Four Meroterpenoids with Novel Aminoglycoside Moiety from the Basidiomycete Clitocybe clavipes with Cytotoxic Activity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. Extraction and Isolation
4.4. Acid Hydrolysis and Monosaccharide Identification
4.5. Cytotoxicity Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESI-MS | High resolution electrospray ionization mass spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift Correlation Spectroscopy |
| NOESY | Nuclear Overhauser effect spectroscopy |
| TMS | Tetramethylsilane |
| ODS | Octadecyl silane |
| HPLC | High performance liquid chromatography |
| CH2Cl2 | Dichloromethane |
| EtOAc | Ethyl acetate |
| MeOH | Methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
References
- Matsuda, Y.; Abe, I. Biosynthesis of fungal meroterpenoids. Nat. Prod. Rep. 2016, 33, 26–53. [Google Scholar] [CrossRef]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063–1094. [Google Scholar] [CrossRef]
- Elissawy, A.M.; Ebada, S.S.; Ashour, M.L.; Ozkaya, F.C.; Ebrahim, W.; Singab, A.B.; Proksch, P. Spiroarthrinols A and B, two novel meroterpenoids isolated from the sponge-derived fungus Arthrinium sp. Phytochem. Lett. 2017, 20, 246–251. [Google Scholar] [CrossRef]
- Matsuda, Y.; Awakawa, T.; Mori, T.; Abe, I. Unusual chemistries in fungal meroterpenoid biosynthesis. Curr. Opin. Chem. Biol. 2016, 31, 1–7. [Google Scholar] [CrossRef]
