Feitosa da Silva, Jefferson Marcio Sanches Lopes, Jordy Neves Cruz, Fabrine Silva Alves, José de Arimatéia Rodrigues do Rego, Marcondes Lima da Costa, Paulo Pimentel de Assumpção, Davi do Socorro Barros Brasil, and André Salim Khayat. 2023. "Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer" Molecules 28, no. 14: 5587. https://doi.org/10.3390/molecules28145587
APA StyleRamos, I. N. d. F., da Silva, M. F., Lopes, J. M. S., Cruz, J. N., Alves, F. S., do Rego, J. d. A. R., Costa, M. L. d., Assumpção, P. P. d., Barros Brasil, D. d. S., & Khayat, A. S. (2023). Extraction, Characterization, and Evaluation of the Cytotoxic Activity of Piperine in Its Isolated form and in Combination with Chemotherapeutics against Gastric Cancer. Molecules, 28(14), 5587. https://doi.org/10.3390/molecules28145587








