Golden Buckwheat Extract–Loaded Injectable Hydrogel for Efficient Postsurgical Prevention of Local Tumor Recurrence Caused by Residual Tumor Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. GBE Content Analysis
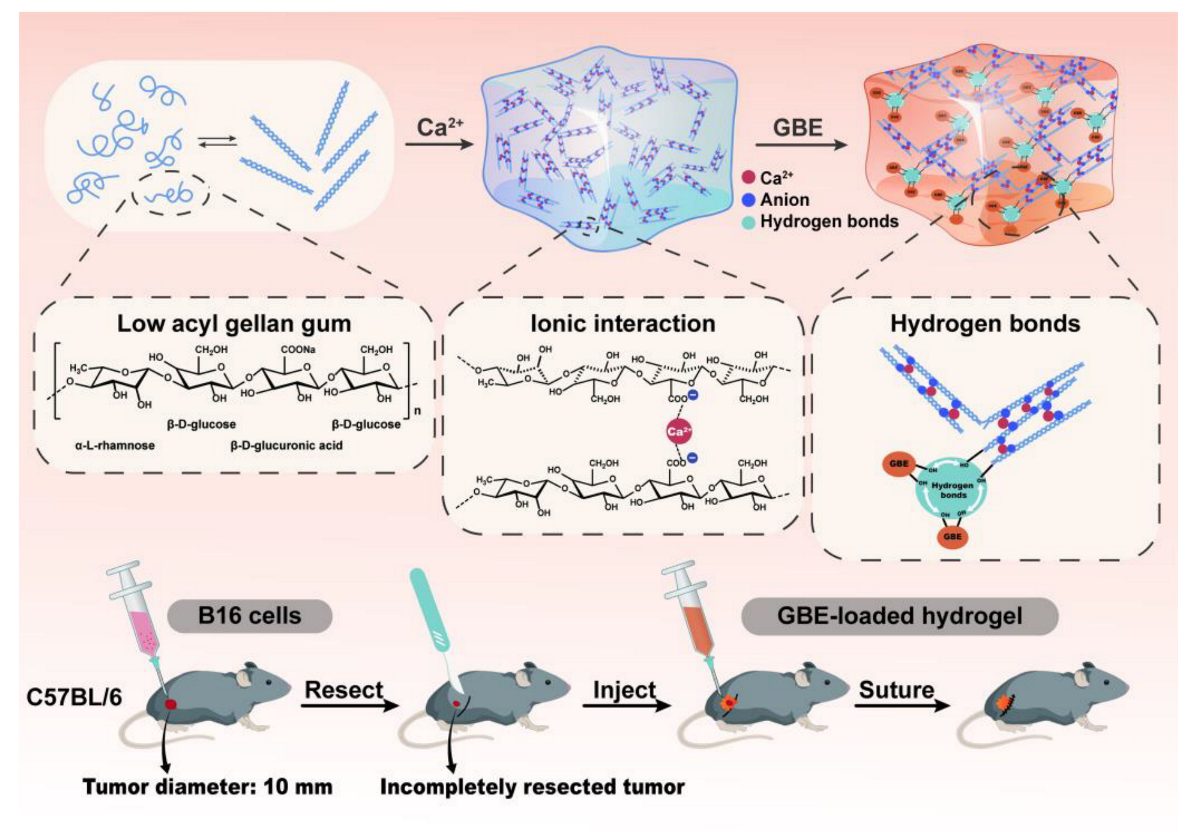
2.2. FT–IR Study of Blank Hydrogels
2.3. TGA Study of Hydrogels
2.4. Morphology Study of Hydrogels
2.5. Drug Release Studies of Hydrogels
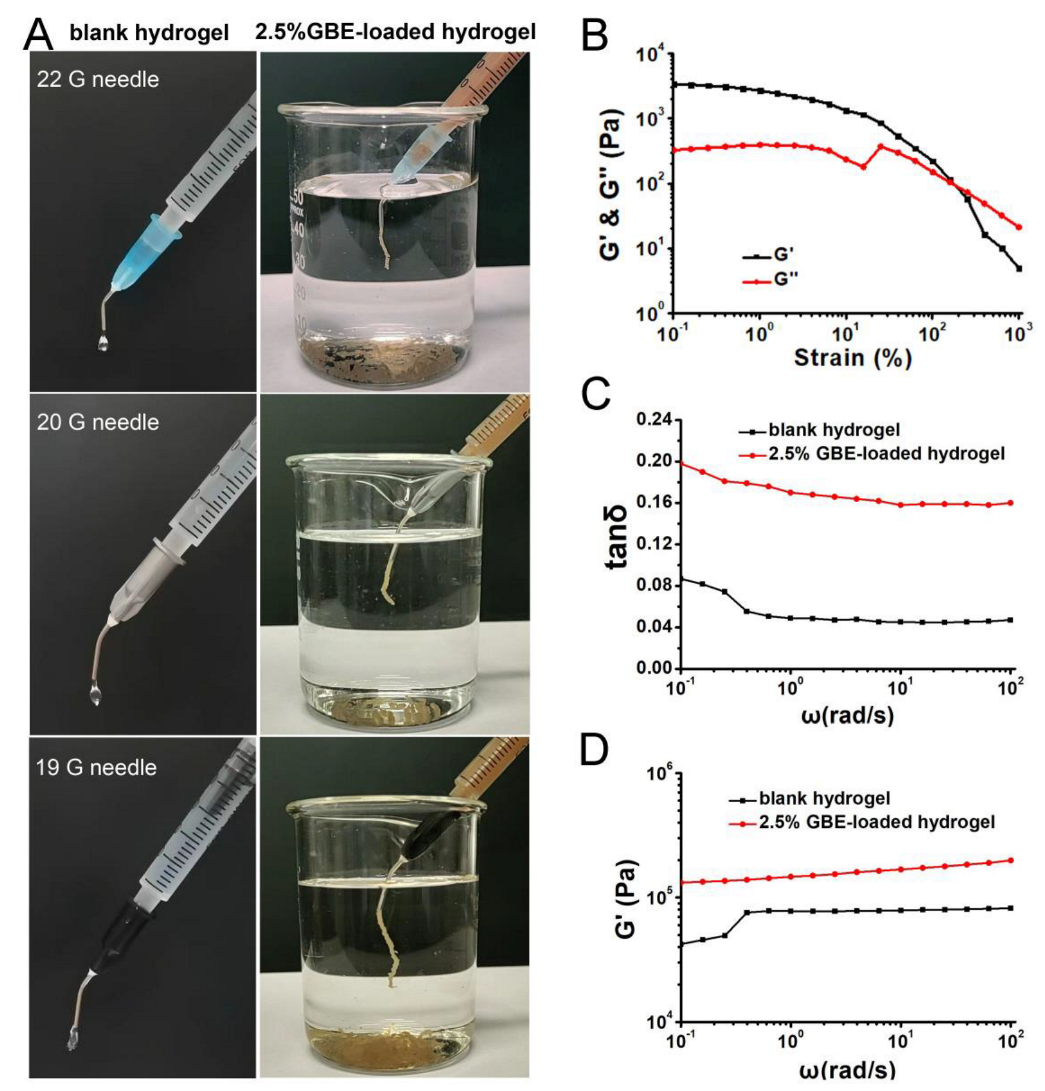
2.6. Injectability, Flexibility and Mechanical Strength Studies of Hydrogels
2.7. In Vitro Biocompatibility of Blank Hydrogels
2.8. In Vitro Hemolysis Assay
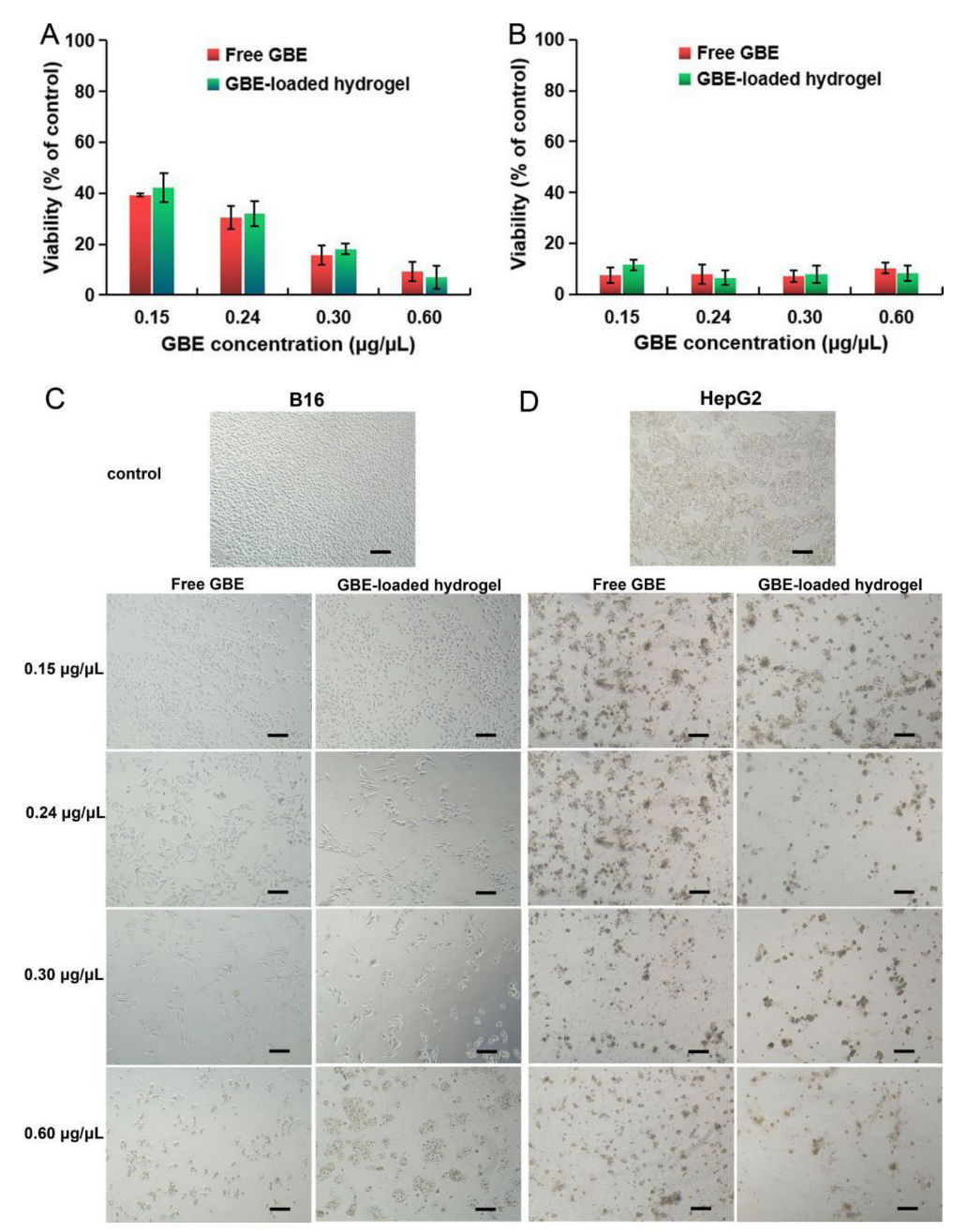
2.9. In Vitro Anti–Tumor Evaluation
2.10. In Vivo Anti–Tumor Evaluation
3. Materials and Methods
3.1. Materials and Reagents
3.2. Methods
3.3. Preparation of Golden Buckwheat Extract (GBE)
3.4. GBE Content Analysis
3.5. Preparation of Hydrogels
3.6. Drug Release Studies of Hydrogels
3.7. DFT Calculation
3.8. Injectability of Hydrogels
3.9. In Vitro Biocompatibility of Blank Hydrogels
3.10. In Vitro Hemolysis Evaluation
3.11. In Vitro Anti–Tumor Evaluation
3.12. In Vivo Anti–Tumor Evaluation
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Zhang, J.; Ding, F.; Pan, G.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. Stressing the Role of DNA as a Drug Carrier: Synthesis of DNA–Drug Conjugates through Grafting Chemotherapeutics onto Phosphorothioate Oligonucleotides. Adv. Mater. 2019, 31, 1807533. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, 1806538. [Google Scholar] [CrossRef] [PubMed]
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release. 2007, 123, 78–99. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Pan, G.; Wang, P.; Li, Y.; Zhu, X.; Zhang, C. Injectable Drug–Conjugated DNA Hydrogel for Local Chemotherapy to Prevent Tumor Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 21441–21449. [Google Scholar] [CrossRef]