- Arnone, A.; Cardillo, R.; Meille, S.V.; Nasini, G.; Tolazzi, M. Secondary mould metabolites. Part 47. Isolation and structure elucidation of kavalactones A-C, new metabolites from the fungus Clitocybe clavipes. J. Chem. Soc. Perkin Trans. 1 1994, 15, 2165–2168. [Google Scholar] [CrossRef]
- Dettrakul, S.; Surerum, S.; Rajviroongit, S.; Kittakoop, P. Biomimetic Transformation and Biological Activities of Globiferin, a Terpenoid Benzoquinone from Cordia globifera. J. Nat. Prod. 2009, 72, 861–865. [Google Scholar] [CrossRef]
- Arnone, A.; Cardillo, R.; Meille, S.V.; Nasini, G.; Tolazzi, M. Recoverable chiral sulfoxide: Remote asymmetric induction in Lewis acid-promoted Diels–Alder reaction of chiral sulfinyl-substituted pyrrolyl α, β-unsaturated enones. J. Chem. Soc. Perkin Trans. 1 1999, 15, 2165–2170. [Google Scholar]
- Merlini, L.; Nasini, G.; Scaglioni, L.; Cassinelli, G.; Lanzi, C. Structure elucidation of clavilactone D: An inhibitor of protein tyrosine kinases. Phytochemistry 2000, 53, 1039–1041. [Google Scholar] [CrossRef]
- Yin, X.; Feng, T.; Li, Z.H.; Dong, Z.J.; Li, Y.; Liu, J.K. Highly Oxygenated Meroterpenoids from Fruiting Bodies of the Mushroom Tricholoma terreum. J. Nat. Prod. 2013, 76, 1365–1368. [Google Scholar] [CrossRef]
- He, Y.; Zheng, M.; Li, Q.; Hu, Z.; Zhu, H.; Liu, J.; Wang, J.; Xue, Y.; Li, H.; Zhang, Y. Asperspiropene A, a novel fungal metabolite as an inhibitor of cancer-associated mutant isocitrate dehydrogenase 1. Org. Chem. Front. 2017, 4, 1137–1144. [Google Scholar] [CrossRef]
