Core–Shell Structured Carbon Nanofiber-Based Electrodes for High-Performance Supercapacitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of Loading Different Transition Metal Sulfides on Electrode Materials
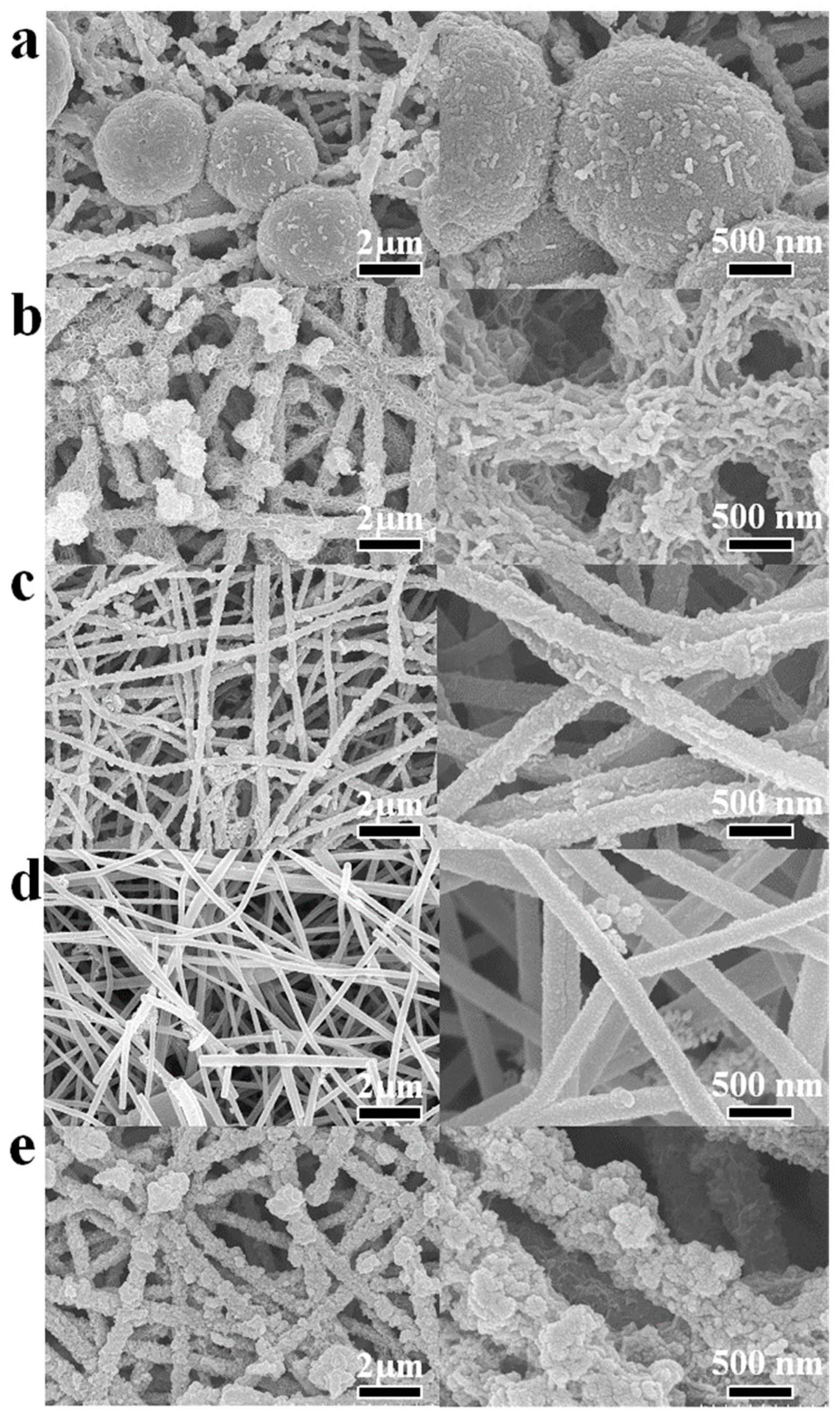
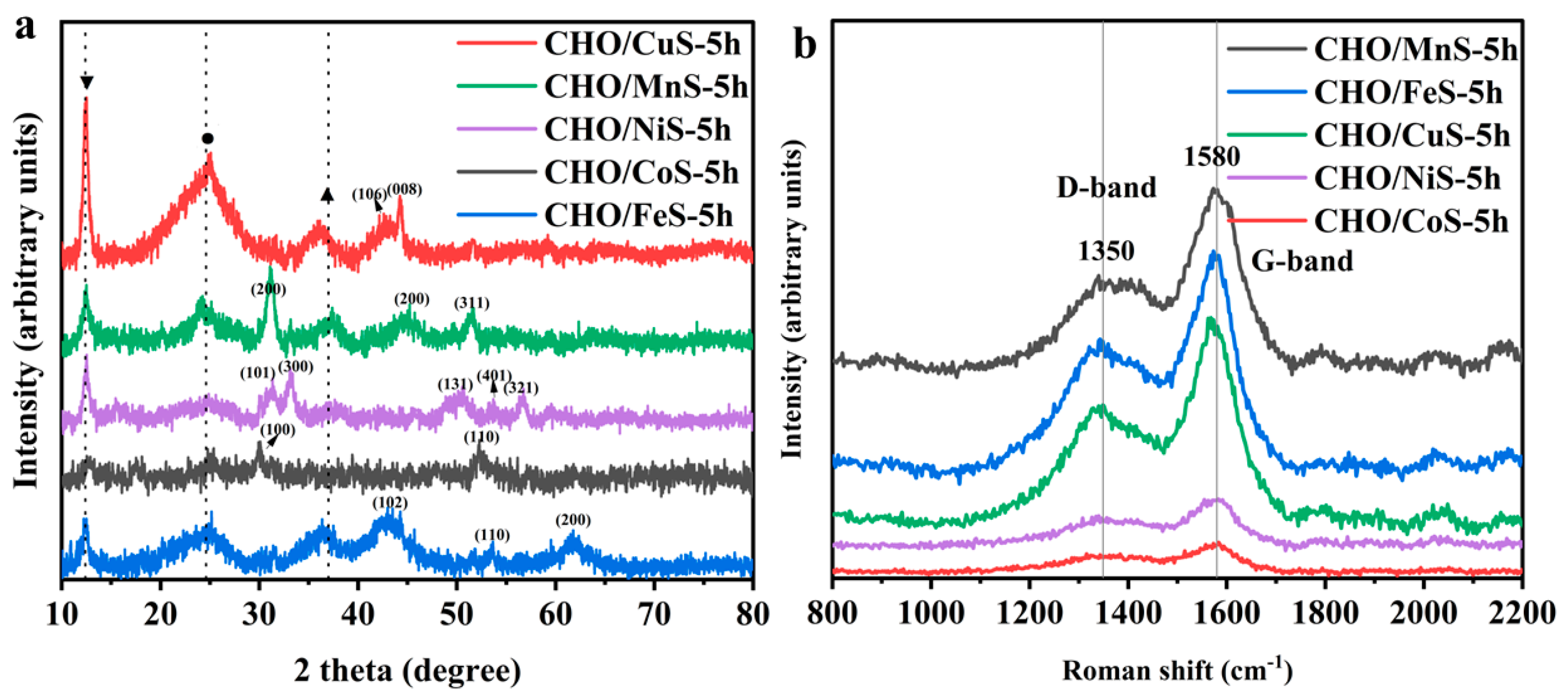
2.1.1. Morphological and Structural Analysis of Electrode Materials
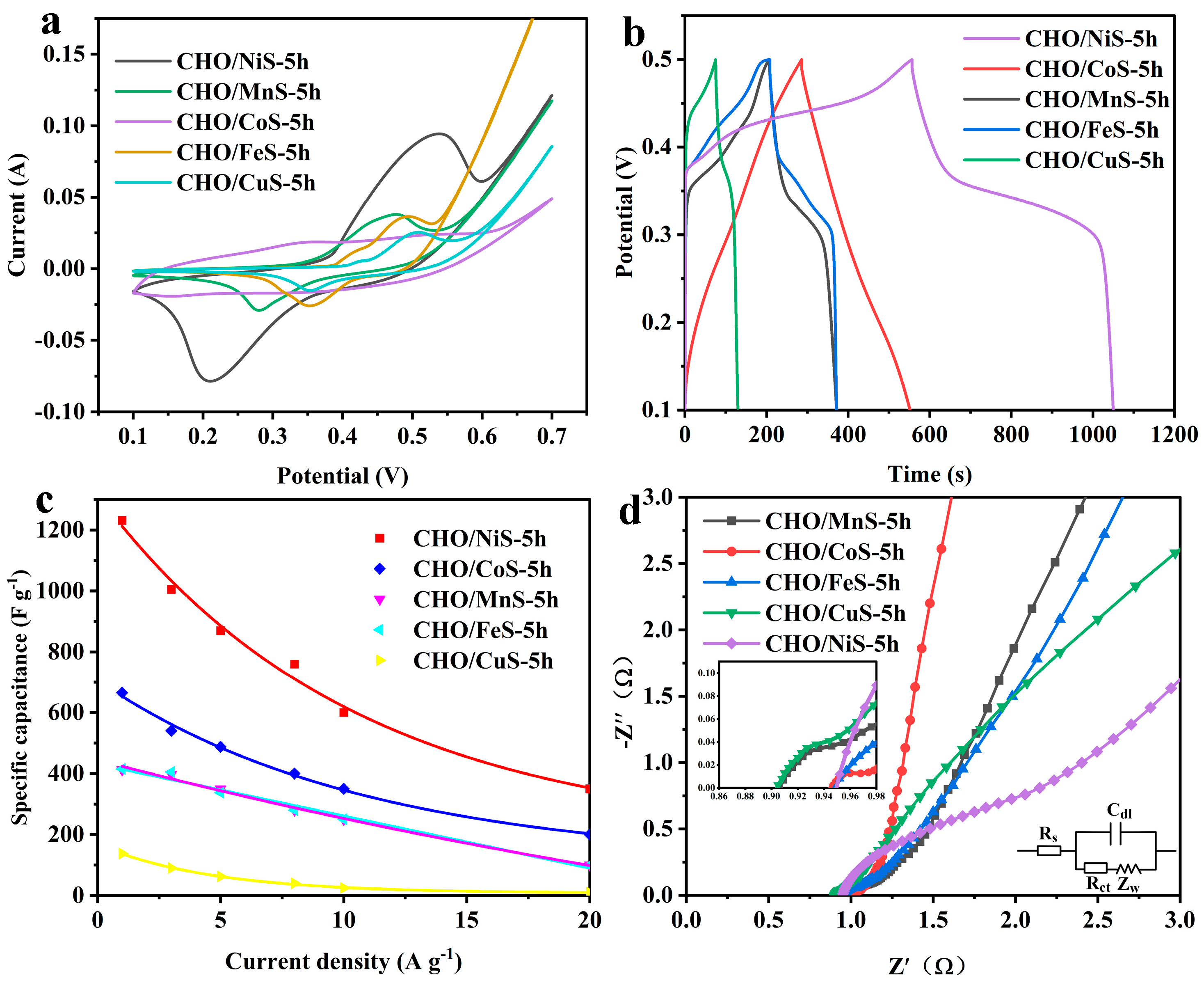
2.1.2. Electrochemical Performance of Electrode Materials
2.2. Effect of Hydrothermal Reaction Duration on Electrode Materials
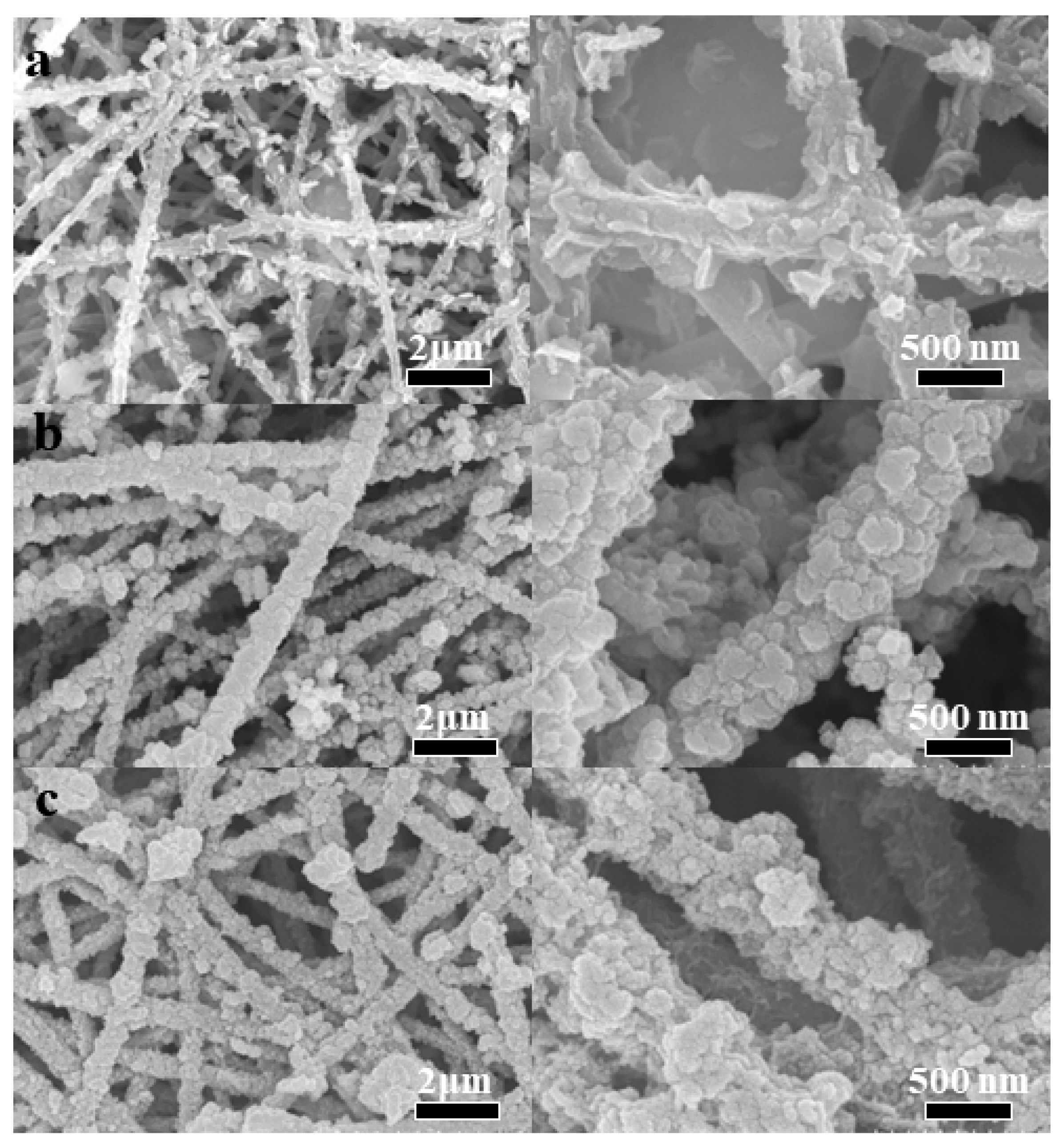
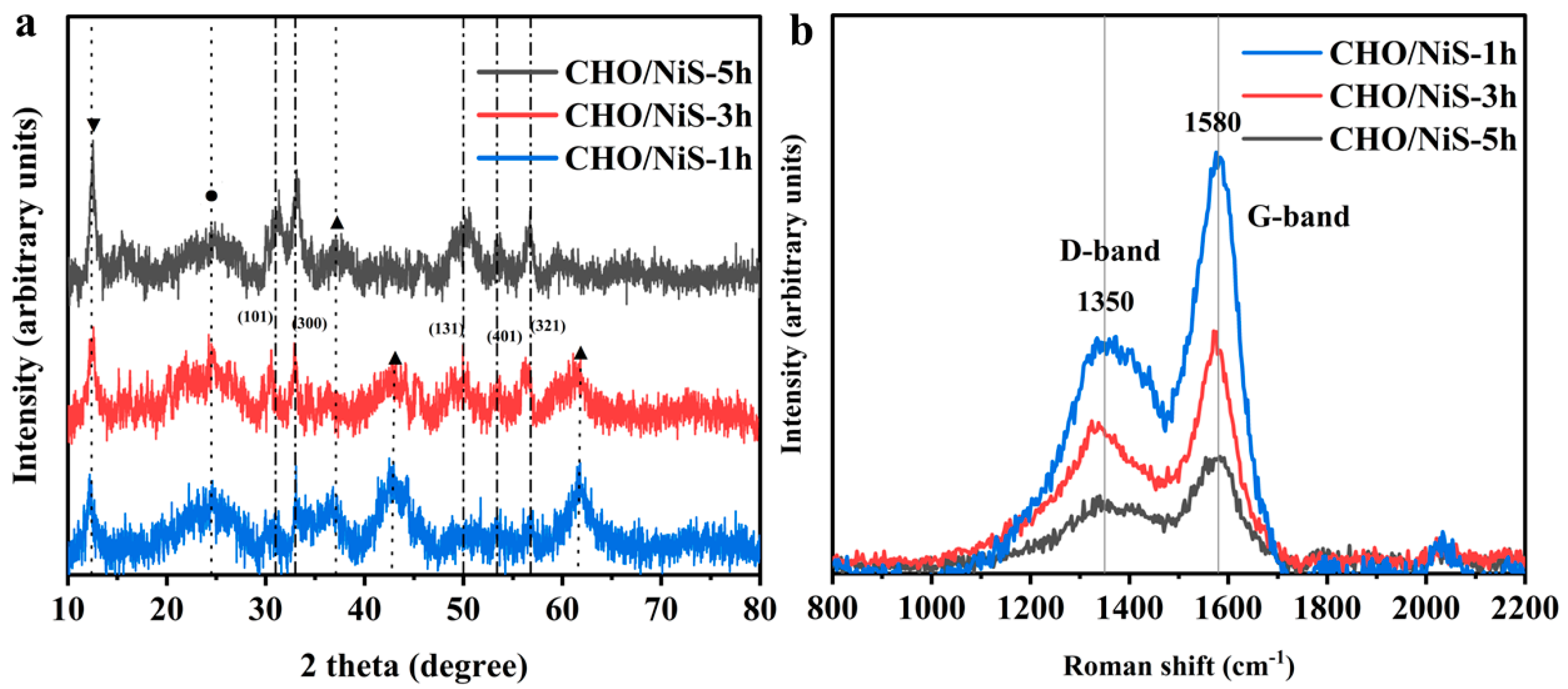
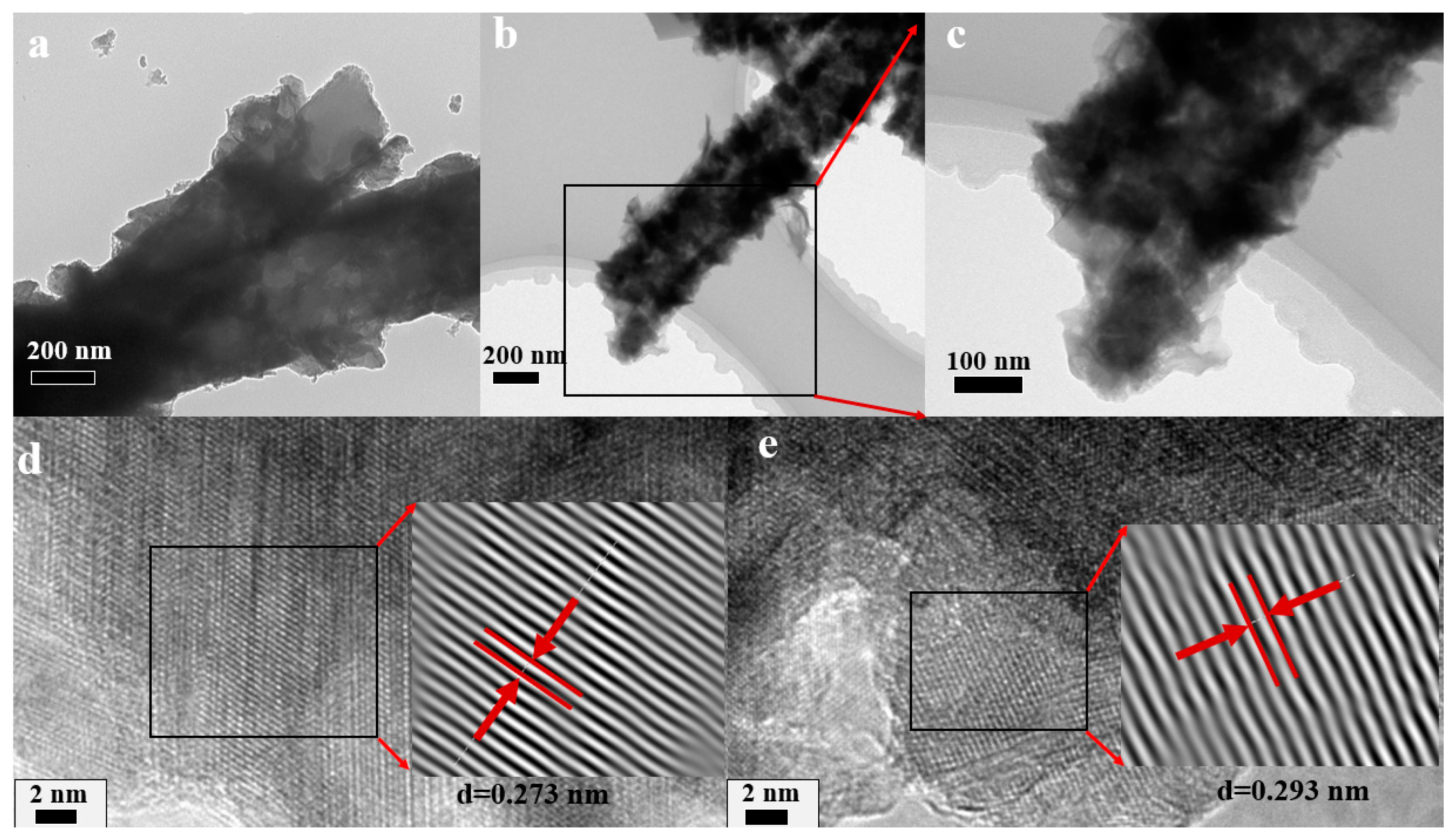
2.2.1. Morphological and Structural Analysis of Electrode Materials
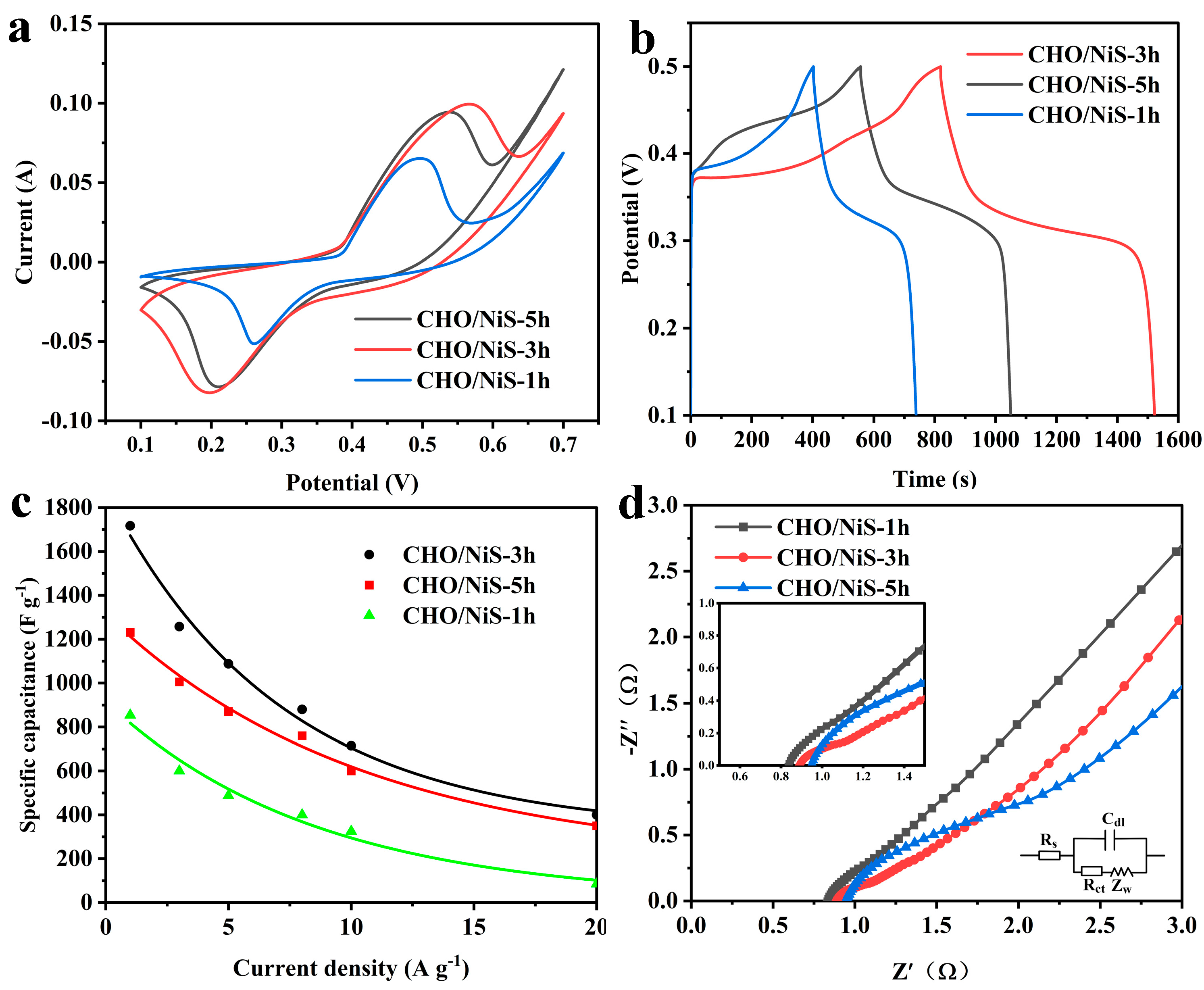
2.2.2. Electrochemical Performance of Electrode Materials
2.3. Characterization and Application of CHO/NiS-3h
2.3.1. Morphological and Structural Analysis of CHO/NiS-3h
2.3.2. Electrochemical Performance of CHO/NiS-3h
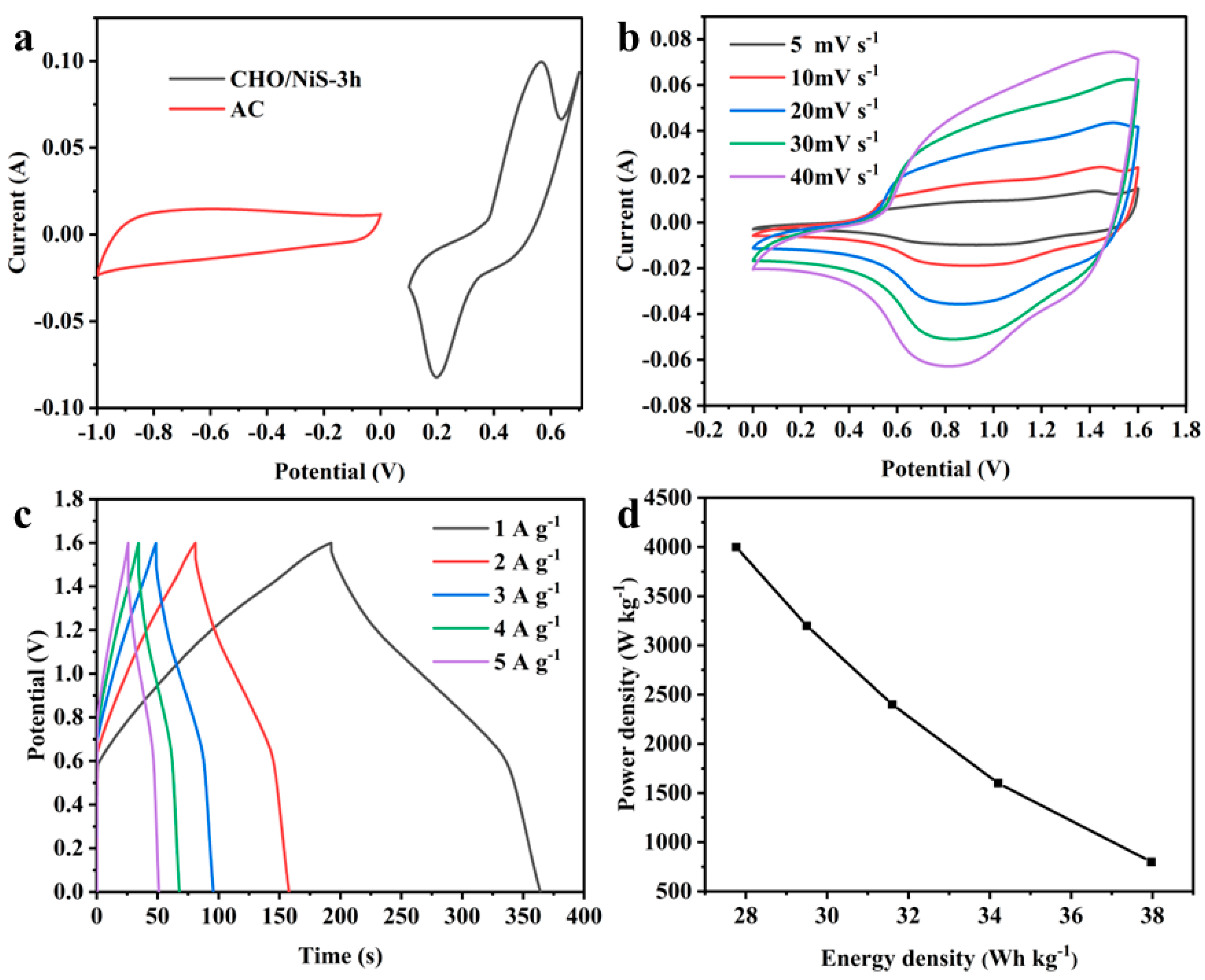
2.3.3. Electrochemical Performance of Asymmetric Supercapacitors
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of CHO
3.4. Growth of Transition Metal Sulfide
3.5. Preparation of Working Electrodes
3.6. Assembly of Asymmetric Supercapacitors