- He, D.; Li, H. Bifunctional Cx43 Mimic Peptide Grafted Hyaluronic Acid Hydrogels Inhibited Tumor Recurrence and Stimulated Wound Healing for Postsurgical Tumor Treatment. Adv. Funct. Mater. 2020, 30, 2004709. [Google Scholar] [CrossRef]
- Sahni, V.; Choudhury, D.; Ahmed, Z. Chemotherapy–associated renal dysfunction. Nat. Revi. Nephrol. 2009, 5, 450–462. [Google Scholar] [CrossRef]
- Zhuang, B.; Chen, T.; Xiao, Z.; Jin, Y. Drug–loaded implantable surgical cavity–adaptive hydrogels for prevention of local tumor recurrence. Int. J. Pharmaceut. 2020, 577, 119048. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wan, C.; Zou, Z.; Zhao, G.; Zhang, L.; Geng, Y.; Chen, T.; Huang, A.; Jiang, F.; Feng, J.-P.; et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano 2018, 12, 3295–3310. [Google Scholar] [CrossRef]
- Zhao, M.; Danhier, F.; Bastiancich, C.; Joudiou, N.; Ganipineni, L.P.; Tsakiris, N.; Gallez, B.; Rieux, A.; Jankovski, A.; Bianco, J.; et al. Post–resection treatment of glioblastoma with an injectable nanomedicine–loaded photopolymerizable hydrogel induces long–term survival. Int. J. Pharmaceut. 2018, 548, 522–529. [Google Scholar] [CrossRef]
- Poláková, L.; Širc, J.; Hobzová, R.; Cocârță, A.-I.; Heřmánková, E. Electrospun nanofibers for local anticancer therapy: Review of in vivo activity. Int. J. Pharmaceut. 2019, 558, 268–283. [Google Scholar] [CrossRef]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug–loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil–mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Ye, Y.; Hu, Q.; Bomba, H.N.; Gu, Z. In situ activation of platelets with checkpoint inhibitors for post–surgical cancer immunotherapy. Nat. Biomed. Eng. 2017, 1, 0011. [Google Scholar] [CrossRef]
