Abstract
A total of 147 oral Kampo prescriptions, which are used clinically in Japan, were evaluated for their anti-glycation activity. Kakkonto demonstrated significant anti-glycation activity, prompting further analysis of its chemical constituents using LC-MS, which revealed the presence of two alkaloids, fourteen flavonoids, two but-2-enolides, five monoterpenoids, and four triterpenoid glycosides. To identify the components responsible for its anti-glycation activity, the Kakkonto extract was reacted with glyceraldehyde (GA) or methylglyoxal (MGO) and analyzed using LC-MS. In LC-MS analysis of Kakkonto reacted with GA, the peak intensity of ephedrine was attenuated, and three products from ephedrine-scavenging GA were detected. Similarly, LC-MS analysis of Kakkonto reacted with MGO revealed two products from ephedrine reacting with MGO. These results indicated that ephedrine was responsible for the observed anti-glycation activity of Kakkonto. Ephedrae herba extract, which contains ephedrine, also showed strong anti-glycation activity, further supporting ephedrine’s contribution to Kakkonto’s reactive carbonyl species’ scavenging ability and anti-glycation activity.
1. Introduction
Protein glycation, which is the non-enzymatic reaction between reducing sugars and proteins, results in the formation of advanced glycation end products (AGEs). In the first step of glycation, reducing sugars react reversibly with free amino groups on the protein through a nucleophilic attack, leading to the formation of Schiff bases. Intermediate products such as 3-deoxyglucosone undergo slow rearrangement to form stable Amadori products or ketoamines [1,2,3,4]. Furthermore, reactive dicarbonyl compounds such as glyoxal and methylglyoxal (MGO), which are primarily produced as by-products of glycolysis, can also cause the formation of AGEs by selectively reacting with the side chains of basic amino acids such as arginine and lysine on proteins [3]. The aforementioned reactive carbonyl species, including glyoxal, MGO, and 3-deoxyglucosone, are highly reactive and represent important precursors of AGEs in the human body [5]. Accumulation of AGEs in tissues leads to the formation of irreversible cross-linked structures with proteins [6], resulting in pathological reactions in tissues and organs and causing a series of diabetic complications [7]. Furthermore, AGEs and reactive carbonyl species are also implicated in aging [8], Alzheimer’s disease [9], and atherosclerosis [10]. While several compounds, including aminoguanidine [11] and metformin [12,13], have been investigated for their ability to inhibit the formation of AGEs and avert diabetic complications, their clinical application has been constrained due to apprehensions regarding their safety and efficacy. Aminoguanidine, for example, failed clinical trials of ACTION II due to its high toxicity in diabetic patients [14]. Hence, it is a critical issue to develop effective and safe anti-AGE drugs to protect people with diabetes from complications.
Kampo medicine is a traditional Japanese medicine that originated in ancient China and underwent its unique development in Japan. There are currently 294 Kampo prescriptions available as over-the-counter (OTC) medications and 148 Kampo prescriptions available as prescription medications in Japan. Kampo medicines are gaining attention as an alternative or complementary treatment option to Western medicines, particularly for chronic conditions that may not be fully addressed using conventional medicine alone. In recent years, there has been increasing research on Kampo medicines, with studies focusing on their efficacy, safety, and mechanism of action [15,16,17]. As part of our ongoing research to scientifically clarify the effectiveness of Kampo medicines [18,19,20,21], this study evaluated the anti-glycation activity of a total of 147 prescriptions of oral Kampo medicines covered by health insurance in Japan. Among them, Kakkonto showed significant activity, and its chemical constituents were further studied using LC-MS analysis. The components responsible for the anti-glycation activity were identified by analyzing the Kakkonto extract that was reacted with glyceraldehyde (GA) or MGO using LC-MS.
2. Results and Discussion
2.1. Evaluation of Anti-Glycation Activity of 147 Oral Kampo Prescriptions
In Japan, a total of 147 oral Kampo prescriptions are covered by health insurance. To evaluate the anti-glycation activity of Kampo medicines, all of these oral prescriptions were screened using the bovine serum albumin (BSA)–D-ribose assay according to a method in the literature with minor modifications [22]. BSA was incubated with D-ribose to induce glycation, and the formation of AGEs was measured using fluorescence spectroscopy. This assay can be evaluated within a short incubation time of 1 h. Of the Kampo medicines tested, 18 prescriptions, including Kakkonto, exhibited anti-glycation activity of more than 50% inhibition at a concentration of 50 μU/mL (Table 1).

Table 1.
Anti-glycation activity of oral Kampo prescriptions.
Subsequently, the anti-glycation activity of the 18 prescriptions was evaluated using a BSA–glyceraldehyde (GA) assay according to the manufacturer’s protocol. GA is a reactive carbonyl species that is produced during sugar metabolism. Out of the 18 prescriptions, 14 showed anti-glycation activity of more than 50% inhibition at a concentration of 400 μU/mL (Table 1). Kakkonto was found to exhibit more than 80% inhibitory activity in both assays and was selected for further study. Further testing of Kakkonto was carried out at different concentrations ranging from 400 to 12.5 μU/mL in the BSA–GA assay. The results showed that Kakkonto inhibited the formation of AGEs in a dose-dependent manner, with an IC50 value of 120 μU/mL (Figure 1A).
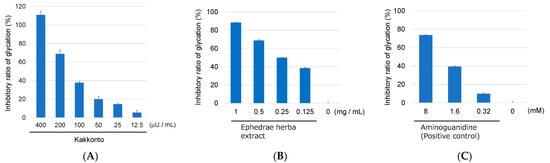
Figure 1.
Anti-glycation activity of Kakkonto (A), Ephedrae herba extract (B), and aminoguanidine (positive control) (C) at different concentrations in the BSA–GA assay.
