Asymmetric Sulfoxidation by a Tyrosinase Biomimetic Dicopper Complex with a Benzimidazolyl Derivative of L-Phenylalanine
Abstract
1. Introduction
2. Results and Discussion
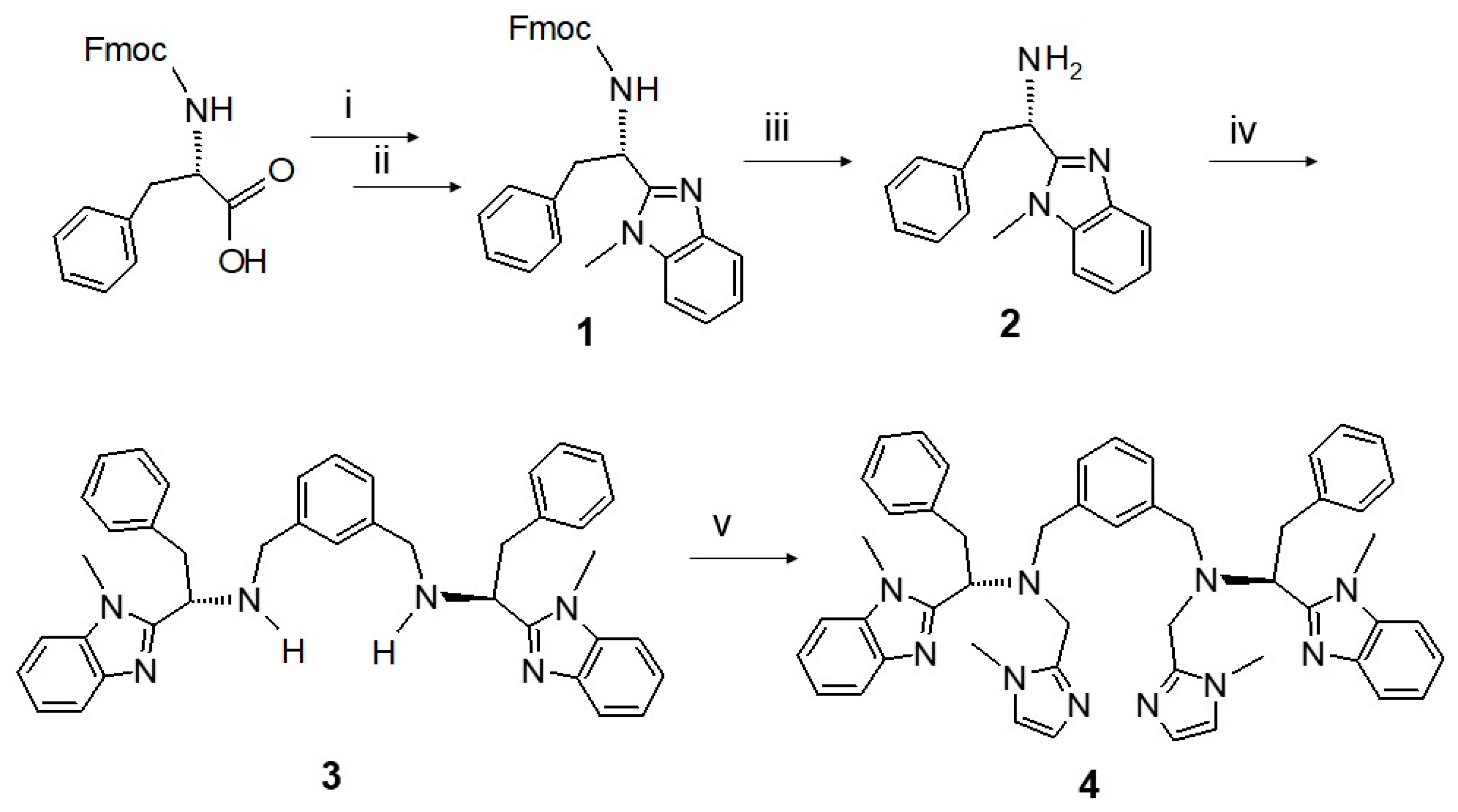
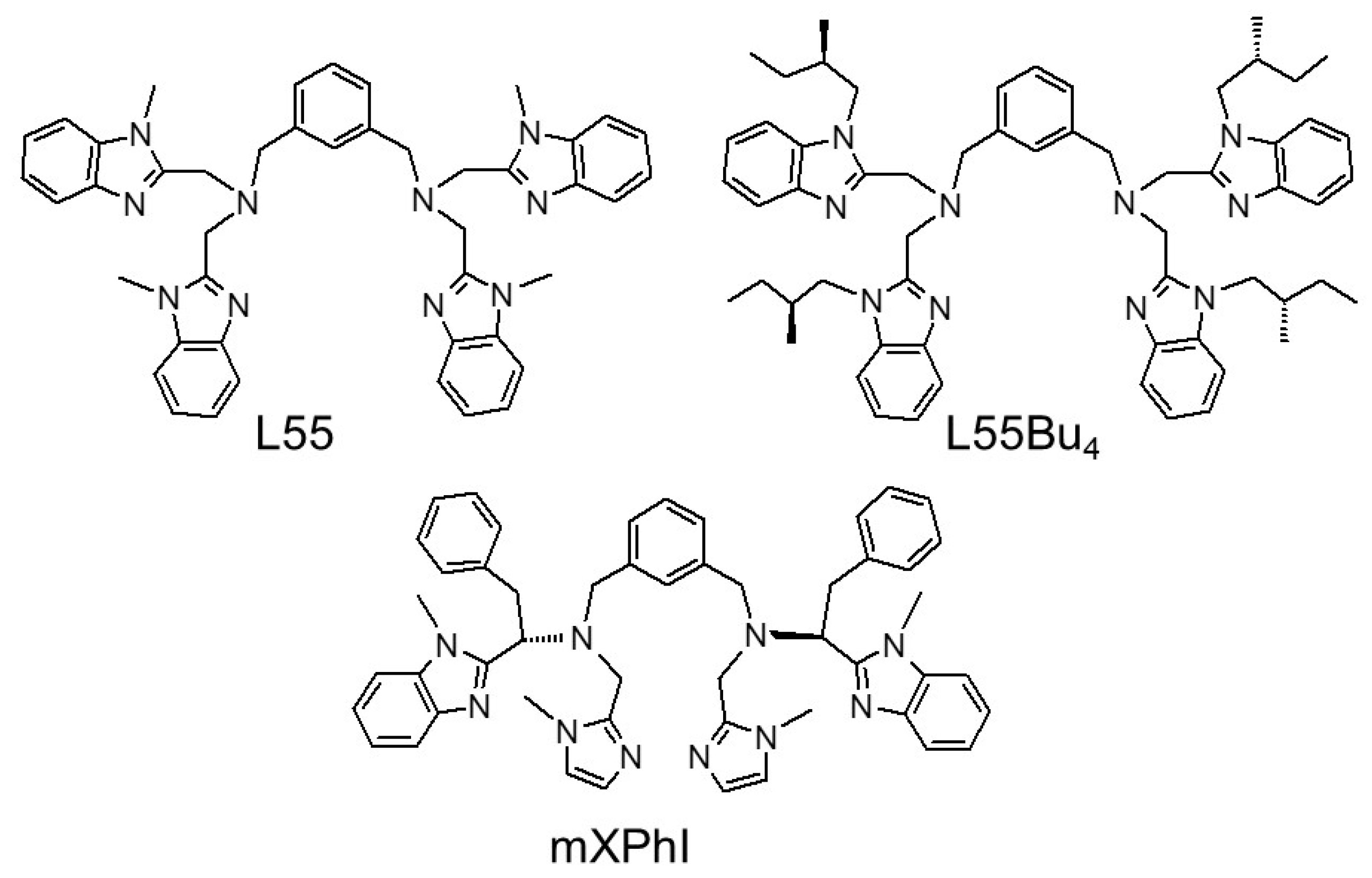
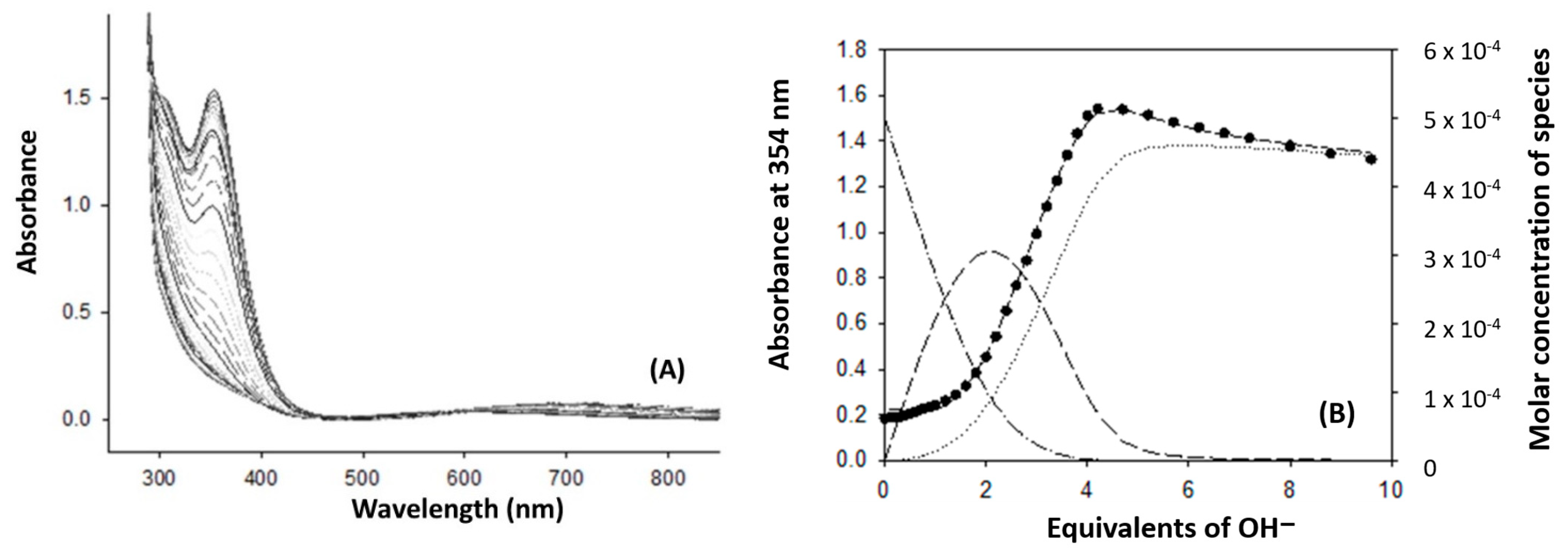
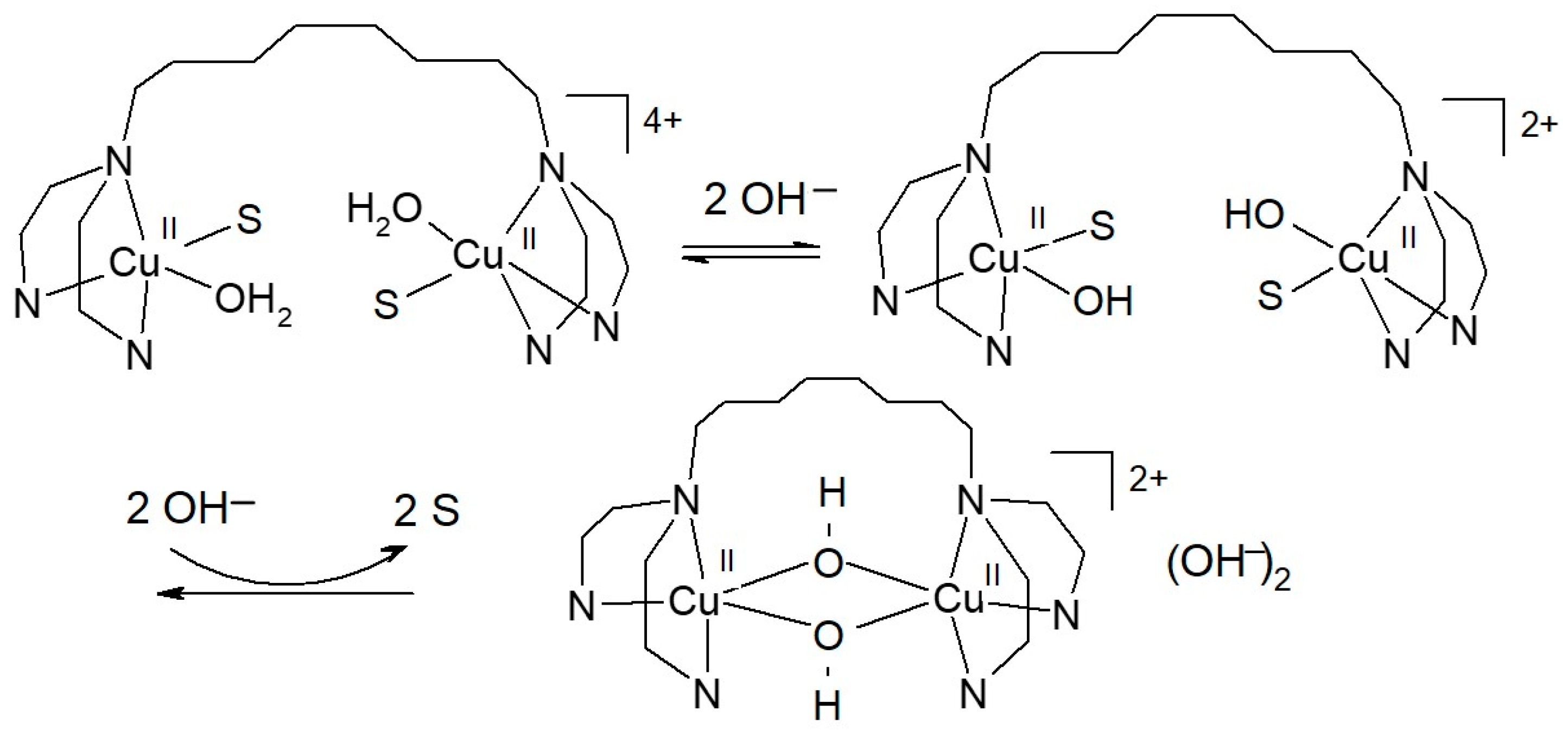
2.1. Synthesis and Characterization
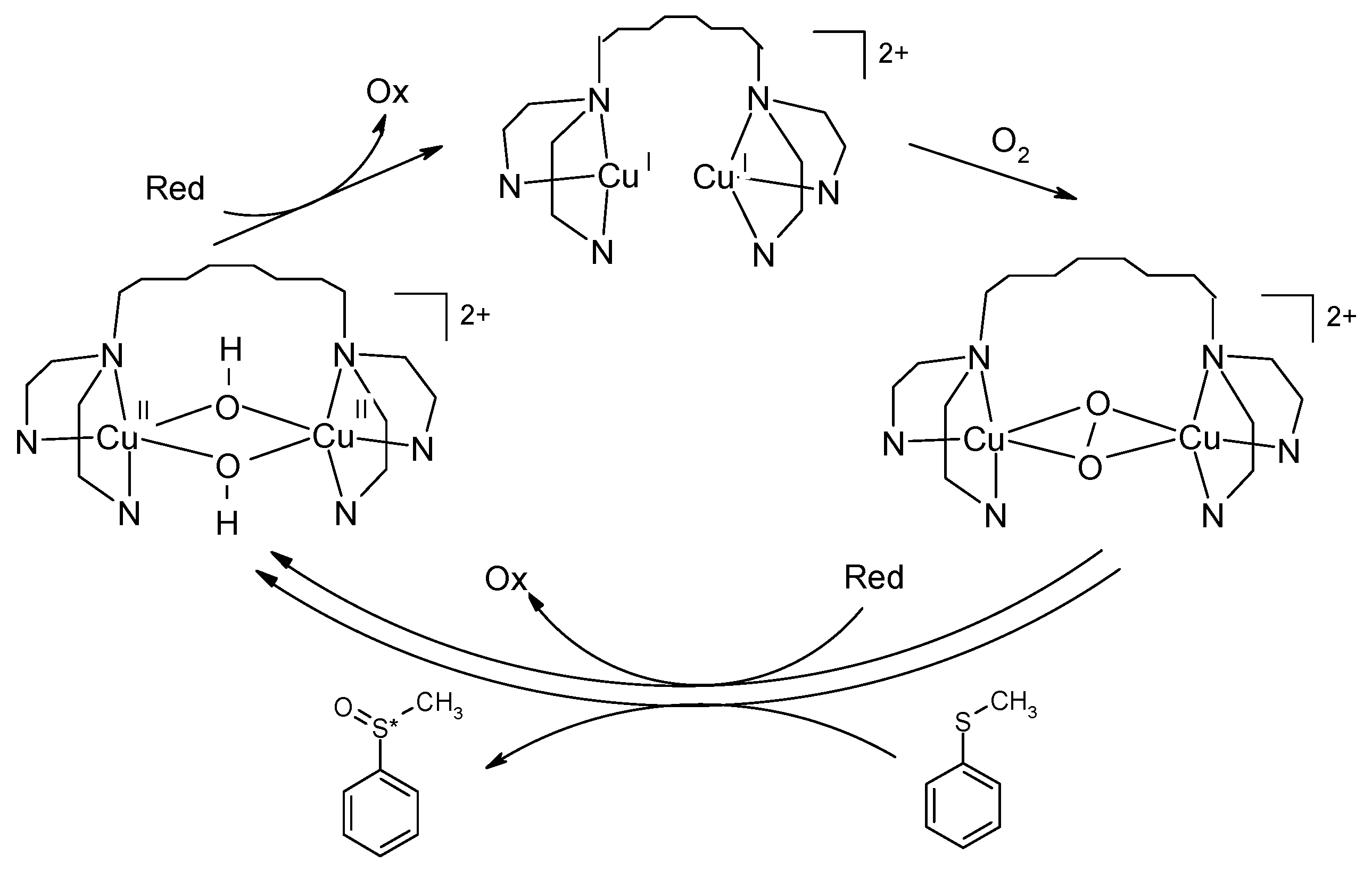
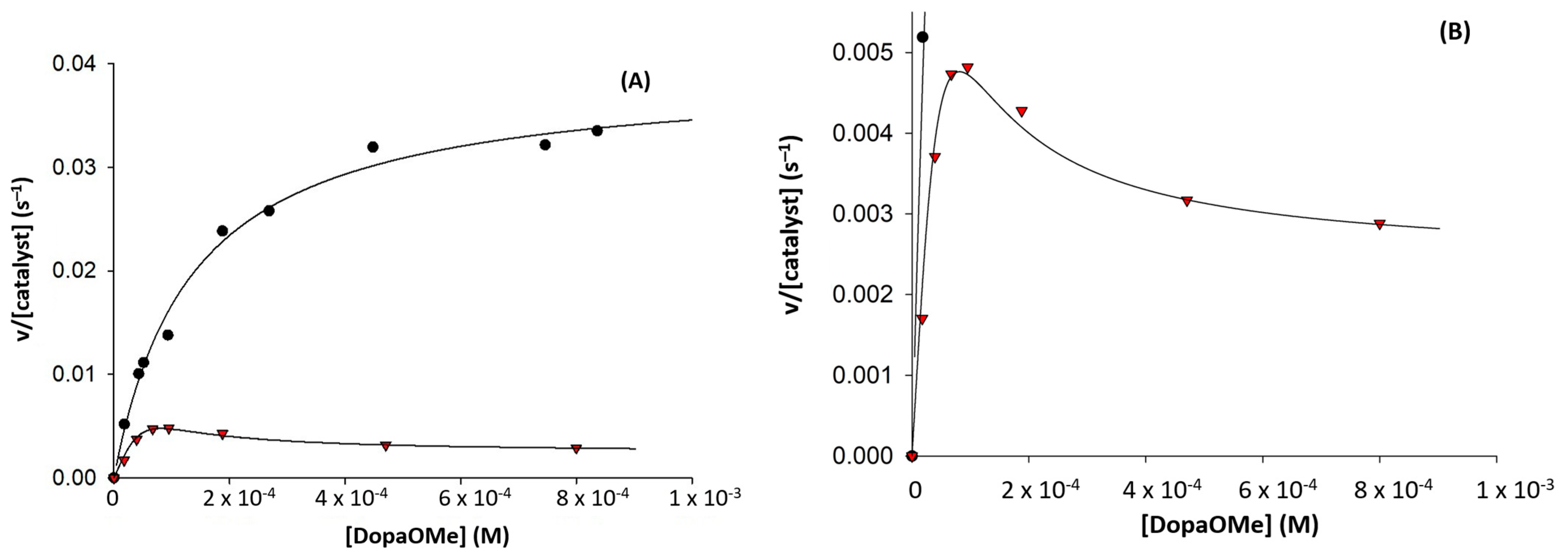
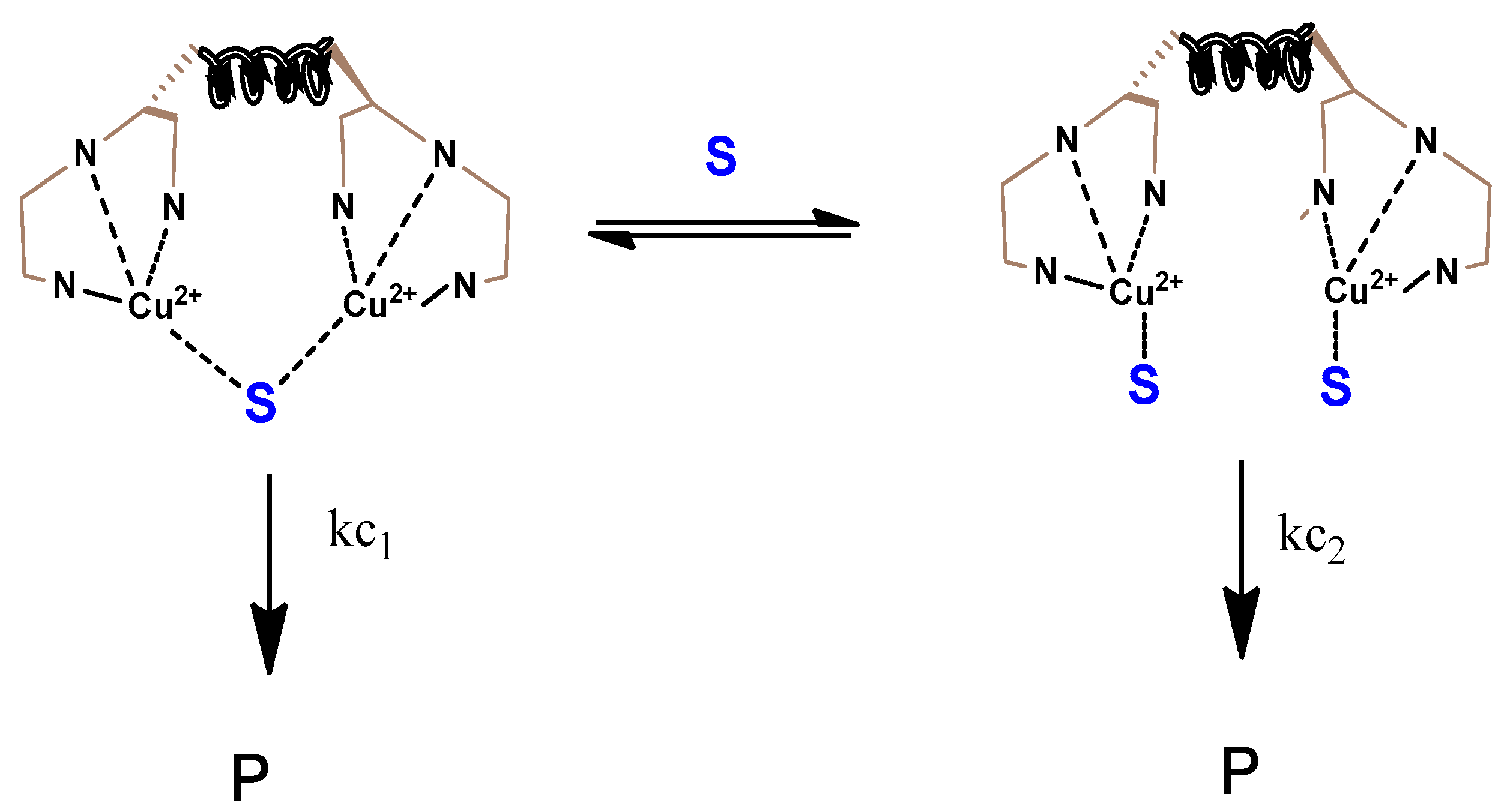
2.2. Enantioselective Oxidation of Sulfides
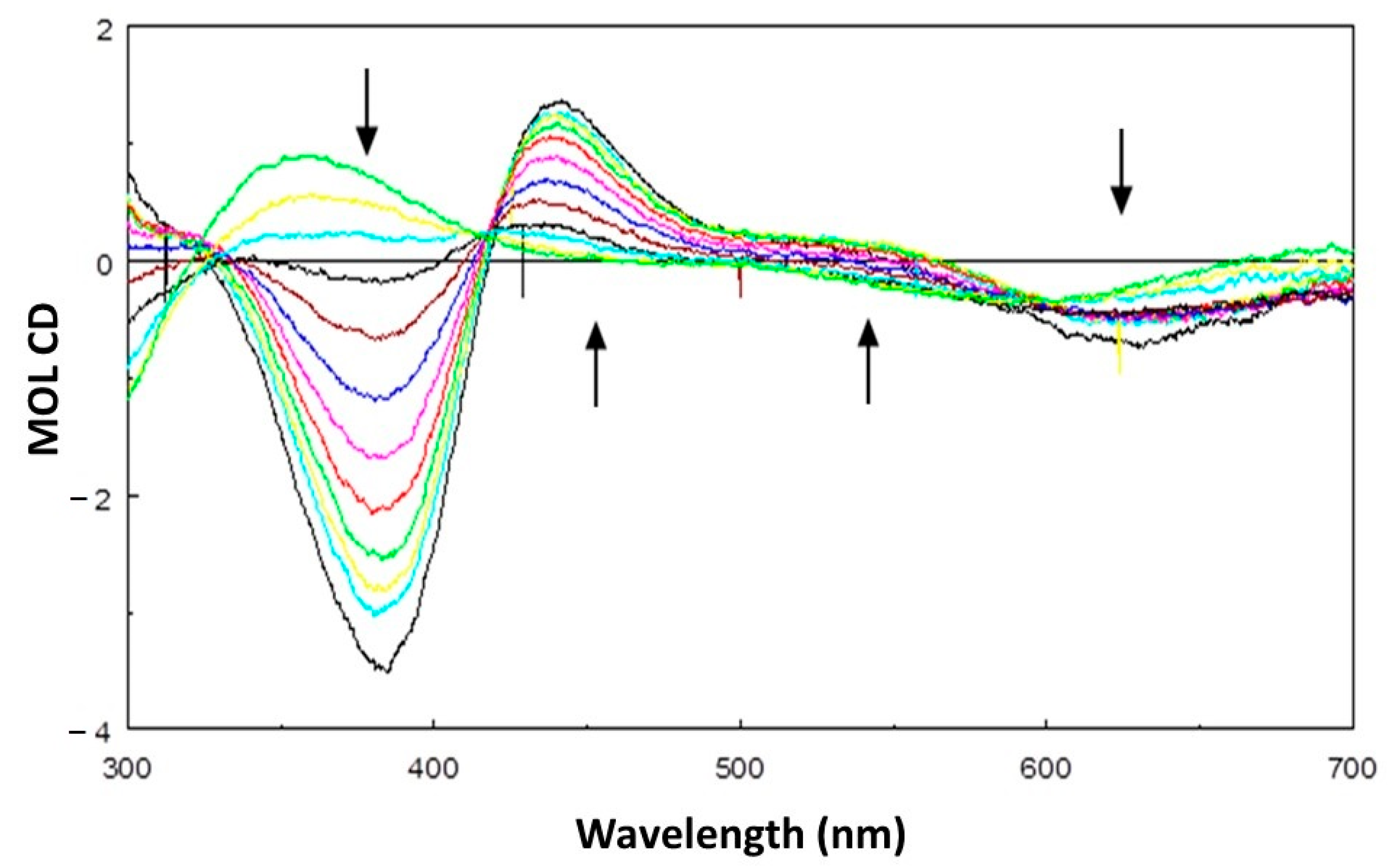
2.3. Oxidation of Chiral Catechols: Enantio-Discriminating Catecholase Activity
3. Materials and Methods
3.1. Synthesis of (9H-fluoren-9-yl)methyl(1-(1-methyl-1H-benzoimidazol-2-yl)-2-phenylethyl)carbamate (1)
3.2. Synthesis of 1-(1-methyl-1H-benzoimidazol-2-yl)-2-phenylethanamine (2)
3.3. Synthesis of N,N′-(1,3-phenylenebis(methylene))bis(1-(1-methyl-1H-benzoimidazol-2-yl)-2-phenylethanamine) (3)
3.4. Synthesis of N,N′-(1,3-phenylenebis(methylene))bis(1-(1-methyl-1H-benzoimidazol-2-yl)-N-((1-methyl-1H-imidazol-2-yl)methyl)-2-phenylethanamine) (4, mXPhI)
