Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
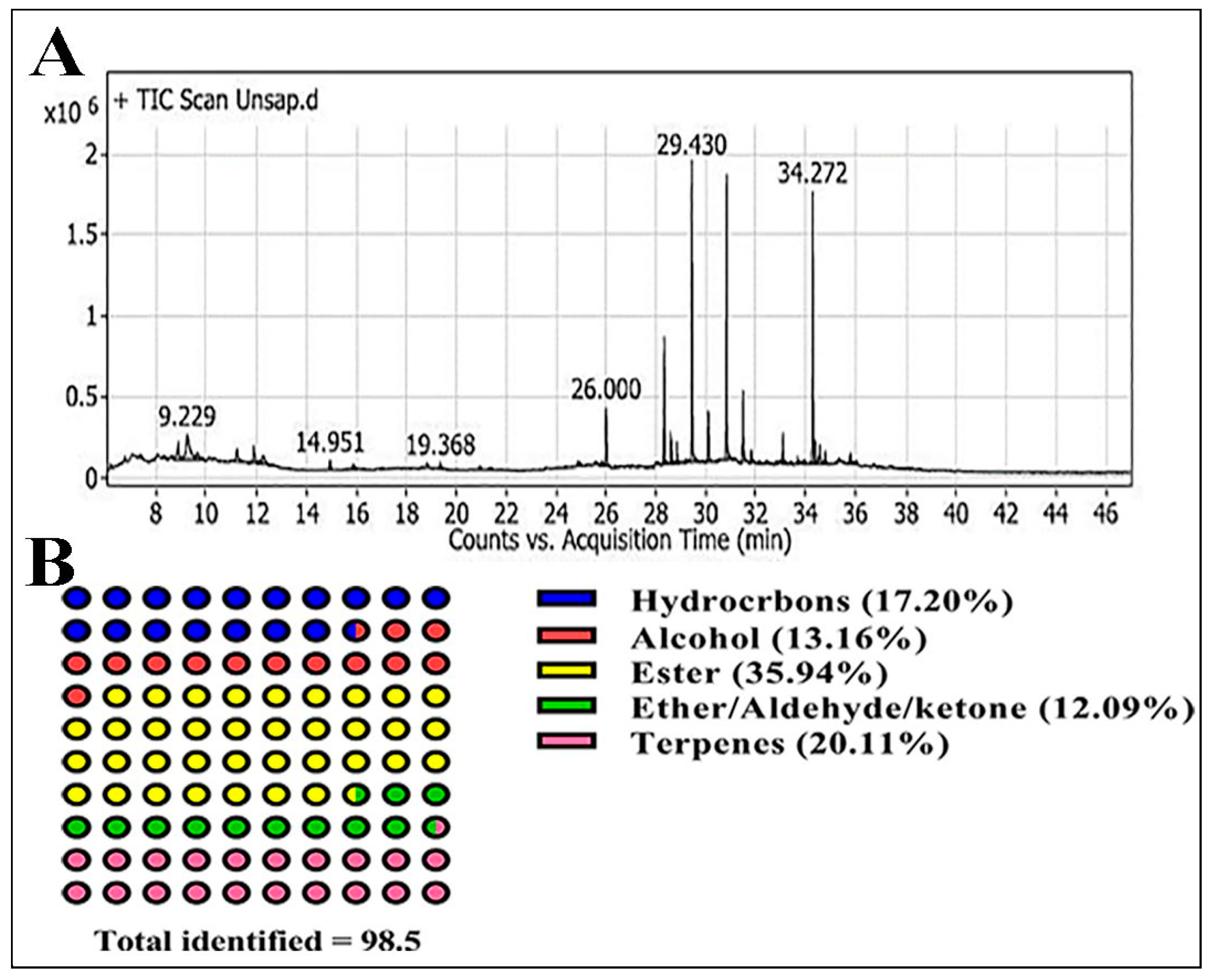
GC-MS Analysis
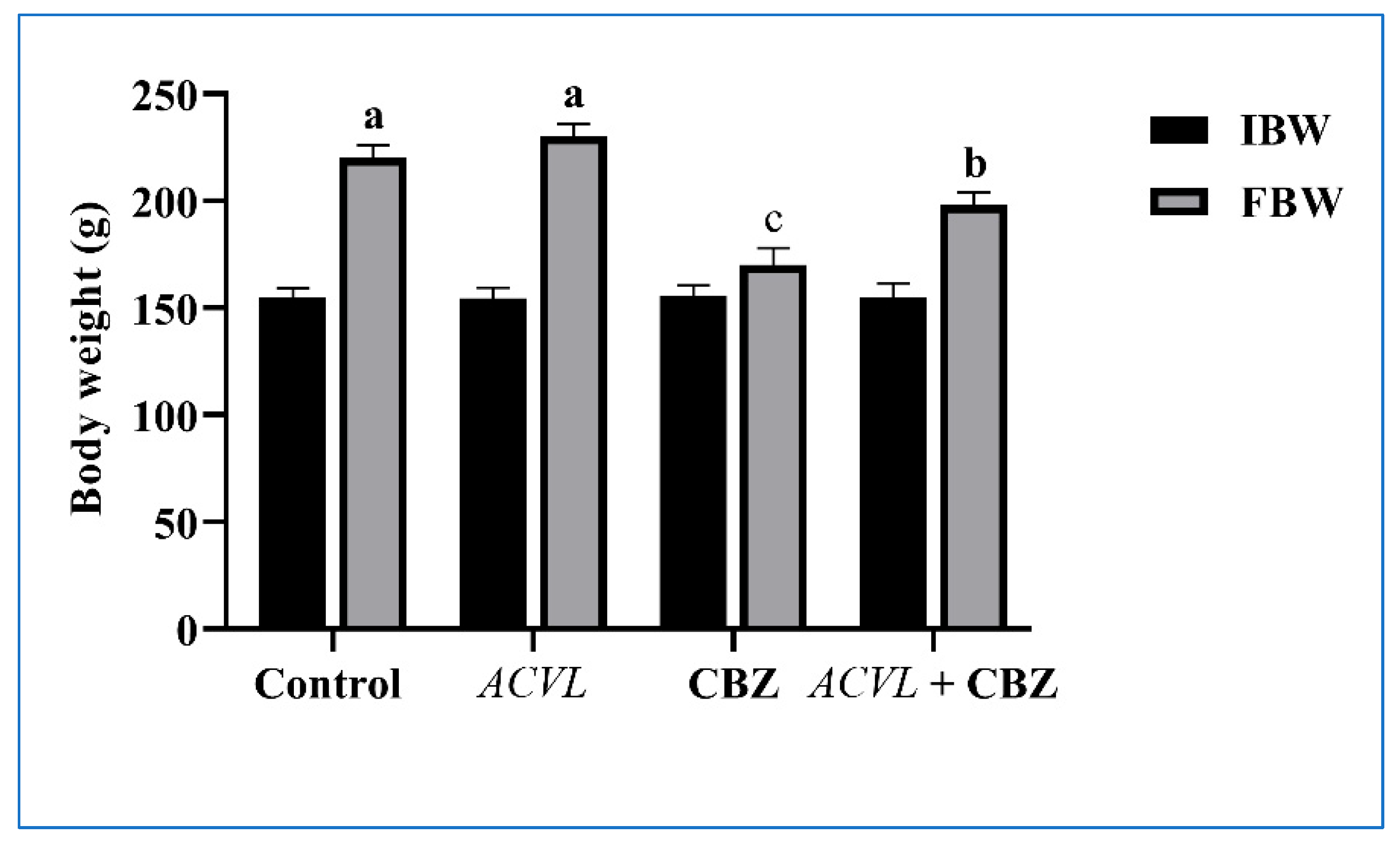
2.2. General Clinical Symptoms, Body Weight, and Liver Weight
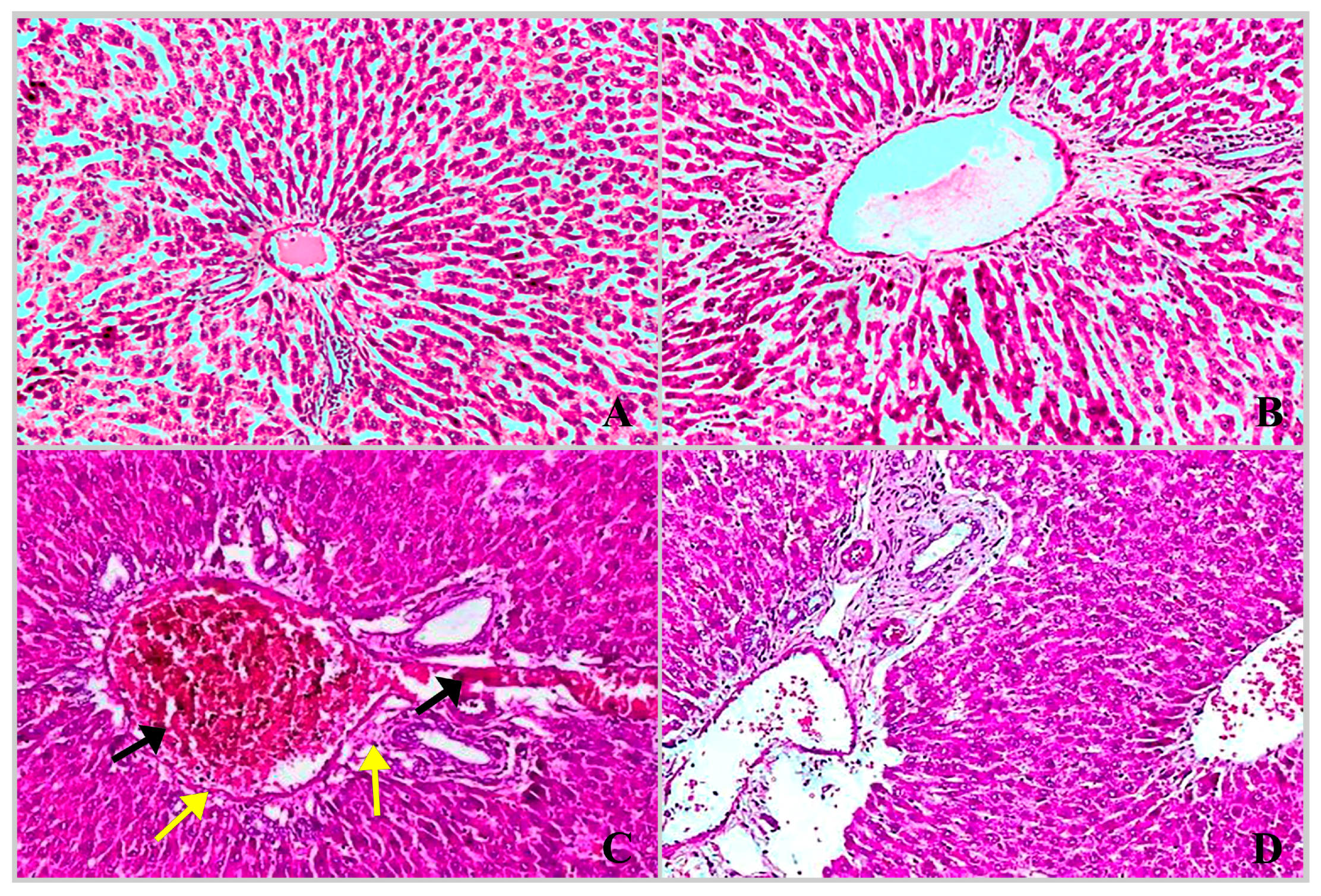
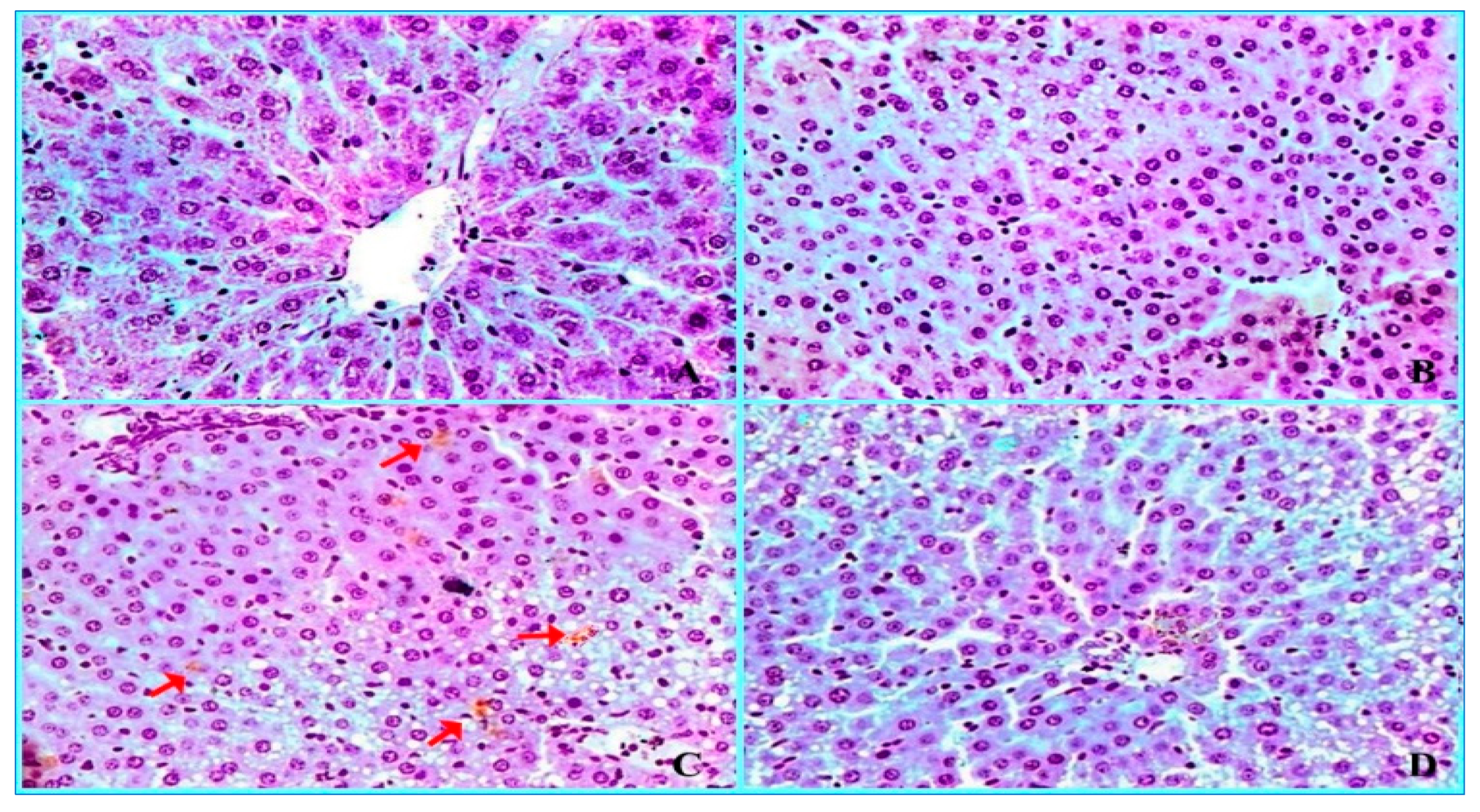
2.3. Histopathological Findings