2.2. Identification of Chemical Constituents in Kakkonto by LC-MS
To identify the chemical constituents in Kakkonto, an LC-MS analysis was conducted. As shown in Figure 2, a total of twenty-seven peaks were detected in the total ion chromatogram, and their retention time and MS fragmentation ions are listed in Table S2. By carefully analyzing the chromatographic behaviors and MS data and referring to the literature [23,24,25,26,27], the presence of two alkaloids (1, 2), fourteen flavonoids (3, 4, 6, 7, 9–11, 13, 14, and 17–21), two but-2-enolides (12, 16), five monoterpenoids (5, 8, 15, 22, 23), and four triterpenoid glycosides (24–27), were identified (Figure 3). The structure elucidation based on MS fragmentation is described briefly as follows.
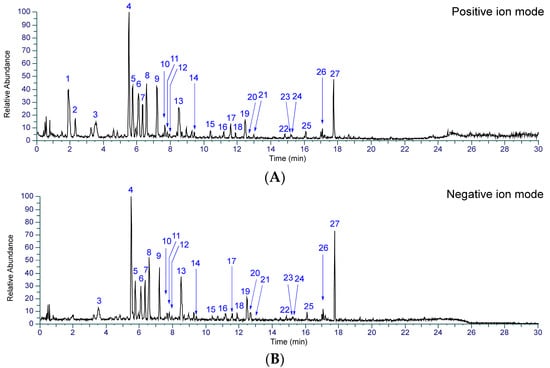
Figure 2.
Total ion chromatograms of Kakkonto in positive ion mode (A) and negative ion mode (B).
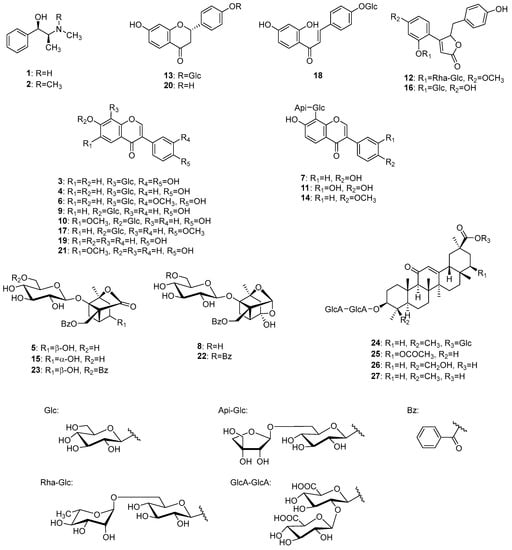
Figure 3.
Structures of identified compounds in Kakkonto.
Peaks 1 (tR 1.92 min) and 2 (tR 2.32 min) were identified as ephedrine (1) and methylephedrine (2), respectively, which are commonly found in Ephedrae herba. MS of both compounds exhibited molecular ions [M+H]+ at m/z 166.1227 and 180.1383, respectively, in positive ion mode, which is consistent with their molecular formulas of C10H15ON and C11H17ON. In MS/MS analysis, they revealed a similar fragmentation pattern, with characteristic product ions due to the desorption of H2O and the methyl amino or dimethyl amino groups from [M+H]+ (Figure 4).
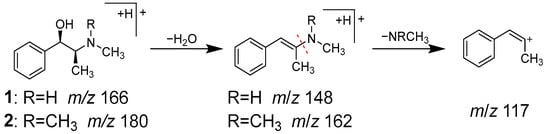
Figure 4.
MS/MS fragmentation pathway of compounds 1 and 2.
Peaks 13 (tR 8.50 min) and 18 (tR 11.88 min) were identified as liquiritin (13) and isoliquiritin (18), and peak 20 (tR 12.71 min) was the aglycone of 13, which was liquiritigenin (20). These compounds are all contained in Glycyrrhizae radix [28]. Peaks 13 and 18 have the same molecular formula of C21H22O9, which was deduced from the ions of m/z 436.1597 [M+HN4]+ and m/z 419.1333 [M+H]+, respectively. In MS/MS, they also exhibited the same product ions [Aglycone+H]+ at m/z 257 resulting from the detachment of the glucose moiety, and ions at m/z 137 and m/z 147 derived from the A ring and B ring, respectively (Figure 5). Although the MS data alone could not distinguish peaks 13 and 18, they were identified based on the retention times [29]. Similarly, peak 20 was most likely to be the aglycone of 13 or 18, as evidenced by its molecular formula of C15H12O4 and the MS/MS product ions of m/z 137 and m/z 147 from the precursor [M+H]+ ion. Due to its slightly longer retention time than 18, peak 20 was identified as liquiritigenin (20) instead of isoliquiritigenin (18a) [30].
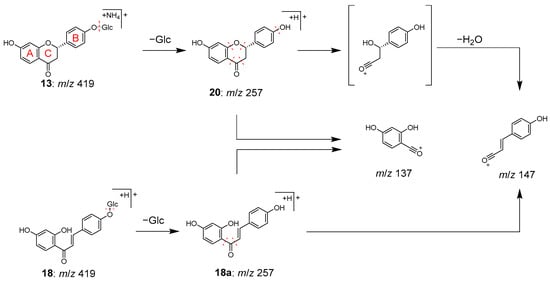
Figure 5.
MS/MS fragmentation pathway of compounds 13, 18, and 20.
Peaks 3, 4, 6, 7, 9–11, 14, 17, 19, and 21 were identified as isoflavones (19, 21) and their O-glycosides (9, 10, 17) and C-glycosides (3, 4, 6, 7, 11, 14).
Peaks 19 (tR 12.47 min) and 21 (tR 12.99 min) were identified as daidzein (19) and glycitein (21), respectively. Peak 19 has been reported to be contained in both Glycyrrhizae radix [31] and Puerariae radix [32], while 20 has been reported to be contained in Puerariae radix [32]. Peaks 19 and 21 have the molecular formulas of C15H10O4 and C16H12O5, which were deduced from the molecular ions of m/z 255.0649 [M+H]+ and m/z 285.0754 [M+H]+, respectively.
Peaks 9 (tR 7.20 min), 10 (tR 7.68 min), and 17 (tR 11.60 min) were identified as daidzin (9), glycitin (10), and ononin (17), respectively, which are isoflavone O-glycosides. Compounds 9 and 17 have been reported to be contained in both Puerariae radix and Glycyrrhizae radix [32,33], while 10 has been found in Puerariae radix [32]. In the MS/MS of [M+H]+, the product ions were observed as [Aglycone+H]+ ions corresponding to 19, 21, and hormononetin (17a), resulting from the detachment of the glucose moiety (Figure 6).