3.5. Synthesis of [Cu2(mXPhI)](ClO4)2
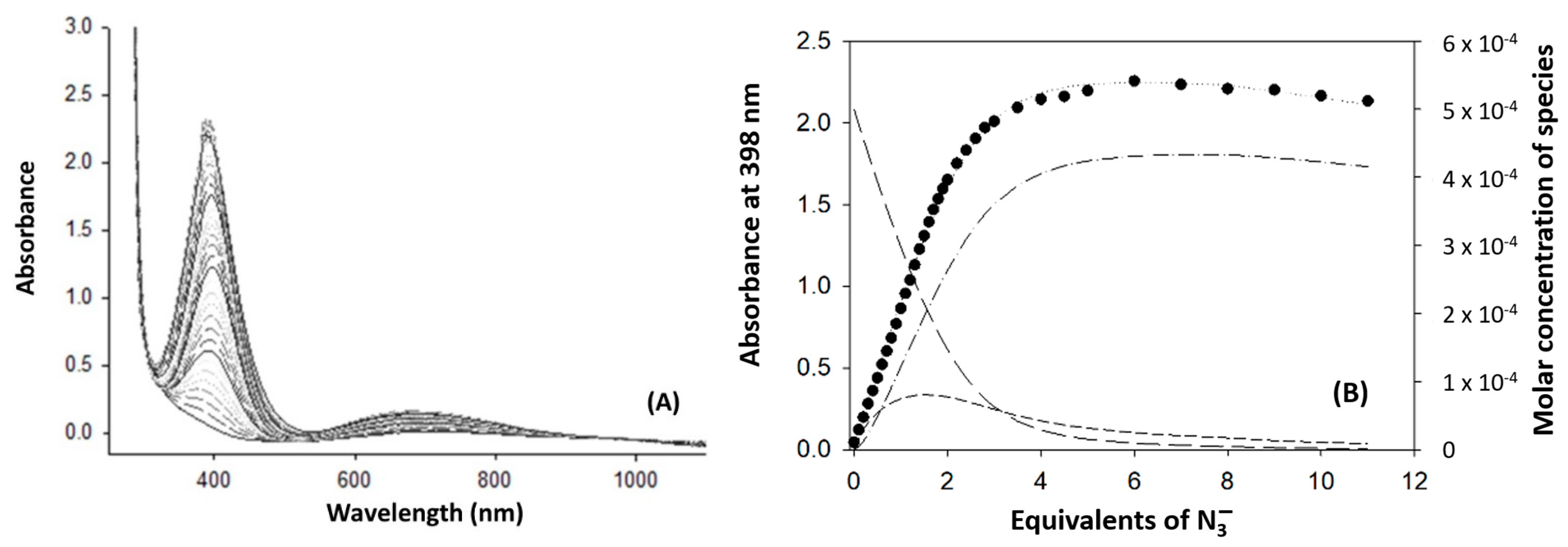
3.6. Spectrophotometric Titration of [Cu2(mXPhI)]4+ with NaN3
3.7. Spectrophotometric Titration of [Cu2(mXPhI)]4+ with NaOH
3.8. Oxidation of Chiral Catechols
3.9. General Procedure for Asymmetric Sulfoxidation
3.10. Determination of Enantiomeric Excess
3.11. Experiment with 18-O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamann, J.N.; Herzigkeit, B.; Jurgeleit, R.; Tuczek, F. Small-molecule models of tyrosinase: From ligand hydroxylation to catalytic monooxygenation of external substrates. Coord. Chem. Rev. 2017, 334, 54–66. [Google Scholar]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [PubMed]
- Elwell, C.E.; Gagnon, N.L.; Neisen, B.D.; Dhar, D.; Spaeth, A.D.; Yee, G.M.; Tolman, W.B. Copper−oxygen complexes revisited: Structures, spectroscopy, and reactivity. Chem. Rev. 2017, 117, 2059–2107. [Google Scholar] [PubMed]
- Trammell, R.; Rajabimoghadam, K.; Garcia-Bosch., I. Copper-promoted functionalization of organic molecules: From biologically relevant Cu/O2 model systems to organometallic transformations. Chem. Rev. 2019, 119, 2954–3031. [Google Scholar] [PubMed]