3.7. Electrochemical Property Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Fan, P.; Ye, C.; Xu, L. One-dimensional nanostructured electrode materials based on electrospinning technology for supercapacitors. Diam. Relat. Mater. 2023, 134, 109803. [Google Scholar] [CrossRef]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Aziz, U. Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices. J. Energy Storage 2022, 46, 103823. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Qiao, Y. NiS/Cu7S4 composites as high-performance supercapacitor electrodes. J. Solid State Electr. 2023, 27, 25–36. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Guan, B.; Li, Y.; Yin, B.; Liu, K.; Wang, D.; Zhang, H.; Cheng, C. Synthesis of hierarchical NiS microflowers for high performance asymmetric supercapacitor. Chem. Eng. J. 2017, 308, 1165–1173. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, S.; Zhang, J.; Wang, J.; She, W.; Wang, K.; Xia, X.; Yang, B.; Meng, X. Facile solid−phase synthesis of layered NiS/rGO nanocomposite for high−performance hybrid supercapacitor. J. Power Sources 2021, 514, 230590. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Cao, S.; Xue, H.; Pang, H. Transition Metal Sulfides Based on Graphene for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Mallick, S.; Mondal, A.; Raj, C.R. Rationally designed mesoporous carbon-supported Ni–NiWO4@NiS nanostructure for the fabrication of hybrid supercapacitor of long-term cycling stability. J. Power Sources 2020, 477, 229038. [Google Scholar] [CrossRef]