- Seib, F.P.; Tsurkan, M.; Freudenberg, U.; Kaplan, D.L.; Werner, C. Heparin–Modified Polyethylene Glycol Microparticle Aggregates for Focal Cancer Chemotherapy. ACS Biomater. Sci. Eng. 2016, 2, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.H.; Nisar, S.; Imtiyaz, K.; Nadeem, M.; Mazumdar, N.; Rizvi, M.M.A.; Ahmad, S. Injectable, Self–Healing, and Biocompatible N,O–Carboxymethyl Chitosan/Multialdehyde Guar Gum Hydrogels for Sustained Anticancer Drug Delivery. Biomacromolecules 2021, 22, 3731–3745. [Google Scholar] [CrossRef]
- Aoki, T.; Nishikawa, R.; Sugiyama, K.; Nonoguchi, N.; Kawabata, N.; Mishima, K.; Adachi, J.-I.; Kurisu, K.; Yamasaki, F.; Tominaga, T. A multicenter phase I/II study of the BCNU implant (Gliadel(®) Wafer) for Japanese patients with malignant gliomas. Neurol. Med. Chir. 2014, 54, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Fung, L.K.; Ewend, M.G.; Sills, A.; Sipos, E.P.; Thompson, R.; Watts, M.; Colvin, O.M.; Brern, H.; Saltzman, W.M. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998, 58, 672–684. [Google Scholar]
- Chen, Q.; Shu, C.; Laurence, A.D.; Chen, Y.; Peng, B.-G.; Zhen, Z.-J.; Cai, J.-Q.; Ding, Y.-T.; Li, L.-Q.; Zhang, Y.-B.; et al. Effect of Huaier granule on recurrence after curative resection of HCC: A multicentre, randomised clinical trial. Gut 2018, 67, 2006–2016. [Google Scholar] [CrossRef]
- Haranaka, K.; Satomi, N.; Sakurai, A.; Haranaka, R.; Okada, N.; Kobayashi, M. Antitumor activities and tumor necrosis factor producibility of traditional Chinese medicines and crude drugs. Cancer Immunol. Immun. 1985, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, J.J.; Wang, X.S.; Lin, H. Evaluation of Traditional Chinese Medicine Herbs in Oncology Clinical Trials. Cancer J. 2019, 25, 367–371. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.; Hu, C.; Jiang, Y.; Qin, L.; Zheng, C. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef]
