Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning
Abstract
1. Introduction
2. Results and Discussion
2.1. Statistical Analysis of Tea Polyphenols and EGCG Content in Different Varieties
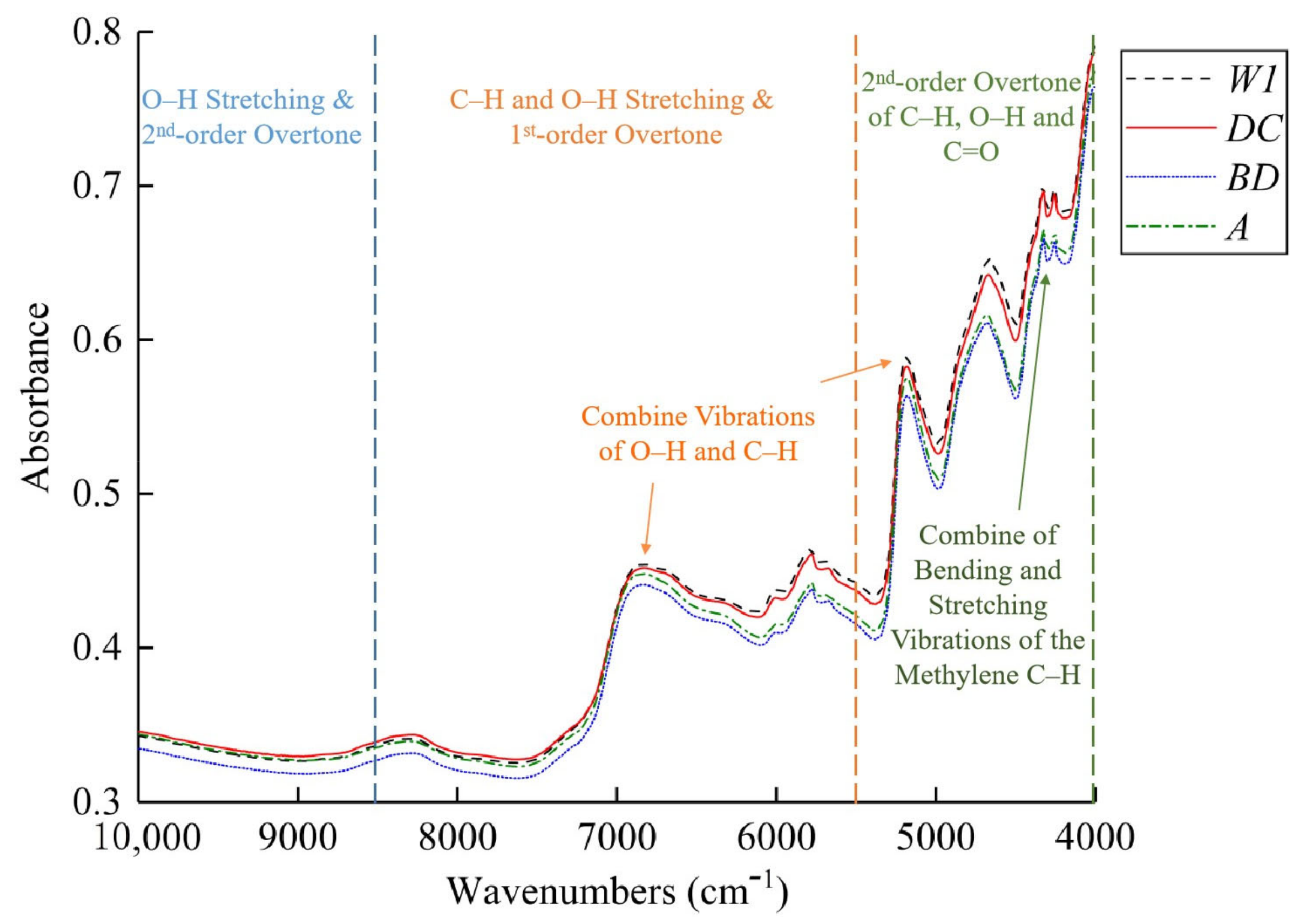
2.2. Analysis of Fourier Transform–Near-Infrared Spectroscopy Curves of Tea Powder
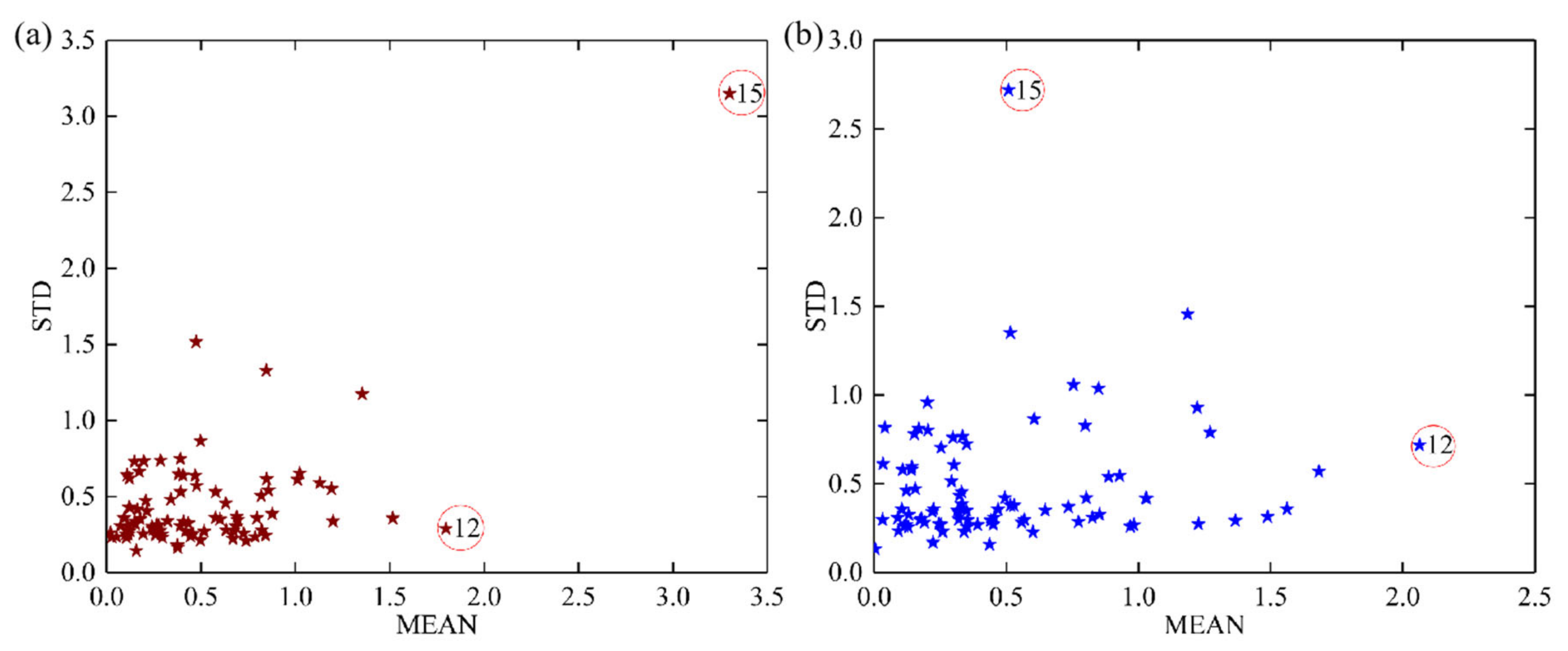
2.3. Outliers Elimination
2.4. Description of Sample for Model Establishment
2.5. Models Establishment for Tea Polyphenol and EGCG Content Prediction
2.5.1. Model Establishment Based on Full Spectrum
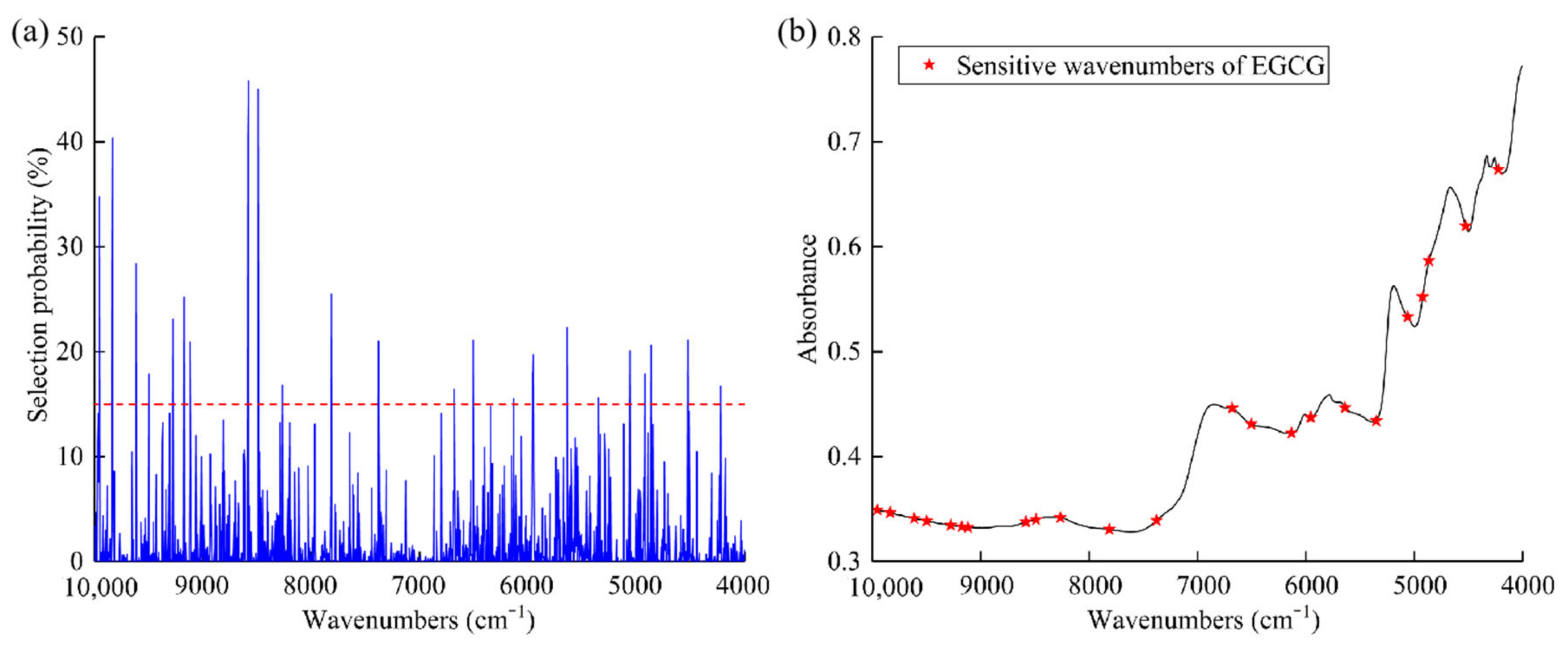
2.5.2. Model Establishment Based on Selected Sensitive Wavenumbers
3. Materials and Methods
3.1. Tea Powder Samples Preparation
3.2. Fourier Transform Near-Infrared Spectroscopy Data Collection
3.3. Determination of Tea Polyphenol and EGCG Content
3.4. Data Analysis
3.4.1. Preprocessing Methods
3.4.2. Prediction Models’ Establishment
3.4.3. Models Performance Evaluation
3.4.4. Software and Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kerio, L.C.; Wachira, F.N.; Wanyoko, J.K.; Rotich, M.K. Total Polyphenols, Catechin Profiles and Antioxidant Activity of Tea Products from Purple Leaf Coloured Tea Cultivars. Food Chem. 2013, 136, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential Benefits of Green Tea Polyphenol EGCG in the Prevention and Treatment of Vascular Inflammation in Rheumatoid Arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, A.K.; Chanda, S.; Sabhapondit, S.; Sanyal, S.; Tamuly, P.; Tasrin, S.; Sing, D.; Tudu, B.; Bandyopadhyay, R. Quality Assessment of Fresh Tea Leaves by Estimating Total Polyphenols Using near Infrared Spectroscopy. J. Food Sci. Technol. 2018, 55, 4867–4876. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Zhang, Y.-H.; Chen, G.-S.; Yin, J.-F.; Chen, J.-X.; Wang, F.; Xu, Y.