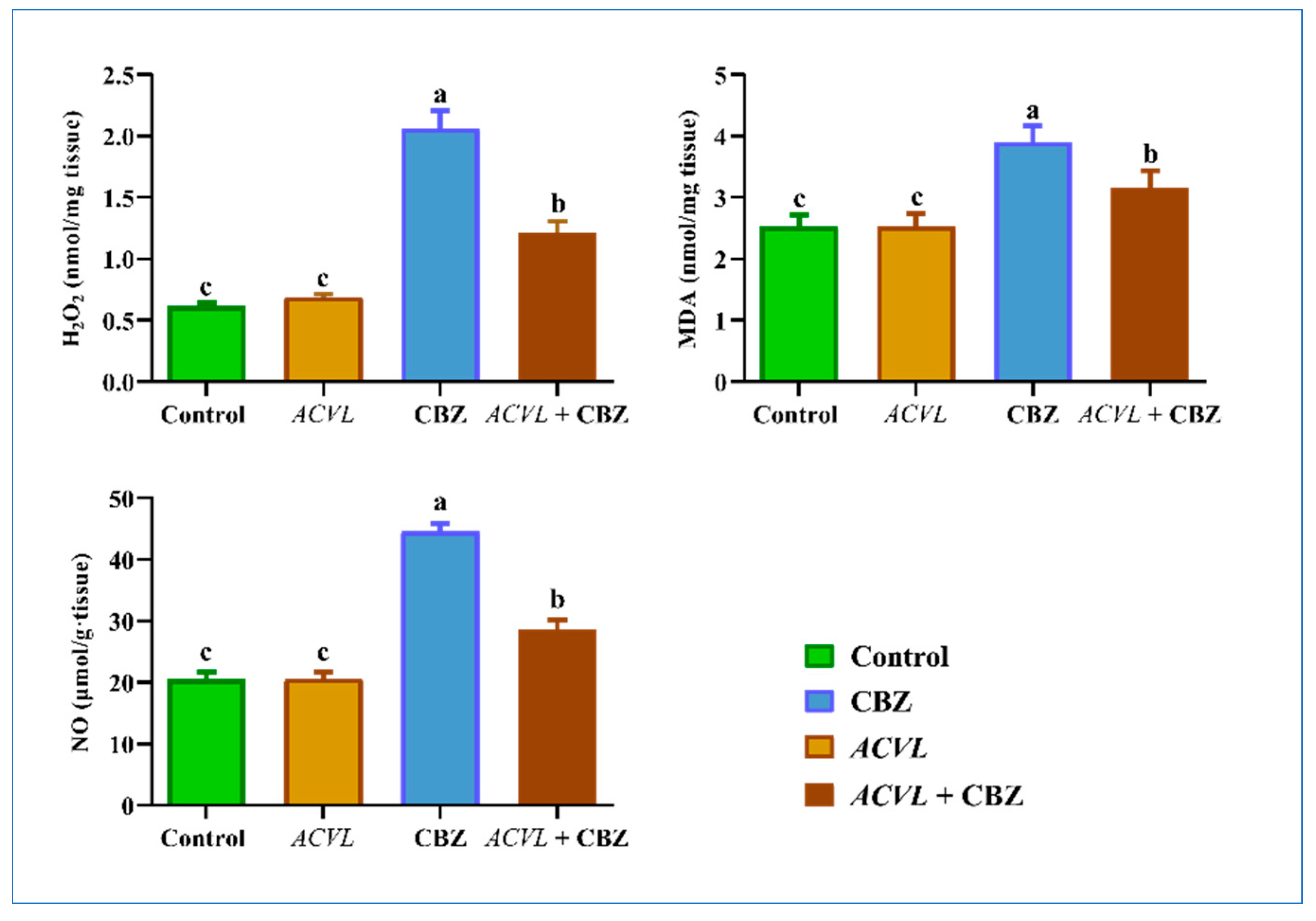
2.4. Oxidative Stress and Antioxidant Biomarkers
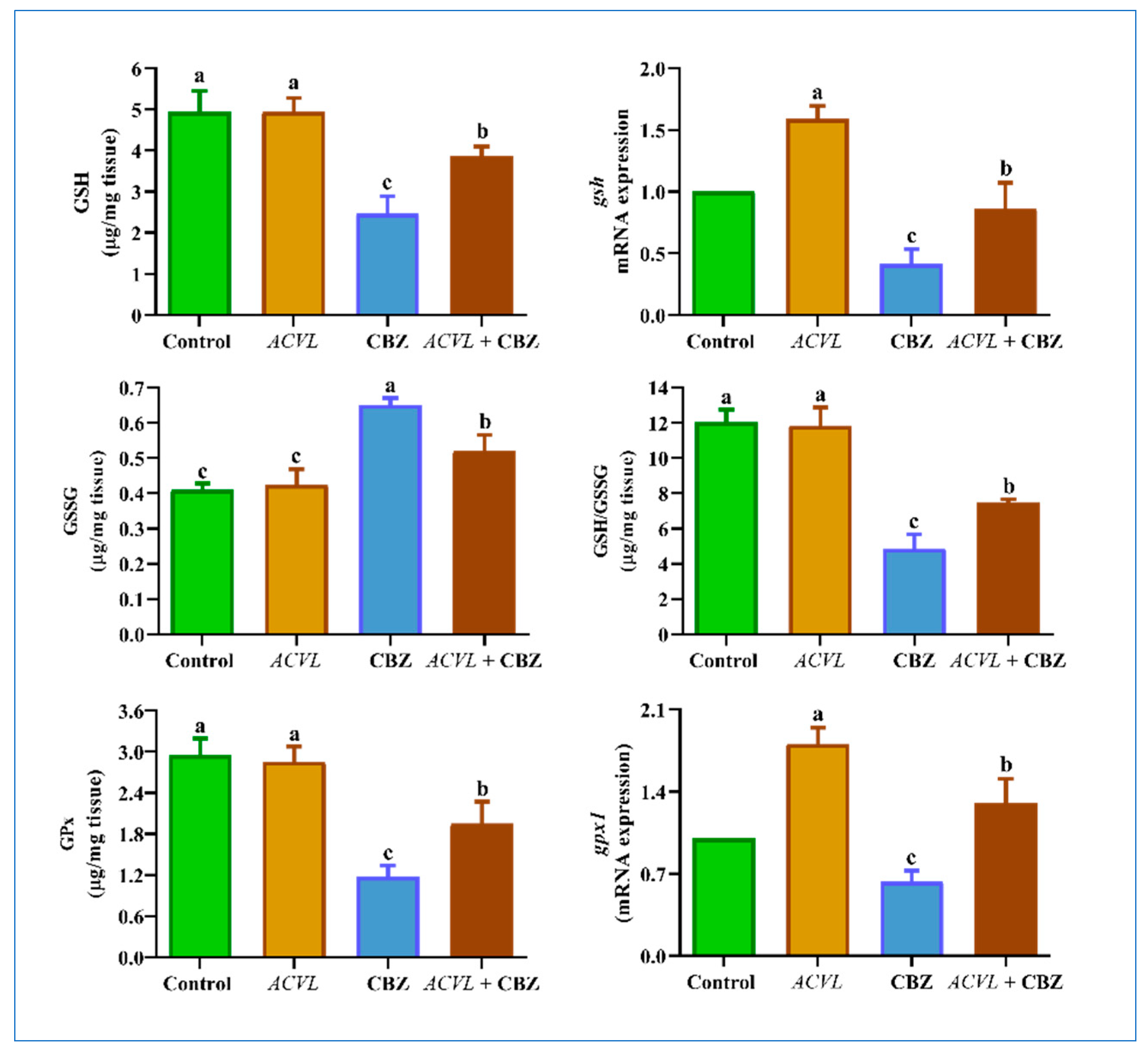
2.5. GSH-Related Antioxidant Biomarkers
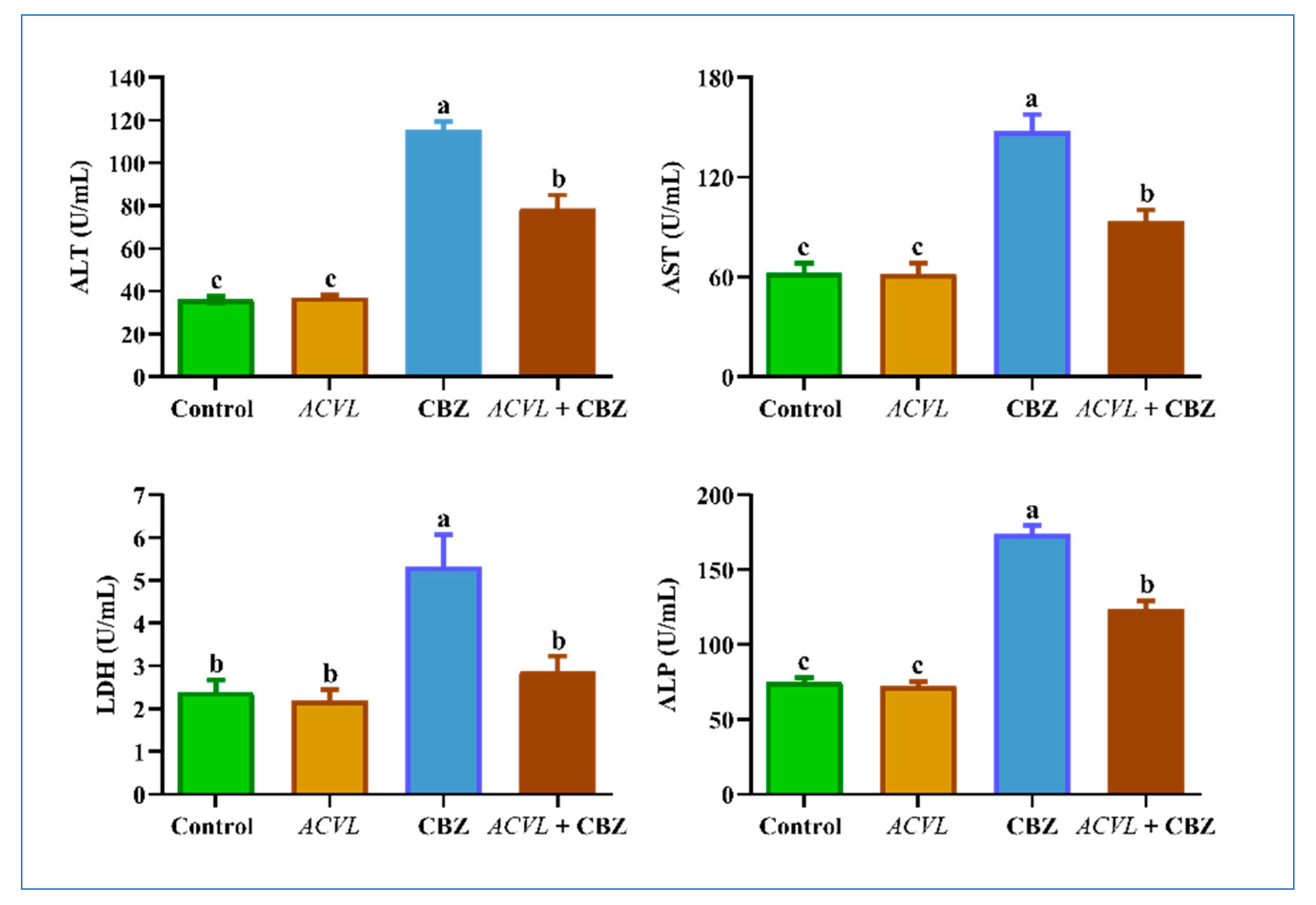
2.6. Serum Hepatic Function Biomarkers
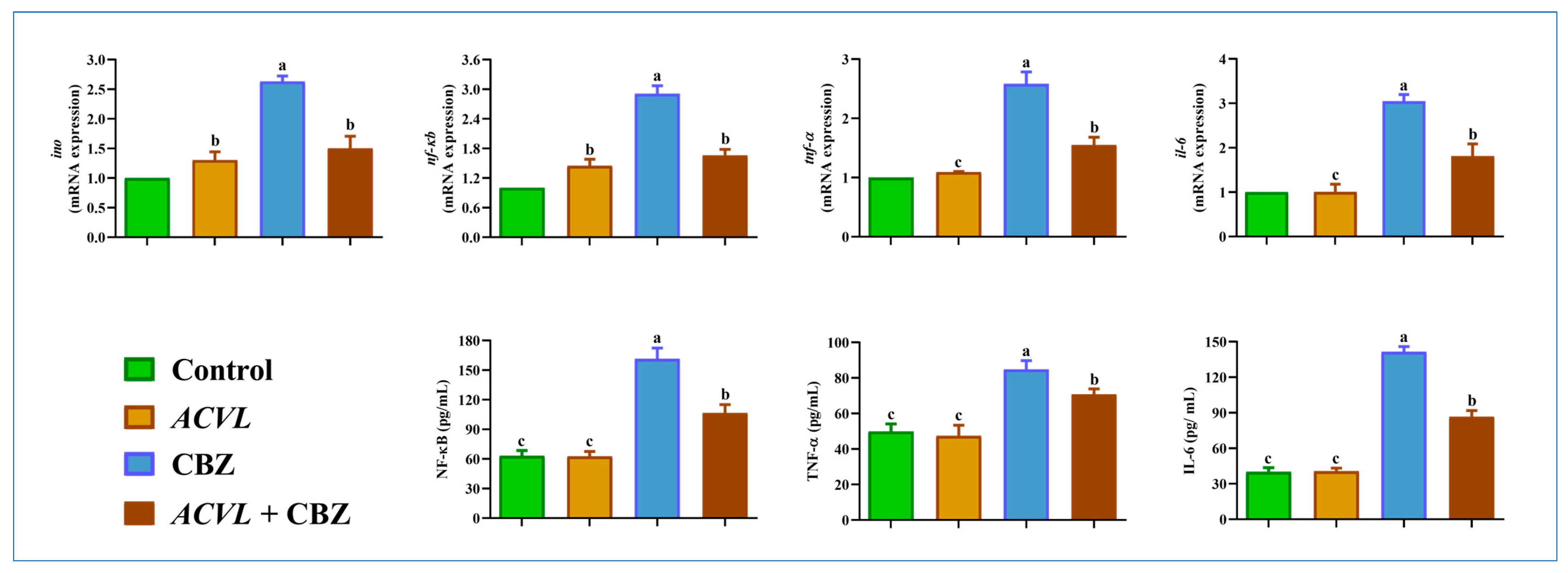
2.7. Hepatic Inflammation Biomarkers