- Bu, L.-L.; Yan, J.; Wang, Z.; Ruan, H.; Chen, Q.; Gunadhi, V.; Bell, R.B.; Gu, Z. Advances in drug delivery for post–surgical cancer treatment. Biomaterials 2019, 219, 119182. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wang, D.; Shi, L.; Zhang, Z.; Qian, J.; Shen, J.; Yu, C.; Zhu, X. A pure molecular drug hydrogel for post–surgical cancer treatment. Biomaterials 2021, 265, 120403. [Google Scholar] [CrossRef]
- Dey, M.; Ghosh, B.; Giri, T.K. Enhanced intestinal stability and pH sensitive release of quercetin in GIT through gellan gum hydrogels. Colloid. Surface B 2020, 196, 111341. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Varshosaz, J.; Hashemibeni, B.; Sarmadi, A. Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies. Mat. Sci. Eng. C-Mater. 2020, 115, 111114. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Włoch, M.; Drożdż, A.; Krok-Borkowicz, M.; Włodarczyk-Biegun, M.; Dobrzyński, P.; Chrzanowski, W.; Pamuła, E. Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Expert Opin. Drug Del. 2016, 13, 613–620. [Google Scholar] [CrossRef]
- Wu, H.Z.; Zhou, J.Y.; Pan, H.L. Study on chemical constituents of Fagopyrum dibotrys (D.Don) Hara. Chin. J. Hosp. Pharm. 2008, 28, 21–26. [Google Scholar]
- Prajapati, V.D.; Jani, G.K.; Zala, B.S.; Khutliwala, T.A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohyd. Polym. 2013, 93, 670–678. [Google Scholar] [CrossRef]
- Nara, M.; Torii, H.; Tasumi, M. Correlation between the Vibrational Frequencies of the Carboxylate Group and the Types of Its Coordination to a Metal Ion: An ab Initio Molecular Orbital Study. J. Phys. Chem. 1996, 100, 19812–19817. [Google Scholar] [CrossRef]
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of gellan—A review. Food Hydrocolloid. 2012, 28, 373–411. [Google Scholar] [CrossRef]