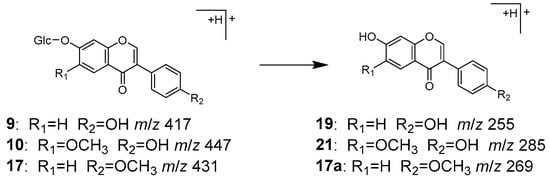
Figure 6.
MS/MS fragmentation pathway of compounds 9, 10, and 17.
Peaks 3 (tR 3.51 min), 4 (tR 5.52 min), 6 (tR 6.10 min), 7 (tR 6.35 min), 11 (tR 7.82 min), and 14 (tR 9.43 min) were identified as 3′-hydroxypuerarin (3), puerarin (4), 3′-methoxypuerarin (6), puerarin apioside (7), 3′-hydroxypuerarin apioside (11), and formononetin 8-C-[β-D-apiofuranosyl-(1→6)]-β-D-glucopyranoside (14), respectively, which are characteristic isoflavone C-glycosides originated from Puerariae radix [34,35,36,37].
Compounds 3, 4, and 6 were considered to have one sugar attached to the isoflavone based on their molecular formula. These compounds exhibited a series of product ions due to the elimination of H2O from the sugar moiety, followed by the characteristic bond cleavage of C-glycosidide via retro-Diels–Alder reaction, as well as elimination of hydride, formyl group, H2O, and CO. On the other hand, it was revealed that 7, 11, and 14 have an apiose moiety bound to 4, 3, and 4′-methoxypuerarin (14a), respectively, since in MS/MS of 7, 11, and 14, the product ions generated by the detachment of terminal sugar corresponded to 4, 3, and 14a (Figure 7).
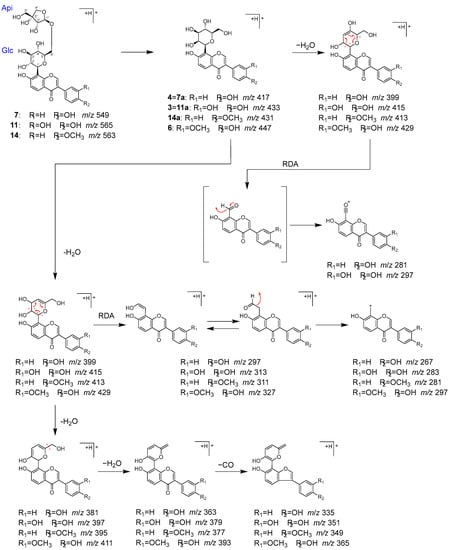
Figure 7.
MS/MS fragmentation pathway of compounds 3, 4, 6, 7, 11, and 14.
Peaks 12 (tR 7.96 min) and 16 (tR 11.19 min) were identified as pueroside A (12) and sophoraside A (16), respectively, which are characteristic but-2-enolide derivatives of Puerariae radix [38].
In MS/MS, 12 underwent fragmentation to generate fragment ion 12a, corresponding to a demethylated 16, through the elimination of terminal rhamnose. Further fragmentation of 12a and 16 generated 12b and 16b by the loss of a glucose, then 12c and 16c by the loss of a hydroxy group. Furthermore, 12d and 12e were generated from 12c via the elimination of benzoyl and hydroxy groups, and 16f from 16c via the elimination of a phenyl group (Figure 8).
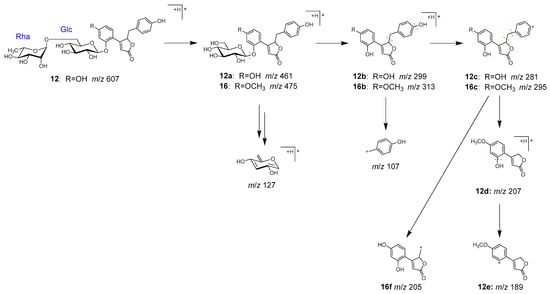
Figure 8.
MS/MS fragmentation pathway of compounds 12 and 16.
Peaks 5 (tR 5.76 min), 8 (tR 6.57 min), 15 (tR 10.38 min), 22 (tR 14.86 min), and 23 (tR 15.20 min) were identified as characteristic monoterpenoids in Paeoniae radix [39].
Peaks 5, 15, and 8 were identified as albiflorin (5), 4-epi-albiflorin (15), and peoniflorin (8), with the same molecular formula (C23H28O11 each), and they were deduced from the ions of m/z 498.1966 [M+NH4]+, 481.1699 [M+H]+, and m/z 481.1704 [M+H]+, respectively. Fragmentation of 5 and 15 generated ions 5a and 15a via the loss of a glucose, followed by the loss of H2O and the benzoyl group to generate product ions 5b and 15b (Figure 9). On the other hand, peoniflorin (8) first produced ion 8a via the loss of H2O, followed by the loss of the benzoyl group and glucose to produce ion 8b. A series of product ions from 5b, 8b, and 15b were generated by the loss of H2O and CO (Figure 9).
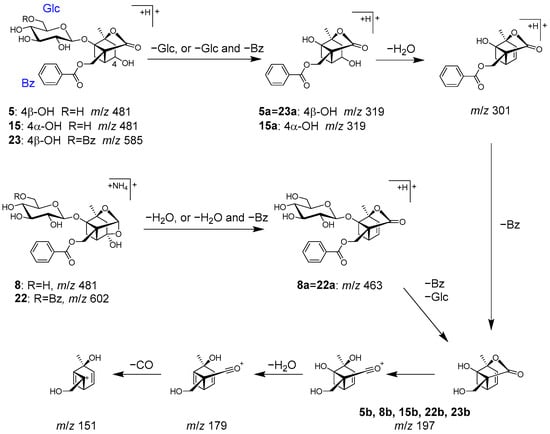
Figure 9.
MS/MS fragmentation pathway of compounds 5, 8, 15, 22, and 23.