-Q. Effects of Phenolic Acids and Quercetin-3-O-Rutinoside on the Bitterness and Astringency of Green Tea Infusion. NPJ Sci. Food 2022, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ying, K.; Bai, J. Savitzky–Golay Smoothing and Differentiation Filter for Even Number Data. Signal Process. 2005, 85, 1429–1434. [Google Scholar] [CrossRef]
- Bi, Y.; Yuan, K.; Xiao, W.; Wu, J.; Shi, C.; Xia, J.; Chu, G.; Zhang, G.; Zhou, G. A Local Pre-Processing Method for Near-Infrared Spectra, Combined with Spectral Segmentation and Standard Normal Variate Transformation. Anal. Chim. Acta 2016, 909, 30–40. [Google Scholar] [CrossRef]
- Syvilay, D.; Wilkie-Chancellier, N.; Trichereau, B.; Texier, A.; Martinez, L.; Serfaty, S.; Detalle, V. Evaluation of the Standard Normal Variate Method for Laser-Induced Breakdown Spectroscopy Data Treatment Applied to the Discrimination of Painting Layers. Spectrochim. Acta Part B At. Spectrosc. 2015, 114, 38–45. [Google Scholar] [CrossRef]
- Chen, P. Effects of Normalization on the Entropy-Based TOPSIS Method. Expert Syst. Appl. 2019, 136, 33–41. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Huang, X.; Chen, M.; Zhao, Z.; Ji, Y.; Li, X.; He, Y.; Zhu, Y.; Chen, X.; Yu, X. Nondestructive Monitoring of Polyphenols and Caffeine during Green Tea Processing Using Vis-NIR Spectroscopy. Food Sci. Nutr. 2020, 8, 5860–5874. [Google Scholar] [CrossRef]
- Lee, M.-S.; Hwang, Y.-S.; Lee, J.; Choung, M.-G. The Characterization of Caffeine and Nine Individual Catechins in the Leaves of Green Tea (Camellia sinensis L.) by Near-Infrared Reflectance Spectroscopy. Food Chem. 2014, 158, 351–357. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.-Y.; Tsai, C.-Y.; Yang, I.-C.; Luo, S.-J.; Chuang, Y.-K. Fermentation Quality Evaluation of Tea by Estimating Total Catechins and Theanine Using Near-Infrared Spectroscopy. Vib. Spectrosc. 2021, 115, 103278. [Google Scholar] [CrossRef]
