Ultrasound-Assisted Mineralization of 2,4-Dinitrotoluene in Industrial Wastewater Using Persulfate Coupled with Semiconductors
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Persulfate Oxidation Simply and Persulfate Integrated with Diverse Semiconductors under Ultrasonic Irradiation
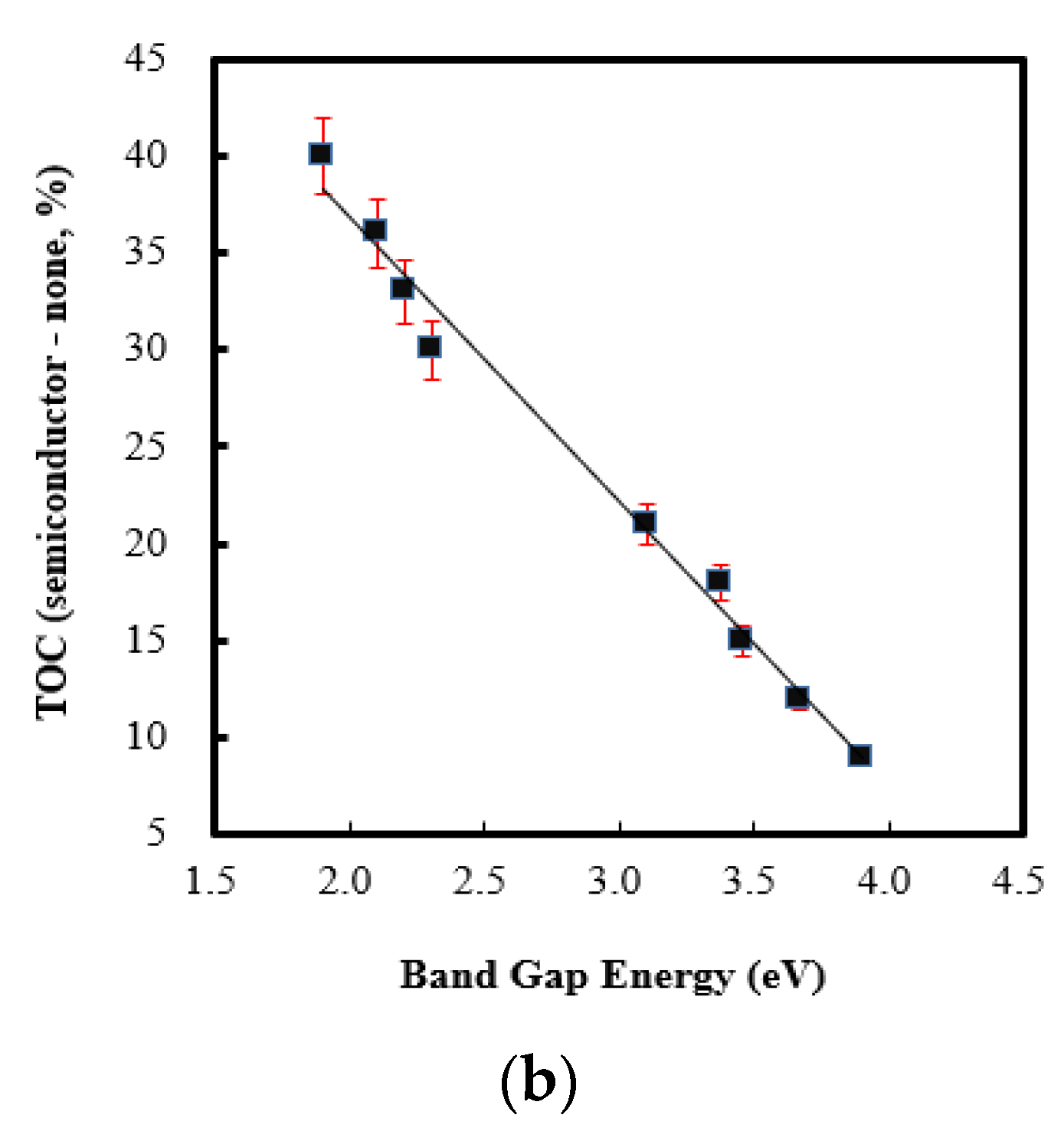
2.2. Effect of Band Gap Energy on Persulfate Integrated with Ag/NiO Semiconductor
2.3. Effect of Scavenger Dosages on Persulfate Integrated with Ag (4 wt%)/NiO under Ultrasonic Irradiation
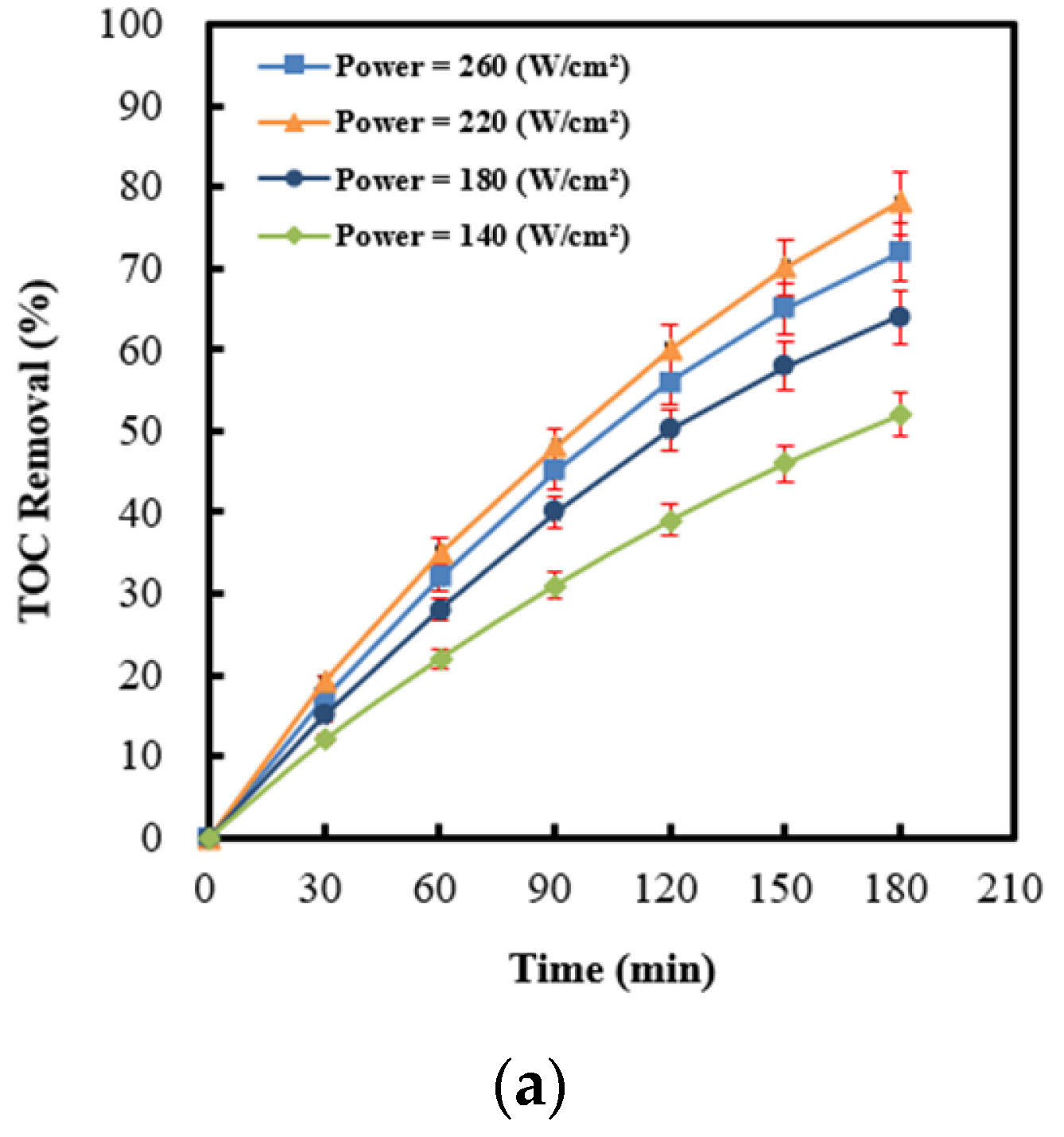
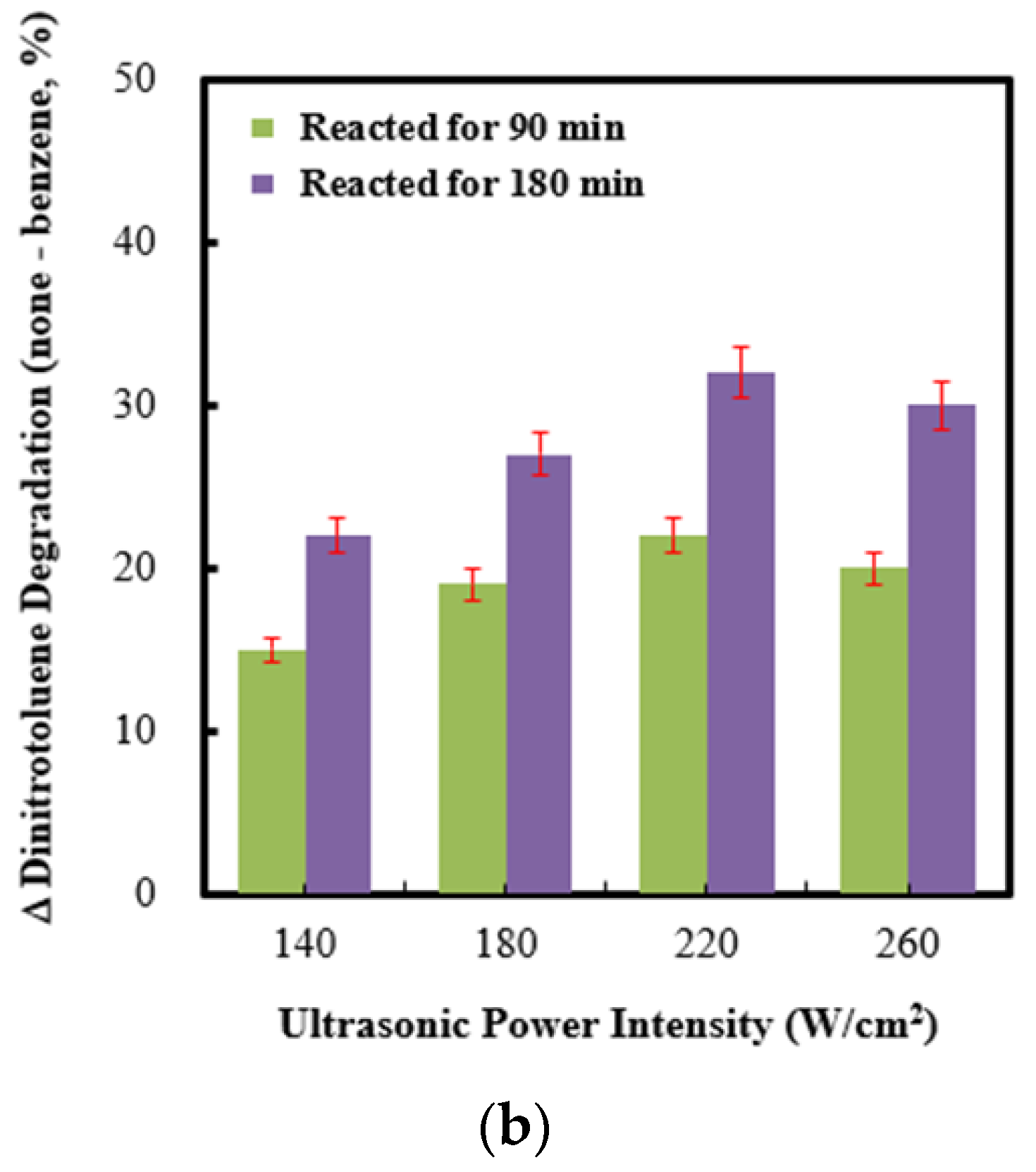
2.4. Effect of Ultrasonic Power Intensity on Persulfate Integrated with Ag (4 wt%)/NiO under Ultrasonic Irradiation
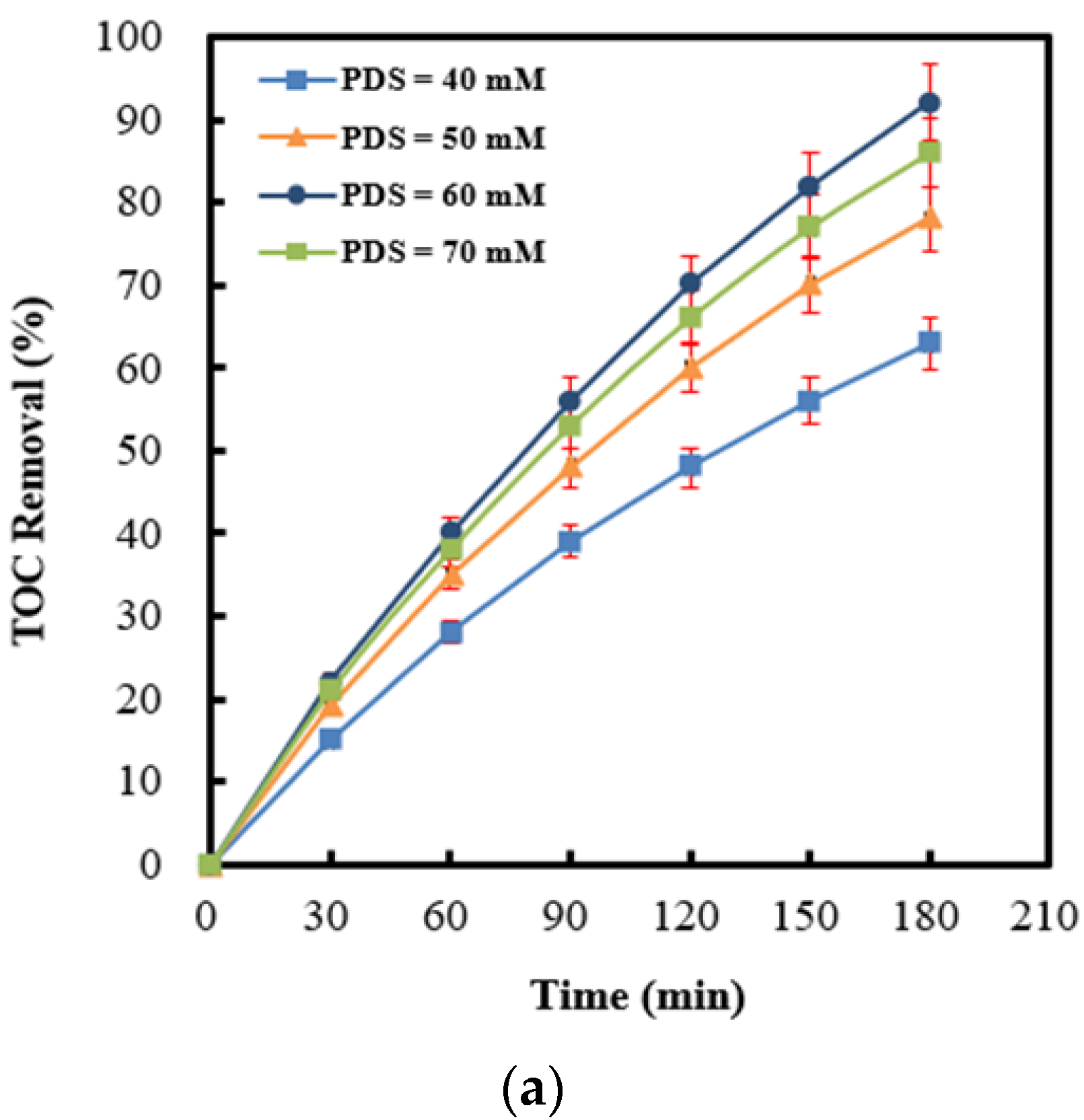
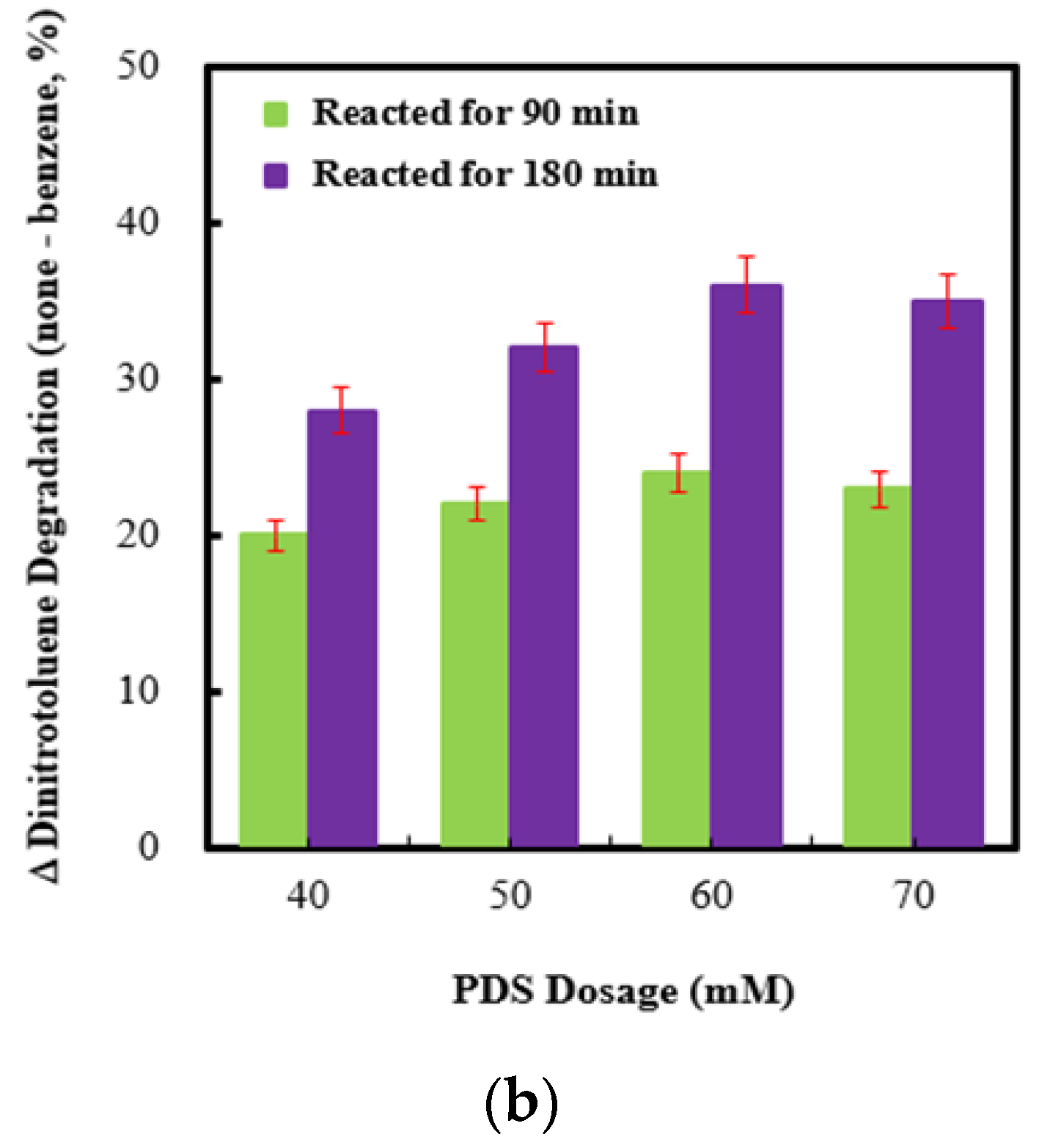
2.5. Effect of Persulfate Concentrations on Persulfate Integrated with Ag (4 wt%)/NiO under Ultrasonic Irradiation
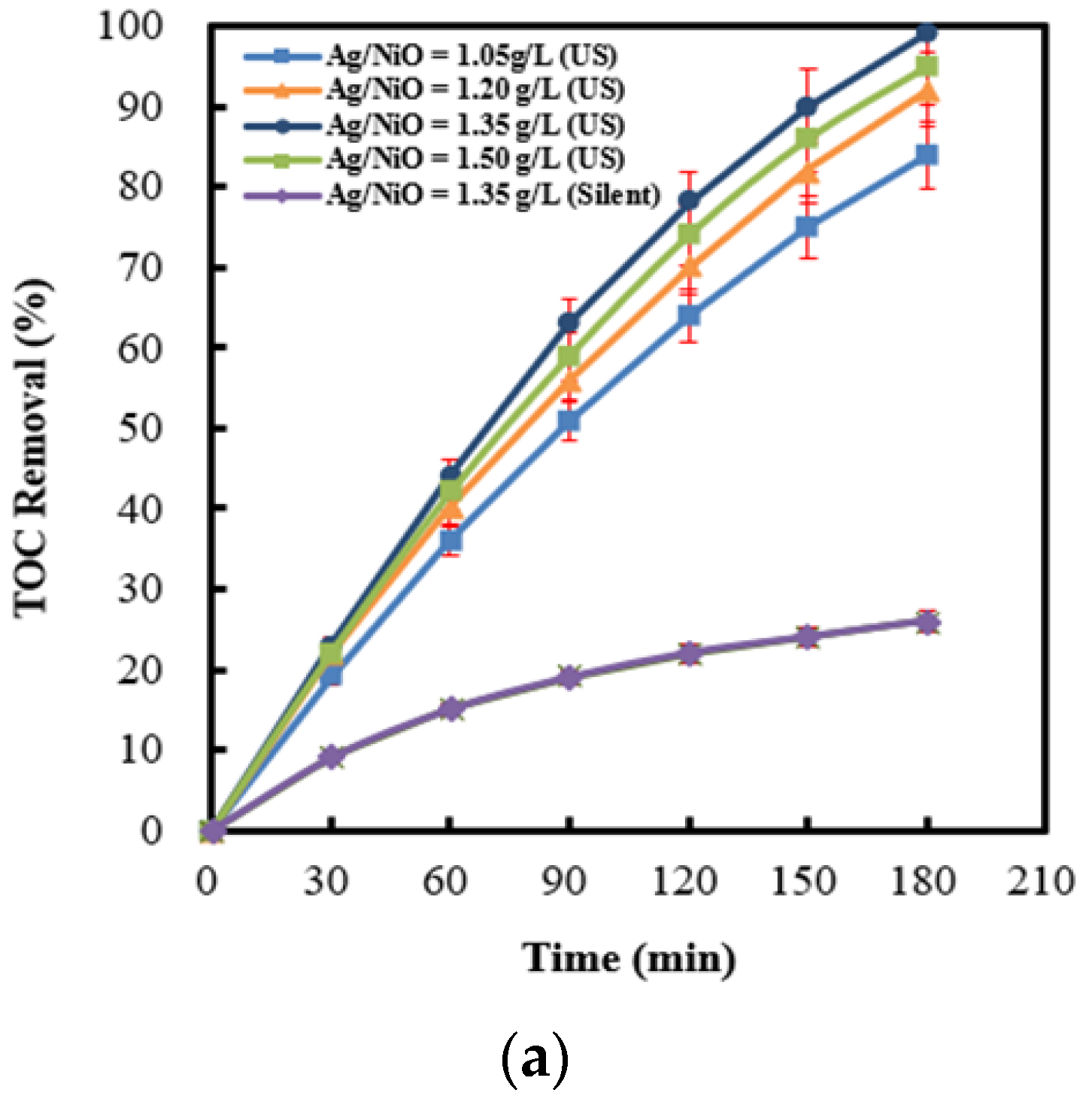
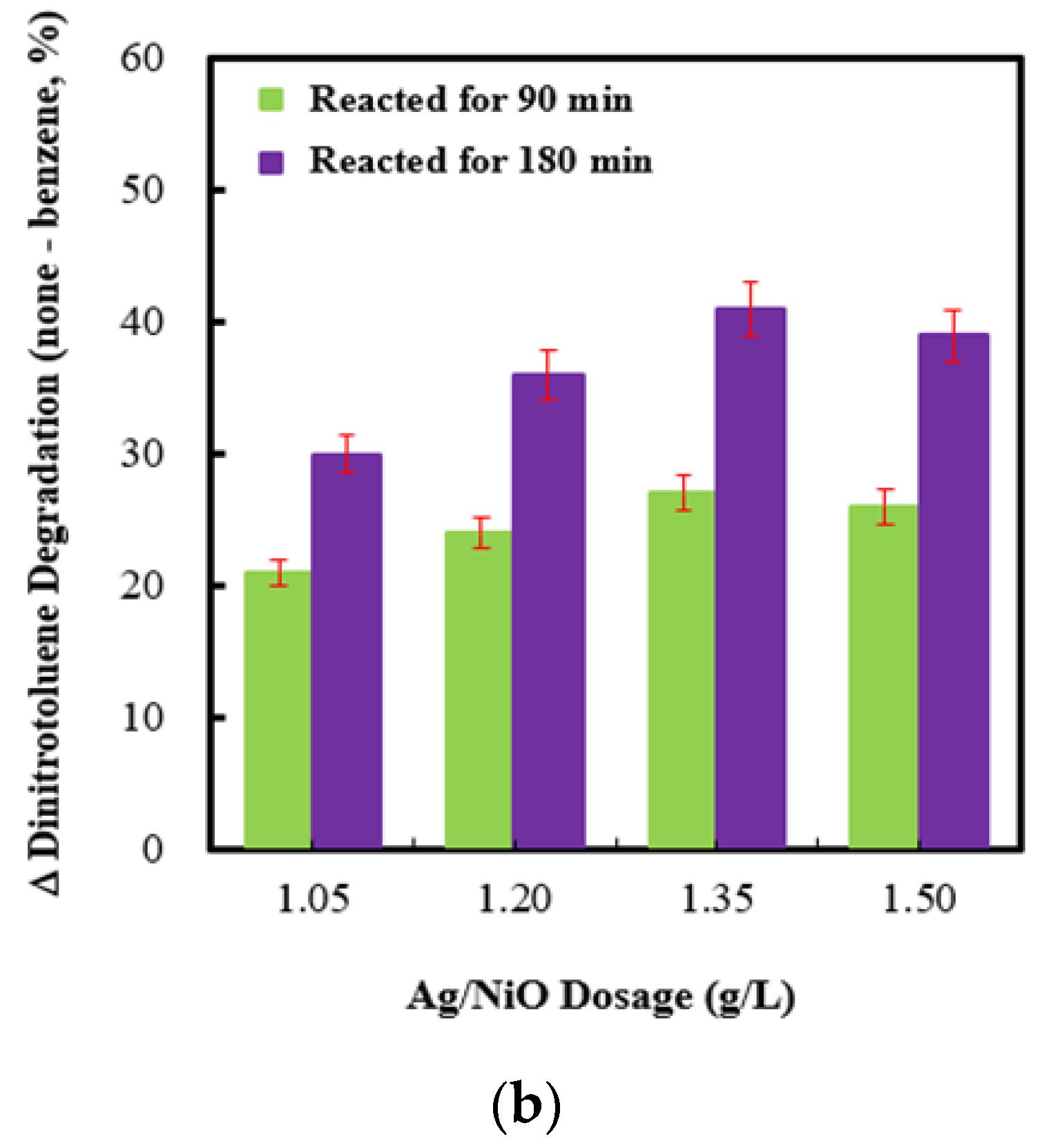
2.6. Effect of Dosages of Ag (4 wt%)/NiO Integrated with Persulfate under Ultrasonic Irradiation
