Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids
Abstract
1. Introduction
2. Results
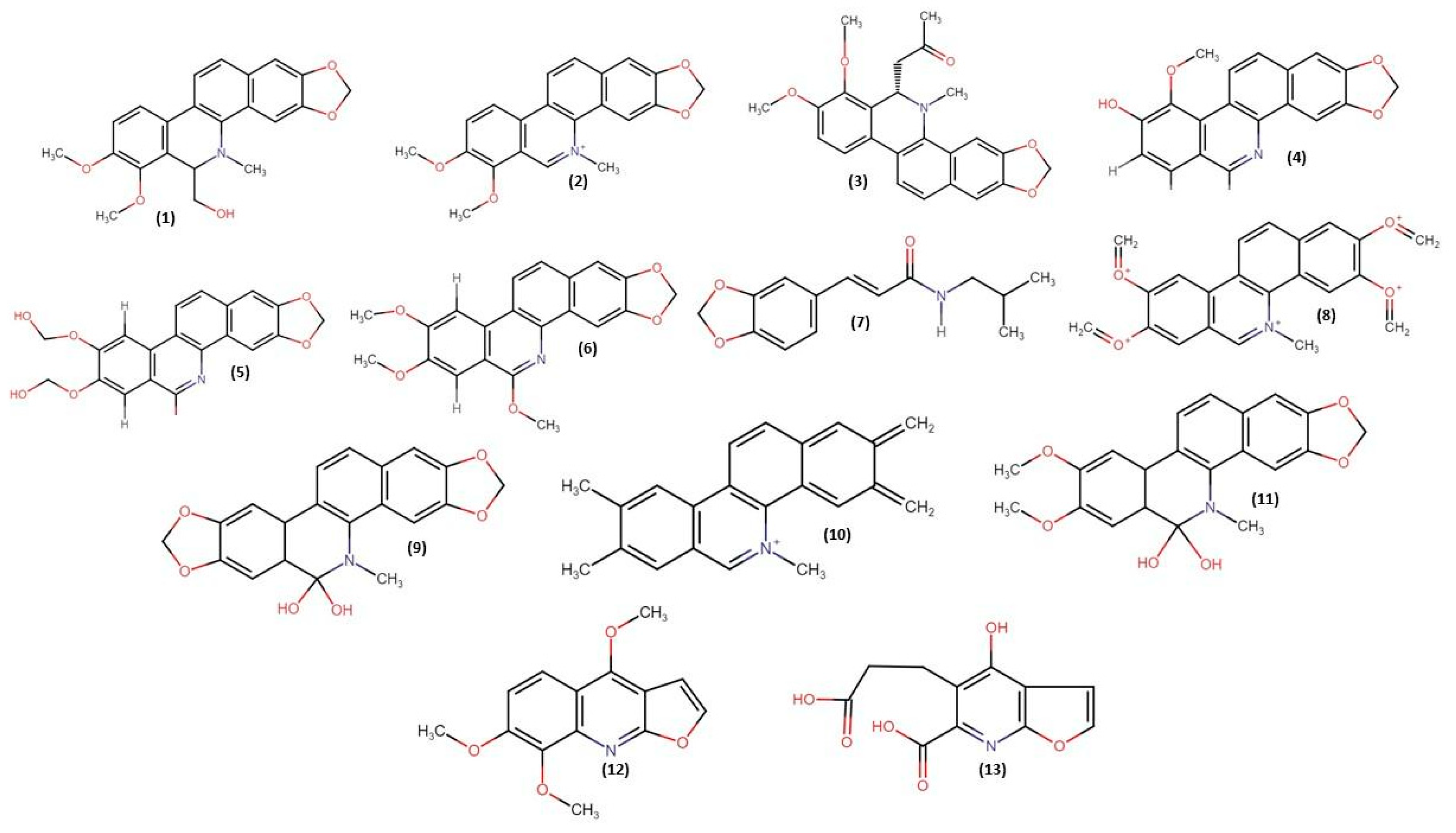
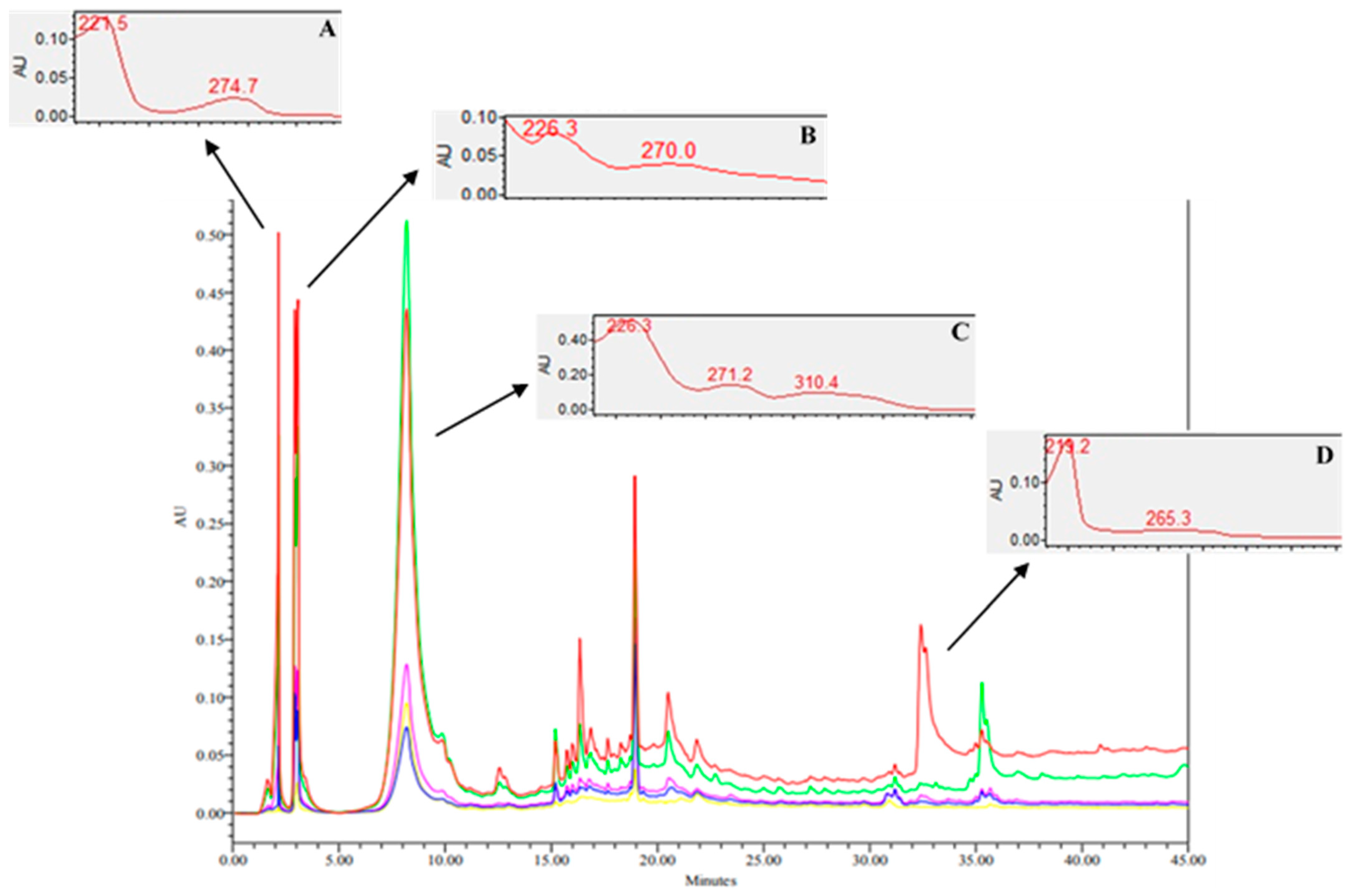
2.1. Phytochemical Studies
2.2. Toxicity of Z. rhoifolium
2.2.1. Cytotoxicity Assays
2.2.2. Allium Cepa Test
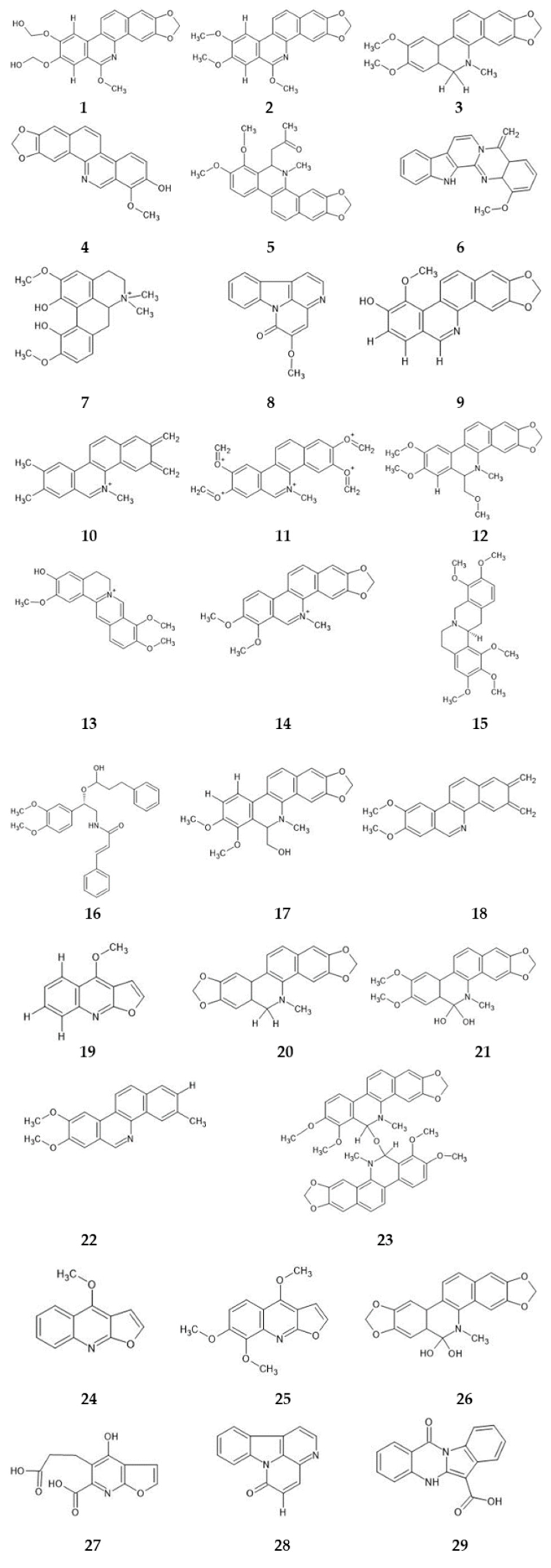
2.2.3. In Silico Studies
2.3. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemical Studies
4.2. Cell Viability Assay with Tetrazolium Salt (MTT)
4.3. Mutagenic Activity through the Allium Cepa Test
4.4. In Silico Studies of Alkaloids
4.5. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lemée, A.M. Flore de la Guyane Française. IV. Végétaux Utiles de la Guyane Francaise; Paul Lechévalier: Paris, France, 1956. [Google Scholar]
- Rodrigues, E.; Mendes, F.R.; Negri, G. Plants indicated by Brazilian Indians for disturbances of the central nervous system: A bibliographical survey. Cent. Nerv. Syst. Agents Med. Chem. Former. Curr. Med. Chem. Cent. Nerv. Syst. Agents 2006, 6, 211–244. [Google Scholar] [CrossRef]
- Figueiró-Leandro, A.C.; Citadini-Zanette, V. Árvores medicinais de um fragmento florestal urbano no município de Criciúma, Santa Catarina, Brasil. Rev. Bras. Plantas Med. 2008, 10, 56–67. [Google Scholar]
- Bieski, I.G.; Rios Santos, F.; de Oliveira, R.M.; Espinosa, M.M.; Macedo, M.; Albuquerque, U.P.; de Oliveira Martins, D.T. Ethnopharmacology of medicinal plants of the pantanal region (Mato Grosso, Brazil). Evid. Based Complement. Altern. Med. 2012, 2012, 272749. [Google Scholar] [CrossRef] [PubMed]
- Arruda, M.S.; Fernandes, J.B.; Vieira, P.C.; Da Silva, M.F.; Pirani, J.R. Chemistry of Zanthoxylum rhoifolium: A new secofuroquinoline alkaloid. Biochem. Syst. Ecol. 1992, 20, 173–178. [Google Scholar] [CrossRef]
- De Moura, N.F.; Ribeiro, H.B.; Machado, E.C.; Ethur, E.M.; Zanatta, N.; Morel, A.F. Benzophenanthridine alkaloids from Zanthoxylum rhoifolium. Phytochemistry 1997, 46, 1443–1446. [Google Scholar] [CrossRef]
- Gonzaga, W.; Weber, A.D.; Giacomelli, S.R.; Dalcol, I.; Hoelzel, S.C.; Morel, A.F. Antibacterial alkaloids from Zanthoxylum rhoifolium. Planta Medica 2003, 69, 371–374. [Google Scholar] [CrossRef]