- Rosi, F.; Daveri, A.; Doherty, B.; Nazzareni, S.; Brunetti, B.G.; Sgamellotti, A.; Miliani, C. On the Use of Overtone and Combination Bands for the Analysis of the CaSO4—H2O System by Mid-Infrared Reflection Spectroscopy. Appl. Spectrosc. 2010, 64, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Mittal, A.; Barnette, A.L.; Kafle, K.; Park, Y.B.; Shin, H.; Johnson, D.K.; Park, S.; Kim, S.H. Cellulose Polymorphism Study with Sum-Frequency-Generation (SFG) Vibration Spectroscopy: Identification of Exocyclic CH2OH Conformation and Chain Orientation. Cellulose 2013, 20, 991–1000. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C. A Review of Mid-Infrared and Near-Infrared Imaging: Principles, Concepts and Applications in Plant Tissue Analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Rocha-Mendoza, I.; Yankelevich, D.R.; Wang, M.; Reiser, K.M.; Frank, C.W.; Knoesen, A. Sum Frequency Vibrational Spectroscopy: The Molecular Origins of the Optical Second-Order Nonlinearity of Collagen. Biophys. J. 2007, 93, 4433–4444. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-S.; Wang, S.-L.; Huang, S.-T.; Tzou, Y.-M.; Huang, J.-H. Biosorption of Cr(VI) by Coconut Coir: Spectroscopic Investigation on the Reaction Mechanism of Cr(VI) with Lignocellulosic Material. J. Hazard. Mater. 2010, 179, 160–165. [Google Scholar] [CrossRef]
- Md Salim, R.; Asik, J.; Sarjadi, M.S. Chemical Functional Groups of Extractives, Cellulose and Lignin Extracted from Native Leucaena Leucocephala Bark. Wood Sci. Technol. 2021, 55, 295–313. [Google Scholar] [CrossRef]
- Oubaha, M.; Etienne, P.; Calas, S.; Sempere, R.; Nedelec, J.M.; Moreau, Y. Spectroscopic Characterization of Sol–Gel Organo-Siloxane Materials Synthesized from Aliphatic and Aromatic Alcoxysilanes. J. Non-Cryst. Solids 2005, 351, 2122–2128. [Google Scholar] [CrossRef]