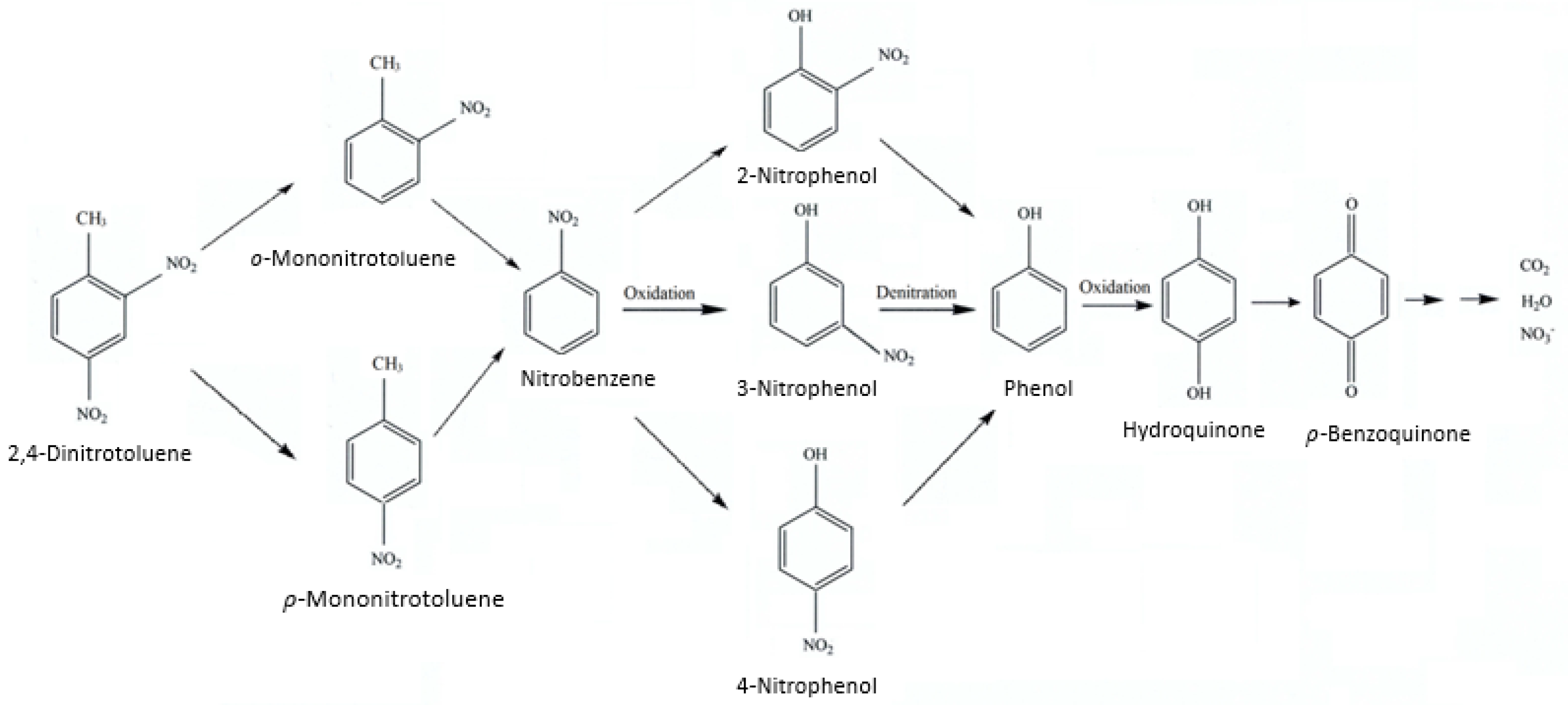
2.7. Reaction Pathway of 2,4-Dinitrotoluene on Persulfate Integrated with Ag (4 wt%)/NiO under Ultrasonic Irradiation
3. Experimental Methods
3.1. Test on Persulfate Integrated with Semiconductors Motivated by Ultrasound
3.2. Total Organic Carbon (TOC) Analysis
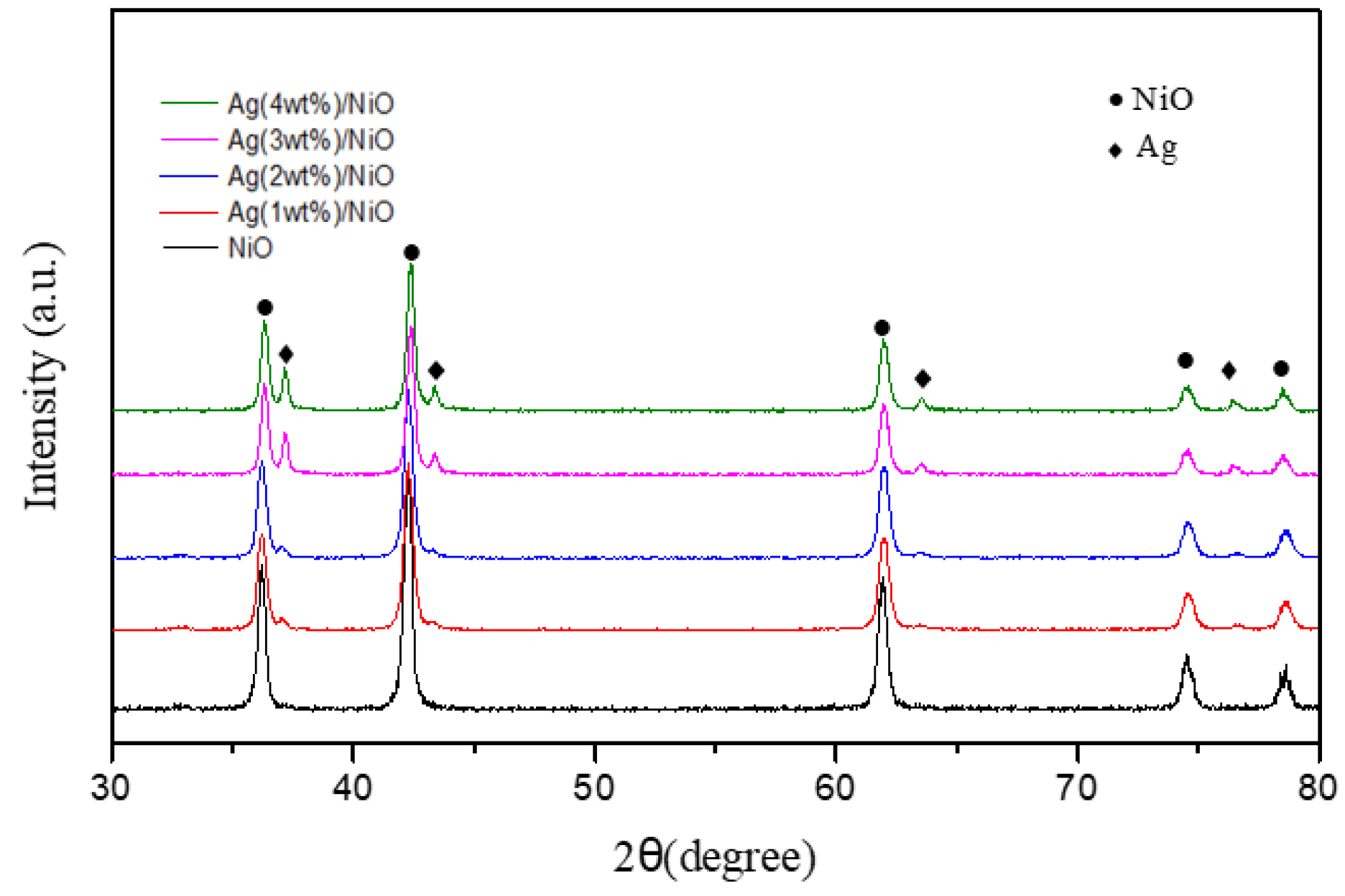
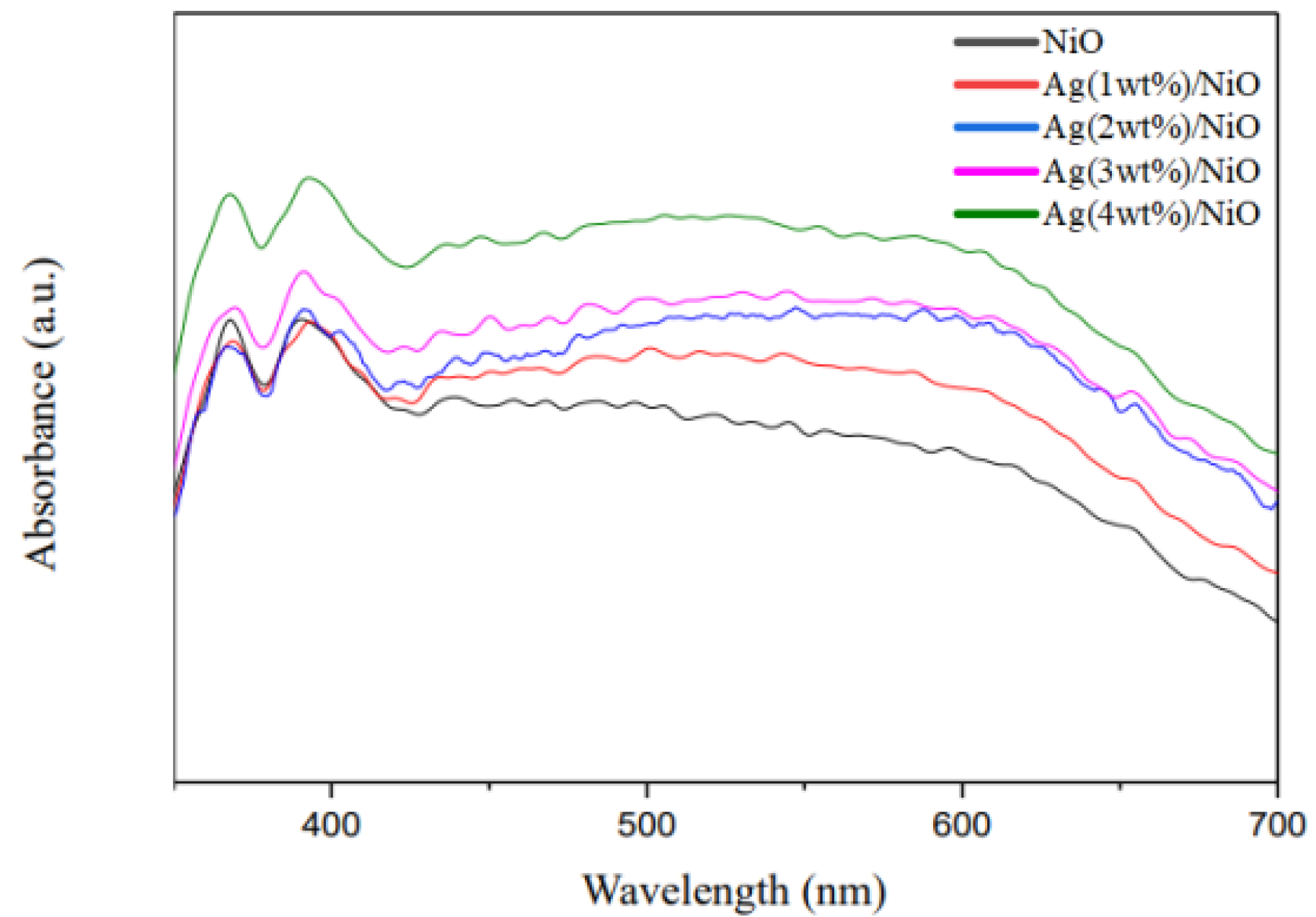
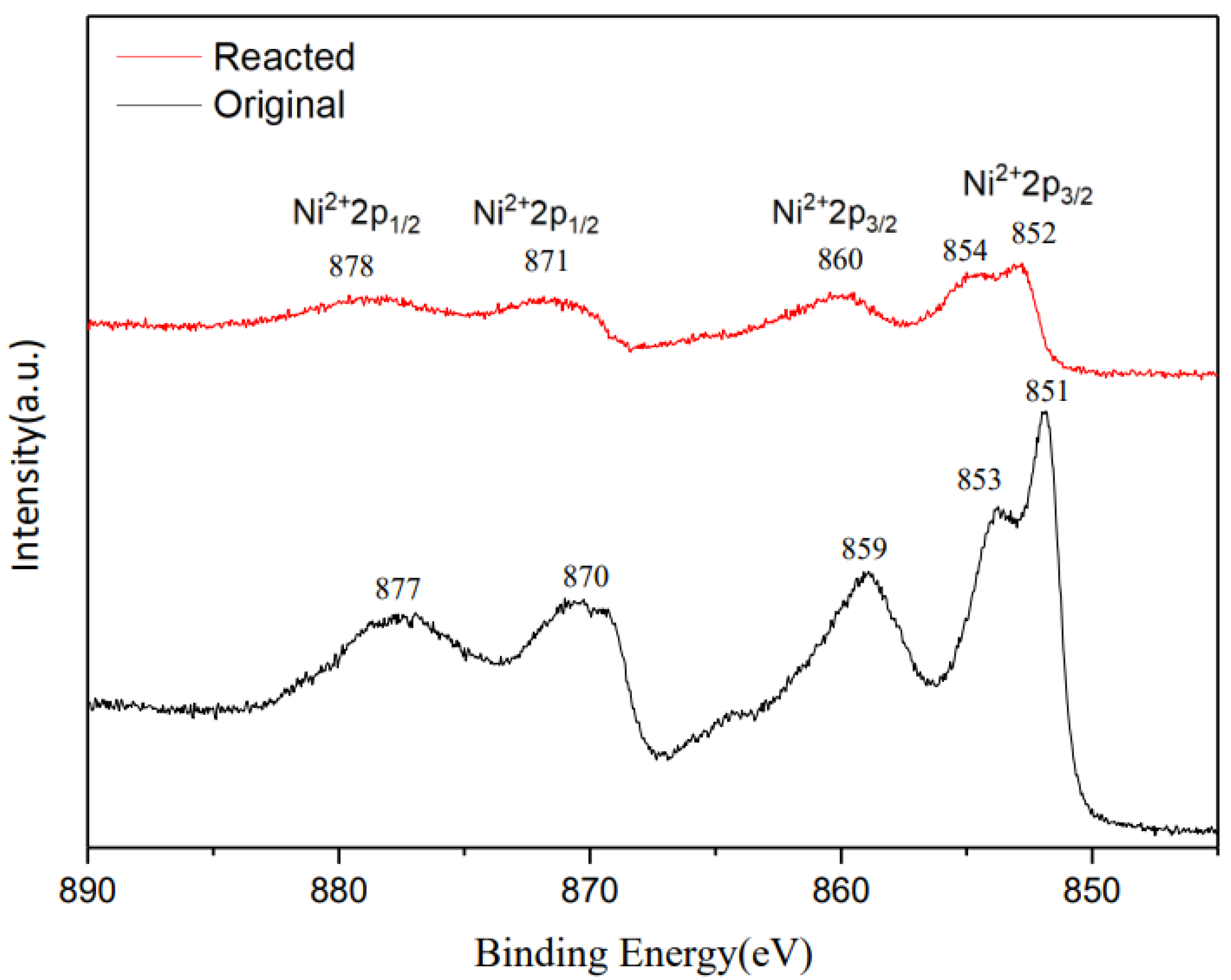
3.3. Physicochemical Characterizations of Ag/NiO
3.4. Scavenging Effects
3.5. Gas Chromatograph–Mass Spectrometer Analysis (GC-MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weissermel, K.; Arpe, H.-J. Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; VCH: Weinheim, Germany, 1991; Volume A17. [Google Scholar]
- LaGrega, M.D.; Buckingham, P.L.; Evans, J.C. Hazardous Waste Management, 2nd ed.; Waveland Press, Inc.: West Salem, WI, USA, 2010. [Google Scholar]
