Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Adsorbents
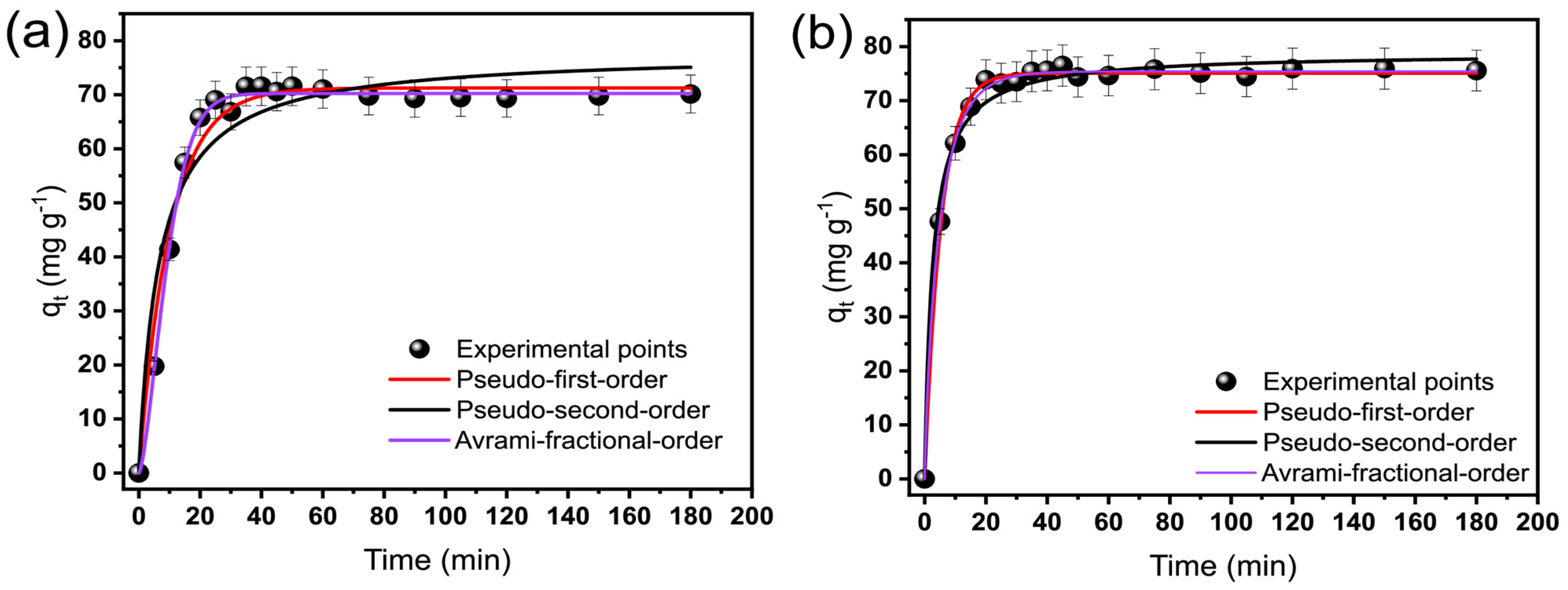
2.2. Kinetic of OME Adsorption on BP and BTM Adsorbents
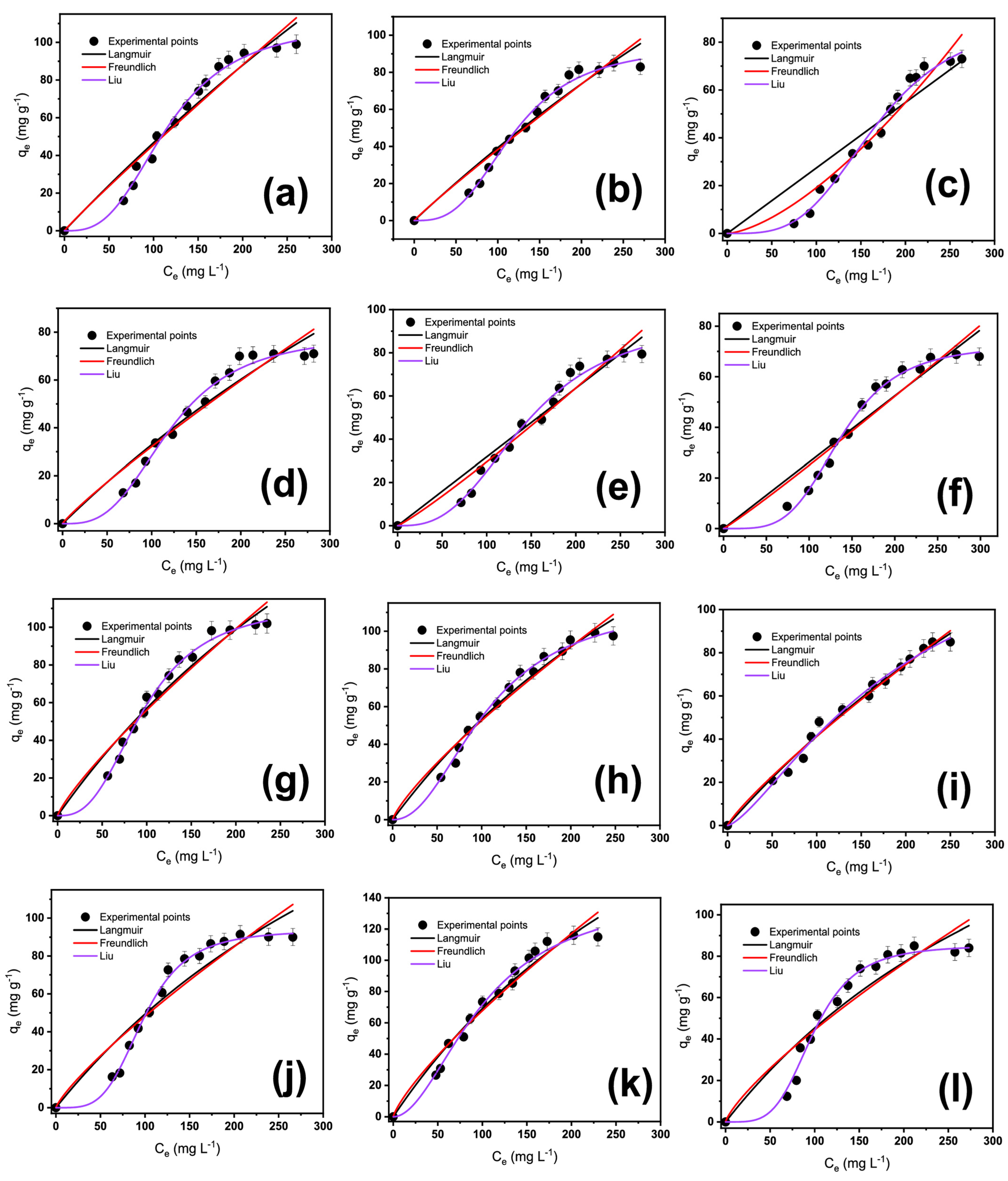
2.3. Equilibrium Isotherms Studies of OME on Biobased Adsorbents
2.4. Thermodynamic Studies of OME on BP and BTM
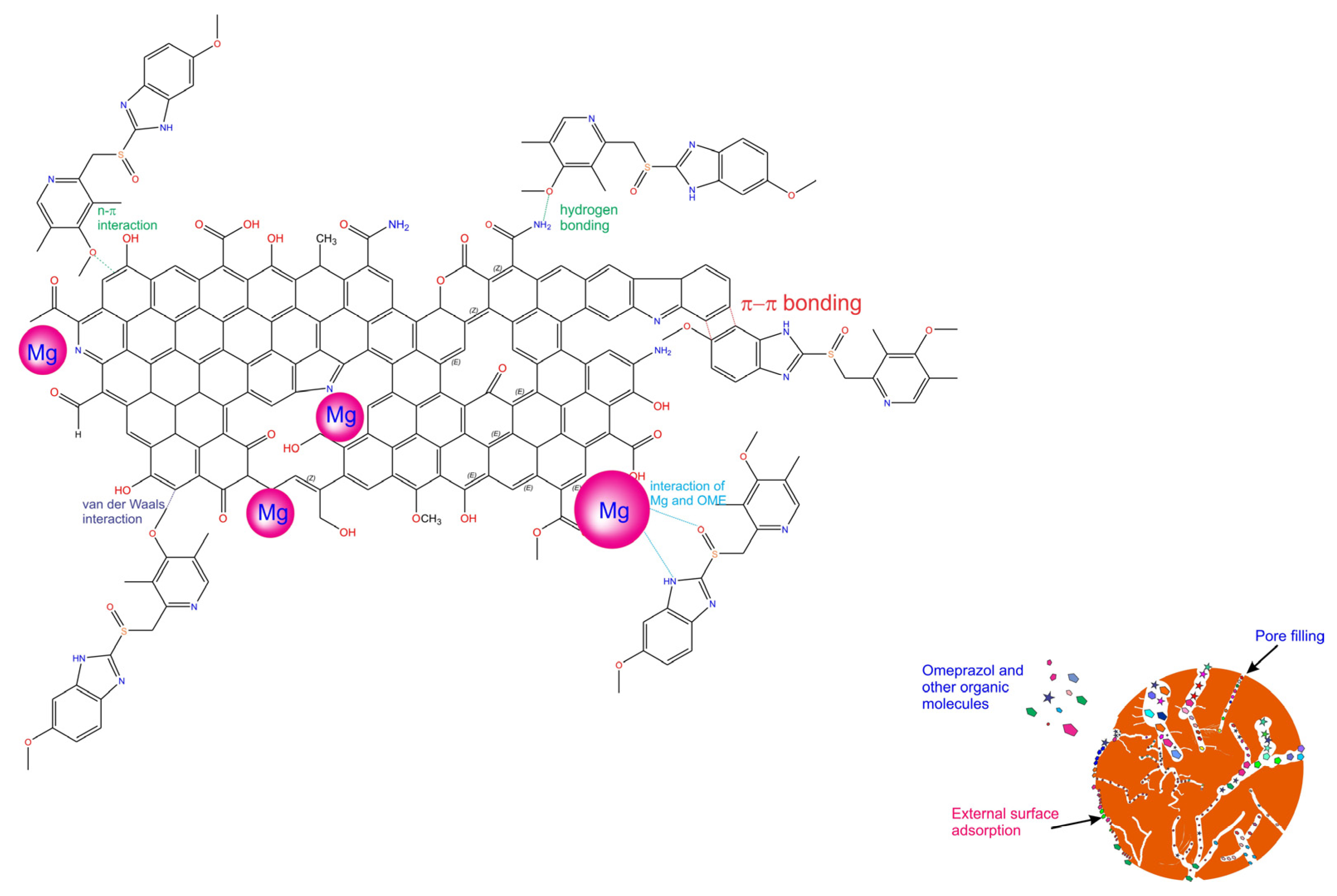
2.5. Mechanism of Adsorption
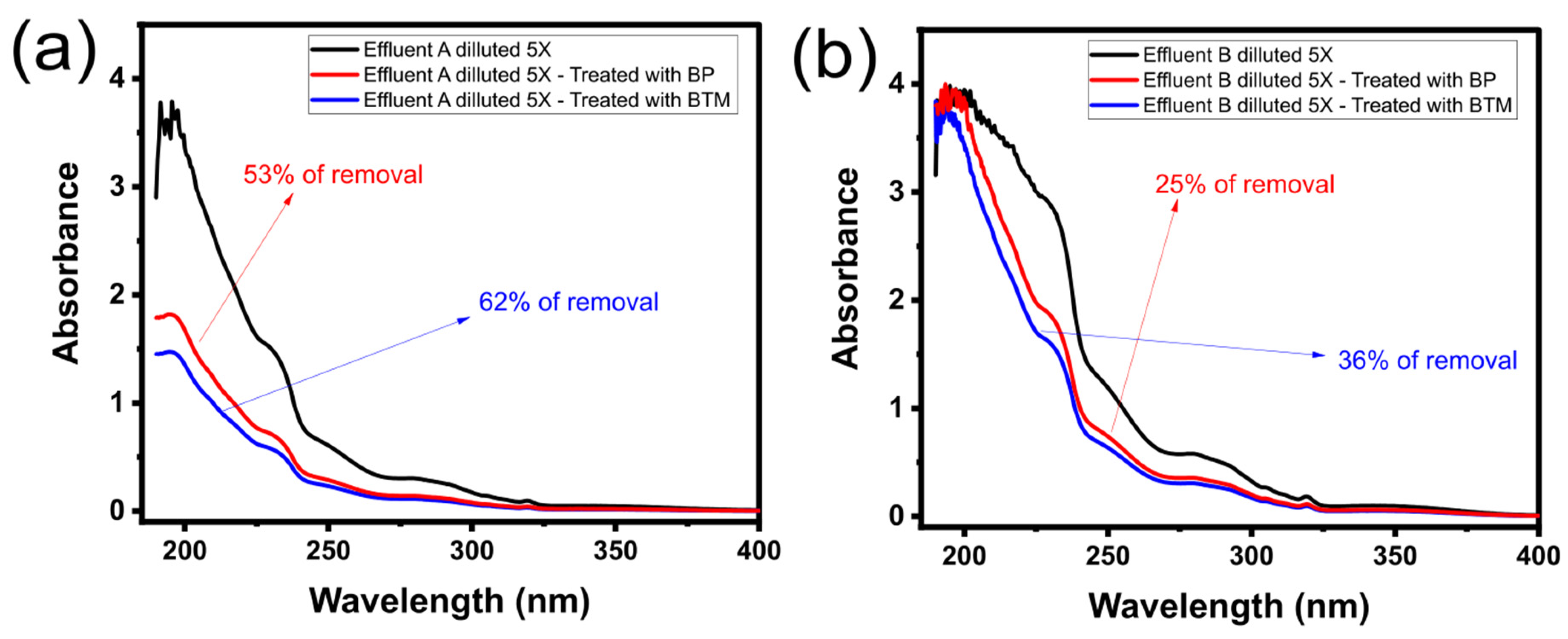
2.6. Synthetic Effluent Treatment Tests
3. Materials and Methods
3.1. Preparation of the Adsorbents-Activated Biochars
3.2. Characterization of the Biochars
3.3. Adsorption Studies
3.4. Kinetics, Equilibrium, and Thermodynamics Studies
3.5. Synthetic Effluents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evgenidou, E.N.; Konstantinou, I.K.; Lambropoulou, D.A. Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: A review. Sci. Total Environ. 2015, 505, 905–926. [Google Scholar] [CrossRef] [PubMed]
- Imwene, K.O.; Ngumba, E.; Kairigo, P.K. Emerging technologies for enhanced removal of residual antibiotics from source-separated urine and wastewaters: A review. J. Environ. Manag. 2022, 322, 116065. [Google Scholar] [CrossRef] [PubMed]
- Carmalin, S.A.; Lima, E. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar]
- Li, X.; Wang, B.; Liu, F.; Yu, G. Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review. Water 2023, 15, 1517. [Google Scholar] [CrossRef]
- Ibanez, M.; Gracia-Lor, E.; Bijlsma, L.; Morales, E.; Pastor, L.; Hernández, F. Removal of emerging contaminants in sewage water subjected to advanced oxidation with ozone. J. Hazard. Mater. 2013, 260, 389–398. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.F.; Fia, R.; Rodrigues, F.N.; Fia, F.R.L.; de Matos, M.P.; Siniscalchi, L.A.B.; Sanson, A.L. Quantification, removal and potential ecological risk of emerging contaminants in different organic loads of swine wastewater treated by integrated biological reactors. Chemosphere 2020, 260, 127516. [Google Scholar] [CrossRef]
- Isac, L.; Cazan, C.; Andronic, L.; Enesca, A. CuS-Based Nanostructures as Catalysts for Organic Pollutants Photodegradation. Catalysts 2022, 12, 1135. [Google Scholar] [CrossRef]