- Li, X.; Jin, J.; Sun, C.; Ye, D.; Liu, Y. Simultaneous Determination of Six Main Types of Lipid-Soluble Pigments in Green Tea by Visible and Near-Infrared Spectroscopy. Food Chem. 2019, 270, 236–242. [Google Scholar] [CrossRef]
- Yan, J.; Huang, X.-P.; Zhu, W.-W. Simultaneous Determination of Antioxidant Properties and Total Phenolic Content of Siraitia Grosvenorii by Near Infrared Spectroscopy. Food Meas. 2020, 14, 2300–2309. [Google Scholar] [CrossRef]
- Bess, E.N.; Guptill, D.M.; Davies, H.M.L.; Sigman, M.S. Using IR Vibrations to Quantitatively Describe and Predict Site-Selectivity in Multivariate Rh-Catalyzed C–H Functionalization. Chem. Sci. 2015, 6, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, W.; Sanaeifar, A.; Wang, X.; Luo, W.; Zhan, B.; Liu, X.; Li, R.; Zhang, H.; Li, X. Development of Simple Identification Models for Four Main Catechins and Caffeine in Fresh Green Tea Leaf Based on Visible and Near-Infrared Spectroscopy. Comput. Electron. Agric. 2020, 173, 105388. [Google Scholar] [CrossRef]
- Boronat, M.; Concepcion, P.; Corma, A.; Renz, M.; Valencia, S. Determination of the Catalytically Active Oxidation Lewis Acid Sites in Sn-Beta Zeolites, and Their Optimisation by the Combination of Theoretical and Experimental Studies. J. Catal. 2005, 234, 111–118. [Google Scholar] [CrossRef]
- Bauschlicher, C.W.; Langhoff, S.R. The Calculation of Accurate Harmonic Frequencies of Large Molecules: The Polycyclic Aromatic Hydrocarbons, a Case Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1997, 53, 1225–1240. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; Van Der Houwen, J.A.M.; Silva, M.J. Lack of OH in Nanocrystalline Apatite as a Function of Degree of Atomic Order: Implications for Bone and Biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sun, J.; Zhou, X.; Hu, Y.; Wu, X.; Zhang, X.; Wang, P. Visualizing Distribution of Moisture Content in Tea Leaves Using Optimization Algorithms and NIR Hyperspectral Imaging. Comput. Electron. Agric. 2019, 160, 153–159. [Google Scholar] [CrossRef]