2.8. Detection of the NF-κB (IHC)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Kits
4.2. Plant Materials and Extract Preparation
4.3. Phytochemical Analysis
4.3.1. Quantitative Estimation of Phenolic and Flavonoid Contents
4.3.2. Evaluation of the Radical Scavenging Abilities of ACVL
ABTS Assay
DPPH Free Radical Scavenging Assay
4.3.3. Identified the Hydrocarbon and Fatty Acid Contents by GC/MS
Gas Chromatography-Mass Spectrometry Analysis (GC/MS)
Gas Chromatography for Fatty Acids Methyl Ester (FAME)
4.4. Biological Evaluation of ACVL Extract
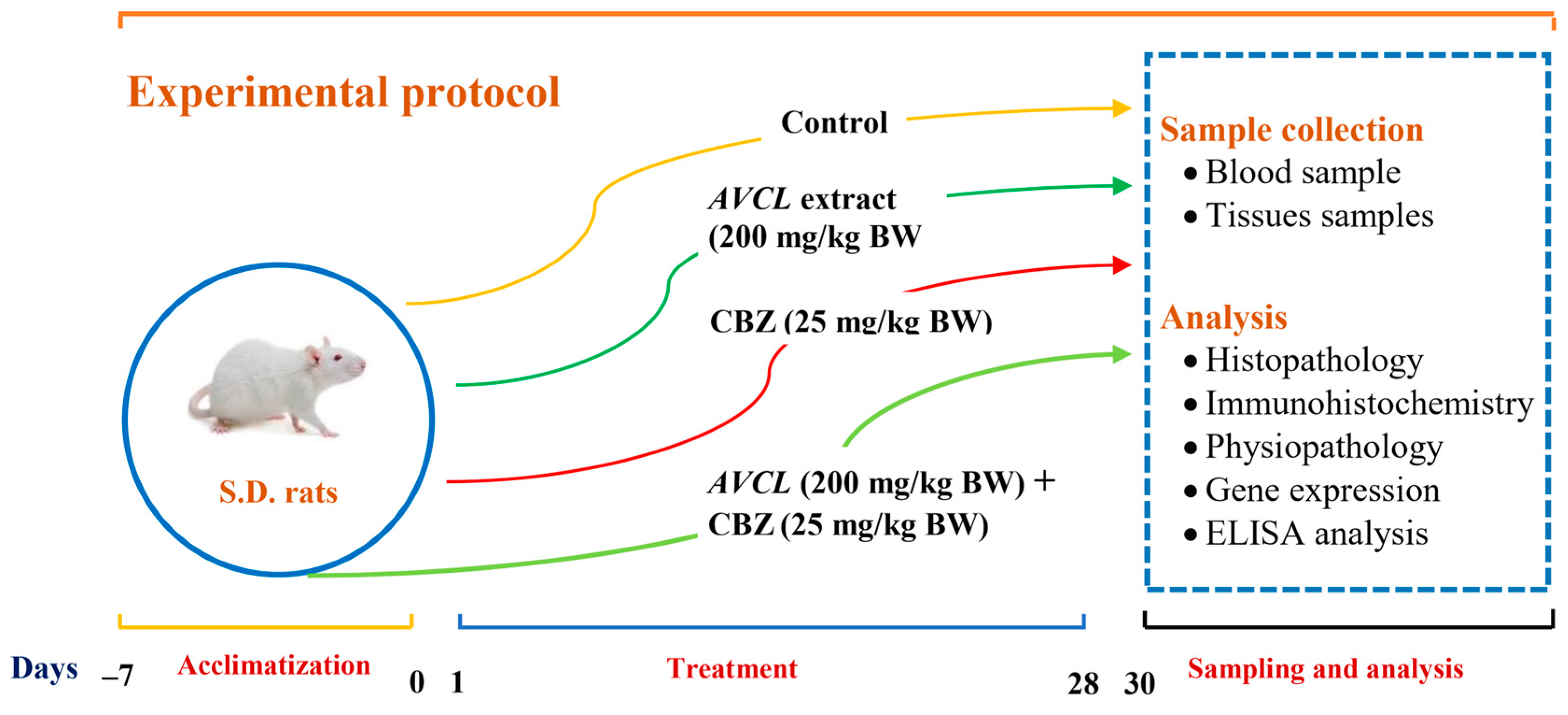
4.4.1. Animals and Treatments
- The control group: animals served as controls and were given normal saline.