- Tchounwou, P.B.; Wilson, B.A.; Ishaque, A.B.; Schneider, J. Transcriptional activation of stress genes and cytotoxicity in human liver carcinoma cells (HepG2) exposed to 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and 2,6-dinitrotoluene. Environ. Toxicol. 2001, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Al-Madanat, O.; Al Salka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 photocatalysis for the transformation of aromatic water pollutants into fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, G.C. Sonochemical decomposition of dinitrotoluenes and trinitrotoluene in wastewater. J. Hazard. Mater. 2009, 169, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Huang, Y.L. Removal of dinitrotoluenes and trinitrotoluene from wastewater by ultrasound enhanced with titanium dioxide. Ultrason. Sonochem. 2011, 18, 1232–1240. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, S.C. Sonophotocatalytic degradation of dinitrotoluenes and trinitrotoluene in industrial wastewater. Chem. Eng. J. 2011, 172, 944–951. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, C.P. Decomposition of nitrotoluenes in wastewater by sonoelectrochemical and sonoelectro-Fenton oxidation. Ultrason. Sonochem. 2014, 21, 840–845. [Google Scholar] [CrossRef]
- Schmelling, D.C.; Gray, K.A. Photocatalytic transformation and mineralization of 2,4,6-trinitrotoluene (TNT) in TiO2 slurries. Water Res. 1995, 29, 2651–2662. [Google Scholar] [CrossRef]
- Vohra, M.S.; Tanaka, K. Photocatalytic degradation of trinitrotoluene in aqueous TiO2 suspension. Water Res. 2002, 36, 59–64. [Google Scholar] [CrossRef]
- Li, Z.M.; Shea, P.J.; Comfort, S.D. Nitrotoluene destruction by UV-catalyzed Fenton oxidation. Chemosphere 1998, 36, 1849–1865. [Google Scholar] [CrossRef]
- Liou, M.-J.; Lu, M.-C.; Ohen, J.-N. Oxidation of explosives by Fenton and photo-Fenton process. Water Res. 2003, 37, 3172–3179. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Encinar, J.M.; Alonso, M.A. Nitroaromatic hydrocarbon ozonation in water. 1. Single ozonation. Ind. Eng. Chem. Res. 1998, 37, 25–31. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Encinar, J.M.; Alonso, M.A. Nitroaromatic hydrocarbon ozonation in water. 2. Combined ozonation with hydrogen peroxide or UV radiation. Ind. Eng. Chem. Res. 1998, 37, 32–40. [Google Scholar] [CrossRef]