- Ravikanth, L.; Singh, C.B.; Jayas, D.S.; White, N.D.G. Classification of Contaminants from Wheat Using Near-Infrared Hyperspectral Imaging. Biosyst. Eng. 2015, 135, 73–86. [Google Scholar] [CrossRef]
- Debba, P.; Carranza, E.J.M.; Van Der Meer, F.D.; Stein, A. Abundance Estimation of Spectrally Similar Minerals by Using Derivative Spectra in Simulated Annealing. IEEE Trans. Geosci. Remote Sens. 2006, 44, 3649–3658. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, J.; Yao, X.; Cao, W.; Zhu, Y. Laboratory Assessment of Three Quantitative Methods for Estimating the Organic Matter Content of Soils in China Based on Visible/near-Infrared Reflectance Spectra. Geoderma 2013, 202–203, 161–170. [Google Scholar] [CrossRef]
- Ferragina, A.; De Los Campos, G.; Vazquez, A.I.; Cecchinato, A.; Bittante, G. Bayesian Regression Models Outperform Partial Least Squares Methods for Predicting Milk Components and Technological Properties Using Infrared Spectral Data. J. Dairy Sci. 2015, 98, 8133–8151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-H.; Sun, D.-W. Partial Least Squares Regression (PLSR) Applied to NIR and HSI Spectral Data Modeling to Predict Chemical Properties of Fish Muscle. Food Eng. Rev. 2017, 9, 36–49. [Google Scholar] [CrossRef]
- Li, Y.; Shao, X.; Cai, W. A Consensus Least Squares Support Vector Regression (LS-SVR) for Analysis of Near-Infrared Spectra of Plant Samples. Talanta 2007, 72, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, K.; Nie, J.; De Klerk, A.; Prasad, V. Least Squares-Support Vector Regression for Determining Product Concentrations in Acid-Catalyzed Propylene Oligomerization. Ind. Eng. Chem. Res. 2018, 57, 13156–13176. [Google Scholar] [CrossRef]