- Gonzaga, W.; Weber, A.D.; Giacomelli, S.R.; Simionatto, E.; Dalcol, I.; Dessoy, E.C.; Morel, A.F. Composition and antibacterial activity of the essential oils from Zanthoxylum rhoifolium. Planta Medica 2003, 69, 773–775. [Google Scholar]
- Taborda, M.E.; Suárez, L.E. Un nuevo alcaloide carbozolico de Zanthoxylum rhoifolium. Sci. Tech. 2007, 13, 191–192. [Google Scholar]
- Jullian, V.; Bourdy, G.; Georges, S.; Maurel, S.; Sauvain, M. Validation of use of a traditional antimalarial remedy from French Guiana, Zanthoxylum rhoifolium Lam. J. Ethnopharmacol. 2006, 106, 348–352. [Google Scholar] [CrossRef]
- Tavares, L.C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.P.; Da Cruz, I.B.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-Activity Relationship of Benzophenanthridine Alkaloids from Zanthoxylum rhoifolium Having Antimicrobial Activity. PLoS ONE 2014, 9, e97000. [Google Scholar] [CrossRef]
- da Costa, J.G.M.; Campos, A.R.; Brito, S.A.; Pereira, C.K.; Souza, E.O.; Rodrigues, F.F. Biological screening of araripe basin medicinal plants using Artemia salina Leach and pathogenic bacteria. Pharmacogn. Mag. 2010, 6, 331. [Google Scholar] [CrossRef]
- Krause, M.S.; Bonettia, A.d.F.; Turnes, J.d.M.; Dias, J.d.F.G.; Miguel, O.G.; Duarte, M.d.R. Phytochemistry and biological activities of Zanthoxylum rhoifolium Lam., Rutaceae-Mini review. Visão Acad. 2013, 14. [Google Scholar] [CrossRef]
- Melo Neto, B.; Leitao, J.M.; Oliveira, L.G.; Santos, S.E.; Carneiro, S.M.; Rodrigues, K.A.; Chaves, M.H.; Arcanjo, D.D.; Carvalho, F.A. Inhibitory effects of Zanthoxylum rhoifolium Lam. (Rutaceae) against the infection and infectivity of macrophages by Leishmania amazonensis. An. Da Acad. Bras. Ciênc. 2016, 88, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Taíse, P.; Luciana Ferreira, D.; Almeida, G.N.D.; Ayres, M.C.C.; Moreira, E.L.T.; Cruz, A.C.F.D.; Bittencourt, T.C.B.D.S.C.D.; Almeida, M.A.O.D.; Batatinha, M.J.M. Atividade anti-helmíntica do extrato aquoso das folhas de Zanthoxylum rhoifolium Lam. (Rutaceae). Rev. Bras. Parasitol. Vet. 2009, 18, 43–48. [Google Scholar]
- Moura, N.F.; Giacomelli, S.R.; Macha ECd Morel, A.F.; Silveira, C.S.; Bittencourt, C.F. Antibacterial activity of Zanthoxylum rhoifolium. Fitoterapia 1998, 69, 271–272. [Google Scholar]