- Casella, L.; Monzani, E.; Gullotti, M.; Cavagnino, D.; Cerina, G.; Santagostini, L.; Ugo, R. Functional Modeling of Tyrosinase. Mechanism of Phenol ortho-Hydroxylation by Dinuclear Copper Complexes. Inorg. Chem. 1996, 35, 7516–7525. [Google Scholar]
- Battaini, G.; Casella, L.; Gullotti, M.; Monzani, E.; Nardin, A.; Perotti, A.; Randaccio, L.; Santagostini, L.; Heinemann, F.W.; Schindler, S. Structure and reactivity studies on dinuclear copper complexes of the ligand α,α′-bis{bis[1-(1′-methyl-2′-benzimidazolyl)-methyl]amino}-m-xylene. Eur. J. Inorg. Chem. 2003, 2003, 1197–1205. [Google Scholar] [CrossRef]
- Granata, A.; Monzani, E.; Casella, L. Mechanistic insight into the catechol oxidase activity by a biomimetic dinuclear copper complex. J. Biol. Inorg. Chem. 2004, 9, 903–913. [Google Scholar] [CrossRef]
- Palavicini, S.; Granata, A.; Monzani, E.; Casella, L. Hydroxylation of phenolic compounds by a peroxodicopper(II) complex: Further insight into the mec hanism of tyrosinase. J. Am. Chem. Soc. 2005, 127, 18031–18036. [Google Scholar] [CrossRef]
