Multi-Responsive Molecular Encapsulation and Release Based on Hydrogen-Bonded Azo-Macrocycle
Abstract
1. Introduction
2. Results and Discussion
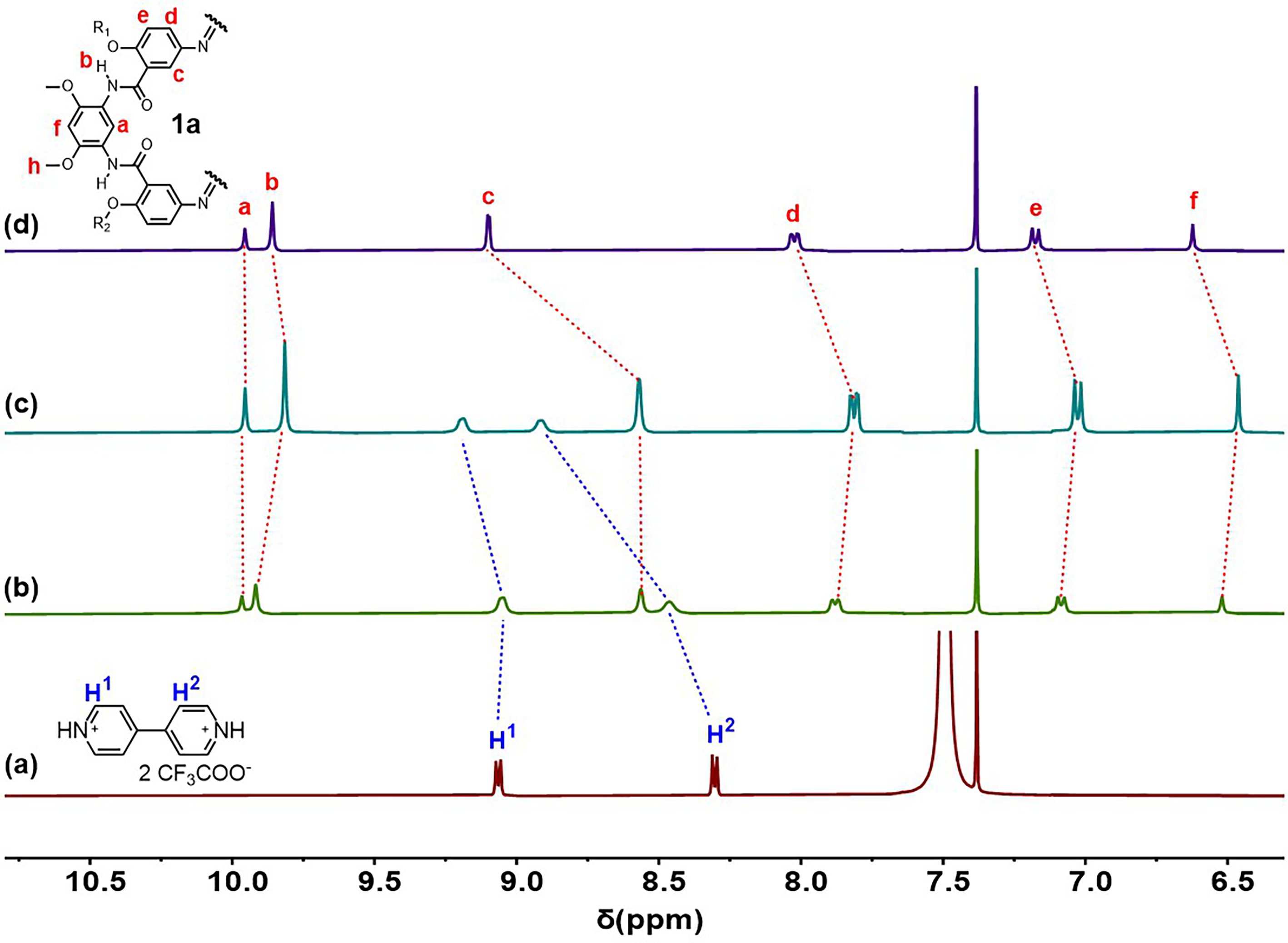
2.1. Host–Guest Complexation
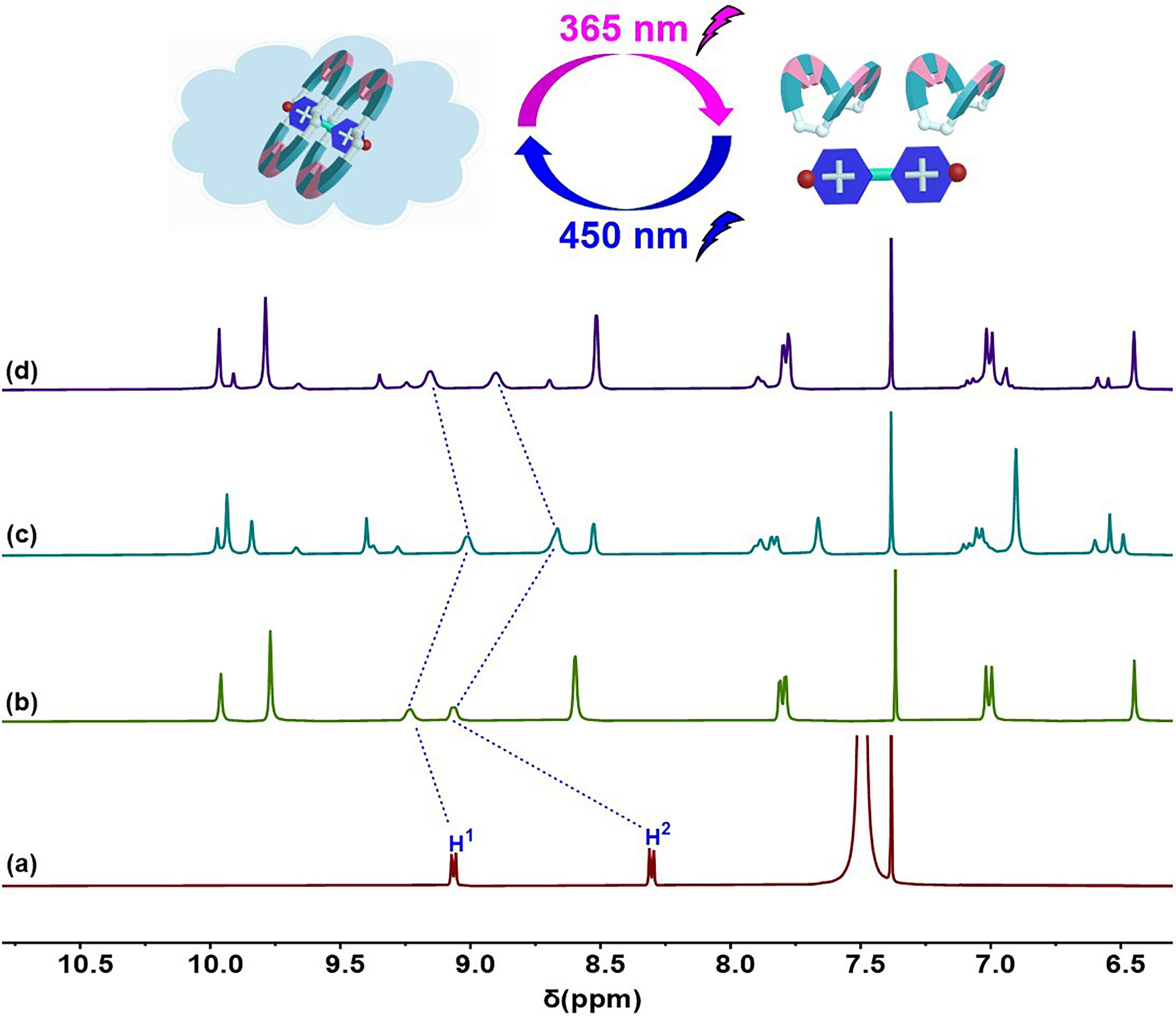
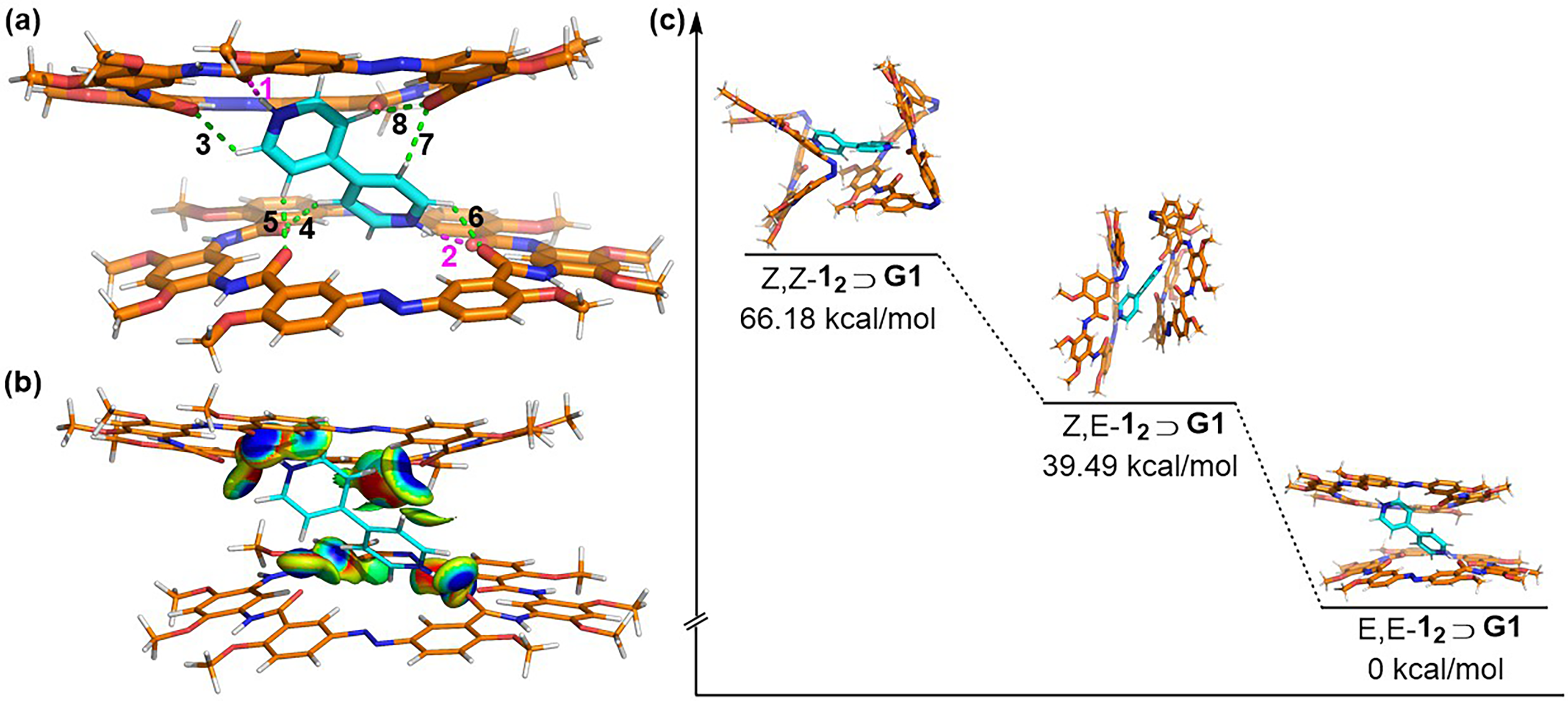
2.2. Photo-Switchable Complexation
2.3. Acid/Base-Switchable Complexation
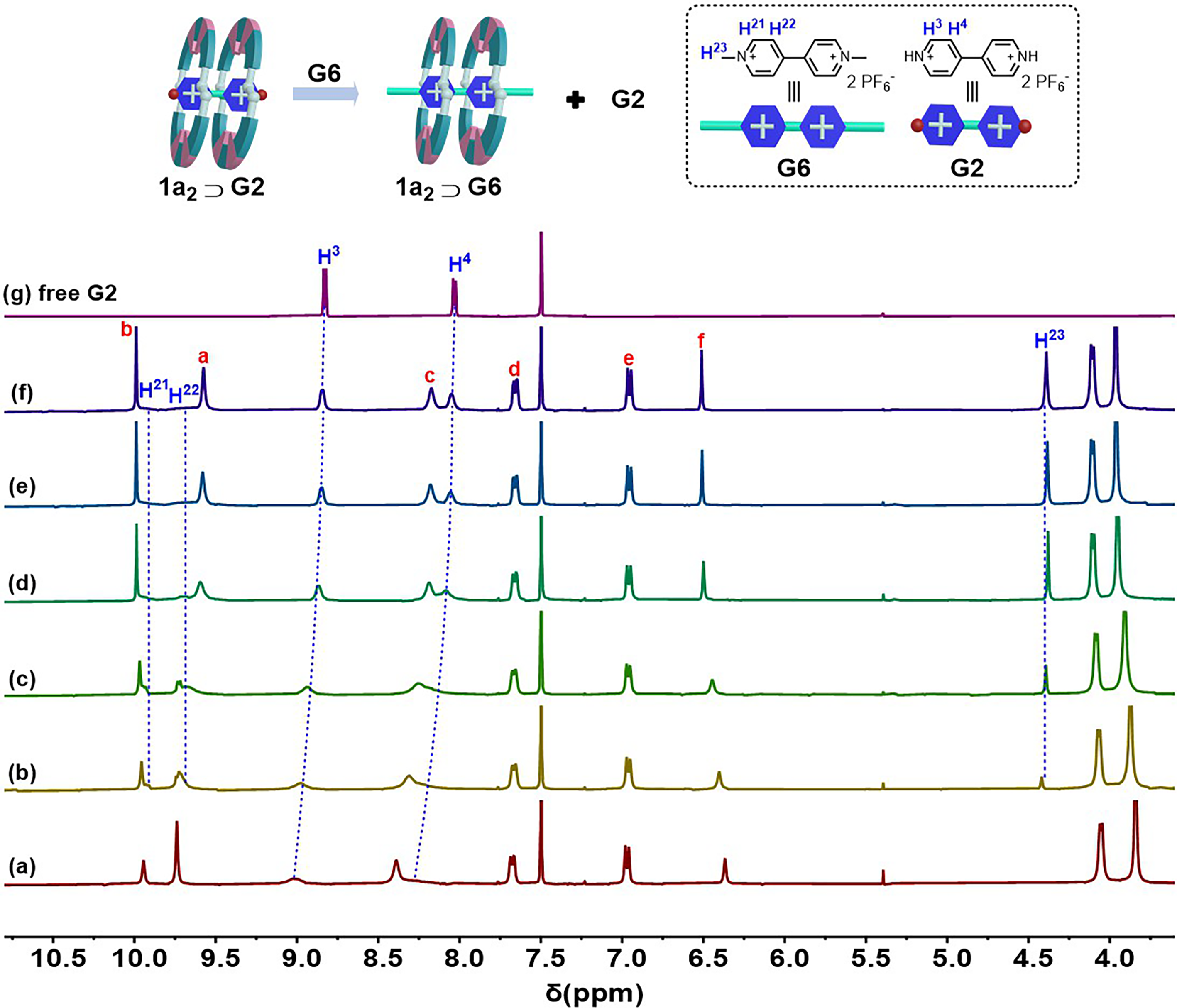
2.4. Competitive Ion Complexation
3. Materials and Methods
3.1. Materials and Reagents
3.2. Experimental Methods
3.3. Synthesis of Azo-Macrocycles 1 (1a and 1b) and Guests G1–G6
3.4. DFT Calculations
3.5. Visualization of Noncovalent Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Du, N.; Ye, F.; Sun, J.; Liu, K. Stimuli-Responsive Natural Proteins and Their Applications. ChemBioChem 2022, 23, e20210041. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Shen, J.; Fan, C.; Li, Q.; Yang, X. DNA Assembly-Based Stimuli-Responsive Systems. Adv. Sci. 2021, 8, 2100328. [Google Scholar] [CrossRef]
- Xiong, R.; Xu, R.X.; Huang, C.; De Smedt, S.; Braeckmans, K. Stimuli-Responsive Nanobubbles for Biomedical Applications. Chem. Soc. Rev. 2021, 50, 5746–5776. [Google Scholar] [CrossRef] [PubMed]
- Braegelman, A.S.; Webber, M.J. Integrating Stimuli-Responsive Properties in Host-Guest Supramolecular Drug Delivery Systems. Theranostics 2019, 9, 3017–3040. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Cao, Y.; Li, Y.; Hu, X.; Xiao, T.; Lin, C.; Pan, Y.; Wang, L. pH-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and Ferrocene Derivative for Drug Delivery. J. Am. Chem. Soc. 2013, 135, 10542–10549. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Wu, D.; Li, M.; Yang, F.; Li, Z.; An, W.; Jiang, S.; Zheng, X.; Niu, C.; Qu, D. An Acid-Base Responsive Linear-Cyclic Polymer Rotaxane Molecular Shuttle with Fluorescence Signal Output. Chin. Chem. Lett. 2022, 33, 881–884. [Google Scholar] [CrossRef]
- Han, X.-N.; Zong, Q.-S.; Han, Y.; Chen, C.-F. Pagoda[5]arene with Large and Rigid Cavity for the Formation of 1:2 Host-Guest Complexes and Acid/Base-Responsive Crystalline Vapochromic Properties. CCS Chem. 2022, 4, 318–330. [Google Scholar] [CrossRef]
- Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Photoresponsive Host–Guest Functional Systems. Chem. Rev. 2015, 115, 7543–7588. [Google Scholar] [CrossRef]