- Silva, S.L.d.; Figueredo, P.M.; Yano, T. Antibacterial and antifungal activities of volatile oils from Zanthoxylum Rhoifolium. Leaves. Pharm. Biol. 2006, 44, 657–659. [Google Scholar] [CrossRef]
- Correa-Barbosa, J.; Sodré, D.F.; Nascimento, P.H.C.; Dolabela, M.F. Activity of the genus Zanthoxylum against diseases caused by protozoa: A systematic review. Front. Pharmacol. 2023, 13, 873208. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.L.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Choi, H.J. Evaluation of Antiviral Activity of Zanthoxylum Species Against Picornaviruses. Osong Public Health Res. Perspect. 2016, 7, 400–403. [Google Scholar] [CrossRef]
- Antonaccio, L.D.; Gottlieb, O.R. Sesamin and lupeol in the bark of two Brazilian Fagara species. An. Ass. Bras. Quim. 1959, 18, 183–184. [Google Scholar]
- Zee-Cheng, R.K.; Cheng, C.C. Preparation and antileukemic activity of some alkoxybenzo [c] phenanthridinium salts and corresponding dihydro derivatives. J. Med. Chem. 1975, 18, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.V.D.; Faitanin, R.D.; Brasileiro, B.G.; Silveira, D.; Jamal, C.M. Triagem fitoquímica e avaliação das atividades trombolítica e citotóxica de Cecropia hololeuca Miq. (Urticaceae), Lippia alba (Mill.) N.E.Br. ex P. Wilson (Verbenaceae) e Zanthoxylum rhoifolium Lam. (Rutaceae). Infarma Ciênc. Farm. 2016, 20, 10. [Google Scholar] [CrossRef]
- Chagas, C.K.S.; Mota, T.C.; Barbosa, J.C.; Brígido, H.P.C.; Bahia, M.O.; Dolabela, M.F. Avaliação da Citotoxicidade da Zanthoxylum Rhoifolium lam para Linhagem de Célula Epitelial Gástrica Normal. In Proceedings of the Anais da 29ª Semana do ICB: ICB 50 Anos, uma História em Construção, Goiania, Brazil, 24–28 September 2018. [Google Scholar]
- Leitão, J.M.; Melo-Neto, B.; Carneiro, S.M.; Silva, J.D.S.; Alves, M.M.; Arcanjo, D.D.; Cavalcante, A.A.C.; Carvalho, F.A.A. Evaluation of Potential Toxic, Cytotoxic and Mutagenic Effects induced by Zanthoxylum rhoifolium Lam. in Eukaryotic Cells. J. Glob. Innov. 2020, 2, 1. [Google Scholar]
- Silva, M.T.; de Oliveira, M.G.; de Paula, J.R.; da Silva, V.B.; Neves, K.D.; Machado, M.B.; Nunomura, R.D. qNMR quantification and in silico analysis of isobrucein B and neosergeolide from Picrolemma sprucei as potential inhibitors of SARS-CoV-2 protease (3CLpro) and RNA-dependent RNA polymerase (RdRp) and pharmacokinetic and toxicological properties. Res. Soc. Dev. 2021, 10, e69101623220. [Google Scholar] [CrossRef]