- Azad, M.; Hussain, Z.; Baig, M.M. MWCNTs/NiS2 decorated Ni foam based electrode for high-performance supercapacitors. Electrochim. Acta 2020, 345, 136196. [Google Scholar] [CrossRef]
- Fan, P.; Xu, L. Core-Shell Carbon Nanofibers@Ni(OH)2/NiO Composites for High-Performance Asymmetric Supercapacitors. Materials 2022, 15, 8377. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Liu, F.; Wen, J.; Zhang, N.; Wang, S.; Liu, K. Enhanced performance of multi-dimensional CoS nanoflake/NiO nanosheet architecture with synergetic effect for asymmetric supercapacitor. Nanotechnology 2018, 29, 455401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, T.; Li, Y.; Yang, J.; Huang, B.; Ou, S.; Meng, C.; Zhang, S.; Tong, Y. Consecutive chemical bonds reconstructing surface structure of silicon anode for high-performance lithium-ion battery. Energy Storage Mater. 2021, 39, 354–364. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Meng, T.; Yang, J.; Huang, B.; Gu, F.L.; Zhang, S.; Meng, C.; Tong, Y. Boosting Electron Transfer with Heterointerface Effect for High-Performance Lithium-Ion Storage. Energy Storage Mater. 2021, 36, 365–375. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Guo, Y.; Wang, Y. Self-supported Co-Ni-S@CoNi-LDH electrode with a nanosheet-assembled core-shell structure for a high-performance supercapacitor. J. Alloys Compd. 2022, 908, 164635. [Google Scholar] [CrossRef]