- Lo Presti, E.L.; Monzani, E.; Santagostini, L.; Casella, L. Building biomimetic model compounds of dinuclear and trinuclear copper clusters for stereoselective oxidations. Inorg. Chim. Acta 2018, 481, 47–55. [Google Scholar] [CrossRef]
- Perrone, M.L.; Lo Presti, E.; Dell’Acqua, S.; Monzani, E.; Santagostini, L.; Casella, L. Synthesis, characterization, and stereoselective oxidations of the dinuclear copper(II) complex derived from a chiral diamino-m-xylenetetra(benzimidazole) ligand. Eur. J. Inorg. Chem. 2015, 21, 3493–3500. [Google Scholar] [CrossRef]
- Perrone, M.L.; Salvadeo, E.; Lo Presti, E.; Pasotti, L.; Monzani, E.; Santagostini, L.; Casella, L. A dinuclear biomimetic Cu complex derived from L-histidine: Synthesis and stereoselective oxidations. Dalton Trans. 2017, 46, 4018–4029. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, E.; Perrone, M.L.; Santagostini, L.; Casella, L.; Monzani, E. A Stereoselective tyrosinase model compound derived from an m-xylyl-L-histidine ligand. Inorg. Chem. 2019, 58, 7335–7344. [Google Scholar] [CrossRef]