- Griffiths, M.R.; Strobel, B.W.; Hama, J.R.; Cedergreen, N. Toxicity and risk of plant-produced alkaloids to Daphnia magna. Environ. Sci. Eur. 2021, 33, 10. [Google Scholar] [CrossRef]
- Reina, M.; Ruiz-Mesia, W.; Ruiz-Mesia, L.; Martínez-Díaz, R.; González-Coloma, A. Indole alkaloids from Aspidosperma rigidum and A. schultesii and their antiparasitic effects. Z. Naturforschung C 2011, 66, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Granada, A.; Nemen, D.; Dora, C.L.; Neckel, G.L.; Lemos-Senna, E. O emprego de sistemas de liberação como estratégia para melhorar as propriedades terapêuticas de fármacos de origem natural: O exemplo da camptotecina e seus derivados. Rev. Ciênc. Farm. Básica Apl. 2007, 28, 129–139. [Google Scholar]
- Hsiang, Y.H.; Hertzberg, R.; Hecht, S.; Liu, L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [CrossRef]
- Paul Gleeson, M.; Hersey, A.; Hannongbua, S. In-silico ADME models: A general assessment of their utility in drug discovery applications. Curr. Top. Med. Chem. 2011, 11, 358–381. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Singh, B.; Kumar, N. Quantitative and structural analysis of amides and lignans in Zanthoxylum armatum by UPLCDAD-ESI-QTOF–MS/MS. J. Pharm. Biomed. Anal. 2014, 94, 23–29. [Google Scholar] [CrossRef]
- Deng, A.J.; Qin, H.L. Cytotoxic dihydrobenzophenanthridine alkaloids from the roots of Macleaya microcarpa. Phytochemistry 2010, 71, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Boehme, A.K.; Noletto, J.A.; Haber, W.A.; Setzer, W.N. Bioactivity and chemical composition of the leaf essential oils of Zanthoxylum rhoifolium and Zanthoxylum setulosum from Monteverde, Costa Rica. Nat. Prod. Res. 2008, 22, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Vanhaecke, P.; Persoone, G.; Claus, C.; Sorgeloos, P. Research on the Development of a Short Term Standard Toxicity Test with Artemia Nauplii. Agris 2000, 1, 263. [Google Scholar]
- Blinova, I.; Kanarbik, L.; Irha, N.; Kahru, A. Ecotoxicity of Nanosized Magnetite to Crustacean Daphnia magna and Duckweed Lemna Minor. Hydrobiologia 2017, 798, 141–149. [Google Scholar] [CrossRef]