- Ferreiro, V.; Gómez-Motos, I.; Lombrana, J.I.; de Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of emerging concern removal in the effluent of wastewater treatment plant under biological and continuous mode ultrafiltration treatment. Water 2020, 12, 725. [Google Scholar] [CrossRef]
- Thue, P.S.; Umpierres, C.S.; Lima, E.C.; Lima, D.R.; Machado, F.M.; dos Reis, G.S.; da Silva, R.S.; Pavan, F.A.; Tran, H.N. Single-step pyrolysis for producing magnetic activated carbon from tucumã (Astrocaryum aculeatum) seed and nickel(II) chloride and zinc(II) chloride. Application for removal of Nicotinamide and Propanolol. J. Hazard Mater. 2020, 398, 122903. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Guy, M.; Mathieu, M.; Jebrane, M.; Lima, E.C.; Thyrel, M.; Dotto, G.L.; Larsson, S.H. A Comparative Study of Chemical Treatment by MgCl2, ZnSO4, ZnCl2, and KOH on Physicochemical Properties and Acetaminophen Adsorption Performance of Biobased Porous Materials from Tree Bark Residues. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128626. [Google Scholar] [CrossRef]
- González-Hourcade, M.; Simões dos Reis, G.; Grimm, A.; Dinh, V.M.; Lima, E.C.; Larsson, S.H.; Gentili, F.G. Microalgae Biomass as a Sustainable Precursor to Produce Nitrogen-Doped Biochar for Efficient Removal of Emerging Pollutants from Aqueous Media. J. Clean. Prod. 2022, 348, 131280. [Google Scholar] [CrossRef]
- Lima, E.C.; Naushad, M.; dos Reis, G.S.; Dotto, G.L.; Pavan, F.A.; Guleria, A.; Seliem, M.K.; Sher, F. Production of carbon-based adsorbents from lignocellulosic biomass. In Biomass-Derived Materials for Environmental Applications; Anastopoulos, I., Lima, E.C., Meili, L., Giannakoudakis, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 169–191. ISBN 978-0-323-91914-2. [Google Scholar]
- Ahmad, T.; Manzar, M.S.; Georgin, J.; Franco, D.S.P.; Khan, S.; Meili, L.; Ullah, N. Development of a new hyper crosslinked resin based on polyamine-isocyanurate for the efficient removal of endocrine disruptor bisphenol-A from water. J. Water Proc. Eng. 2023, 53, 103623. [Google Scholar] [CrossRef]