- Li, C.W. The Preparation Method and Medical Application of a New Meroterpenoid Compound. CN Patent 105152895A, 16 December 2015. [Google Scholar]
- Liu, Z.; Liu, H.; Chen, Y.; She, Z. A new anti-inflammatory meroterpenoid from the fungus Aspergillus terreus H010. Nat. Prod. Res. 2017, 32, 2652–2656. [Google Scholar] [CrossRef]
- Chan, S.T.; Pearce, A.N.; Januario, A.H.; Page, M.J.; Kaiser, M.; McLaughlin, R.J.; Harper, J.L.; Webb, V.L.; Barker, D.; Copp, B.R. Anti-inflammatory and antimalarial meroterpenoids from the New Zealand ascidian Aplidium scabellum. J. Org. Chem. 2011, 76, 9151–9156. [Google Scholar] [CrossRef]
- Sabry, O.M.; Andrews, S.; Mcphail, K.L.; Goeger, D.E.; Yokochi, A.; LePage, K.T.; Murray, T.F.; Gerwick, W.H. Neurotoxic meroditerpenoids from the tropical marine brown alga Stypopodium flabelliforme. J. Nat. Prod. 2005, 68, 1022–1030. [Google Scholar] [CrossRef]
- Cassinelli, G.; Lanzi, C.; Pensa, T.; Gambetta, R.A.; Nasini, G.; Cuccuru, G.; Cassinis, M.; Pratesi, G.; Polizzi, D.; Tortoreto, M.; et al. Clavilactones, a novel class of tyrosine kinase inhibitors of fungal origin. Biochem. Pharmacol. 2000, 59, 1539–1547. [Google Scholar] [CrossRef]