- Itoyama, M.M.; Bicudo, H.E.; Cordeiro, J.A. Effects of caffeine on mitotic index in Drosophila prosaltans (Diptera). Braz. J. Genet. 1997, 20, 655–658. [Google Scholar] [CrossRef]
- Grisolia, C.K.; Takahashi, C.S.; Ferriani, I. In vivo and in vitro tests in humans confirm that the antimalarial drugs mefloquine is not mutagenic. Braz. J. Genet. 1995, 18, 611–614. [Google Scholar]
- Dias, F.D. Cytogenetic evaluation of the effect of aqueous extracts of the medicinal plants Alpinia nutans Rosc.(Zingiberaceae) and Pogo-stemun heyneanus Benth. (Labiatae) on Wistar rats and Allium cepa Linn. (Liliaceae) root tip cells. Brazil. J. Genet. 1994, 17, 175–180. [Google Scholar]
- Burim, R.V.; Canalle, R.; Lopes, J.L.; Takahashi, C.S. Genotoxic action of the sesquiterpene lactone glaucolide B on mammalian cells in vitro and in vivo. Genet. Mol. Biol. 1999, 22, 401–406. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. Rev. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Ames, B.N. Identifying environmental chemicals causing mutations and cancer. The Biological Revolution: Applications of Cell Biology to Public Welfare. Science 1979, 204, 587–593. [Google Scholar] [CrossRef]
- Martins, B.O.; Lavallée, C.; Silkoset, A. Drone Use for COVID-19 Related Problems: Techno-solutionism and its Societal Implications. Glob. Policy 2021, 12, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.R.; Olivi, P.; Botta, C.M.; Espindola, E.L. A toxicidade em ambientes aquáticos: Discussão e métodos de avaliação. Quím. Nova 2008, 31, 1820–1830. [Google Scholar] [CrossRef]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas; Springer Science & Business Media: Berlin, Germany, 1996. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.G.; Quaresma, A.C.S.; Brandão, D.L.D.N.; Marinho, A.M.D.R.; Siqueira, J.E.D.S.; Correa, K.L.; Silva-Júnior, J.O.C.; Percario, S.; Dolabela, M.F. Evaluation of Genotoxicity and Toxicity of Annona muricata L. Seeds and In Silico Studies. Molecules 2022, 28, 231. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.S.; Dolabela, M.F.; Póvoa, M.M.; Oliveira, D.J.; Müller, A.H. Estudos farmacognósticos, fitoquímicos, atividade antiplasmódica e toxicidade em Artemia salina de extrato etanólico de folhas de Montrichardia linifera (Arruda) Schott, Araceae. Rev. Bras. Farmacogn. 2009, 19, 834–838. [Google Scholar] [CrossRef]