- The ACVL group: animals were treated orally with plant extract (200 mg kg−1 BW).
- The ACVL+ CBZ group: animals were given ACVL extract (200 mg kg−1 BW) and CBZ (25 mg kg−1 BW) orally.
4.4.2. Histopathological Inspection
4.4.3. Oxidative Stress Markers
Preparation of Liver Tissue Homogenates
Oxidative Stress Biomarkers
4.4.4. Hepatic Functions Biomarkers
4.4.5. Antioxidant Enzymatic Markers
4.4.6. GSH System Markers
4.4.7. Determination NF-κB, TNF-α, and IL-6 in Liver Tissue
4.4.8. Detection of the NF-κB (IHC)
4.4.9. qPCR Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Russell, P. The development of commercial disease control. Plant Pathol. 2006, 55, 585–594. [Google Scholar] [CrossRef]
- Seif, M.; Deabes, M.; El-Askary, A.; El-Kott, A.F.; Albadrani, G.M.; Seif, A.; Wang, Z. Ephedra sinica mitigates hepatic oxidative stress and inflammation via suppressing the TLR4/MyD88/NF-κB pathway in fipronil-treated rats. Environ. Sci. Pollut. Res. 2021, 28, 62943–62958. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Bakırcı, G.T.; Acay, D.B.Y.; Bakırcı, F.; Ötleş, S. Pesticide residues in fruits and vegetables from the Aegean region, Turkey. Food Chem. 2014, 160, 379–392. [Google Scholar] [CrossRef]
- Chakraborty, U.; Kaur, G.; Chaudhary, G.R. Development of Environmental Nanosensors for Detection Monitoring and Assessment. In New Frontiers of Nanomaterials in Environmental Science; Springer: Berlin/Heidelberg, Germany, 2021; pp. 91–143. [Google Scholar]
- Hathout, A.; Amer, M.; Mossa, A.-T.H.; Hussain, O.; Yassen, A.A.; Elgohary, M.R. Estimation of the Most Widespread Pesticides in Agricultural Soils Collected from Some Egyptian Governorates. Egypt. J. Chem. 2022, 65, 35–44. [Google Scholar] [CrossRef]
- Gad Alla, S.; Almaz, M.M.; Thabet, W.M.; Nabil, M.M. Evaluation of pesticide residues in some Egyptian fruits. Int. J. Environ. 2015, 4, 87–97. [Google Scholar]
- Liu, H.; Xie, L.; Wang, Y.; Liu, Y.; Fu, R.; Cui, Y.; Zhao, Q.; Wang, C.; Jiao, B.; He, Y. Construction of a portable immunosensor for the sensitive detection of carbendazim in agricultural products using a personal glucose meter. Food Chem. 2023, 407, 135161. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Li, B.; Tang, L. Carbendazim residues in vegetables in China between 2014 and 2016 and a chronic carbendazim exposure risk assessment. Food Control 2018, 91, 20–25. [Google Scholar] [CrossRef]
- Adekomi, D.A. Madagascar periwinkle (Catharanthus roseus) enhances kidney and liver functions in Wistar rats. Int. J. Biomed. Health Sci. 2021, 6, 245–254. [Google Scholar]
- Cortés-Iza, S.C.; Rodríguez, A.I. Oxidative stress and pesticide disease: A challenge for toxicology. Rev. Fac. Med. 2018, 66, 261–267. [Google Scholar] [CrossRef]
- Abdel-Rahman, G.N.; Fouzy, A.S.; Amer, M.M.; Saleh, E.M.; Hamed, I.A.; Sabry, B.A. Control of carbendazim toxicity using banana peel powder in rats. Biotechnol. Rep. 2022, 36, e00773. [Google Scholar] [CrossRef]
- Zhu, M.; Li, H.; Bai, L.; Wang, L.; Zou, X. Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult. Sci. 2020, 99, 3215–3228. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.M.; Yoon, J.-H.; Lim, J.; Shin, J.-W.; Cho, A.Y.; Heo, J.; Lee, K.B.; Lee, J.-H.; Lee, W.J.; Kim, H.-J. Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem Cell Rep. 2018, 10, 600–614. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Madboli, A.E.-N.A.; Seif, M.M. Immunohistochemical, histopathological, and biochemical studies of the NF-κB P65 marker in rat ovaries experimentally intoxicated by cadmium and the protective effect of the purslane plant extract. Environ. Sci. Pollut. Res. 2021, 28, 17613–17626. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.; El-Aziz, A.; Sayed, M.; Wang, Z. Zingiber officinale ethanolic extract attenuates oxidative stress, steroidogenic gene expression alterations, and testicular histopathology induced by sodium arsenite in male rats. Environ. Sci. Pollut. Res. 2021, 28, 19783–19798. [Google Scholar] [CrossRef]
- Seif, M.M.; Madboli, A.-N.; Marrez, D.A.; Aboulthana, W.M. Hepato-renal protective effects of Egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Ibraheim, Z.Z.; Ahmed, A.S.; Gouda, Y.G. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm. J. 2011, 19, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Boukada, F.; Sitayeb, S.; Khadem, H.; Meddah, B.; Zohra, S.F. Chemical composition, antioxidant and antibacterial activity of Adiantum capillus-veneris L. extract from Algeria. Kragujev. J. Sci. 2022, 44, 91–101. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. Reversed phase HPLC-DAD profiling of carotenoids, chlorophylls and phenolic compounds in Adiantum capillus-veneris leaves. Front. Chem. 2017, 5, 29. [Google Scholar] [CrossRef]
- Ishaq, M.S.; Hussain, M.M.; Siddique Afridi, M.; Ali, G.; Khattak, M.; Ahmad, S. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris. Sci. World J. 2014, 2014, 269793. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Lavate, R.; Raole, V.; Rajput, K.; Adiantum, L. Overview on Taxonomy, Distribution, Conservation Status, Ethnomedicinal Uses, Phytochemistry, and Pharmacognosy from India. In Ferns: Biotechnology, Propagation, Medicinal Uses and Environmental Regulation; Springer: Berlin/Heidelberg, Germany, 2022; pp. 553–570. [Google Scholar]
- Ahmadpoor, J.; Chahardahcheric, S.V.; Setorki, M. The Protective effect of hydroalcoholic extract of the southern maidenhair fern (adiantum capillus-veneris) on the depression and anxiety caused by chronic stress in adult male mice: An experimental randomized study. Iran. Red Crescent Med. J. 2019, 21, e86750. [Google Scholar] [CrossRef]
- Hadi, I.; Mashkoor Hussein, H. Antimicrobial Activity and spectral chemical analysis of methanolic leaves extract of Adiantum capillus-veneris using GC-MS and FT-IR spectroscopy. Int. J. Pharm. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Omidi, S.; Sedaghat, S.; Tahvildari, K.; Derakhshi, P.; Motiee, F. Biosynthesis of silver nanoparticles with Adiantum capillus-veneris L leaf extract in the batch process and assessment of antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 544–551. [Google Scholar] [CrossRef]