- Kawagishi, H.; Miyazawa, T.; Kume, H.; Arimoto, Y.; Inakuma, T. Aldehyde dehydrogenase inhibitors from the mushroom Clitocybe clavipes. J. Nat. Prod. 2002, 65, 1712–1714. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, X.; Liang, H.; Xia, X.; Ma, G.; Shi, L. Five New Meroterpenoids from the Fruiting Bodies of the Basidiomycete Clitocybe clavipes with Cytotoxic Activity. Molecules 2019, 24, 4015. [Google Scholar]
- Hou, Y.; Li, Q.; Chen, M.; Wu, H.; Yang, J.; Sun, Z.; Xu, X.; Ma, G. Novel geranylhydroquinone derived meroterpenoids from the fungus Clitocybe clavipes and their cytotoxic activity. Fitoterapia 2022, 161, 105251. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, D.; Li, L.; Hou, Y.; Chen, M.; Huang, G.; Ma, G.; Li, Z. Clavipyrrine A, a unique polycyclic nitrogenous meroterpenoid with promising anti-glioma effects isolated from the fungus Clitocybe clavipes. Bioorg. Chem. 2021, 117, 105468. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, N.L.; Zhou, M.; Huo, X.; Wu, H.; Tian, Y.; Yang, J.; Ma, G.; Yang, Y.-L.; Xu, X. Clavipines A-C, antiproliferative meroterpenoids with a fused azepine skeleton from the basidiomycete Clitocybe clavipes. Org. Chem. Front. 2019, 6, 3759–3765. [Google Scholar] [CrossRef]
- Takao, K.I.; Nemoto, R.; Mori, K.; Namba, A.; Yoshida, K.; Ogura, A. Total Synthesis and Structural Revision of Clavilactone D. Chemistry 2017, 23, 3828–3831. [Google Scholar] [CrossRef]
- Chen, J.J.; Hung, H.C.; Sung, P.J.; Chen, I.S.; Kuo, W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry 2011, 72, 523–532. [Google Scholar] [CrossRef]