- Bhagwan, J.; Han, J.I. Promotive Effect of MWCNTs on α-NiS Microstructure and Their Application in Aqueous Asymmetric Supercapacitor. Energy Fuels 2022, 36, 15210–15220. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, L.; Wei, T.; Liu, X. MoS2/NiS Yolk–Shell Microsphere-Based Electrodes for Overall Water Splitting and Asymmetric Supercapacitor. Small 2019, 15, 1803639. [Google Scholar] [CrossRef]
- Ansari, S.A.; Kotb, H.M.; Ahmad, M.M. Wrinkle-Shaped Nickel Sulfide Grown on Three-Dimensional Nickel Foam: A Binder-Free Electrode Designed for High-Performance Electrochemical Supercapacitor Applications. Crystals 2022, 12, 757. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Guo, X.; Liu, Y.; Zheng, Y.; Zhang, M.; Li, R.; Peng, Z.; Zhang, Y.; Zhang, T. One-step microwave-hydrothermal preparation of NiS/rGO hybrid for high-performance symmetric solid-state supercapacitor. Appl. Surf. Sci. 2020, 514, 146080. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, X.; Zhan, J.; Sui, Y.; Qi, J.; Wei, F.; Meng, Q.; He, Y.; Ren, Y.; Zhan, Z.; et al. Hierarchical NiS@CoS with Controllable Core-Shell Structure by Two-Step Strategy for Supercapacitor Electrodes. Adv. Mater. Interfaces 2020, 7, 1901618. [Google Scholar] [CrossRef]
- Qi, M.; Zhu, W.; Lu, Z.; Zhang, H.; Ling, Y.; Ou, X. Synthesis of nickel sulfide–graphene oxide composite microflower structures to enhance supercapacitor performance. J. Mater. Sci. Mater. Electron. 2020, 31, 12536–12545. [Google Scholar] [CrossRef]