- Morris, E.R.; Gothard, M.G.E.; Hember, M.W.N.; Manning, C.E.; Robinson, G. Conformational and rheological transitions of welan, rhamsan and acylated gellan. Carbohyd. Polym. 1996, 30, 165–175. [Google Scholar] [CrossRef]
- Rawat, P.; Singh, R.N. Synthesis and study on aroylhydrazones having cyanovinylpyrrole. Arab. J. Chem. 2019, 12, 2384–2397. [Google Scholar] [CrossRef]
- Butler, M.; Mañez, P.A.; Cabrera, G.M.; Maître, P. Gas Phase Structure and Reactivity of Doubly Charged Microhydrated Calcium(II)–Catechol Complexes Probed by Infrared Spectroscopy. J. Phys. Chem. A 2014, 118, 4942–4954. [Google Scholar] [CrossRef]
- Shen, J.; Chang, R.; Chang, L.; Wang, Y.; Deng, K.; Wang, D.; Qin, J. Light emitting CMC–CHO based self–healing hydrogel with injectability for in vivo wound repairing applications. Carbohyd. Polym. 2022, 281, 119052. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Shelton, R.M.; Perrie, Y.; Harris, J.J. An initial evaluation of gellan gum as a material for tissue engineering applications. J. Biomater. Appl. 2007, 22, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Giri, T. Hydrogels based on gellan gum in cell delivery and drug delivery. J. Drug Deliv. Sci.Tec. 2020, 56, 101586. [Google Scholar] [CrossRef]
- Li, Z.; Liu, L.; Chen, Y. Dual dynamically crosslinked thermosensitive hydrogel with self–fixing as a postoperative anti–adhesion barrier. Acta Biomater. 2020, 110, 119–128. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06–class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Accounts Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self–Consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. J. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Bauernschmitt, R.; Ahlrichs, R. Stability analysis for solutions of the closed shell Kohn–Sham equation. J. Chem. Phys. 1996, 104, 9047–9052. [Google Scholar] [CrossRef]