- Zhang, J.; Larissa, V.; Ponomareva, N.; Nandurkar, Y.; Lei, F.; Zhan, C.G.; Thorson, J. Influence of Sugar Amine Regiochemistry on Digitoxigenin Neoglycoside Anticancer Activity. ACS Med. Chem. Lett. 2015, 6, 1053–1058. [Google Scholar] [CrossRef]
- Hu, M.G.; Li, Y.; Sun, Z.; Huo, X.; Zhu, N.; Sun, Z.; Liu, Y.; Wu, H.; Xu, X.; Ma, G.X.; et al. New polyoxypregnane glycosides from Aspidopterys obcordata vines with antitumor activity. Fitoterapia 2018, 129, 203–209. [Google Scholar] [CrossRef]
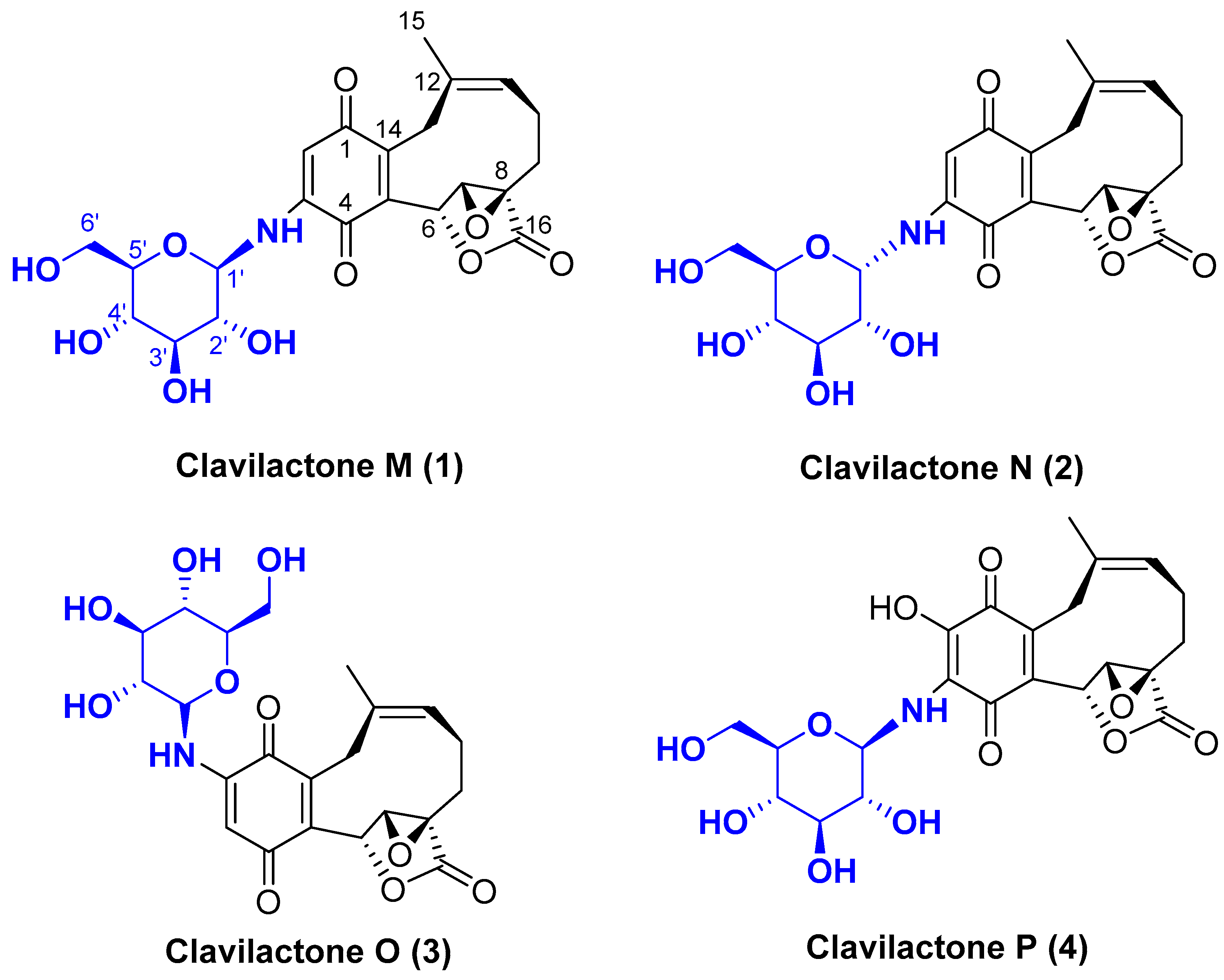
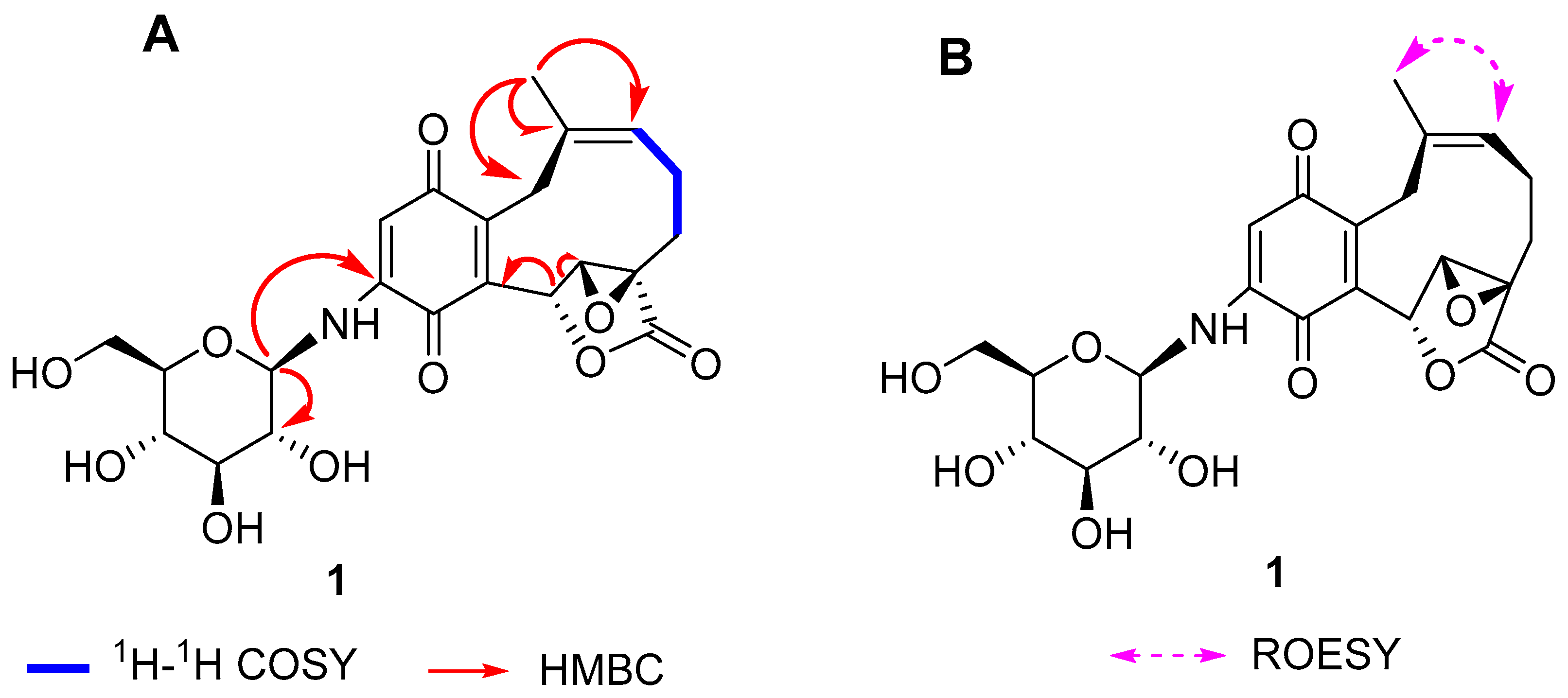
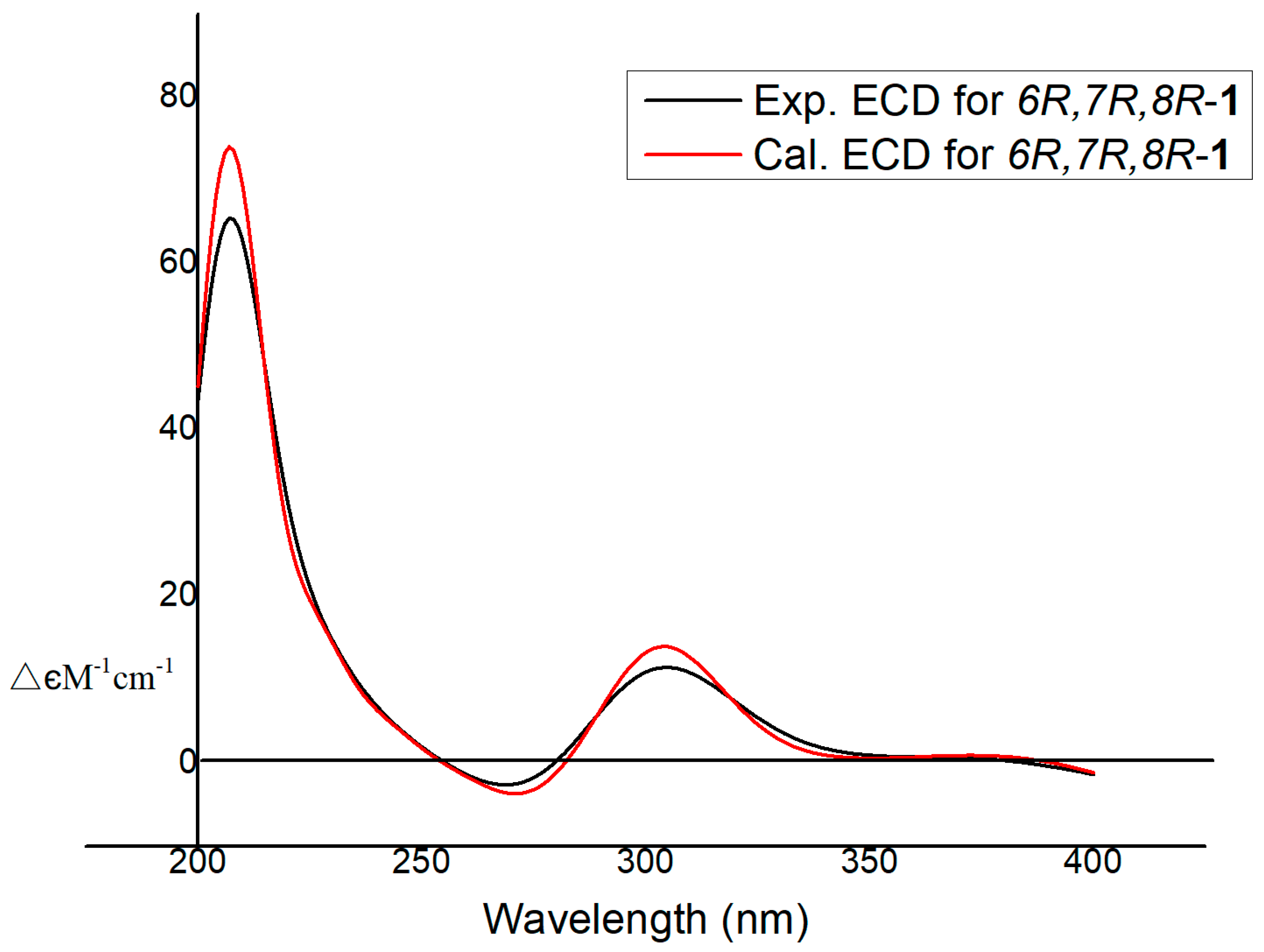
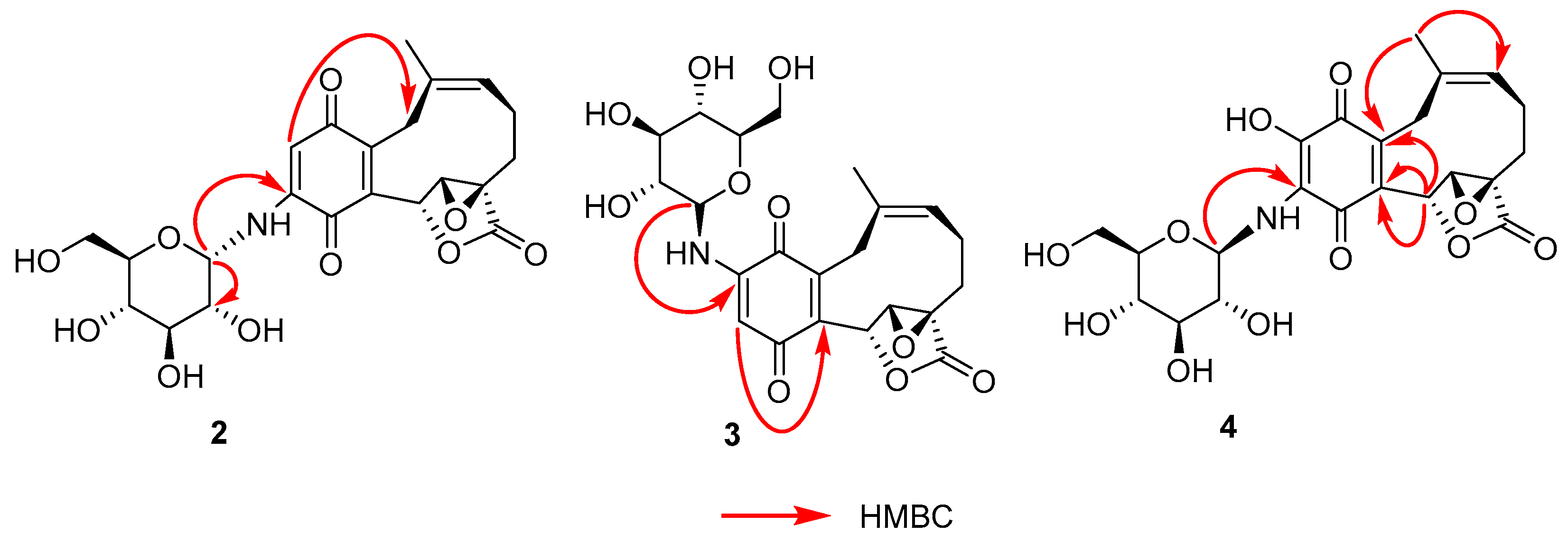
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δH(J in Hz) | δc | δH(J in Hz) | δc | δH(J in Hz) | δc | |
| 1 | - | 186.1 | - | 185.8 | - | 186.1 |
| 2 | 5.94(s) | 103.0 | 6.27(s) | 103.8 | - | 148.1 |
| 3 | - | 148.1 | - | 147.5 | 5.92(s) | 102.7 |
| 4 | - | 183.0 | - | 182.8 | - | 183.0 |
| 5 | - | 151.9 | - | 151.8 | - | 146.1 |
| 6 | 5.98(s) | 74.1 | 5.97(s) | 73.5 | 5.94(s) | 74.0 |
| 7 | 4.24(s) | 64.2 | 4.23(s) | 64.1 | 4.24(s) | 64.3 |
| 8 | - | 61.8 | - | 61.6 | - | 61.9 |
| 9 | 1.30(m), 2.60(d,13.2), | 25.7 | 1.29(m), 2.58(m), | 25.5 | 1.30(m), 2.60(d,12.0), | 25.7 |
| 10 | 2.18(m), 2.35(m), | 23.8 | 2.21(m), 2.35(m), | 23.6 | 2.22(m), 2.35(m), | 23.8 |