- Guilhermino, L.; Diamantino, T.; Silva, M.C.; Soares, A.M. Acute toxicity test with Daphnia magna: An alternative to mammals in the prescreening of chemical toxicity? Ecotoxicol. Environ. Saf. 2000, 46, 357–362. [Google Scholar] [CrossRef]
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–364. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, O.A.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef]
- Ferreira, G.G.; Brandão, D.L.D.N.; Dolabela, M.F. Predição do comportamento farmacocinético, toxicidade e de atividades biológicas de alcaloides isolados de Geissospermum laeve (Vell.) Miers. Res. Soc. Dev. 2020, 9, e27991211056. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef] [PubMed]
- Ruthenburg, A.J. Type II Topoisomerase-DNA Interactions: Structure, Mechanism, and Covalent Trapping. Master’s Thesis, Harvard University, Cambridge, MA, USA, 2005. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Hevener, K.E.; Zhao, W.; Ball, D.M.; Babaoglu, K.; Qi, J.; White, S.W.; Lee, R.E. Validation of molecular docking programs for virtual screening against dihydropteroate synthase. J. Chem. Inf. Model. 2009, 49, 444–460. [Google Scholar] [CrossRef] [PubMed]

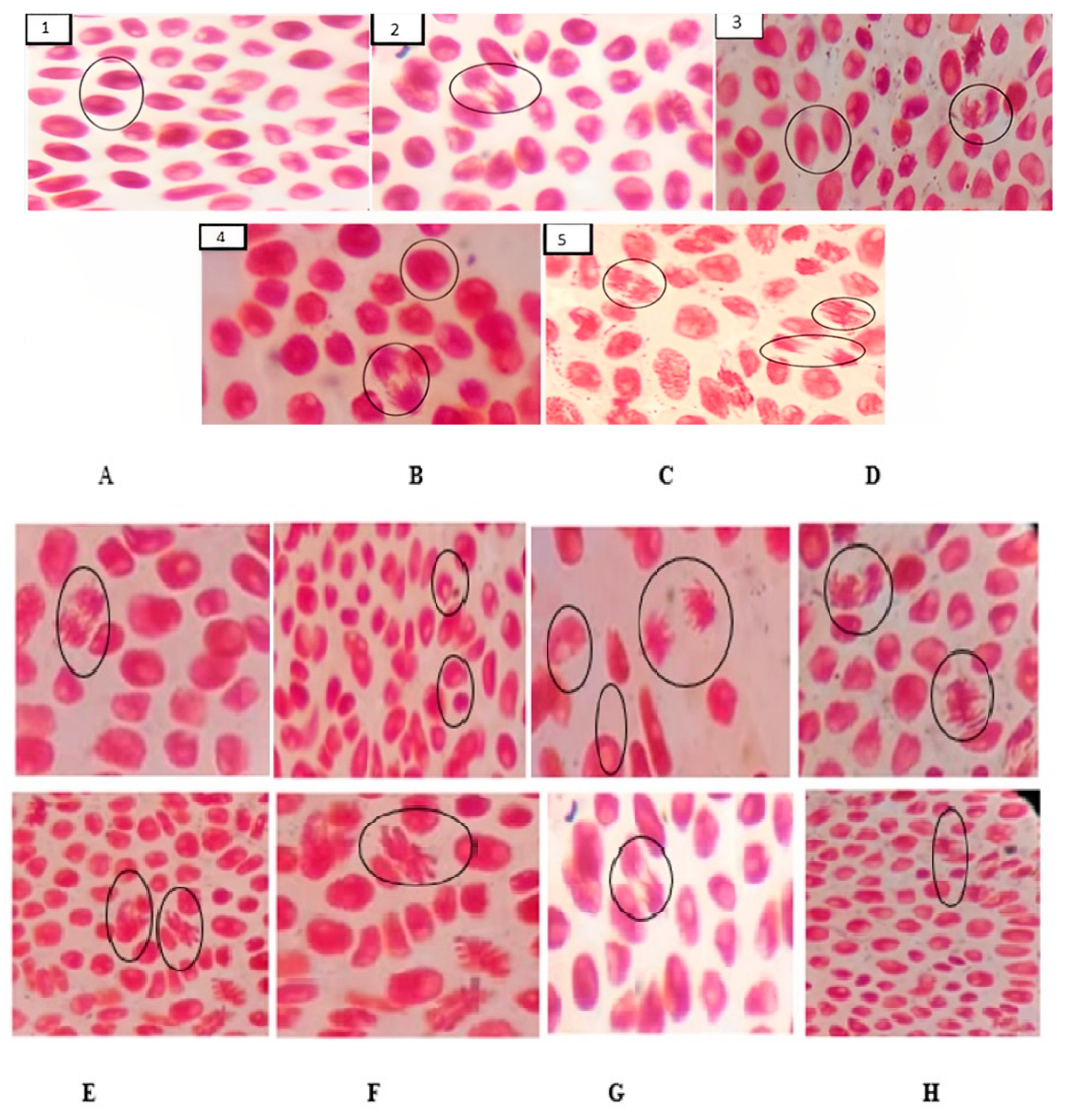
| Samples (µg/mL) | Mitotic Index (%) | Aberration Index (%) | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| NC | 8.5 | 5.3 | 3.8 | 0.06 | 0.1 | 0.2 |
| PC | ||||||
| 160 | ND | ND | ND | ND | ND | ND |
| 80 | 38.0 * | 28.0 * | 20.9 * | 0.56 * | 1.14 * | 1.44 * |
| 40 | 29.0 * | 24.9 * | 16.5 * | 0.52 * | 1.06 * | 1.36 * |
| 20 | 26.3 * | 22.0 * | 12.1 * | 0.48 * | 0.96 * | 124 * |
| 10 | 23.6 * | 19.9 * | 11.9 * | 0.42 * | 0.88 * | 1.14 * |
| EEZR | ||||||
| 340 | 37.1 * | 29.0 * | 19.0 * | 0.52 * | 0.72 * | 1.18 * |
| 170 | 33.7 * | 24.8 * | 17.1 * | 0.44 * | 0.68 * | 1.10 * |
| 85 | 29.0 * | 22.3 * | 15.0 * | 0.40 * | 0.64 * | 1.00 * |
| 42.5 | 25.0 * | 20.6 * | 12.7 * | 0.38 * | 0.58 * | 0.88 * |
| 21.25 | 24.7 * | 18.2 * | 10.0 * | 0.34 * | 0.54 * | 0.78 * |
| FNZR | ||||||
| 840 | ND | ND | ND | ND | ND | ND |
| 420 | 36.7 * | 30.0 * | 19.0 * | 0.46 * | 0.78 * | 1.18 * |