- Forghani, M.; Donne, S.W. Method Comparison for Deconvoluting Capacitive and Pseudo-Capacitive Contributions to Electrochemical Capacitor Electrode Behavior. J. Electrochem. Soc. 2018, 165, A664. [Google Scholar] [CrossRef]
- Jayababu, N.; Jo, S.; Kim, Y.; Kim, D. Preparation of NiO decorated CNT/ZnO core-shell hybrid nanocomposites with the aid of ultrasonication for enhancing the performance of hybrid supercapacitors. Ultrason. Sonochem. 2021, 71, 105374. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zang, S.; Liang, W.; Wu, D.; Lian, Z.; Xu, F.; Jiang, K.; Gao, Z. Enhanced faradic activity by construction of p-n junction within reduced graphene oxide@cobalt nickel sulfide@nickle cobalt layered double hydroxide composite electrode for charge storage in hybrid supercapacitor. J. Colloid Interf. Sci. 2021, 590, 114–124. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Q.; Chen, Y.; Liu, Q.; Yu, Q.; Wu, H.B. Interface-Induced Pseudocapacitance in Nonporous Heterogeneous Particles for High Volumetric Sodium Storage. Adv. Funct. Mater. 2020, 30, 2002019. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Yang, B.; Shi, H.; Wang, K.; Han, L.; Wang, S.; Wang, Y. Targeted synthesis of NiS and NiS2 nanoparticles for high−performance hybrid supercapacitor via a facile green solid−phase synthesis route. J. Energy Storage 2020, 32, 101852. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, W.; Zhao, Q.; Lu, S.; Wang, X.; Zhou, M.; Tao, B.; Xie, Q.; Ruan, Y. NiS Nanoflake-Coated Carbon Nanofiber Electrodes for Supercapacitors. ACS Appl. Nano Mater. 2022, 5, 6192–6200. [Google Scholar] [CrossRef]