- Cimirro, N.F.G.M.; Lima, E.C.; Cunha, M.R.; Grimm, P.S.T.A.; dos Reis, G.S.; Rabiee, N.; Saeb, M.R.; Keivanimehr, F.; Habibzadeh, S. Removal of diphenols using pine biochar. Kinetics, equilibrium, thermodynamics, and mechanism of uptake. J. Mol. Liq. 2022, 364, 119979. [Google Scholar] [CrossRef]
- Lima, D.R.; Lima, E.C.; Thue, P.S.; Dias, S.L.P.; Machado, F.M.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Saeb, M.R.; Rinklebe, J. Comparison of acidic leaching using a conventional and ultrasound-assisted method for preparation of magnetic-activated biochar. J. Environ. Chem. Eng. 2021, 9, 105865. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; Mathieu, M.; Thyrel, M.; Pham, T.N. Application of design of experiments (DoE) for optimised production of micro- and mesoporous Norway spruce bark activated carbons. Biomass Convers. Biorefinery 2021, 1–19. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Bergna, D.; Tuomikoski, S.; Grimm, A.; Lima, E.C.; Thyrel, M.; Skoglund, N.; Lassi, U.; Larsson, S.H. Preparation and characterization of pulp and paper mill sludge-activated biochars using alkaline activation: A box–Behnken design approach. ACS Omega 2022, 7, 32620–32630. [Google Scholar] [CrossRef]
- Lee, Y.-E.; Jeong, Y.; Shin, D.-C.; Ahn, K.-H.; Jung, J.-H.; Kim, I.-T. Fabrication of Mg-Doped Sargassum Biochar for Phosphate and Ammonium Recovery. Sustainability 2021, 13, 12752. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Y.; Ma, R.; Feng, X.; Yan, L.; Jia, D.; Xu, M.; Ai, L.; Guo, N.; Wang, L. Nitrogen-Doped Hierarchical Porous Carbon Derived from Coal for High-Performance Supercapacitor. Molecules 2023, 28, 3660. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; Thyrel, M.; Pham, T.N.; Claudio Lima, E.; de Oliveira, H.P.; Dotto, G.L. Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents. Coatings 2021, 11, 772. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Lima, D.R.; da Silva, R.S.; Thue, P.S.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Larsson, S.H. Removal of captopril pharmaceutical from synthetic pharmaceutical-industry wastewaters: Use of activated carbon derived from Butia catarinensis. J. Environ. Chem. Eng. 2020, 8, 104506. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Alagbe, E.; Odika, B.; Babalola, R.; Oladimeji, T.E.; Abatan, O.G.; Yusuf, E.O. Preparation and characterization of activated carbon from plantain peel and coconut shell using biological activators. J. Phys. Conf. Ser. 2019, 1378, 032035. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J. Physisorption of gases, with special reference to the evaluation of the surface area and pore size distribution (IUPAC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Deng, Y.; Li, X.; Ni, F.; Liu, Q.; Yang, Y.; Wang, M.; Ao, T.; Chen, W. Synthesis of Magnesium Modified Biochar for Removing Copper, Lead, and Cadmium in Single and Binary Systems from Aqueous Solutions: Adsorption Mechanism. Water 2021, 13, 599. [Google Scholar] [CrossRef]
- Guy, M.; Mathieu, M.; Anastopoulos, I.P.; Martínez, M.G.; Rousseau, F.; Dotto, G.L.; de Oliveira, H.P.; Lima, E.C.; Thyrel, M.; Larsson, S.H.; et al. Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules 2022, 27, 456. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Smith, E.; Dent, G. Modern Raman Spectroscopy—A Practical Approach; John Wiley and Sons: Hoboken, NJ, USA, 2005; pp. 1–210. [Google Scholar]
- Song, X.; Ma, X.; Li, Y.; Ding, L.; Jiang, R. Tea waste-derived microporous active carbon with enhanced double-layer supercapacitor behaviors. Appl. Surf. Sci. 2019, 487, 189–197. [Google Scholar] [CrossRef]
- Huang, G.G.; Liu, Y.F.; Wu, X.-X.; Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Mansuri, I.; Farzana, R.; Rajarao, R.; Sahajwalla, V. Carbon Dissolution Using Waste Biomass—A Sustainable Approach for Iron-Carbon Alloy Production. Metals 2018, 8, 290. [Google Scholar] [CrossRef]
- Han, Y.; Lin, N.; Xu, T.; Li, T.; Tian, J.; Zhu, Y.; Qian, Y. An amorphous Si material with a sponge-like structure as an anode for Li-ion and Na-ion batteries. Nanoscale 2018, 10, 3153. [Google Scholar] [CrossRef]