| 210 | 31.9 * | 25.0 * | 15.9 * | 0.44 * | 0.72 * | 1.08 * |
| 105 | 27.8 * | 21.8 * | 12.9 * | 0.40 * | 0.66 * | 0.96 * |
| 52.5 | 24.5 * | 18.0 * | 10.8 * | 0.34 * | 0.56 * | 0.86 * |
| FAZR | ||||||
| 120 | ND | ND | ND | ND | ND | ND |
| 60 | 38.0 * | 28.9 * | 19.6 * | 0.38 * | 0.70 * | 1.12 * |
| 30 | 30.6 * | 25.0 * | 16.7 * | 0.32 * | 0.66 * | 1.04 * |
| 15 | 26.0 * | 22.0 * | 13.4 * | 0.30 * | 0.60 * | 0.92 * |
| 7.5 | 23.0 * | 19.1 * | 11.0 * | 0.28 * | 0.50 * | 0.80 * |
| Mol. | Algae | Daphnia | Fish | Ames | Carcinogenicity | Herg Inhibition | ||
|---|---|---|---|---|---|---|---|---|
| Medaka | Minnow | Mouse | Rat | |||||
| 1 | T | T | VT | VT | NM | N | P | + |
| 2 | T | T | VT | VT | M | N | P | + |
| 3 | T | T | VT | VT | M | N | N | + |
| 4 | T | T | VT | VT | M | N | P | + |
| 5 | T | T | VT | VT | NM | N | N | + |
| 6 | T | T | VT | VT | M | P | N | + |
| 7 | T | NT | VT | VT | NM | N | N | + |
| 8 | T | T | VT | VT | M | P | N | + |
| 9 | T | T | VT | VT | M | N | P | + |
| 10 | T | T | T | VT | NM | P | N | + |
| 11 | T | T | VT | VT | NM | P | N | + |
| 12 | T | T | VT | VT | NM | N | N | + |
| 13 | T | T | VT | VT | NM | N | N | + |
| 14 | T | T | VT | VT | NM | P | N | + |
| 15 | T | T | VT | VT | NM | N | N | + |
| 16 | T | T | T | VT | NM | N | N | + |
| 17 | T | T | VT | VT | NM | N | P | + |
| 18 | T | T | VT | VT | M | N | P | + |
| 19 | T | T | VT | VT | M | P | N | + |
| 20 | T | T | VT | VT | M | N | N | + |
| 21 | T | T | VT | VT | M | N | N | + |
| 22 | T | T | T | VT | M | N | P | + |
| 23 | NT | T | VT | VT | NM | N | P | + |
| 24 | T | T | VT | VT | NM | P | P | + |
| 25 | T | T | VT | VT | NM | N | P | + |
| 26 | T | T | VT | VT | M | P | N | + |
| 27 | T | VT | VT | VT | M | N | P | + |
| 28 | T | T | VT | VT | M | P | N | + |
| 29 | T | T | VT | VT | M | N | N | + |
| Molecules | LD50 (mg/Kg) | Classification | Toxic Effect |
|---|---|---|---|
| 1 | 1250 | N | I |
| 2 | 1250 | N | I |
| 3 | 167 | T | I |
| 4 | 1000 | N | I |
| 5 | 778 | N | I |
| 6 | 445 | N | I |
| 7 | 401 | N | I |
| 8 | 1000 | N | I |
| 9 | 778 | N | I |
| 10 | 940 | NT | NTE |
| 11 | 347 | N | I |
| 12 | 1000 | N | I |
| 13 | 200 | T | I |
| 14 | 778 | N | I |
| 15 | 580 | N | I |
| 16 | 650 | N | I |
| 17 | 296 | T | I |
| 18 | 640 | N | I |