- Qi, X.; Li, W.; Shi, B.; Zhang, Y.; Yao, H.; Lin, Q.; Wei, T. Fabricating a Novel Supramolecular Light-Activated Platform Based on Internal-Driven Forces Induced by the UV-Light. Chin. Chem. Lett. 2022, 33, 5065–5068. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Miao, X.; Ren, Y.; Zhang, B.; Wang, P.; Yu, Y.; Li, B.; Isaacs, L.; Cao, L. Shape-Controllable and Fluorescent Supramolecular Organic Frameworks Through Aqueous Host-Guest Complexation. Angew. Chem. Int. Ed. 2018, 57, 729–733. [Google Scholar] [CrossRef]
- Li, Q.; Sun, J.; Zhou, J.; Hua, B.; Shao, L.; Huang, F. Barium Cation-Responsive Supra-Amphiphile Constructed by a New Twisted Cucurbit[15]uril/Paraquat Recognition Motif in Water. Organ. Chem. Front. 2018, 5, 1940–1944. [Google Scholar] [CrossRef]
- Blanco-Gomez, A.; Corton, P.; Barravecchia, L.; Neira, I.; Azos, E.; Peinador, C.; Garcia, M.D. Controlled Binding of Organic Guests by Stimuli-Responsive Macrocycles. Chem. Soc. Rev. 2020, 49, 3834–3862. [Google Scholar] [CrossRef]
- Engel, S.; Moeller, N.; Ravoo, B.J. Stimulus-Responsive Assembly of Nanoparticles using Host-Guest Interactions of Cyclodextrins. Chem.-Eur. J. 2018, 24, 4741–4748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Zhang, N.-Y.; Xiao, K.; Yu, Q.; Liu, Y. Photo-Controlled Reversible Microtubule Assembly Mediated by Paclitaxel-Modified Cyclodextrin. Angew. Chem. Int. Ed. 2018, 57, 8649–8653. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Q.; Zhao, W.; Dong, S.; Li, T.; Stang, P.J. Thermo/Anion Dual-Responsive Supramolecular Organoplatinum-Crown Ether Complex. Org. Lett. 2020, 22, 4289–4293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H.W. Stimuli-Responsive Host-Guest Systems Based on the Recognition of Cryptands by Organic Guests. Acc. Chem. Res. 2014, 47, 1995–2005. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.-M.; Li, H.; Zhang, J.; Zhou, Y.; Liu, G.; Xu, X.; Liu, Y. Synergistic Activation of Photoswitchable SupraMolecular Assembly Based on Sulfonated Crown Ether and Dithienylethene Derivative. Chin. Chem. Lett. 2022, 33, 2447–2450. [Google Scholar] [CrossRef]
- Gao, J.; Yu, H.; Chen, F.-Y.; Hu, X.-Y.; Wang, Y.; Guo, D.-S. A Hyaluronidase/ATP Tandem Stimuli-Responsive Supramolecular Assembly. Chem. Commun. 2019, 55, 14387–14390. [Google Scholar] [CrossRef]
- Shetty, D.; Skorjanc, T.; Olson, M.A.; Trabolsi, A. Self-Assembly of Stimuli-Responsive Imine-Linked Calix[4]arene Nanocapsules for Targeted Camptothecin Delivery. Chem. Commun. 2019, 55, 8876–8879. [Google Scholar] [CrossRef]