- Cavalcante, E.H.M.; Candido, I.C.M.; de Oliveira, H.P.; Silveira, K.B.; Alvares, T.V.S.; Lima, E.C.; Thyrel, M.; Larsson, S.H.; dos Reis, G.S. 3-Aminopropyl-triethoxysilane functionalized tannin-rich grape biomass for the adsorption of methyl orange dye: Synthesis, characterization, and the adsorption mechanism. ACS Omega 2022, 7, 18997–19009. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, G.S.; Pinto, D.; Lima, E.C.; Knani, S.; Grimm, A.; Silva, L.F.O.; Cadaval Jr, T.R.S.; Dotto, G. Lanthanum uptake from water using chitosan with different configurations. React. Funct. Polym. 2022, 180, 105395. [Google Scholar] [CrossRef]
- Grimm, A.; dos Reis, G.S.; Dinh, V.M.; Larsson, S.H.; Mikkola, J.P.; Lima, E.C.; Xiong, S.J. Hardwood spent mushroom substrate-based activated biochar as a sustainable bioresource for removal of emerging pollutants from wastewater. Biomass. Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Oladoja, N.A. A critical review of the applicability of Avrami fractional kinetic equation in adsorption-based water treatment studies. Desalin. Water Treat. 2015, 57, 15813–15825. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.A.; Dugos, N.P.; Wan, M.W. Adsorption of sulfones from actual oxidized diesel oil in the frame of oxidative desulfurization: A process optimization study using activated clay. J. Clean. Prod. 2022, 363, 132357. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.A.; Dugos, N.P.; Wan, M.W. Adsorption of benzothiophene sulfone over clay mineral adsorbents in the frame of oxidative desulfurization. Fuel 2017, 205, 153–160. [Google Scholar] [CrossRef]
- Choi, A.E.S.; Roces, S.A.; Dugos, N.P.; Arcega, A.; Wan, M.W. Adsorptive removal of dibenzothiophene sulfone from fuel oil using clay material adsorbents. J. Clean. Prod. 2017, 161, 267–276. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Ahmed, W.; Mehmood, S.; Qaswar, M.; Ali, S.; Khan, Z.H.; Ying, H.; Chen, D.Y.; Núñez-Delgado, A. Oxidized biochar obtained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 105104. [Google Scholar] [CrossRef]
- Ranguin, R.; Ncibi, M.C.; Cesaire, T.; Lavoie, S.; Jean-Marius, C.; Grutzmacher, H.; Gaspard, S. Development and characterization of nanostructured hybrid material with vitamin B12 and bagasse-derived activated carbon for anaerobic chlordecone (Kepone) removal. Environ. Sci. Pollut. Res. Int. 2020, 27, 41122–41131. [Google Scholar] [CrossRef]