- Madboli, A.E.-N.A.; Seif, M.M. Adiantum capillus-veneris Linn protects female reproductive system against carbendazim toxicity in rats: Immunohistochemical, histopathological, and pathophysiological studies. Environ. Sci. Pollut. Res. 2021, 28, 19768–19782. [Google Scholar] [CrossRef] [PubMed]
- Rautray, S.; Panikar, S.; Sofia, A.; Rajananthini, A.U. Anti-oxidant and anti-microbial study of Adiantum capillus veneris and Pteris quadriureta L. J. Med. Plants Res. 2018, 12, 359–368. [Google Scholar]
- Zakir, M.; Ashraf, N.; Minhajuddin, M.A.A.; Javed, G.; Ashraf, N. Ethnomedicinal properties of parsioshan (Adiantum capillus-veneris L.): An important herb of the unani system of medicine. J. Clin. Otorhinolaryngol. Head Neck Surg. 2022, 26, 21–36. [Google Scholar]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.; Azab, A.; Quwaydir, F. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant-and Animal-Based Antioxidants’ Structure, Efficacy, Mechanisms, and Applications: A Review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochemicals: Source of Antioxidants and Role in Disease Prevention. BoD Books Demand 2018, 7, 49–74. [Google Scholar]
- Tiloke, C.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer. Biomed. Pharmacother. 2018, 108, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahi, I.; Talebi, E.; Bashardoost, Z. In-vitro study of chemical composition, antimicrobial and antioxidant properties of Adiantum capillus-veneris L. essential oil. Preprints 2022. [Google Scholar] [CrossRef]
- Roy, S.C.; Sajeeb, B.; Muhit, M.A.; Bachar, S.C. Evaluation of antioxidant and cytotoxic activities of aerial parts of Adiantum capillus-veneris L. growing in Bangladesh. Dhaka Univ. J. Pharm. Sci. 2019, 18, 217–222. [Google Scholar] [CrossRef]
- Patil, N.; Lonare, M.; Sharma, M.; Lalhriatpuia, P.; Saini, S.; Rampal, S. Hemato-biochemical alterations mediated by carbendazim exposure and protective effect of quercetin in male rats. Toxicol. Int. 2018, 25, 7–18. [Google Scholar]
- Selmanoğlu, G.; Barlas, N.; Songür, S.; KocSkaya, E. Carbendazim-induced haematological, biochemical and histopathological changes to the liver and kidney of male rats. Hum. Exp. Toxicol. 2001, 20, 625–630. [Google Scholar] [CrossRef]
- Salihu, M.; Ajayi, B.O.; Adedara, I.A.; Farombi, E.O. 6-Gingerol-rich fraction from Zingiber officinale prevents hematotoxicity and oxidative damage in kidney and liver of rats exposed to carbendazim. J. Diet. Suppl. 2016, 13, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Mo, E.; Ebedy, Y.A.; Ibrahim, M.A.; Farroh, K.Y.; Hassanen, E.I. Newly synthesized chitosan-nanoparticles attenuate carbendazim hepatorenal toxicity in rats via activation of Nrf2/HO1 signalling pathway. Sci. Rep. 2022, 12, 9986. [Google Scholar] [CrossRef]
- Abolaji, A.; Awogbindin, I.; Adedara, I.; Farombi, E. Insecticide chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. Hum. Exp. Toxicol. 2017, 36, 483–493. [Google Scholar] [CrossRef]
- Agrahari, S.; Pandey, K.C.; Gopal, K. Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic. Biochem. Physiol. 2007, 88, 268–272. [Google Scholar] [CrossRef]
- Ramos-Tovar, E.; Muriel, P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage. J. Appl. Toxicol. 2020, 40, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Nwozo, S.O.; Najophe, E.S. Quercetin abates induction of hepatic and renal oxidative damage, inflammation, and apoptosis in carbendazim-treated rats. Toxicol. Res. Appl. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Patil, N.V.; Lonare, M.K.; Sharma, M.; Deshmukh, S.; Gupta, K.; Sharma, S.K. Ameliorative Effect of Quercetin on Neurotoxicogical Alterations Induced by Carbendazim: Oxidative Stress, Biochemicals, and Histopathology. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 93, 351–364. [Google Scholar] [CrossRef]
- Leutner, S.; Eckert, A.; Müller, W. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001, 108, 955–967. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathi, A.; Tripathi, P.; Singh, R.; Singh, S.; Singh, R. Studies on lipid peroxidation and non-enzymatic antioxidant status as indices of oxidative stress in patients with chronic myeloid leukaemia. Singap. Med. J. 2010, 51, 110. [Google Scholar]
- Keita, M.; Vincendeau, P.; Buguet, A.; Cespuglio, R.; Vallat, J.-M.; Dumas, M.; Bouteille, B. Inducible nitric oxide synthase and nitrotyrosine in the central nervous system of mice chronically infected with Trypanosoma brucei brucei. Exp. Parasitol. 2000, 95, 19–27. [Google Scholar] [CrossRef]
- Scalavino, V.; Liso, M.; Cavalcanti, E.; Gigante, I.; Lippolis, A.; Mastronardi, M.; Chieppa, M.; Serino, G. miR-369-3p modulates inducible nitric oxide synthase and is involved in regulation of chronic inflammatory response. Sci. Rep. 2020, 10, 15942. [Google Scholar] [CrossRef]
- Hunto, S.T.; Kim, H.G.; Baek, K.-S.; Jeong, D.; Kim, E.; Kim, J.H.; Cho, J.Y. Loratadine, an antihistamine drug, exhibits anti-inflammatory activity through suppression of the NF-kB pathway. Biochem. Pharmacol. 2020, 177, 113949. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Liu, B.; Liu, J.; Liu, S.; Zhao, Z.; Li, W.; Tian, B.; Zhao, R.; He, F. Guiding Transition Metal-Doped Hollow Cerium Tandem Nanozymes with Elaborately Regulated Multi-Enzymatic Activities for Intensive Chemodynamic Therapy. Adv. Mater. 2022, 34, 2107054. [Google Scholar] [CrossRef]
- Gate, L.; Paul, J.; Ba, G.N.; Tew, K.; Tapiero, H. Oxidative stress induced in pathologies: The role of antioxidants. Biomed. Pharmacother. 1999, 53, 169–180. [Google Scholar] [CrossRef]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a micronutrient that modulates cardiovascular health via redox enzymology. Nutrients 2021, 13, 3238. [Google Scholar] [CrossRef] [PubMed]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Kaplowitz, N. Glutathione in liver diseases and hepatotoxicity. Mol. Asp. Med. 2009, 30, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Therapeutic properties of medicinal plants: A review of plants with antioxidant activity (part 1). Int. J. Pharmacol. Toxicol. 2015, 6, 159–182. [Google Scholar]