- Cen, R.; Liu, M.; Lu, J.; Zhang, W.; Dai, J.; Zeng, X.; Tao, Z.; Xiao, X. Synthesis and Characterization of a Sensitive and Selective Fe3+ Fluorescent Sensor Based on Novel Sulfonated Calix[4]arene-Based Host-Guest Complex. Chin. Chem. Lett. 2022, 33, 2469–2472. [Google Scholar] [CrossRef]
- Zhu, P.; Luo, W.; Qian, J.; Meng, C.; Shan, W.; Xu, Z.; Zhang, W.; Liu, X.; Ling, Y. GSH/ROS Dual-Responsive Supramolecular Nanoparticles Based on Pillar[6]arene and Betulinic Acid Prodrug for Chemo–Chemodynamic Combination Therapy. Molecules 2021, 26, 5900. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.-A.; Ogoshi, T.; Ogoshi, T. Stimuli-Responsive Supramolecular Assemblies Constructed from Pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Sun, G.; Zuo, M.; Xu, Z.; Wang, K.; Wang, L.; Hu, X.-Y. Orthogonal Design of Supramolecular Prodrug Vesicles via Water-Soluble Pillar[5]arene and Betulinic Acid Derivative for Dual Chemotherapy. ACS Appl. Bio Mater. 2022, 5, 3320–3328. [Google Scholar] [CrossRef]
- Tang, R.; Ye, Y.; Zhu, S.; Wang, Y.; Lu, B.; Yao, Y. Pillar[6]arenes: From Preparation, Host-Guest Property to Self-Assembly and Applications. Chin. Chem. Lett. 2023, 34, 107734. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Q.; Gao, Z.-Z.; Huang, Y.; Xiao, X.; Tao, Z. Stimuli-Responsive Supramolecular Assemblies between Twisted Cucurbit[14]uril and Hemicyanine Dyes and Their Analysis Application. J. Phys. Chem. B 2017, 121, 11119–11123. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Jonkheijm, P. Stimuli-Responsive Cucurbit[n]uril-Mediated Host-Guest Complexes on Surfaces. Isr. J. Chem. 2018, 58, 314–325. [Google Scholar] [CrossRef]
- Xiao, T.; Qi, L.; Zhong, W.; Lin, C.; Wang, R.; Wang, L. Stimuli-Responsive Nanocarriers Constructed from Pillar[n]arene-Based Supra-amphiphiles. Mater. Chem. Front. 2019, 3, 1973–1993. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, X.-Y.; Li, Y.; Zou, X.; Xiong, S.; Lin, C.; Shen, Y.-Z.; Wang, L. Multistimuli-Responsive Supramolecular Vesicles Based on Water-Soluble Pillar[6]arene and SAINT Complexation for Controllable Drug Release. J. Am. Chem. Soc. 2014, 136, 10762–10769. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, S.; Mao, W.; Li, P.; Zhou, F.; Ma, D. Smart Supramolecular Vesicles Based on Glutathione-Reactive Pillar[6]arene and Acid-Labile Prodrug: Dual Drug Loading and Sequential Release. Chin. Chem. Lett. 2022, 33, 209–212. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Zhang, Y.; Zhang, C.-W.; Chen, L.-J.; Wang, C.; Tan, H.; Yu, Y.; Li, X.; Yang, H.-B. Cross-Linked Supramolecular Polymer Gels Constructed from Discrete Multi-pillar[5]arene Metallacycles and Their Multiple Stimuli-Responsive Behavior. J. Am. Chem. Soc. 2014, 136, 8577–8589. [Google Scholar] [CrossRef]
- Samanta, S.K.; Quigley, J.; Vinciguerra, B.; Briken, V.; Isaacs, L. Cucurbit[7]uril Enables Multi-Stimuli-Responsive Release from the Self-Assembled Hydrophobic Phase of a Metal Organic Polyhedron. J. Am. Chem. Soc. 2017, 139, 9066–9074. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, J.; Wang, C.; Yang, J.; Wang, Y.; Yang, Y.-W. Stimuli-Responsive Fluorescent Supramolecular Polymer Network Based on a Monofunctionalized Leaning Tower[6]arene. Chin. Chem. Lett. 2019, 30, 2299–2303. [Google Scholar] [CrossRef]
- Peng, L.; Feng, A.; Huo, M.; Yuan, J. Ferrocene-Based Supramolecular Structures and Their Applications in Electrochemical Responsive Systems. Chem. Commun. 2014, 50, 13005–13014. [Google Scholar] [CrossRef]
- Liu, M.; Yan, X.; Hu, M.; Chen, X.; Hang, M.; Zheng, B.; Hu, X.; Shao, S.; Huang, F. Photoresponsive Host–Guest Systems Based on a New Azobenzene-Containing Crytpand. Org. Lett. 2010, 12, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.J.; del Barrio, J.; Suardíaz, R.; Ryan, D.F.; Rosta, E.; Scherman, O.A. A Dynamic and Responsive Host in Action: Light-Controlled Molecular Encapsulation. Angew. Chem. Int. Ed. 2016, 55, 16096–16100. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Cen, W.; Queenan, J.A.; Long, L.; Lynch, V.M.; Khashab, N.M.; Sessler, J.L. Azobenzene-Bridged Expanded “Texas-sized” Box: A Dual-Responsive Receptor for Aryl Dianion Encapsulation. J. Am. Chem. Soc. 2019, 141, 6468–6472. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Y.; Zhang, L.; Anamimoghadam, O.; Hen, D.; Liu, Z.; Cai, K.; Pezzato, C.; Stern, C.L.; Liu, Y.; et al. A Dynamic Tetracationic Macrocycle Exhibiting Photoswitchable Molecular Encapsulation. J. Am. Chem. Soc. 2019, 141, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Liu, P.; Zhu, H.; Shi, B.; Hong, X.; Huang, F. Azobenzene-Based Macrocyclic Arenes: Synthesis, Crystal Structures, and Light-Controlled Molecular Encapsulation and Release. Angew. Chem. Int. Ed. 2021, 60, 5766–5770. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Crespi, S.; Chen, S.; Zhang, X.-K.; Xu, T.-Y.; Ma, C.-S.; Zhou, S.-W.; Shi, Z.-T.; Tian, H.; et al. Motorized Macrocycle: A Photo-Responsive Host with Switchable and Stereoselective Guest Recognition. Angew. Chem. Int. Ed. 2021, 60, 16129–16138. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, Z.; Wang, L.; Chen, L.; Cai, Y.; Deng, P.; Feng, W.; Li, X.; Yuan, L. A Dynamic Hydrogen-Bonded Azo-Macrocycle for Precisely Photo-Controlled Molecular Encapsulation and Release. Angew. Chem. Int. Ed. 2019, 58, 12519–12523. [Google Scholar] [CrossRef]
- Gong, B.; Shao, Z. Self-Assembling Organic Nanotubes with Precisely Defined, Sub-nanometer Pores: Formation and Mass Transport Characteristics. Acc. Chem. Res. 2013, 46, 2856–2866. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Q.; Zeng, H. Rapid Construction of Shape-persistent H-bonded Macrocycles via One-pot H-bonding-Assisted Macrocyclization. J. Incl. Phenom. Mol. Recognit. Chem. 2013, 76, 1–11. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Zhao, X.; Hou, J.-L.; Li, Z.-T. Aromatic Amide Foldamers: Structures, Properties, and Functions. Chem. Rev. 2012, 112, 5271–5316. [Google Scholar] [CrossRef]
- Hu, J.; Chen, L.; Ren, Y.; Deng, P.; Li, X.; Wang, Y.; Jia, Y.; Luo, J.; Yang, X.; Feng, W.; et al. Nonaggregational Shape-Persistent Cyclo[6]aramide and Its Macrocyclic Effect toward Binding Secondary Ammonium Salts in Moderately Polar Media. Org. Lett. 2013, 15, 4670–4673. [Google Scholar] [CrossRef]
- Chen, L.; Peng, Z.; Liu, S.; Li, X.; Chen, R.; Ren, Y.; Feng, W.; Yuan, L. Cyclo[6]aramide-Tropylium Charge Transfer Complex as a Colorimetric Chemosensor for Differentiation of Intimate and Loose Ion Pairs. Org. Lett. 2015, 17, 5950–5953. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Chen, L.; Feng, W.; Zou, S.; Yang, Y.; Liu, N.; Yuan, L. Shape-Persistent Macrocycles: Efficient Extraction Towards Lanthanide and Actinide Elements. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 367–373. [Google Scholar] [CrossRef]
- Fu, H.; Liu, Y.; Zeng, H. Shape-Persistent H-bonded Macrocyclic Aromatic Pentamers. Chem. Commun. 2013, 49, 4127–4144. [Google Scholar] [CrossRef]
- Ren, C.; Shen, J.; Zeng, H. One-Pot Synthesis of Strained Macrocyclic Pyridone Hexamers and Their High Selectivity toward Cu2+ Recognition. Org. Lett. 2015, 17, 5946–5949. [Google Scholar] [CrossRef]