Paeonivayin (23) and benzoylpaeoniflorin (22) possess structures with an additional benzoyl moiety attached at C-6 of the glucose of 5 and 8, respectively. In MS/MS, product ions from 23 and 22 were observed to be the same as those from 5 and 8 (Figure 9). Notably, 5, 8, 15, 22, and 23 can also be distinguished by their retention times [26].
Peaks 24 (tR 15.24 min), 25 (tR 16.09 min), 26 (tR 17.08 min), and 27 (tR 17.77 min) were identified as licoricesaponin A3 (24), 22β-acetoxyglycyrrhizic acid (25), licorice saponin G2 (26), and glycyrrhizin (27), which are contained in Glycyrrhizae radix [40].
Peak 27 exhibited a higher peak intensity and was identified as glycyrrhizin (27) based on the observed [M+H]+ ion at m/z 823.4097, which corresponds to the molecular formula (C42H62O16). In addition, the molecular formulas of 24, 25, and 26 were deduced from the [M+H]+ ions at m/z 985.4637, 881.4155, and 839.4055, respectively. This suggested that 24 (C48H72O21) is a glucosylated 27, 25 (C44H64O18) is an acetoxylated 27, and 26 (C42H62O17) is a hydroxyated 27. In MS/MS of 24–27, consecutive fragmentation generated the product ions of 24a–27a [Aglycone+H]+ via deglycosylation and 24c–27c via dehydration. Further fragmentation resulted in the cleavage of the C-ring structure by a Retro-Diels–Alder reaction, which led to the observation of some important ions for structural identification, including 24d–27d derived from the AB ring, and 24d–27d and 24f–27f from the CD ring (Figure 10).
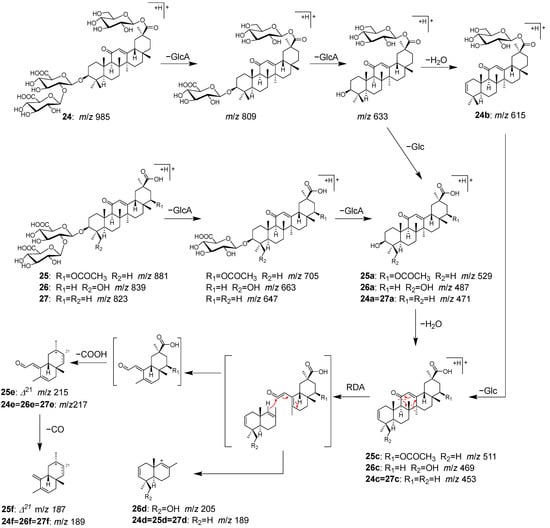
Figure 10.
MS/MS fragmentation pathway of compounds 24–27.
Additionally, LC-MS analysis revealed that Kakkonto contains ephedrin (1), puerarin (4), albiflorin (5), 3′-methoxypuerarin (6), puerarin apioside (7), paeoniflorin (8), daidzin (9), liguiritin (13), ononin (17), daidzein (19), and glycyrrhizin (27) in relatively high contents.
2.3. Identification of the Components That Contribute to the Anti-Glycation Activity
To identify the components contributing to the anti-glycation activity, the methanol fraction prepared from Kakkonto was reacted with GA and analyzed using LC-MS (Figure 11A). Comparison of the total ion chromatograms of Kakkonto extract with and without GA addition showed a decrease in the peak intensity of ephedrine (1) with GA addition, while three new peaks (EM1–EM3) were detected. The new peaks were most similar to products generated by the trapping of GA by 1, as indicated by their molecular formulas of C12H18O3N (EM1 and EM2) and C13H20O3N (EM3), which were deduced from the molecular ions [M+H]+ observed in MS. Further analysis of MS/MS revealed that EM1–EM3 are derivatives with an oxazoline ring generated through nucleophilic addition reaction and condensation between 1 with GA [41]. In MS/MS of EM1–EM3, a series of product ions were observed, which were formed through stepwise loss of H2O, ring opening, and loss of C3H3N (Table S2, Figure 12). Since EM1 and EM2 showed the same molecular formula and fragment pattern, they were considered to be a pair of diastereomers.
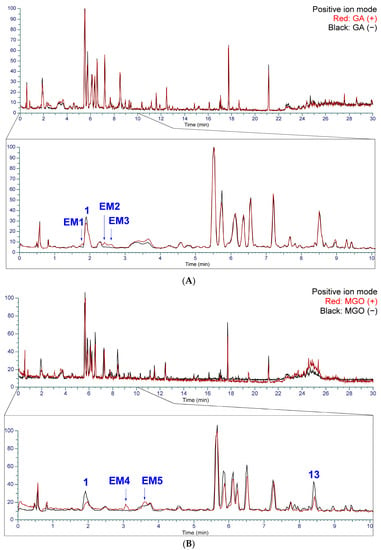
Figure 11.
The total ion chromatogram of Kakkonto reacted with glyceraldehyde (GA) and methyl glyoxal (MGO) in positive ion mode. (A) Superposition of Kakkonto extract reacted with GA (red) and without GA (black). (B) Superposition of Kakkonto extract reacted with MGO (red) and without MGO (black).
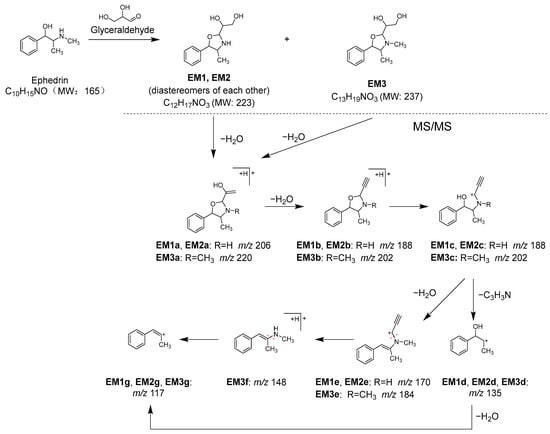
Figure 12.
Reaction between ephedrine (1) and glyceraldehyde (GA), and MS/MS fragmentation pathway of the reaction products.