- Chandra, I.K.; Ju, Y.-H.; Ayucitra, A.; Ismadji, S. Evans blue removal from wastewater by rarasaponin-bentonite. Int. J. Environ. Sci. Technol. 2013, 10, 359–370. [Google Scholar] [CrossRef]
- Kefif, F.; Ezziane, K.; Bahmani, A.; Bettahar, N.; Mayouf, S. Evans Blue dye removal from contaminated water on calcined and uncalcined box {Cu-Al-CO}3 Cu-Al-CO3 layered double hydroxide materials prepared by coprecipitation. Bull. Mater. Sci. 2019, 42, 14. [Google Scholar] [CrossRef]
- Lima, V.V.C.; Dalla Nora, F.B.; Peres, E.C.; Reis, G.S.; Lima, E.C.; Oliveira, M.L.S.; Dotto, G.L. Synthesis and characterization of biopolymers functionalized with APTES (3-aminopropyltriethoxysilane) for the adsorption of sunset yellow dye. J. Environ. Chem. Eng. 2019, 7, 103410. [Google Scholar] [CrossRef]
- Reis, G.S.; Bergna, D.; Grimm, A.; Lima, E.C.; Hu, T.; Naushad, M.; Lassi, U. Preparation of highly porous nitrogen-doped biochar derived from birch tree wastes with superior dye removal performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131493. [Google Scholar] [CrossRef]
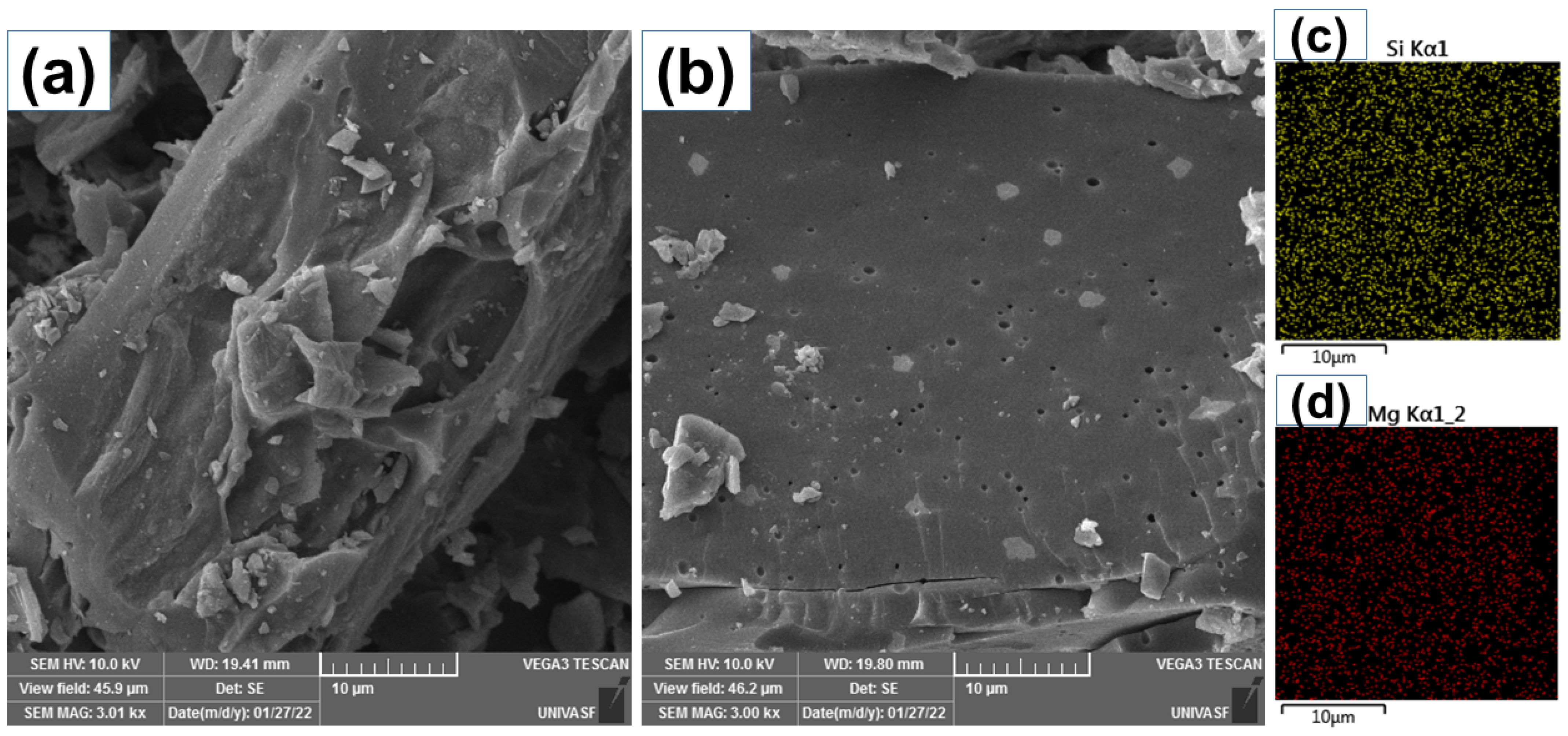
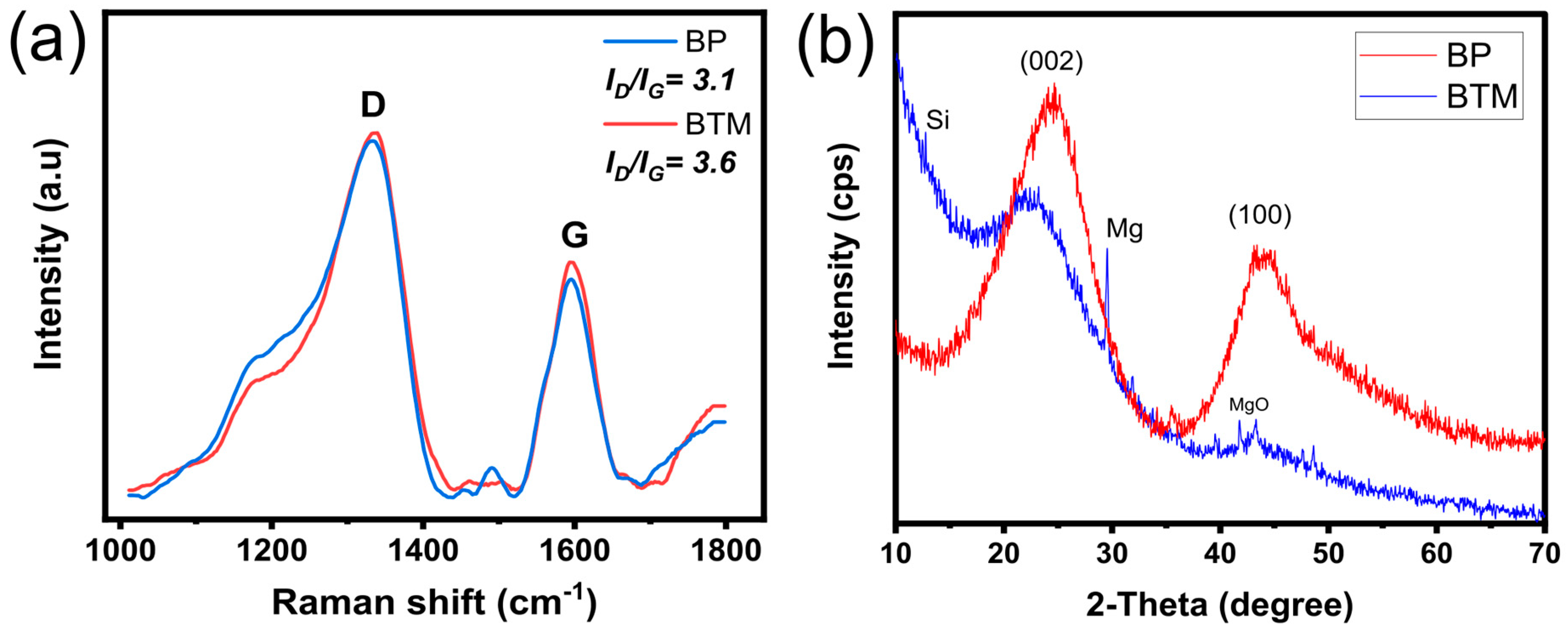
| Samples | SSA (m2 g−1) | AMic (m2 g−1) | AMes (m2 g−1) | Pore Vol. (cm3 g−1) |
|---|---|---|---|---|
| BP | 205 | 191 | 14 | 0.24 |
| BTM | 202 | 168 | 33 | 0.22 |
| BP | BTM | |
|---|---|---|
| Pseudo-first order | ||
| qe (mg g−1) | 71.25 | 75.09 |
| k1 (min−1) | 0.09760 | 0.1874 |
| t1/2 (min) | 7.105 | 3.699 |
| t0.95 (min) | 30.71 | 15.99 |
| R2 adjusted | 0.9782 | 0.9967 |
| SD (mg g−1) | 8.861 | 1.121 |
| BIC | 45.82 | 8.610 |
| Pseudo-second order | ||
| qe (mg g−1) | 77.86 | 78.82 |
| k2 (g mg−1 min−1) | 1.941 × 10−3 | 4.840 × 10−3 |
| t1/2 (min) | 6.613 | 2.622 |
| t0.95 (min) | 125.7 | 49.83 |
| R2 adjusted | 0.9200 | 0.9869 |
| SD (mg g−1) | 32.55 | 4.440 |
| BIC | 69.24 | 33.37 |
| Avrami fractional order | ||
| qe (mg g−1) | 70.23 | 75.42 |
| kAV (min−1) | 0.09433 | 0.1977 |
| nAV | 1.515 | 0.8409 |
| t1/2 (min) | 8.320 | 3.270 |