- Helsel, A.J.; Brown, A.L.; Yamato, K.; Feng, W.; Yuan, L.; Clements, A.J.; Harding, S.V.; Szabo, G.; Shao, Z.; Gong, B. Highly Conducting Transmembrane Pores Formed by Aromatic Oligoamide Macrocycles. J. Am. Chem. Soc. 2008, 130, 15784–15785. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, G.; Shen, Y.; Zhong, Y.; Liu, R.; Yang, N.; Al-Mkhaizim, F.Y.; Kline, M.A.; He, L.; Li, M.; et al. Persistent Organic Nanopores Amenable to Structural and Functional Tuning. J. Am. Chem. Soc. 2016, 138, 2749–2754. [Google Scholar] [CrossRef]
- Pan, W.; Mao, L.; Shi, M.; Fu, Y.; Jiang, X.; Feng, W.; He, Y.; Xu, D.; Yuan, L. The Cytochrome c–Cyclo[6]aramide Complex as a Supramolecular Catalyst in Methanol. New J. Chem. 2018, 42, 3857–3866. [Google Scholar] [CrossRef]
- Kang, K.; Lohrman, J.A.; Nagarajan, S.; Chen, L.; Deng, P.; Shen, X.; Fu, K.; Feng, W.; Johnson, D.W.; Yuan, L. Convergent Ditopic Receptors Enhance Anion Binding upon Alkali Metal Complexation for Catalyzing the Ritter Reaction. Org. Lett. 2019, 21, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, B.; Chen, L.; Hu, J.; Wen, C.; Zheng, Q.; Wu, L.; Zeng, H.; Gong, B.; Yuan, L. Liquid-Crystalline Mesogens Based on Cyclo[6]aramides: Distinctive Phase Transitions in Response to Macrocyclic Host-Guest Interactions. Angew. Chem. Int. Ed. 2015, 54, 11147–11152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Yuan, L. Hydrogen-Bonded Aromatic Amide Macrocycles: Synthesis, Properties and Functions. Org. Biomol. Chem. 2022, 20, 9023–9051. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, L.; Shen, J.; Luo, J.; Deng, P.; Ren, Y.; Zeng, H.; Feng, W.; Yuan, L. Convergent Heteroditopic Cyclo[6]aramides as Macrocyclic Ion-Pair Receptors for Constructing [2]Pseudorotaxanes. Chem. Comm. 2014, 50, 8024–8027. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, M.; Gao, R.; Li, X.; Li, F.; Wu, X.; Xu, D.; Zeng, H.; Yuan, L. Two-Component Supramolecular Gels Derived from Amphiphilic Shape-Persistent Cyclo[6]aramides for Specific Recognition of Native Arginine. Angew. Chem. Int. Ed. 2014, 53, 11834–11839. [Google Scholar] [CrossRef]
- Xu, M.; Chen, L.; Jia, Y.; Mao, L.; Feng, W.; Ren, Y.; Yuan, L. A Rare Case for Binding a Diquat Salt by Two Cyclo[6]aramides. Supramol. Chem. 2015, 27, 436–443. [Google Scholar] [CrossRef]