- Son, Y.-H.; Lee, H.-R.; Akhtar, M.S.; Yang, O.B. Electrochemical behavior of uniformly decorated electrospun nickel on carbon nanofibers. Mol. Catal. 2021, 504, 111458. [Google Scholar] [CrossRef]
- Fan, P.; Ye, C.; Xu, L. Core-shell Nanofiber-based Electrodes for High-performance Asymmetric Supercapacitors. ChemistrySelect 2023, 8, e202204669. [Google Scholar] [CrossRef]



| Samples | Elemental Content of Each Sample (Atomic %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | N | O | Ni | Mn | Co | Fe | Cu | S | |
| CHO/MnS-5h | 53.49 | 6.30 | 20.37 | 7.34 | 9.61 | \ | \ | \ | 2.89 |
| CHO/CoS-5h | 67.78 | 7.39 | 13.63 | 3.70 | \ | 3.09 | \ | \ | 4.41 |
| CHO/FeS-5h | 79.15 | 7.50 | 6.66 | 2.72 | \ | \ | 0.71 | \ | 3.26 |
| CHO/CuS-5h | 86.51 | 4.42 | 4.33 | 3.03 | \ | \ | \ | 0.22 | 1.49 |
| CHO/NiS-5h | 59.87 | 7.15 | 15.07 | 14.96 | \ | \ | \ | \ | 2.95 |
| Samples | Elemental Content of Each Sample (Atomic %) | ||||
|---|---|---|---|---|---|
| C | N | O | Ni | S | |
| CHO/NiS-5h | 59.87 | 7.15 | 15.07 | 14.96 | 2.95 |
| CHO/NiS-3h | 47.96 | 12.72 | 27.07 | 10.38 | 1.86 |
| CHO/NiS-1h | 58.87 | 11.75 | 24.05 | 8.44 | 1.09 |
| SA (m2 g−1) | VT (cm3 g−1) | VS (cm3 g−1) | VL (cm3 g−1) | Wavg (nm) |
|---|---|---|---|---|