| t0.95 (min) | 21.87 | 18.65 |
| R2 adjusted | 0.9969 | 0.9981 |
| SD (mg g−1) | 1.266 | 0.6329 |
| BIC | 12.53 | 0.04500 |
| BP | ||||||
| Temperature in K | 283 | 293 | 298 | 303 | 313 | 318 |
| Langmuir | ||||||
| Qmax (mg g−1) | 730.9 | 582.9 | 511.0 | 351.2 | 417 | 133 |
| KL (L mg−1) | 68.31 × 10−5 | 7.24 × 10−4 | 5.36 × 10−8 | 1.04 × 10−3 | 7.63 × 10−7 | 1.98 × 10−5 |
| R2adj | 0.9288 | 0.9278 | 0.8723 | 0.9208 | 0.9369 | 0.8992 |
| SD (mg g−1) | 73.30 | 56.63 | 85.57 | 46.20 | 45.32 | 55.98 |
| BIC | 70.40 | 66.53 | 72.72 | 63.47 | 63.18 | 66.35 |
| Freundlich | ||||||
| KF (mg g−1 (mg L−1)−1/nF) | 0.5770 | 0.4990 | 0.0170 | 0.5260 | 0.1780 | 0.1870 |
| nF | 1.054 | 1.061 | 0.659 | 1.119 | 0.901 | 0.9410 |
| R2adj | 0.9234 | 0.9220 | 0.9458 | 0.9114 | 0.9427 | 0.9012 |
| SD (mg g−1) | 78.79 | 61.22 | 36.29 | 51.69 | 41.16 | 54.85 |
| BIC | 71.48 | 67.70 | 59.85 | 65.16 | 61.74 | 66.05 |
| Liu | ||||||
| Qmax (mg g−1) | 110.2 | 94.62 | 91.54 | 79.04 | 96.21 | 72.70 |
| Kg (L mg−1) | 0.008600 | 0.008400 | 0.005900 | 0.008300 | 0.006900 | 0.007270 |
| nL | 2.951 | 2.974 | 3.508 | 3.041 | 2.795 | 4.080 |
| R2adj | 0.9906 | 0.9891 | 0.9855 | 0.9852 | 0.9857 | 0.9925 |
| SD (mg g−1) | 9.648 | 8.518 | 9.739 | 8.627 | 10.23 | 4.140 |
| BIC | 41.48 | 39.62 | 41.63 | 39.81 | 42.37 | 28.80 |
| BTM | ||||||
| Temperature in K | 283 | 293 | 298 | 303 | 313 | 318 |
| Langmuir | ||||||
| Qmax (mg g−1) | 260.1 | 208.9 | 310.9 | 220.7 | 256.9 | 263.8 |
| KL (L mg−1) | 3.59 × 10−3 | 4.45 × 10−3 | 1.92 × 10−3 | 4.37 × 10−3 | 4.54 × 10−3 | 3.23 × 10−3 |
| R2adj | 0.9839 | 0.9426 | 0.9909 | 0.9567 | 0.9784 | 0.9602 |
| SD (mg g−1) | 17.74 | 59.45 | 7.251 | 46.00 | 29.10 | 43.19 |
| BIC | 49.12 | 67.25 | 35.70 | 63.41 | 56.54 | 62.46 |
| Freundlich | ||||||
| KF (mg g−1 (mg L−1)−1/nF) | 2.603 | 3.207 | 1.314 | 3.1309 | 3.4704 | 2.2674 |
| nF | 1.416 | 1.549 | 1.267 | 1.515 | 1.479 | 1.384 |
| R2adj | 0.9721 | 0.9149 | 0.9922 | 0.9326 | 0.9649 | 0.9427 |
| SD (mg g−1) | 30.73 | 88.14 | 6.181 | 71.59 | 47.26 | 62.17 |
| BIC | 57.36 | 73.16 | 33.30 | 70.04 | 63.81 | 67.93 |
| Liu | ||||||