- Li, X.; Yuan, X.; Deng, P.; Chen, L.; Ren, Y.; Wang, C.; Wu, L.; Feng, W.; Gong, B.; Yuan, L. Macrocyclic Shape-Persistency of Cyclo[6]aramide Results in Enhanced Multipoint Recognition for the Highly Efficient Template-Directed Synthesis of Rotaxanes. Chem. Sci. 2017, 8, 2091–2100. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, X.; Wang, Z.; Luo, Y.; Huang, W.; Zhang, S.; Yuan, W.; Qin, S.; Tao, G.; Yuan, L. A Redox-Responsive Complex System Based on 2 D Shape-Persistent Cyclo[6]aramide and Ferrocenium. Asian J. Org. Chem. 2016, 5, 966–970. [Google Scholar] [CrossRef]
- Kothapalli, S.S.K.; Kannekanti, V.K.; Ye, Z.; Yang, Z.; Chen, L.; Cai, Y.; Zhu, B.; Feng, W.; Yuan, L. Light-Controlled Switchable Complexation by a Non-photoresponsive Hydrogen-bonded Amide Macrocycle. Org. Chem. Front. 2020, 7, 846–855. [Google Scholar] [CrossRef]
- Wang, X.; Ji, J.; Liu, Z.; Cai, Y.; Tang, J.; Shi, Y.; Yang, C.; Yuan, L. Chiroptical Sensing of Amino Acid Derivatives by Host–Guest Complexation with Cyclo[6]aramide. Molecules 2021, 26, 4064. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, J.; Liu, Z.; Xu, W.; Liu, M.; Jia, A.; Liu, Y.; Xiao, X.; Li, X.; Yuan, L. Threading of Three Rings on Two Stations: A Convergent Approach to [4]Rotaxane. Chem. Commun. 2021, 57, 13506–13509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mei, L.; Guo, C.; Huang, S.; Shi, W.-Q.; Li, X.; Feng, W.; Li, X.; Yang, C.; Yuan, L. Supramolecular Shish Kebabs: Higher Order Dimeric Structures from Ring-in-Rings Complexes with Conformational Adaptivity. Angew. Chem. 2023, 135, e202216690. [Google Scholar] [CrossRef]
- Baroncini, M.; Silvi, S.; Credi, A. Photo- and Redox-Driven Artificial Molecular Motors. Chem. Rev. 2020, 120, 200–268. [Google Scholar] [CrossRef]
- Corra, S.; de Vet, C.; Groppi, J.; La Rosa, M.; Silvi, S.; Baroncini, M.; Credi, A. Chemical On/Off Switching of Mechanically Planar Chirality and Chiral Anion Recognition in a [2]Rotaxane Molecular Shuttle. J. Am. Chem. Soc. 2019, 141, 9129–9133. [Google Scholar] [CrossRef]
- Curcio, M.; Nicoli, F.; Paltrinieri, E.; Fois, E.; Tabacchi, G.; Cavallo, L.; Silvi, S.; Baroncini, M.; Credi, A. Chemically Induced Mismatch of Rings and Stations in [3]Rotaxanes. J. Am. Chem. Soc. 2021, 143, 8046–8055. [Google Scholar] [CrossRef]
- Han, Y.; Meng, Z.; Chen, C.-F. Acid/base controllable complexation of a triptycene-derived macrotricyclic host and protonated 4,4′-bipyridinium/pyridinium salts. Chem. Commun. 2016, 52, 590–593. [Google Scholar] [CrossRef]