Additionally, when the Kakkonto methanol fraction was reacted with MGO and analyzed using LC-MS, a decrease in the peak intensity of ephedrine (1) was also observed, along with two new peaks EM4 and EM5 (Figure 11B). Peaks EM4 and EM5 were identified as a pair of diastereomers formed between 1 and MGO through nucleophilic addition, and their structures were determined based on their molecular formulas, which were obtained from the molecular ions [M+H]+ observed in MS, as well as the MS/MS product ions generated through the loss of H2O, CO, and C3H7N (Table S3, Figure 13).
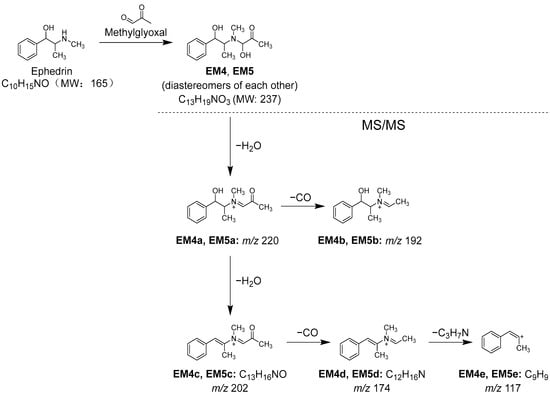
Figure 13.
Reaction between ephedrine (1) and methylglyoxal (MGO), and MS/MS fragmentation pathway of the reaction products.
2.4. Evaluation of Ephedrae Herba Extract for Anti-Glycation Activity
In Japan, ephedrine (1) is a regulated substance by law, which made it impossible to investigate the anti-glycation activity of 1 as a single compound. However, the inhibitory activity of an Ephedrae herba extract, which contained 1 as the main component, was evaluated using the BSA–GA assay. As a result, the extract dose-dependently inhibited glycation in the concentration range of 10–0.125 mg/mL, with an IC50 of 0.22 mg/mL (Figure 1B). Its activity was comparable to that of the positive control, aminoguanidine (IC50 2.6 mM, equivalent to 0.19 mg/mL) (Figure 1C). Based on these results, it can be concluded that ephedrine (1) is the key component responsible for the anti-glycation activity of Kakkonto, and its possible mechanism of action is trapping the reactive carbonyl species.
So far, there have been no reports on the anti-glycation activity of ephedrine or Ephedra herba, but there have been several reports that they suppress hyperglycemia in vivo. For example, it has been reported that crude extracts of Ephedra sinica or E. foeminea and l-ephedrine showed suppression of hyperglycemia in mice with diabetes induced by streptozotocin [42,43]. Furthermore, extracts of E. sinica have been shown to promote the regeneration of atrophied pancreatic islets. It has also been suggested that E. sinica could improve hyperglycemia by regenerating atrophied pancreas islets and restoring insulin secretion [42]. In addition, the anti-obesity effect and anti-hyperglycemic action of Ephedra sinica were evaluated in mice fed a high-fat diet, and Ephedra sinica was found to reduce weight gain and fasting blood glucose levels, as well as improve HDL cholesterol levels [44]. Furthermore, it was suggested that E. sinica could improve obesity and hyperglycemia by increasing PPAR-α and adiponectin and decreasing TNF-α [44]. In these reports, it was concluded that the decrease in blood sugar levels was due to improved metabolism, but considering our research results as well, it is possible that the direct trapping of sugar by ephedrine may also be involved.
3. Materials and Methods
3.1. General Methods
LC-MS analysis was performed on a Vanquish UHPLC system combined with a Q-Exactive Hybrid Quadrupole Orbitrap mass spectrometer. Fluorescence intensity was measured using a multi-label plate reader from EnSpire 2300 (Perkinelmer Japan Co., Ltd., Kanagawa, Japan). A Diaion HP-20 for column chromatography was purchased from Mitsubishi Chemical Co. (Tokyo, Japan).
3.2. Materials and Chemicals
A total of 147 oral Kampo prescriptions, which are covered by health insurance in Japan, were purchased from Ohsugi Pharmaceutical Co., Ltd. (Osaka, Japan), Kracie Holdings, Ltd. (Tokyo, Japan), Kotaro Pharmaceutical Co., Ltd. (Osaka, Japan), Sanwa Shoyaku Co., Ltd. (Tochigi, Japan), Taikoseido Pharmaceutical Co., Ltd. (Hyogo, Japan), Tsumura & Co. (Tokyo, Japan), and Toyo-Kampo Pharmaceutical Co., Ltd. (Osaka, Japan), respectively. The Albumin Glycation Assay Kit, Glyceraldehyde (Cat. No. AAS-AGE-K01) was purchased from Cosmo Bio Co., Ltd. (Tokyo, Japan). Sodium azide was purchased from Sigma-Aldrich Japan Co. (Tokyo, Japan). Ephedrae herba was purchased from Tanuma Shokai Co., Ltd. (Chiba, Japan) (Lot. 131017). Methylglyoxal and Glyceraldehyde were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). An amount of 0.1 moL/L Phosphate buffer, acetic acid, and dimethyl sulfoxide were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). LC-MS-grade acetonitrile, methanol, formic acid, and distilled water were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan).
3.3. Sample Preparation of Kampo Solutions
We defined the amount of the daily dose as 1 unit (U) for Kampo prescriptions. A Kampo prescription (2 mU) was suspended in 1 mL of purified water and extracted using sonication at room temperature for 15 min. The mixture was then centrifuged at 4800 rpm for 15 min, and the supernatant was used as the 2 mU/mL sample solution.
3.4. Assay for the Anti-Glycation Activity Using D-Ribose
An assay of AGE formation inhibitory activity between BSA and D-ribose was conducted according to a method from the literature with minor modifications [22]. The solution containing BSA solution (final concentration 10 mg/mL), each sample solution (final concentration 50 μU/mL), and D-ribose solution (final concentration 0.5 M) were added to each well of a 96-well microplate, and then fluorescence intensity (excitation wavelength: 370 nm, fluorescence wavelength: 440 nm) was measured using a fluorescence plate reader after 1 h of incubation. The glycation value of the vehicle control (0 μU/mL) was assumed to be 100%. The glycation value of each well relative to the control was determined as the glycation ratio.