| 11 | 5.34(br, s) | 124.9 | 5.33(br, s) | 124.7 | 5.34(br, s) | 125.2 |
| 12 | - | 136.9 | - | 136.7 | - | 139.0 |
| 13 | 2.84(d,12.0), 3.67(d,12.0), | 27.8 | 2.84(d,14.4), 3.69(d,14.4), | 27.6 | 2.84(d,12.0), 3.67(d,12.0), | 27.5 |
| 14 | - | 135.6 | - | 135.5 | - | 136.4 |
| 15 | 1.53(3H, s) | 23.4 | 1.41(3H, s) | 23.1 | 1.53(3H, s) | 23.2 |
| 16 | - | 173.8 | - | 173.6 | - | 173.8 |
| 1′ | 4.52(d,8.4) | 84.4 | 5.11(d,4.8) | 82.3 | 4.53(d,8.4) | 84.3 |
| 2′ | 3.35~3.70 (4H, m) | 74.1 | 3.35–3.76 (4H, m) | 74.0 | 3.4~3.70 (4H, m) | 74.1 |
| 3′ | 78.8 | 78.6 | 78.8 | |||
| 4′ | 71.5 | 71.6 | 71.5 | |||
| 5′ | 79.6 | 79.4 | 79.6 | |||
| 6′ | 3.68(dd, 2.4, 12.0) 3.86(dd, 5.4, 12.0) | 62.7 | 3.68(m) 3.73(m) | 62.3 | 3.68(dd, 5.4, 12.0) 3.86(dd, 2.4, 12.0) | 62.7 |
| No. | δH(J in Hz) | δc | No. | δH(J in Hz) | δc |
|---|---|---|---|---|---|
| 1 | - | 183.9 | 12 | - | 134.1 |
| 2 | - | 149.2 | 13 | 2.66(d,12.0) 3.57(d,12.0) | 26.6 |
| 3 | - | 146.9 | |||
| 4 | - | 181.9 | 14 | - | 135.1 |
| 5 | - | 133.3 | 15 | 1.47(3H, s) | 21.8 |
| 6 | 5.87(s) | 72.7 | 16 | - | 172.1 |
| 7 | 4.5(s) | 62.9 | 1′ | 4.39(t, 7.2) | 83.1 |
| 8 | - | 60.6 | 2′ | 3.10–3.41 (4H, m) | 78.6 |
| 9 | 2.54(br, s) 1.30(m) | 24.3 | 3′ | 77.2 | |
| 4′ | 72.1 | ||||
| 10 | 2.19(br, s) 2.08(m) | 22.5 | 5′ | 70.2 | |
| 6′ | 3.66(dd, 4.2, 12.0) 3.42(m) | 61.2 | |||
| 11 | 5.29(br, s) | 123.4 |
| Compounds | IC50 (µM) | ||