| 19 | 1000 | N | NTE |
| 20 | 167 | T | I |
| 21 | 167 | T | I |
| 22 | 2300 | P | I |
| 23 | 2000 | N | I |
| 24 | 1000 | N | NTE |
| 25 | 1000 | N | I |
| 26 | 700 | N | I |
| 27 | 305 | N | NTE |
| 28 | 1200 | N | NTE |
| 29 | 3000 | P | NTE |
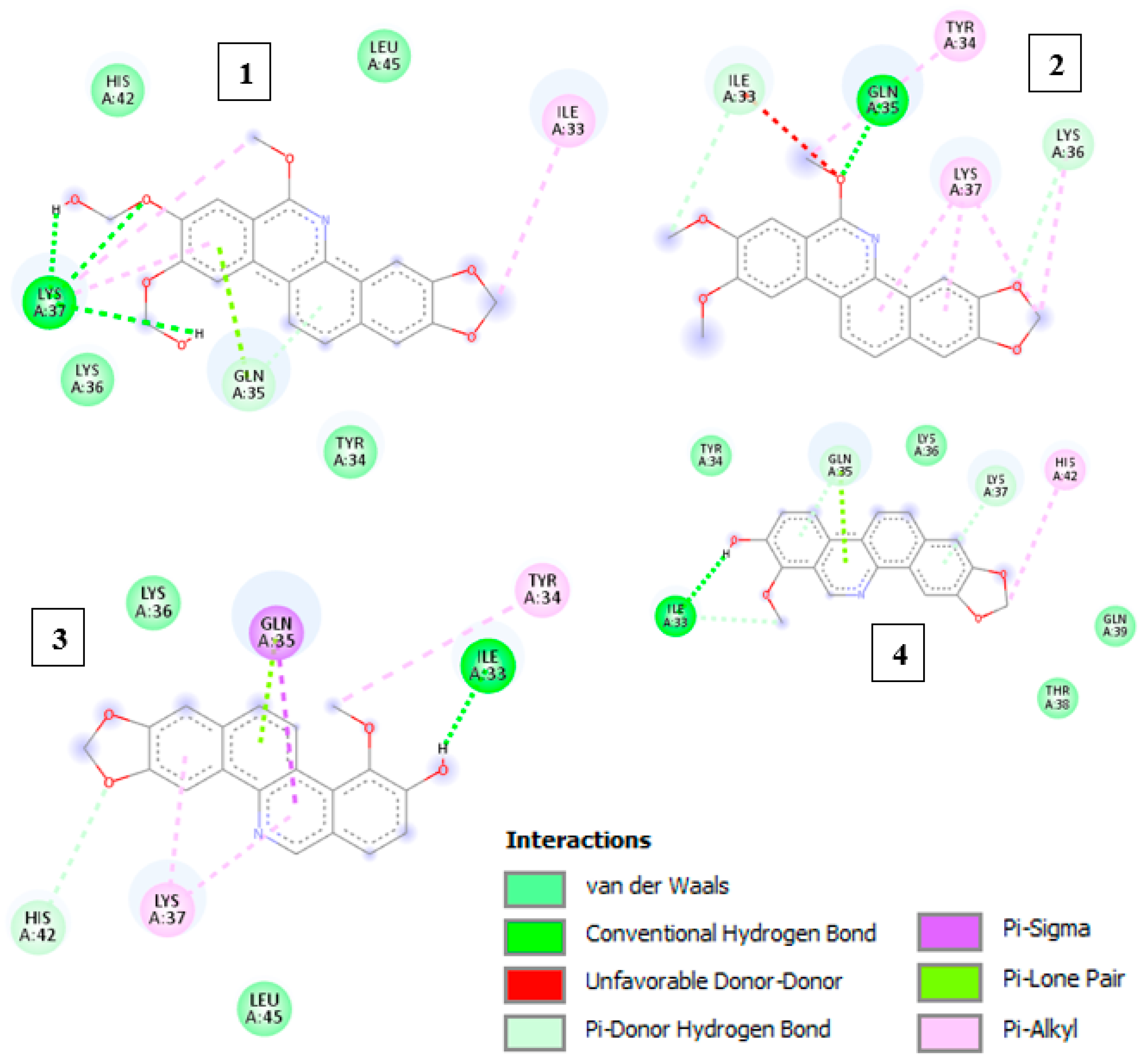
| Binder | ΔG Kcal/mol | Ki µM | Hydrogen Bridges | Hydrophobic Bonds | π-Bond |
|---|---|---|---|---|---|
| Rhoifoline A | 4.58 | 439.97 | Lys37 | His42, Leu45, Tyr34, Gln35 | Ile33 |
| Rhoifoline B | 5.4 | 110.97 | Gln35 | Ile33, Lys36 | Tyr34, Lys37 |
| Decarine | 5.0 | 217.56 | Ile33 | Tyr34, Gln35, Lys37, Gln39, Thr38 | His42, Gln35, Lys37 |
| Zanthoxyline | 5.08 | 189.94 | Ile33 | Lys36, Leu45, His42 | Gln35, Tyr34, Lys37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azonsivo, R.; Albuquerque, K.C.O.d.; Castro, A.L.G.; Correa-Barbosa, J.; Souza, H.J.R.d.; Almada-Vilhena, A.O.d.; Ferreira, G.G.; Souza, A.A.d.; Marinho, A.M.d.R.; Percario, S.; et al. Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids. Molecules 2023, 28, 5336. https://doi.org/10.3390/molecules28145336
Azonsivo R, Albuquerque KCOd, Castro ALG, Correa-Barbosa J, Souza HJRd, Almada-Vilhena AOd, Ferreira GG, Souza AAd, Marinho AMdR, Percario S, et al. Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids. Molecules. 2023; 28(14):5336. https://doi.org/10.3390/molecules28145336
Chicago/Turabian StyleAzonsivo, Rufine, Kelly Cristina Oliveira de Albuquerque, Ana Laura Gadelha Castro, Juliana Correa-Barbosa, Helena Joseane Raiol de Souza, Andryo Orfi de Almada-Vilhena, Gleison Gonçalves Ferreira, Anderson Albuquerque de Souza, Andrey Moacir do Rosario Marinho, Sandro Percario, and et al. 2023. "Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids" Molecules 28, no. 14: 5336. https://doi.org/10.3390/molecules28145336
APA StyleAzonsivo, R., Albuquerque, K. C. O. d., Castro, A. L. G., Correa-Barbosa, J., Souza, H. J. R. d., Almada-Vilhena, A. O. d., Ferreira, G. G., Souza, A. A. d., Marinho, A. M. d. R., Percario, S., Nagamachi, C. Y., Pieczarka, J. C., & Dolabela, M. F. (2023). Cytotoxicity and Genotoxicity Evaluation of Zanthoxylum rhoifolium Lam and In Silico Studies of Its Alkaloids. Molecules, 28(14), 5336. https://doi.org/10.3390/molecules28145336