3.5. Assay for the Anti-Glycation Activity Using Glyceraldehyde
The anti-glycation activity using glyceraldehyde was evaluated with an Albumin Glycation Assay Kit, Glyceraldehyde (Cosmo Bio Co., Ltd. (Tokyo, Japan), Cat. No. AAS-AGE-K01). The assay kit buffer was used, with sodium azide (3 mM) added. Each concentration of sample solution was prepared from 2 mU/mL sample solution via dilution using the assay kit buffer to each concentration (final concentrations 400, 200, 100, 50, 25, and 12.5 μU/mL).
BSA solution (50 μL), each concentration sample solution (40 μL), and glyceraldehyde solution (500 mM, 10 μL) were added to each well of a 96-well microplate, and then fluorescence intensity A (excitation wavelength: 370 nm, fluorescence wavelength: 440 nm) was measured using a fluorescence plate reader. After 24 h of incubation, fluorescence intensity B was measured. The glycation value (fluorescence intensity B—fluorescence intensity A) of the vehicle control (0 μU/mL) was assumed to be 100%. The glycation value of each well relative to the control was determined as the glycation ratio.
3.6. UHPLC-MS Conditions
UHPLC was conducted using a Vanquish UHPLC system (Thermo Scientific, Waltham, MA, USA). Chromatographic peaks were separated on a TSKgel ODS-120H (100 × 2.0 mm I.D., 1.9 μm) at a flow rate of 0.4 mL/min at 40 °C with a column temperature oven. A mobile phase consisted of eluent A (distilled water with 0.1% formic acid) and B (acetonitrile with 0.1% formic acid) programmed as follows: 0–2.5 min, 10% B, 2.5–15 min,10→35% B, 15–20 min, 35→60% B, 20–25 min, 60→100% B, 25–30 min 100% B, and then the column was re-equilibrated at 10% B for 5 min before the next injection. The injection volume was 2 μL for analysis.
A Q-Exactive hybrid quadrupole orbitrap high-resolution accurate mass spectrometer system (Thermo Scientific, Waltham, MA, USA) with an ESI source was operated in the positive and negative ion modes. The calibration solutions were used to calibrate the ESI-MS to increase mass accuracy. The optimized parameters of mass spectrometry were illustrated below: spray voltage, +3.5 kV (for positive ion mode) or −2.5 kV (for negative ion mode); capillary temperature, 262.5 °C; sheath gas flow rate, 50 units; AUX gas flow rate, 12.5 units; sweep gas flow rate, 2.63 units; S-lens RF level, 50 units; and probe heater temperature, 425 °C. Data were collected in the full MS modes and full MS/data-dependent (dd)-MS/MS. In-source CID was set at 0 eV. The resolution was 70,000 for full MS and 35,000 for full MS/dd-MS/MS. The AGC was set at 1E6 for full MS and 1E5 for dd-MS/MS. Maximum IT was set at 200 ms for full MS. Scan range was set at 150 to 2000 m/z for full MS. Data-dependent scan was performed using high-energy collision with normalized collision energy (NCE) at 10 eV; 30 eV; and stepped NCE at 10, 25, and 40 eV.
3.7. Preparation of Kakkonto Solution for LC-MS
Kakkonto formulation (2.5 g) was suspended in 25 mL of purified water and extracted via sonication at room temperature for 30 min. The mixture was then centrifuged at 4800 rpm for 15 min, and the supernatant was subjected to Diaion HP-20 column chromatography to yield the water eluted part (300 mL) and methanol eluted part (300 mL). The methanol eluted part was evaporated in vacuo to yield a methanol fraction (0.29 g). The methanol fraction in methanol (1 mg/mL) was filtrated with a 0.22 mm filter and then analyzed using LC-MS.
3.8. Evaluation of the GA and MGO Trapping Capacity of Kakkonto
Kakkonto methanol fraction (10 mg) was incubated with GA (50 mM or 0 mM) in PBS buffer (pH 7.4, 100 mM) at 37 °C. After 24 h of incubation, the reaction was stopped by adding 200 μL of acetic acid. Purified water (10 mL) was added to the reaction mixture, followed by Diaion HP-20 column chromatography to yield the water eluted part (100 mL) and the methanol eluted part (100 mL). The methanol eluted part was evaporated in vacuo to yield a methanol fraction. The methanol fraction in 10 mL of methanol was filtrated with 0.22 μm filter and then analyzed using LC-MS. Kakkonto (10 mg) and MGO (50 mM or 0 mM) mixtures were reacted and analyzed using LC-MS using a similar method to that described above.
3.9. Extraction and Assay for the Anti-Glycation Activity of Ephedrae Herba
Ephedrae herba (1 g) was suspended in 25 mL of methanol and extracted via sonication at room temperature for 20 min. The mixture was then filtrated, and the filtrate was evaporated in vacuo to yield methanol extract (98 mg). The methanol extract (8 mg) was dissolved with DMSO (80 mL) and 2.5, 1.25, 0.625, and 0.3125 mg/mL of solution (final concentrations 1, 0.5, 0.25, and 0.125 mg/mL) were prepared with the buffer of the Albumin Glycation Assay Kit, Glyceraldehyde. An assay for the anti-glycation activity was evaluated using the same method as that described above.
4. Conclusions
In this study, Kakkonto, one of the prescribed Kampo medicines, revealed significant in vitro anti-glycation activity, and ephedrine was identified as the key component responsible for this activity. Kakkonto is clinically used in Japan for the early stages of colds, inflammatory diseases, shoulder stiffness, upper body neuralgia, and hives. Our findings suggest the potential for Kakkonto to be used for the treatment of diabetic complications based on its anti-glycation activity, but further investigation is needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28114409/s1. Table S1: Mass and MS/MS spectrometric data of compounds identified in Kakkonto extract. Table S2: Mass spectrometric data of products detected in Kakkonto extract reacted with glyceraldehyde (GA). Table S3: Mass spectrometric data of products detected in Kakkonto extract reacted with methylglyoxal (MGO).