|---|---|---|---|
| Hela | SGC-7901 | SHG-44 | |
| 1 | 22.8 ± 0.9 | 53.5 ± 1.2 | >100 |
| 2 | 19.7 ± 1.1 | 38.4 ± 0.8 | 29.5 ± 0.8 |
| 3 | 55.2 ± 0.9 | 34.8 ± 1.3 | 35.8 ± 1.0 |
| 4 | 44.5 ± 0.7 | 47.9 ± 1.6 | >100 |
| Cisplatin | 2.4 ± 0.02 | 2.0 ± 0.04 | 1.5 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Ma, Y.; Zhang, J.; Ma, G.; Wu, H.; Shi, L.; Sun, Z.; Xu, X. Four Meroterpenoids with Novel Aminoglycoside Moiety from the Basidiomycete Clitocybe clavipes with Cytotoxic Activity. Molecules 2023, 28, 5456. https://doi.org/10.3390/molecules28145456
Sun Z, Ma Y, Zhang J, Ma G, Wu H, Shi L, Sun Z, Xu X. Four Meroterpenoids with Novel Aminoglycoside Moiety from the Basidiomycete Clitocybe clavipes with Cytotoxic Activity. Molecules. 2023; 28(14):5456. https://doi.org/10.3390/molecules28145456
Chicago/Turabian StyleSun, Zhonghao, Yongben Ma, Jiawen Zhang, Guoxu Ma, Haifeng Wu, Leiling Shi, Zhaocui Sun, and Xudong Xu. 2023. "Four Meroterpenoids with Novel Aminoglycoside Moiety from the Basidiomycete Clitocybe clavipes with Cytotoxic Activity" Molecules 28, no. 14: 5456. https://doi.org/10.3390/molecules28145456
APA StyleSun, Z., Ma, Y., Zhang, J., Ma, G., Wu, H., Shi, L., Sun, Z., & Xu, X. (2023). Four Meroterpenoids with Novel Aminoglycoside Moiety from the Basidiomycete Clitocybe clavipes with Cytotoxic Activity. Molecules, 28(14), 5456. https://doi.org/10.3390/molecules28145456