- Potter, A.J.; Ray, S.; Gueritz, L.; Nunns, C.L.; Bryant, C.J.; Scrace, S.F.; Matassova, N.; Baker, L.; Dokurno, P.; Robinson, D.A.; et al. Structure-guided design of alpha-amino acid-derived Pin1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 586–590. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Casella, L.; Carugo, O.; Gullotti, M.; Garofani, S.; Zanello, P. Hemocyanin and tyrosinase models. Synthesis, azide binding, and electrochemistry of dinuclear copper(II) complexes with poly(benzimidazole) ligands modeling the met forms of the proteins. Inorg. Chem. 1993, 32, 2056–2067. [Google Scholar] [CrossRef]
- Pate, J.E.; Thamann, T.J.; Solomon, E.I. Resonance Raman studies of the coupled binuclear copper active site in met azide hemocyanin. Spectrochim. Acta Part A 1986, 42, 313–318. [Google Scholar] [CrossRef]
- Pate, J.E.; Ross, P.K.; Thamann, T.J.; Reed, C.A.; Karlin, K.D.; Sorrell, T.N.; Solomon, E.I. Spectroscopic Studies of the Charge Transfer and Vibrational Features of Binuclear Copper(II) Azide Complexes: Comparison to the Coupled Binuclear Copper Active Site in Met Azide Hemocyanin and Tyrosinase. J. Am. Chem. Soc. 1989, 111, 5198–5205. [Google Scholar] [CrossRef]