- Hoseinifar, S.H.; Jahazi, M.A.; Mohseni, R.; Raeisi, M.; Bayani, M.; Mazandarani, M.; Yousefi, M.; Van Doan, H.; Mozanzadeh, M.T. Effects of dietary fern (Adiantum capillus-veneris) leaves powder on serum and mucus antioxidant defence, immunological responses, antimicrobial activity and growth performance of common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 2020, 106, 959–966. [Google Scholar] [CrossRef]
- Dehdari, S.; Hajimehdipoor, H. Medicinal properties of Adiantum capillus-veneris Linn. in traditional medicine and modern phytotherapy: A review article. Iran. J. Public Health 2018, 47, 188. [Google Scholar]
- Vadi, R.; Manisha, V.; Swati, K. Hansraj (Adiantum capillus veneris Linn.): A systematic review on its ethnobotany, phytochemical and pharmacological profile. Int. J. Ayurveda Pharma Res. 2017, 5, 5–21. [Google Scholar]
- Khodaie, L.; Esnaashari, S.; Moghaddam, S.B. Essential oil of arial parts of Adiantum capillus-veneris: Chemical composition and antioxidant activity. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e21968. [Google Scholar] [CrossRef]
- Rabiei, Z.; Setorki, M. Effect of ethanol Adiantum capillus-veneris extract in experimental models of anxiety and depression. Braz. J. Pharm. Sci. 2019, 55, e18099. [Google Scholar] [CrossRef]
- Vithoulkas, G. An integrated perspective on transmutation of acute inflammation into chronic and the role of the microbiome. J. Med. Life 2021, 14, 740. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martínez de Marañón, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Khoramian, L.; Sajjadi, S.-E.; Minaiyan, M. Anti-inflammatory effect of Adiantum capillus-veneris hydroalcoholic and aqueous extracts on acetic acid-induced colitis in rats. Avicenna J. Phytomed. 2020, 10, 492. [Google Scholar] [PubMed]
- Yuan, Q.; Zhang, X.; Liu, Z.; Song, S.; Xue, P.; Wang, J.; Ruan, J. Ethanol extract of Adiantum capillus-veneris L. suppresses the production of inflammatory mediators by inhibiting NF-Κb Activation. J. Ethnopharmacol. 2013, 147, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Maheshwari, N.; Khan, F.H.; Mahmood, R. Carbendazim toxicity in different cell lines and mammalian tissues. J. Biochem. Mol. Toxicol. 2022, 36, e23194. [Google Scholar] [CrossRef]
- Contreras-Zentella, M.L.; Hernández-Muñoz, R. Is liver enzyme release really associated with cell necrosis induced by oxidant stress? Oxidative Med. Cell. Longev. 2016, 2016, 3529149. [Google Scholar] [CrossRef]
- Peltenburg, H.G.; Hermens, W.T.; Willems, G.M.; Flendrig, J.G.; Schmidt, E. Estimation of the fractional catabolic rate constants for the elimination of cytosolic liver enzymes from plasma. Hepatology 1989, 10, 833–839. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Jiang, M.Z.; Yan, H.; Wen, Y.; Li, X.-M. In vitro and in vivo studies of antioxidant activities of flavonoids from Adiantum capillus-veneris L. Afr. J. Pharm. Pharmacol. 2011, 5, 2079–2085. [Google Scholar] [CrossRef]
- Kanwal, Q.; Qadir, A.; Iqbal, H.H.; Munir, B. Healing potential of Adiantum capillus-veneris L. plant extract on bisphenol A-Induced Hepatic Toxicity in Male Albino Rats. Environ. Sci. Pollut. Res. 2018, 25, 11884–11892. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. The chemical constituents and pharmacological effects of Adiantum capillus-veneris-A review. Asian J. Pharm. Sci. Technol. 2015, 5, 106–111. [Google Scholar]
- Nagao, K.; Yanagita, T. Conjugated fatty acids in food and their health benefits. J. Biosci. Bioeng. 2005, 100, 152–157. [Google Scholar] [CrossRef]
- Cerón, M.C.; García-Malea, M.d.C.; Rivas, J.; Acien, F.; Fernández, J.M.; Del Río, E.; Guerrero, M.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 74, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Nengroo, Z.R.; Rauf, A. Fatty acid composition and antioxidant activities of five medicinal plants from Kashmir. Ind. Crops Prod. 2019, 140, 111596. [Google Scholar] [CrossRef]
- Ngamakeue, N.; Chitprasert, P. Encapsulation of holy basil essential oil in gelatin: Effects of palmitic acid in carboxymethyl cellulose emulsion coating on antioxidant and antimicrobial activities. Food Bioprocess Technol. 2016, 9, 1735–1745. [Google Scholar] [CrossRef]
- Hemendra, S.C.; Alekh, N.S.; Sushil, K.S. Fatty acid composition, antioxidant, anti-inflammatory and antibacterial activities of seed oil from Crotalaria juncea Linn. J. Med. Plants Res. 2011, 5, 984–991. [Google Scholar]
- De Morais, S.M.; do Nascimento, J.E.T.; de Sousa Silva, A.A.; Junior, J.E.R.H.; Pinheiro, D.C.S.N.; de Oliveira, R.V. Fatty acid profile and anti-inflammatory activity of fixed plant oils. Acta Sci. Vet. 2017, 45, 8. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir-Knezevic, S. Quantitative analysis of the flavonoids in raw propolis from northern Croatia. Acta Pharm. 2004, 54, 65–72. [Google Scholar]
- Alara, O.; Abdurahman, N.; Mudalip, S.A.; Olalere, O. Effect of drying methods on the free radicals scavenging activity of Vernonia amygdalina growing in Malaysia. J. King Saud Univ. Sci. 2019, 31, 495–499. [Google Scholar] [CrossRef]
- Sakr, S.A.; Shalaby, S.Y. Carbendazim-induced testicular damage and oxidative stress in albino rats: Ameliorative effect of licorice aqueous extract. Toxicol. Ind. Health 2014, 30, 259–267. [Google Scholar] [CrossRef]
- Toor, H.K.; Sangha, G.K.; Khera, K.S. Imidacloprid induced histological and biochemical alterations in liver of female albino rats. Pestic. Biochem. Physiol. 2013, 105, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Keisari, Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J. Immunol. Methods 1980, 38, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Archer, S. Measurement of nitric oxide in biological models. FASEB J. 1993, 7, 349–360. [Google Scholar] [CrossRef]
- Reitman, S. Liver enzymes (AST and ALT); Reitman and Frankel calorimetric method. Am. J. Univ. Path. 1957, 28, 56. [Google Scholar] [CrossRef]
- Belfield, A.; Goldberg, D.M. Normal ranges and diagnostic value of serum 5′ nucleotidase and alkaline phosphatase activities in infancy. Arch. Dis. Child. 1971, 46, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, N.; Lin, D.; Zang, Y. Curcumin protects against hepatic ischemia/reperfusion induced injury through inhibiting TLR4/NF-κB pathway. Oncotarget 2017, 8, 65414. [Google Scholar] [CrossRef]