| Qmax (mg g−1) | 146.9 | 107.6 | 249.0 | 112.2 | 152.9 | 118.2 |
| Kg (L mg−1) | 0.009500 | 0.01260 | 1.109 × 10−8 | 0.01280 | 0.01120 | 0.01140 |
| nL | 1.564 | 2.565 | 0.7892 | 2.404 | 1.566 | 2.276 |
| R2adj | 0.9915 | 0.9906 | 0.9916 | 0.9955 | 0.9855 | 0.9918 |
| SD (mg g−1) | 9.336 | 9.692 | 6.696 | 4.817 | 19.46 | 8.864 |
| BIC | 40.99 | 41.55 | 36.01 | 31.07 | 52.01 | 40.21 |
| Temperature (K) | 283 | 293 | 298 | 303 | 313 | 318 |
|---|---|---|---|---|---|---|
| Liu model | ||||||
| BP | ||||||
| 2980.8 | 2897.9 | 2055.1 | 2853.004 | 2379.8 | 2763.2 | |
| ∆G° (kJ mol−1) | −18.82 | −19.42 | −18.89 | −20.04 | −20.23 | −20.95 |
| ∆H° (kJ mol−1) | - | - | −1.581 | - | - | - |
| ∆S° (J K−1 mol−1) | - | - | 60.90 | - | - | - |
| R2 | - | - | 0.9930 | - | - | - |
| R2adj | - | - | 0.9895 | - | - | - |
| BTM | ||||||
| 3274.4 | 4348.6 | 663.2 | 4403.9 | 3865.0 | 3947.9 | |
| ∆G° (kJ mol−1) | −19.04 | −20.41 | −16.10 | −21.14 | −21.49 | −21.89 |
| ∆H° (kJ mol−1) | - | - | 4.022 | - | - | - |
| ∆S° (J K−1 mol−1) | - | - | 81.50 | - | - | - |
| R2 | - | - | 0.9997 | - | - | - |
| R2adj | - | - | 0.9995 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, R.A.; Thue, P.S.; Lima, É.C.; Grimm, A.; Naushad, M.; Dotto, G.L.; dos Reis, G.S. Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies. Molecules 2023, 28, 4591. https://doi.org/10.3390/molecules28124591
Teixeira RA, Thue PS, Lima ÉC, Grimm A, Naushad M, Dotto GL, dos Reis GS. Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies. Molecules. 2023; 28(12):4591. https://doi.org/10.3390/molecules28124591
Chicago/Turabian StyleTeixeira, Roberta A., Pascal S. Thue, Éder C. Lima, Alejandro Grimm, Mu. Naushad, Guilherme L. Dotto, and Glaydson S. dos Reis. 2023. "Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies" Molecules 28, no. 12: 4591. https://doi.org/10.3390/molecules28124591
APA StyleTeixeira, R. A., Thue, P. S., Lima, É. C., Grimm, A., Naushad, M., Dotto, G. L., & dos Reis, G. S. (2023). Adsorption of Omeprazole on Biobased Adsorbents Doped with Si/Mg: Kinetic, Equilibrium, and Thermodynamic Studies. Molecules, 28(12), 4591. https://doi.org/10.3390/molecules28124591