Author Contributions
Conceived and designed the experiments: T.K., K.K. and W.L.; Performed the experiments: K.I. (Kaori Ito), T.K., K.I. (Kanako Ikube) and K.O.; Analyzed the data: K.I. (Kaori Ito), T.K., K.I. (Kanako Ikube), K.O. and W.L.; Wrote the paper: K.I. (Kaori Ito), T.K. and W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study and samples of the compounds are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Welsh, K.J.; Kirkman, M.S.; Sacks, D.B. Role of glycated proteins in the diagnosis and management of diabetes: Research gaps and future directions. Diabetes Care 2016, 39, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Fedele, D.; Reitano, R.; Bonfante, L.; Guizzo, M.; Seraglia, R.; Tubaro, M.; Traldi, P. Mass spectrometric study of in vivo production of advanced glycation endproducts/peptides. J. Mass Spectrom. 2005, 40, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Lapolla, A.; Fedele, D.; Seraglia, R.; Traldi, P. The role of mass spectrometry in the study of non-enzymatic protein glycation in diabetes: An update. Mass Spectrom. Rev. 2006, 25, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A Perspective on the maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Sjoblom, N.M.; Kelsey, M.M.G.; Scheck, R.A. A Systematic study of selective protein glycation. Angew. Chem. Int. Ed. 2018, 57, 16077–16082. [Google Scholar] [CrossRef]
- Nagaraj, R.H.; Shipanova, I.N.; Faust, F.M. Protein crosslinking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem. 1996, 271, 19338–19345. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Baynes, J.W. The role of AGEs in aging: Causation or correlation. Exp. Gerontol. 2001, 36, 1527–1537. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kikuchi, S.; Sasaki, N.; Suzuki, T.; Watai, T.; Iwaki, M.; Bucala, R.; Yamagishi, S. Involvement of advanced glycation end-products (AGEs) in Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 39–46. [Google Scholar] [CrossRef]
- Basta, G.; Schmidt, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Beisswenger, P.; Ruggiero-Lopez, D. Metformin inhibition of glycation processes. Diabetes Metab. 2003, 29, 6S95–6S103. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef]
- Freedman, B.I.; Wuerth, J.-P.; Cartwright, K.; Bain, R.P.; Dippe, S.; Hershon, K.; Mooradian, A.D.; Spinowitz, B.S. Design and baseline characteristics for the aminoguanidine Clinical Trial in Overt Type 2 Diabetic Nephropathy (ACTION II). Control. Clin. Trials 1999, 20, 493–510. [Google Scholar] [CrossRef]
- Tsuge, A.; Watanabe, A.; Kodama, Y.; Hisaka, S.; Nose, M. Orengedokuto exerts anti-allergic effects via inhibition of effector T cell activation in a murine model of contact hypersensitivity. J. Nat. Med. 2022, 76, 144–151. [Google Scholar] [CrossRef]
- Toume, K.; Hou, Z.; Yu, H.; Kato, M.; Maesaka, M.; Bai, Y.; Hanazawa, S.; Ge, Y.; Andoh, T.; Komatsu, K. Search of anti-allodynic compounds from Plantaginis Semen, a crude drug ingredient of Kampo formula “Goshajinkigan”. J. Nat. Med. 2019, 73, 761–768. [Google Scholar] [CrossRef]
- Takiyama, M.; Matsumoto, T.; Sanechika, S.; Watanabe, J. Pharmacokinetic study of traditional Japanese Kampo medicine shimotsuto used to treat gynecological diseases in rats. J. Nat. Med. 2021, 75, 361–371. [Google Scholar] [CrossRef]
- Onoda, T.; Li, W.; Higai, K.; Koike, K. Evaluation of 147 Kampo prescriptions as novel protein tyrosine phosphatase 1B (PTP1B) inhibitory agents. BMC Complement. Altern. Med. 2014, 14, 64. [Google Scholar] [CrossRef]
- Sato, N.; Li, W.; Takemoto, H.; Takeuchi, M.; Nakamura, A.; Tokura, E.; Akahane, C.; Ueno, K.; Komatsu, K.; Kuriyama, N.; et al. Comprehensive evaluation of antioxidant effects of Japanese Kampo medicines led to identification of Tsudosan formulation as a potent antioxidant agent. J. Nat. Med. 2019, 73, 163–172. [Google Scholar] [CrossRef]
- Onoda, T.; Li, W.; Sasaki, T.; Miyake, M.; Higai, K.; Koike, K. Identification and evaluation of magnolol and chrysophanol as the principle protein tyrosine phosphatase-1B inhibitory compounds in a Kampo medicine, Masiningan. J. Ethnopharmacol. 2016, 186, 84–90. [Google Scholar] [CrossRef]
- Onoda, T.; Ishikawa, C.; Fukazawa, T.; Li, W.; Obayashi, M.; Koike, K. Inhibitory activities of selected Kampo formulations on human aldose reductase. BMC Complement. Altern. Med. 2014, 14, 435/1–435/6. [Google Scholar] [CrossRef] [PubMed]
- Derbre, S.; Gatto, J.; Pelleray, A.; Coulon, L.; Seraphin, D.; Richomme, P. Automating a 96-well microtiter plate assay for identification of AGEs inhibitors or inducers: Application to the screening of a small natural compounds library. Anal. Bioanal. Chem. 2010, 398, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Kikuchi, A.; Yamaya, M.; Deng, X.; Sugawara, M.; Takayama, S.; Nagatomi, R.; Ishii, T. Kakkonto inhibits cytokine production induced by rhinovirus infection in primary cultures of human nasal epithelial cells. Front. Pharmacol. 2021, 12, 687818. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.-J.; Wang, Q.; Bo, T.; Ye, M.; Qiao, X.; Yang, W.-Z.; Xiang, C.; Guan, X.-Y.; Guo, D.-A. Rapid characterization of chemical constituents and rats metabolites of the traditional Chinese patent medicine Gegen-Qinlian-Wan by UHPLC/DAD/qTOF-MS. J. Pharm. Biomed. Anal. 2013, 72, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Jiang, Y.; Li, W.; Cai, B. Rapid characterization and determination of isoflavones and triterpenoid saponins in Fu-Zhu-Jiang-Tang tablets using UHPLC-Q-TOF/MS and HPLC-UV. Anal. Methods 2016, 8, 4211–4219. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; Toume, K.; Wang, Z.; Batkhuu, J.; Komatsu, K. Characterization and quantification of monoterpenoids in different types of peony root and the related Paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2016, 129, 581–592. [Google Scholar] [CrossRef]