- Nishikimi, M.; Roa, N.; Yogi, K. Improved method for the determination of red blood cell superoxide activity. Biochem. Biop. Res. Common 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Santini, D.; Schiavon, G.; Vincenzi, B.; Gaeta, L.; Pantano, F.; Russo, A.; Ortega, C.; Porta, C.; Galluzzo, S.; Armento, G. Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS ONE 2011, 6, e19234. [Google Scholar] [CrossRef]
- Haines, D.M.; Clark, E.G. Enzyme immunohistochemical staining of formalin-fixed tissues for diagnosis in veterinary pathology. Can. Vet. J. 1991, 32, 295. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | ABTS (mg TE/g Extract) | DPPH (IC50 µg/mL) | |
|---|---|---|---|---|
| ACVL extract | 86.16 ± 0.41 | 74.24 ± 0.37 | 58.52 ± 0.8 | 92.05 ± 0.42 |
| Ascorbic acid | - | - | - | 30.08 ± 0.02 |
| No. | Rt (min) | rRT | Metabolites | Molecular Formula | Area Sum % | Class |
|---|---|---|---|---|---|---|
| 1 | 8.87 | 0.30 | 4-ethenyl-3,8-Dioxatricyclo [5.1.0.0(2,4)] octane | C8H10O2 | 2.76 | Diepoxides |
| 2 | 9.22 | 0.31 | Hexadecanal | C16H32O | 7.46 | Aldehydes |
| 3 | 9.62 | 0.32 | 2,4-Hexadien-1-ol | C6H10O | 1.67 | Alcohols |
| 4 | 11.22 | 0.38 | 6-methyl-Octadecane | C19H40 | 1.78 | Hydrocarbons |
| 5 | 11.89 | 0.40 | Pentadecan-8-ol | C15H32O | 1.89 | HC alcohol |
| 6 | 12.24 | 0.42 | Valeric acid, 2-pentadecyl ester | C20H40O2 | 1.9 | Ester |
| 7 | 14.95 | 0.51 | Glycerol | C3H8O3 | 0.73 | Alcohol |
| 8 | 15.89 | 0.54 | 2-methoxy-2-methyl-Propane | C5H12O | 0.46 | Ether |
| 9 | 19.36 | 0.66 | (E)-5-Tetradecen-3-yne | C14H24 | 0.42 | Hydrocarbons |
| 10 | 20.95 | 0.71 | Bicyclo [2.2.0] hex-1-yl-methanol | C7H12O | 0.22 | Bicyclic alcohol |
| 11 | 24.86 | 0.85 | 5β,7βH,10α-Eudesm-11-en-1α-ol | C15H26O | 0.77 | Monoterpene hydrocarbons |
| 12 | 25.84 | 0.88 | Phytol | C20H40O | 0.67 | Acyclic diterpene alcohol |
| 13 | 26 | 0.88 | 2-tert-Butyl-4-methylphenol | C11H16O | 3.94 | Phenylpropanes |
| 14 | 28.32 | 0.96 | 1-chloro-Octadecane | C18H37Cl | 6.4 | Hydrocarbons |
| 15 | 28.60 | 0.97 | Undec-10-ynoic acid, dodecyl ester | C23H42O2 | 1.8 | Ester |
| 16 | 28.83 | 0.98 | 4-(3-tert-butyl-4-hydroxy-5-methylbenzyl)-2-tert-butyl-6-methylphenol | C23H34O2 | 1.16 | Phenol |
| 17 | 29.19 | 0.99 | Undec-10-ynoic acid, dodecyl ester | C23H42O2 | 0.29 | Ester |
| 18 | 29.43 | 1 | 1-Monopalmitin | C19H38O4 | 16.14 | Ester |
| 19 | 29.63 | 1.01 | 1b,5,5,6a-Tetramethyl-octahydro-1-oxa-cyclopropa[a]inden-6-one | C13H20O2 | 0.49 | Epoxy/keto |
| 20 | 30.09 | 1.02 | Heptacosane | C27H56 | 3.29 | Hydrocarbons |
| 21 | 30.81 | 1.05 | 2,3-dihydroxypropyl stearate | C21H42O4 | 15.57 | Ester |
| 22 | 31.47 | 1.07 | Docosane | C22H46 | 4.54 | Hydrocarbons |
| 23 | 31.81 | 1.08 | (Z, Z)-9,12-Octadecadienoyl chloride | C18H31ClO | 0.92 | Ketone |
| 24 | 32.13 | 1.09 | γ-Tocopherol | C28H48O2 | 0.25 | Chroman-6-ol |
| 25 | 33.07 | 1.12 | 1-Octacosanol | C28H58O | 2.25 | fatty alcohol |
| 26 | 33.67 | 1.14 | 12-Methyl-E, E-2,13-octadecadienoic-1-ol | C19H36O | 0.38 | fatty alcohol |
| 27 | 34.27 | 1.16 | Campesterol | C28H48O | 15.36 | Sterols |
| 28 | 34.37 | 1.17 | 4-methyl-, (3β,4α)-Cholesta-8,24-dien-3-ol | C28H46O | 1.79 | Sterol |
| 29 | 34.55 | 1.17 | Stigmasterol | C29H48O | 2.19 | Sterol |
| 30 | 34.77 | 1.18 | Betulin | C30H50O2 | 0.77 | Pentacyclic triterpene |
| 31 | 36.01 | 1.22 | 1-Monolinoleoylglycerol | C21H38O4 | 0.24 | Ester |
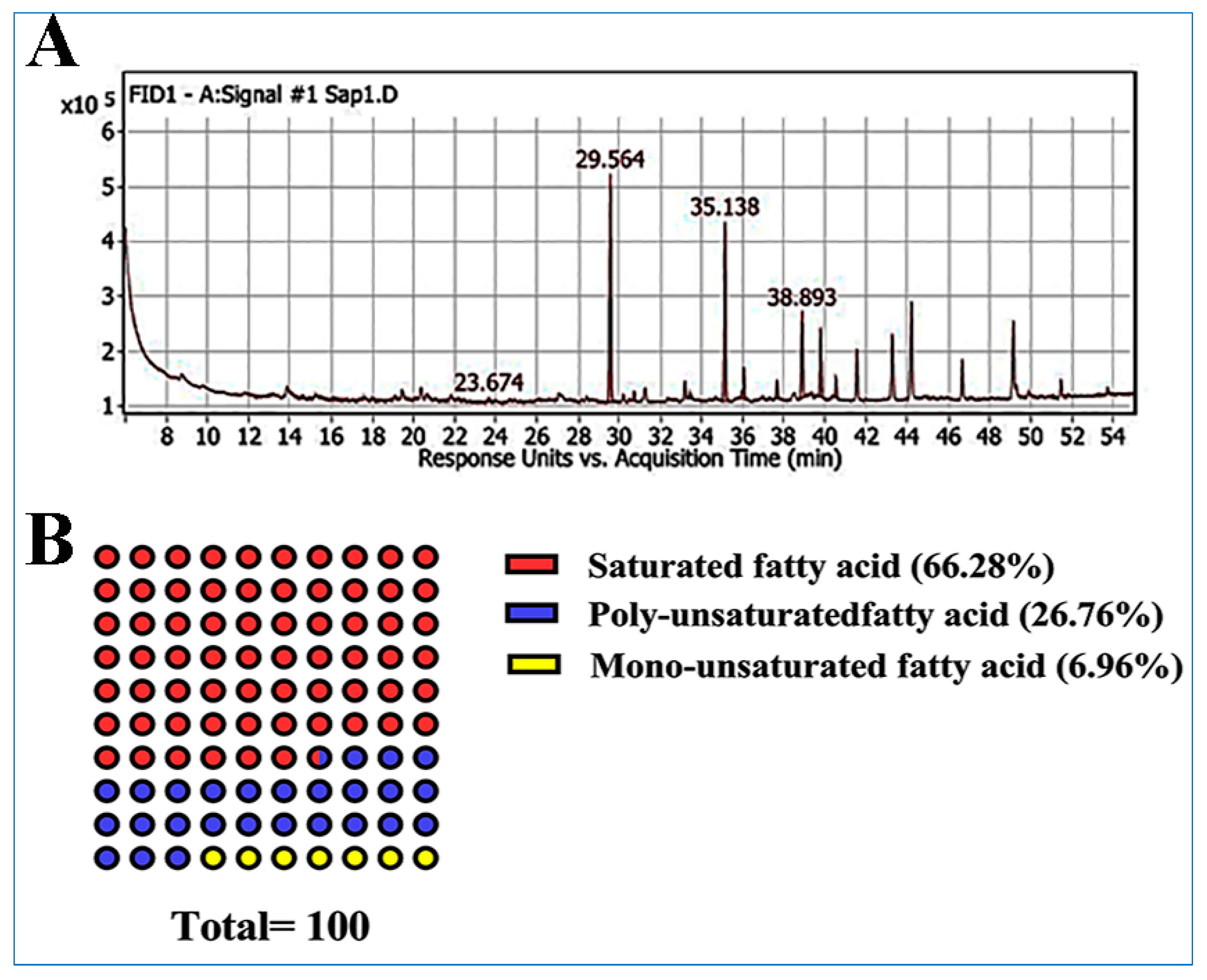
| Peak | RT | rRT | Name | Area % | Class |
|---|---|---|---|---|---|
| 1 | 23.6 | 0.80 | Myristic acid | 0.57 | Saturated fatty acid |
| 2 | 29.5 | 1 | Palmitic acid | 35.91 | Saturated fatty acid |
| 3 | 30.7 | 1.03 | Palmitoleic acid | 1.42 | Monounsaturated fatty acid |
| 4 | 33.4 | 1.13 | cis-10-Heptadecenoic acid | 1.21 | Monounsaturated fatty acid |
| 5 | 35.1 | 1.18 | Stearic acid | 27.32 | Saturated fatty acid |
| 6 | 36.0 | 1.21 | Oleic acid | 4.33 | Monounsaturated fatty acid |
| 7 | 37.6 | 1.27 | Linoleic acid | 2.74 | Polyunsaturated fatty acid |
| 8 | 38.8 | 1.31 | γ-Linolenic acid | 13.8 | Polyunsaturated fatty acid |
| 9 | 39.7 | 1.34 | Linolenic acid | 10.22 | Polyunsaturated fatty acid |
| 10 | 40.5 | 1.37 | Arachidic acid | 2.48 | Saturated fatty acid |
| Gene Description | Target Gene | Accession No. | Sequences (5′—3′) |
|---|---|---|---|
| Cu/Zn Superoxide dismutase | SOD1 | NM_017050.1 | F: CATTCCATCATTGGCCGTACT |
| R: CCACCTTTGCCCAAGTCATC | |||
| Catalase | CAT | NM_012520.2 | F: GTACAGGCCGGCTCTCACA |
| R: ACCCGTGCTTTACAGGTTAGCT | |||
| Glutathione | GSH | NM_053906.1 | F: GGAAGTCAACGGGAAGAAGTTCACTG |
| R: CAATGTAACCGGCACCCACAATAAC | |||
| Glutathione peroxidase | GPx1 | NM_030826.4 | F: GCGCTGGTCTCGTCCATT |
| R: TGGTGAAACCGCCTTTCTTT | |||
| Nuclear factor-kappa B | NF-κB | NM_01276711.1 | F: AATTGCCCCGGCAT |
| R: TCCCGTAACCGCGTA | |||
| Inducible Nitric Oxide | iNOS | NM_012611.3 | F: CACCACCCTCCTTGTTCAAC |
| R: CAATCCACAACTCGCTCCAA | |||
| Tumor necrosis factor-α | TNF-α | NM_012675.3 | F: GATCGGTCCCAACAAGGAGG |
| R: GCTTGGTGGTTTGCTACGAC | |||
| Interleukin-6 | IL-6 | NM_012589.2 | F: AAGCCAGAGTCATTCAGAGCAA |
| R: GGTCCTTAGCCACTCCTTCT | |||
| Glyceraldehyde3-phosphate dehydrogenase | GAPDH | NM_001394060.1 | F: CCACCAACTGCTTAGCCCCC |
| R: GCAGTGATGGCATGGACTGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seif, M.; Aati, H.; Amer, M.; Ragauskas, A.J.; Seif, A.; El-Sappah, A.H.; Aati, A.; Madboli, A.E.-N.A.; Emam, M. Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract. Molecules 2023, 28, 4720. https://doi.org/10.3390/molecules28124720
Seif M, Aati H, Amer M, Ragauskas AJ, Seif A, El-Sappah AH, Aati A, Madboli AE-NA, Emam M. Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract. Molecules. 2023; 28(12):4720. https://doi.org/10.3390/molecules28124720
Chicago/Turabian StyleSeif, Mohamed, Hanan Aati, May Amer, Arthur J. Ragauskas, Amr Seif, Ahmed H. El-Sappah, Abdulrahman Aati, Abd El-Nasser A. Madboli, and Mahmoud Emam. 2023. "Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract" Molecules 28, no. 12: 4720. https://doi.org/10.3390/molecules28124720
APA StyleSeif, M., Aati, H., Amer, M., Ragauskas, A. J., Seif, A., El-Sappah, A. H., Aati, A., Madboli, A. E.-N. A., & Emam, M. (2023). Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract. Molecules, 28(12), 4720. https://doi.org/10.3390/molecules28124720