- Mason, S.F. Molecular Optical Activity and the Chiral Discriminations; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Gullotti, M.; Santagostini, L.; Pagliarin, R.; Palavicini, S.; Casella, L.; Monzani, E.; Zoppellaro, G. Ligand binding, conformational and spectroscopic properties, and biomimetic monooxygenase activity by the trinuclear copper−PHI complex derived from L-histidine. Eur. J. Inorg. Chem. 2008, 2008, 2081–2089. [Google Scholar] [CrossRef]
- Gamba, I.; Palavicini, S.; Monzani, E.; Casella, L. Catalytic sulfoxidation by dinuclear copper complexes. Chem. Eur. J. 2009, 15, 12932–12936. [Google Scholar] [CrossRef]
- Pievo, R.; Gullotti, M.; Monzani, E.; Casella, L. Tyrosinase catalyzes asymmetric sulfoxidation. Biochemistry 2008, 47, 3493–3498. [Google Scholar] [CrossRef]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper–Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. 2020, 132, 13487–13492. [Google Scholar] [CrossRef]
- Kipouros, I.; Solomon, E.I. New mechanistic insights into coupled binuclear copper monooxygenases from the recent elucidation of the ternary intermediate of tyrosinase. FEBS Lett. 2023, 597, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef] [PubMed]