- Farifteh, J.; Van Der Meer, F.; Atzberger, C.; Carranza, E.J.M. Quantitative Analysis of Salt-Affected Soil Reflectance Spectra: A Comparison of Two Adaptive Methods (PLSR and ANN). Remote Sens. Environ. 2007, 110, 59–78. [Google Scholar] [CrossRef]


| Content | n | Rc | RMSEC (%) | Rp | RMSEP (%) | RPD |
|---|---|---|---|---|---|---|
| Tea Polyphenol | 0 | 0.951 | 0.516 | 0.954 | 0.568 | 3.345 |
| 2 | 0.979 | 0.464 | 0.963 | 0.545 | 3.721 | |
| EGCG | 0 | 0.982 | 0.580 | 0.830 | 1.003 | 1.796 |
| 2 | 0.980 | 0.591 | 0.863 | 0.904 | 1.981 |
| Tea Polyphenols Content | EGCG Content | |||
|---|---|---|---|---|
| Calibration Set | Prediction Set | Calibration Set | Prediction Set | |
| N | 55 | 27 | 55 | 27 |
| Range (%) | 11.17–21.96 | 12.62–18.82 | 3.38–18.43 | 5.72–11.68 |
| Mean (%) | 15.88 | 14.64 | 8.90 | 8.18 |
| STD (%) | 2.33 | 1.88 | 3.07 | 1.51 |
| Model | Preprocessing | Rc | RMSEC (%) | Rp | RMSEP (%) | RPD | |
|---|---|---|---|---|---|---|---|
| PLSR | None | 0.979 | 0.464 | 0.963 | 0.545 | 3.721 | |
| SG-Smooth | 0.979 | 0.466 | 0.963 | 0.546 | 3.715 | ||
| SNV | 0.981 | 0.443 | 0.963 | 0.543 | 3.711 | ||
| VN | 0.979 | 0.468 | 0.959 | 0.546 | 3.563 | ||
| MSC | 0.981 | 0.445 | 0.961 | 0.549 | 3.644 | ||
| Tea | FD | 0.992 | 0.280 | 0.963 | 0.571 | 3.724 | |
| Polyphnenol | LS-SVR | None | 0.980 | 0.449 | 0.975 | 0.420 | 4.539 |
| SG-Smooth | 0.980 | 0.449 | 0.975 | 0.420 | 4.540 | ||
| SNV | 0.977 | 0.490 | 0.964 | 0.503 | 3.802 | ||
| VN | 0.980 | 0.449 | 0.972 | 0.438 | 4.322 | ||
| MSC | 0.977 | 0.490 | 0.964 | 0.505 | 3.792 | ||
| FD | 0.999 | 0.443 | 0.955 | 0.578 | 3.389 | ||
| PLSR | None | 0.980 | 0.591 | 0.863 | 0.904 | 1.981 | |
| SG-Smooth | 0.980 | 0.592 | 0.863 | 0.904 | 1.980 | ||
| SNV | 0.982 | 0.569 | 0.913 | 0.661 | 2.461 | ||
| VN | 0.985 | 0.509 | 0.898 | 0.731 | 2.280 | ||
| MSC | 0.983 | 0.555 | 0.909 | 0.678 | 2.402 | ||