- Huang, F.; Gibson, H.W.; Bryant, W.S.; Nagvekar, D.S.; Fronczek, F.R. First Pseudorotaxane-Like [3]Complexes Based on Cryptands and Paraquat: Self-Assembly and Crystal Structures. J. Am. Chem. Soc. 2003, 125, 9367–9371. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Brynn Hibbert, D.; Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 2016, 52, 12792–12805. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. The Nature of the Mechanical Bond: From Molecules to Machines; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cheng, M.; Zhang, J.; Ren, X.; Guo, S.; Xiao, T.; Hu, X.-Y.; Jiang, J.; Wang, L. Acid/base-controllable fluorescent molecular switches based on cryptands and basic N-heteroaromatics. Chem. Commun. 2017, 53, 11838–11841. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chu, L.; Sanakis, Y.; Liu, P. Revisiting the IspH Catalytic System in the Deoxyxylulose Phosphate Pathway: Achieving High Activity. J. Am. Chem. Soc. 2009, 131, 9931. [Google Scholar] [CrossRef] [PubMed]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, B.; Mennucci, G.A.; Petersson, H.; et al. Gaussian 09, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-García, J.; Hénon, E. Accurately Extracting the Signature of Intermolecular Interactions Present in the Nci Plot of the Reduced Density Gradient Versus Electron Density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- The PyMOL Molecular Graphics System, Version 2.3.0; Schrödinger, LLC.: New York, NY, USA, 2019.


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Sun, X.; Li, X.; Li, X.; Feng, W.; Yuan, L. Multi-Responsive Molecular Encapsulation and Release Based on Hydrogen-Bonded Azo-Macrocycle. Molecules 2023, 28, 4437. https://doi.org/10.3390/molecules28114437
Wu J, Sun X, Li X, Li X, Feng W, Yuan L. Multi-Responsive Molecular Encapsulation and Release Based on Hydrogen-Bonded Azo-Macrocycle. Molecules. 2023; 28(11):4437. https://doi.org/10.3390/molecules28114437
Chicago/Turabian StyleWu, Jinyang, Xuan Sun, Xianghui Li, Xiaowei Li, Wen Feng, and Lihua Yuan. 2023. "Multi-Responsive Molecular Encapsulation and Release Based on Hydrogen-Bonded Azo-Macrocycle" Molecules 28, no. 11: 4437. https://doi.org/10.3390/molecules28114437
APA StyleWu, J., Sun, X., Li, X., Li, X., Feng, W., & Yuan, L. (2023). Multi-Responsive Molecular Encapsulation and Release Based on Hydrogen-Bonded Azo-Macrocycle. Molecules, 28(11), 4437. https://doi.org/10.3390/molecules28114437