- Huang, W.-W.; Wang, M.-Y.; Shi, H.-M.; Peng, Y.; Peng, C.-S.; Zhang, M.; Li, Y.; Lu, J.; Li, X.-B. Comparative study of bioactive constituents in crude and processed Glycyrrhizae radix and their respective metabolic profiles in gastrointestinal tract in vitro by HPLC-DAD and HPLC-ESI/MS analyses. Arch. Pharmacal Res. 2012, 35, 1945–1952. [Google Scholar] [CrossRef]
- Cheng, M.; Ding, L.; Kan, H.; Zhang, H.; Jiang, B.; Sun, Y.; Cao, S.; Li, W.; Koike, K.; Qiu, F. Isolation, structural elucidation and in vitro hepatoprotective activity of flavonoids from Glycyrrhiza uralensis. J. Nat. Med. 2019, 73, 847–854. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shin, H.-K.; Seo, C.-S. Chemical interaction between Paeonia lactiflora and Glycyrrhiza uralensis, the components of Jakyakgamcho-tang, using a validated high-performance liquid chromatography method: Herbal combination and chemical interaction in a decoction. J. Sep. Sci. 2014, 37, 2704–2715. [Google Scholar] [CrossRef]
- Xu, T.; Yang, M.; Li, Y.; Chen, X.; Wang, Q.; Deng, W.; Pang, X.; Yu, K.; Jiang, B.; Guan, S.; et al. An integrated exact mass spectrometric strategy for comprehensive and rapid characterization of phenolic compounds in licorice. Rapid Commun. Mass Spectrom. 2013, 27, 2297–2309. [Google Scholar] [CrossRef]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice To Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Guo, N.; Zhuang, G.-D.; Deng, S.-M.; He, W.-J.; Chen, Z.-Q.; Xu, Y.-H.; Tang, D.; Wang, S.-M. Metabolic Profiling of Carbonyl Compounds for Unveiling Protective Mechanisms of Pueraria lobata against Diabetic Nephropathy by UPLC-Q-Orbitrap HRMS/MS Analysis. J. Agric. Food Chem. 2021, 69, 10943–10951. [Google Scholar] [CrossRef]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, H.J.; Lee, C.H.; Kim, H.P. Antiinflammatory activity of isoflavonoids from Pueraria radix and biochanin A derivatives. Arch. Pharmacal Res. 1994, 17, 31–35. [Google Scholar] [CrossRef]
- Maciejewska-Turska, M.; Pecio, L.; Zgorka, G. Isolation of Mirificin and Other Bioactive Isoflavone Glycosides from the Kudzu Root Lyophilisate Using Centrifugal Partition and Flash Chromatographic Techniques. Molecules 2022, 27, 6227. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Wang, S.-S.; Feng, J.-T.; Xue, X.-Y.; Liang, X.-M. Two new isoflavone glycosides from Pueraria lobata. J. Asian Nat. Prod. Res. 2008, 10, 719–723. [Google Scholar] [CrossRef]
- Ahn, S.-Y.; Jo, M.S.; Lee, D.; Baek, S.-E.; Baek, J.; Yu, J.S.; Jo, J.; Yun, H.; Kang, K.S.; Yoo, J.-E.; et al. Dual effects of isoflavonoids from Pueraria lobata roots on estrogenic activity and anti-proliferation of MCF-7 human breast carcinoma cells. Bioorg. Chem. 2019, 83, 135–144. [Google Scholar] [CrossRef]
- Nohara, T.; Kinjo, J.; Furusawa, J.; Sakai, Y.; Inoue, M.; Shirataki, Y.; Ishibashi, Y.; Yokoe, I.; Komatsu, M. But-2-enolides from Pueraria lobata and revised structures of puerosides A, B and sophoroside A. Phytochemistry 1993, 33, 1207–1210. [Google Scholar] [CrossRef]
- Shi, Y.-H.; Zhu, S.; Ge, Y.-W.; He, Y.-M.; Kazuma, K.; Wang, Z.; Yoshimatsu, K.; Komatsu, K. Monoterpene derivatives with anti-allergic activity from red peony root, the root of Paeonia lactiflora. Fitoterapia 2016, 108, 55–61. [Google Scholar] [CrossRef]
- Zheng, Y.-F.; Wei, J.-H.; Fang, S.-Q.; Tang, Y.-P.; Cheng, H.-B.; Wang, T.-L.; Li, C.-Y.; Peng, G.-P. Hepatoprotective triterpene saponins from the roots of Glycyrrhiza inflata. Molecules 2015, 20, 6273–6283. [Google Scholar] [CrossRef]
- Beckett, A.H.; Jones, G.R. Identification and stereochemistry of (2S,4S,5R)- and (2R,4S,5R)-2,3,4-trimethyl-5-phenyloxazolidine, degradation products of ephedrine. Tetrahedron 1977, 33, 3313–3316. [Google Scholar] [CrossRef]
- Xiu, L.-M.; Miura, A.B.; Yamamoto, K.; Kobayashi, T.; Song, Q.-H.; Kitamura, H.; Cyong, J.-C. Pancreatic islet regeneration by ephedrine in mice with streptozotocin-induced diabetes. Am. J. Chin. Med. 2001, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Hajleh, M.N.A.; Khleifat, K.M.; Alqaraleh, M.; Al-Hraishat, E.A.; Al-limoun, M.O.; Qaralleh, H.; Al-Dujaili, E.A.S. Antioxidant and Antihyperglycemic Effects of Ephedra foeminea Aqueous Extract in Streptozotocin-Induced Diabetic Rats. Nutrients 2022, 14, 2338. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-K.; Um, J.-Y.; Jang, H.-J.; Lee, B.-C. Beneficial effect of dietary Ephedra sinica on obesity and glucose intolerance in high-fat diet-fed mice. Exp. Ther. Med. 2012, 3, 707–712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).