| 12.3815 | 0.068 | 0.003 | 0.065 | 12.845 |
| Electrode Material | Preparation Method | Morphology | Specific Capacitance (F g−1) | Electrolyte | Ref. |
|---|---|---|---|---|---|
| CHO/NiS-3h | Electrospinning, carbonization, hydrothermal growth | Core–shell fibers | 1717 (1 A g−1) | 3M KOH | This work |
| NiS | Hydrothermal growth | Multistage NiS micro flower | 1122.7 (1 A g−1) | 3 M KOH | [6] |
| NiS/Cu7S4-DT | Hydrothermal growth | Nanoparticles | 1674 (1 A g−1) | 6 M KOH | [4] |
| Co-Ni-S@CoNi-LDH | Hydrothermal growth | Core–shell nanosheet array | 2414 (1 A g−1) | 3 M KOH | [16] |
| α-NiS@MWCNT | Hydrothermal growth, muffle furnace heating | Microsphere | 2057 (1 A g−1) | 2 M KOH | [17] |
| MoS2/NiS | Hydrothermal growth | Egg yolk shell microsphere | 1194 (1 A g−1) | 6 M KOH | [18] |
| NiS-3D-Nf | Hydrothermal growth | Granular NiS | 770 (1 A g−1) | 3 M KOH | [19] |
| NiS/rGO | Microwave-hydrothermal method | Granular hybrid | 1745.67 (1 A g−1) | 2 M KOH | [20] |
| NiS@CoS | Hydrothermal growth, electrodeposition | Core–shell structure | 1210 (1 A g−1) | 2 M KOH | [21] |
| NixSy–TRGO | Hydrothermal growth | Microflorate | 1602.2 (1 A g−1) | 2 M KOH | [22] |
| Assembly Materials | Specific Capacitance (F g−1) | Maximum Power Density (W kg−1) | Energy Density at Maximum Power Density (Wh kg−1) | Voltage Window (V) | Ref. |
|---|---|---|---|---|---|
| CHO/NiS-3h//AC | 106.8 (1 A g−1) | 4000 | 27.76 | 1.6 | This work |