| [NH2OH], mM | Substrate | Yield % | e.e. % | TON a |
|---|---|---|---|---|
| 0.3 | thioanisole | 35% | 18% | 10.5 |
| 0.5 | thioanisole | 13% | 32% | 6.5 |
| 1 | thioanisole | 6% | 43% | 6 |
| 5 | thioanisole | 0.5% | 36% | 2.5 |
| 10 | thioanisole | trace | 19% | trace |
| 0.3 | p-tolyl methyl sulfide | ND b | ~40% | ND |
| 0.5 | p-tolyl methyl sulfide | 6 | 39% | 3 |
| 1 | p-tolyl methyl sulfide | 3 | 38% | 3 |
| 5 | p-tolyl methyl sulfide | 0.3 | 27% | 1.5 |
| 10 | p-tolyl methyl sulfide | trace | racemic | trace |
| Substrate | KM (mM) | kcat (s−1) | kcat/KM (M−1 s−1) | R(kcat) % | R(kcat/KM) % |
|---|---|---|---|---|---|
| L-Dopa | (7.37 ± 0.16) × 10−2 | (2.20 ± 0.13) × 10−3 | 29.9 | 3% | −3% |
| D-Dopa | (6.52 ± 0.89) × 10−2 | (2.14 ± 0.07) × 10−3 | 32.8 | ||
| L-DopaOMe | (1.35 ± 0.14) × 10−1 | (3.93 ± 0.13) × 10−2 | 29.1 | ||
| D-DopaOMe | - | - | - | ||
| R-(-)-norepinephrine | (8.26 ± 0.52) × 10−2 | (2.29 ± 0.04) × 10−3 | 27.7 | 3% | −10% |