- Singh, B.; Dhiman, A. Design of Acacia Gum–Carbopol–Cross–Linked–Polyvinylimidazole Hydrogel Wound Dressings for Antibiotic/Anesthetic Drug Delivery. Ind. Eng. Chem. Res. 2016, 55, 9176–9188. [Google Scholar] [CrossRef]
- Pan, Q.; Xie, L.; Liu, R.; Pu, Y.; Wu, D.; Gao, W.; Luo, K.; He, B. Two birds with one stone: Copper metal–organic framework as a carrier of disulfiram prodrug for cancer therapy. Int. J. Pharmaceut. 2022, 612, 121351. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Liu, R.; Wang, D.; Pan, Q.; Yang, S.; Li, H.; Zhang, X.; Jin, M. Golden Buckwheat Extract–Loaded Injectable Hydrogel for Efficient Postsurgical Prevention of Local Tumor Recurrence Caused by Residual Tumor Cells. Molecules 2023, 28, 5447. https://doi.org/10.3390/molecules28145447
Xie L, Liu R, Wang D, Pan Q, Yang S, Li H, Zhang X, Jin M. Golden Buckwheat Extract–Loaded Injectable Hydrogel for Efficient Postsurgical Prevention of Local Tumor Recurrence Caused by Residual Tumor Cells. Molecules. 2023; 28(14):5447. https://doi.org/10.3390/molecules28145447
Chicago/Turabian StyleXie, Li, Rong Liu, Dan Wang, Qingqing Pan, Shujie Yang, Huilun Li, Xinmu Zhang, and Meng Jin. 2023. "Golden Buckwheat Extract–Loaded Injectable Hydrogel for Efficient Postsurgical Prevention of Local Tumor Recurrence Caused by Residual Tumor Cells" Molecules 28, no. 14: 5447. https://doi.org/10.3390/molecules28145447
APA StyleXie, L., Liu, R., Wang, D., Pan, Q., Yang, S., Li, H., Zhang, X., & Jin, M. (2023). Golden Buckwheat Extract–Loaded Injectable Hydrogel for Efficient Postsurgical Prevention of Local Tumor Recurrence Caused by Residual Tumor Cells. Molecules, 28(14), 5447. https://doi.org/10.3390/molecules28145447