| NiS//AC | 69.1 (1 A g−1) | 8800 | 12.9 | 1.8 | [6] |
| NiS/Cu7S4-DT//AC | 157 (0.5 A g−1) | 7492.5 | 20.8 | 1.5 | [4] |
| Co-Ni-S@CoNi-LDH//AC | 147.27 (1 A g−1) | 8500.93 | 39.91 | 1.7 | [16] |
| α-NiS@MWCNT//AC | 80 (1 A g−1) | 2175 | 12 | 1.5 | [17] |
| N-NiS//PCN | 109 (1 A g−1) | 10,900 | 19.99 | 1.6 | [27] |
| NixSy–TRGO//TRGO | 123.7 (1 A g−1) | 7500 | 23.75 | 1.5 | [22] |
| NiS/CNFs-2//AC | 29.8 mAh g−1 (1 A g−1) | 7500 | 12.69 | 1.6 | [28] |
| NiS@CoS//AC | 75.9 (1 A g−1) | 3849 | 6.875 | 1.5 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Wang, J.; Ding, W.; Xu, L. Core–Shell Structured Carbon Nanofiber-Based Electrodes for High-Performance Supercapacitors. Molecules 2023, 28, 4571. https://doi.org/10.3390/molecules28124571
Fan P, Wang J, Ding W, Xu L. Core–Shell Structured Carbon Nanofiber-Based Electrodes for High-Performance Supercapacitors. Molecules. 2023; 28(12):4571. https://doi.org/10.3390/molecules28124571
Chicago/Turabian StyleFan, Peizhi, Jie Wang, Wenfei Ding, and Lan Xu. 2023. "Core–Shell Structured Carbon Nanofiber-Based Electrodes for High-Performance Supercapacitors" Molecules 28, no. 12: 4571. https://doi.org/10.3390/molecules28124571
APA StyleFan, P., Wang, J., Ding, W., & Xu, L. (2023). Core–Shell Structured Carbon Nanofiber-Based Electrodes for High-Performance Supercapacitors. Molecules, 28(12), 4571. https://doi.org/10.3390/molecules28124571