| EGCG | FD | 0.992 | 0.369 | 0.918 | 0.661 | 2.521 | |
| LS-SVR | None | 0.993 | 0.361 | 0.936 | 0.637 | 2.841 | |
| SG-Smooth | 0.992 | 0.363 | 0.935 | 0.638 | 2.839 | ||
| SNV | 0.993 | 0.337 | 0.922 | 0.682 | 2.587 | ||
| VN | 0.988 | 0.454 | 0.934 | 0.542 | 2.807 | ||
| MSC | 0.994 | 0.320 | 0.916 | 0.709 | 2.506 | ||
| FD | 0.998 | 0.236 | 0.925 | 0.681 | 2.646 |
| Content | Model | Number | Rc | RMSEC (%) | Rp | RMSEP (%) | RPD |
|---|---|---|---|---|---|---|---|
| Tea Polyphenol | SG-Smooth-CARS-LS-SVR | 30 | 0.984 | 0.404 | 0.978 | 0.395 | 4.833 |
| SG-Smooth-RF-LS-SVR | 16 | 0.975 | 0.504 | 0.926 | 0.716 | 2.655 | |
| EGCG | None-CARS-LS-SVR | 20 | 0.995 | 0.306 | 0.901 | 0.796 | 2.315 |
| None-RF-LS-SVR | 27 | 0.996 | 0.267 | 0.944 | 0.937 | 3.049 |
| Time (min) | Mobile Phase A (%) | Mobile Phase B (%) | Injection Volume (μL) |
|---|---|---|---|
| 0.0 | 93 | 7 | 0.4 |
| 1.5 | 93 | 7 | 0.4 |
| 4.5 | 74 | 26 | 0.4 |
| 8.0 | 68 | 32 | 0.4 |
| 10.0 | 93 | 7 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Weng, H.; Xiang, L.; Jia, L.; Xu, J. Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning. Molecules 2023, 28, 5379. https://doi.org/10.3390/molecules28145379
Ye S, Weng H, Xiang L, Jia L, Xu J. Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning. Molecules. 2023; 28(14):5379. https://doi.org/10.3390/molecules28145379
Chicago/Turabian StyleYe, Sitan, Haiyong Weng, Lirong Xiang, Liangquan Jia, and Jinchai Xu. 2023. "Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning" Molecules 28, no. 14: 5379. https://doi.org/10.3390/molecules28145379
APA StyleYe, S., Weng, H., Xiang, L., Jia, L., & Xu, J. (2023). Synchronously Predicting Tea Polyphenol and Epigallocatechin Gallate in Tea Leaves Using Fourier Transform–Near-Infrared Spectroscopy and Machine Learning. Molecules, 28(14), 5379. https://doi.org/10.3390/molecules28145379