| S-(+)-norepinephrine | (6.38 ± 0.41) × 10−2 | (2.17 ± 0.04) × 10−3 | 34.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Presti, E.; Schifano, F.; Bacchella, C.; Santagostini, L.; Casella, L.; Monzani, E. Asymmetric Sulfoxidation by a Tyrosinase Biomimetic Dicopper Complex with a Benzimidazolyl Derivative of L-Phenylalanine. Molecules 2023, 28, 4487. https://doi.org/10.3390/molecules28114487
Lo Presti E, Schifano F, Bacchella C, Santagostini L, Casella L, Monzani E. Asymmetric Sulfoxidation by a Tyrosinase Biomimetic Dicopper Complex with a Benzimidazolyl Derivative of L-Phenylalanine. Molecules. 2023; 28(11):4487. https://doi.org/10.3390/molecules28114487
Chicago/Turabian StyleLo Presti, Eliana, Fabio Schifano, Chiara Bacchella, Laura Santagostini, Luigi Casella, and Enrico Monzani. 2023. "Asymmetric Sulfoxidation by a Tyrosinase Biomimetic Dicopper Complex with a Benzimidazolyl Derivative of L-Phenylalanine" Molecules 28, no. 11: 4487. https://doi.org/10.3390/molecules28114487
APA StyleLo Presti, E., Schifano, F., Bacchella, C., Santagostini, L., Casella, L., & Monzani, E. (2023). Asymmetric Sulfoxidation by a Tyrosinase Biomimetic Dicopper Complex with a Benzimidazolyl Derivative of L-Phenylalanine. Molecules, 28(11), 4487. https://doi.org/10.3390/molecules28114487