- AlSalka, Y.; Al-Madanat, O.; Curti, M.; Hakki, A.; Bahnemann, D.W. Photocatalytic H2 evolution from oxalic acid: Effect of cocatalysts and carbon dioxide radical anion on the surface charge transfer mechanisms. ACS Appl. Energy Mater. 2020, 3, 6678–6691. [Google Scholar] [CrossRef]
- Al-Madanat, O.; Curti, M.; Günnemann, C.; AlSalka, Y.; Dillert, R.; Bahnemann, D.W. TiO2 photocatalysis: Impact of the platinum loading method on reductive and oxidative half-reactions. Catal. Today 2021, 380, 3–15. [Google Scholar] [CrossRef]
- Liang, C.J.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Sun, Y.; Li, M.; Gu, X.; Danish, M.; Shan, A.; Ali, M.; Qiu, Z.; Sui, Q.; Lyu, S. Mechanism of surfactant in trichloroethene degradation in aqueous solution by sodium persulfate activated with chelated-Fe (II). J. Hazard. Mater. 2021, 407, 124814. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; An, N.; Deng, J. Heat-activated persulfate oxidation of diuron in water. Chem Eng. J. 2012, 203, 294–300. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef]
- Pourehie, O.; Saien, J. Treatment of real petroleum refinery wastewater with alternative ferrous-assisted UV/persulfate homogeneous processes. Desalin. Water Treat. 2019, 142, 140–147. [Google Scholar] [CrossRef]
- Chen, W.S.; Jhou, Y.C.; Huang, C.P. Mineralization of dinitrotoluenes in industrial wastewater by electro-activated persulfate oxidation. Chem. Eng. J. 2014, 252, 166–172. [Google Scholar] [CrossRef]
- Hori, H.; Nagano, Y.; Murayama, M.; Koike, K.; Kutsuna, S. Efficient decomposition of perfluoroether carboxylic acids in water with a combination of persulfate oxidant and ultrasonic irradiation. J. Fluor. Chem. 2012, 141, 5–10. [Google Scholar] [CrossRef]
- Chen, W.S.; Su, Y.C. Removal of dinitrotoluenes in wastewater by sono-activated persulfate. Ultrason. Sonochem. 2012, 19, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xue, J.; He, L.; Wu, L.; Ma, Y.; Chen, H.; Li, H.; Peng, P.; Zhang, Z. Review on ultrasound assisted persulfate degradation of organic contaminants in wastewater: Influences, mechanisms and prospective. Chem. Eng. J. 2019, 378, 122146. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, C.P. Mineralization of dinitrotoluenes in aqueous solution by sono-activated persulfate enhanced with electrolytes. Ultrason. Sonochem. 2019, 51, 129–137. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, S.G.; Chiu, P.C. Degradation of 2,4-dinitrotoluene by persulfate activated with zero-valent iron. Sci. Total. Environ. 2010, 408, 3464–3468. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kang, S.G.; Kim, D.W.; Chiu, P.C. Degradation of 2,4-dinitrotoluene by persulfate activated with iron sulfides. Chem. Eng. J. 2011, 172, 641–646. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Jiang, Y.; Xi, B.; Yang, T.; Peng, X.; Lian, X.; Yan, K.; Liu, H. Enhanced degradation of 2,4-dinitrotlouene in groundwater by persulfate activated using iron-carbon micro-electrolysis. Chem. Eng. J. 2017, 311, 183–190. [Google Scholar] [CrossRef]
- Xu, X.; Xi, B.; Zhang, Y.; Xia, F.; Han, X.; Gao, P.; Wan, S.; Jiang, Y.; Yang, Y. A comparative study on the treatment of 2,4-dinitrotoluene contaminated groundwater in the combined system: Efficiencies, intermediates and mechanisms. Sci. Total Environ. 2020, 735, 139161. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Jia, Y.; Lian, X.; Zhang, Y.; Feng, F.; Liu, Q.; Xi, B.; Jiang, Y. Heterogeneous catalytic degradation of 2,4-dinitrotoluene by the combined persulfate and hydrogen peroxide activated by the as-synthesized Fe-Mn binary oxides. Chem. Eng. J. 2019, 374, 776–786. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Sun, Z.; Liu, Y.; Zhang, W.; Wu, B.; Ma, F.; Gu, Q. Degradation of 2,4-dinitrotoluene using ferrous activated persulfate: Kinetics, mechanisms, and effects of natural water matrices. J. Environ. Chem. Eng. 2021, 9, 106048. [Google Scholar] [CrossRef]
- Matula, T.J.; Roy, R.A.; Mourad, P.D.; McNamara Iii, W.B.; Suslick, K.S. Comparison of multibubble and single-bubble sonoluminescence spectra. Phys. Rev. Lett. 1995, 75, 2602–2605. [Google Scholar] [CrossRef]
- Hiller, R.A.; Putterman, S.J.; Weninger, K.R. Time-resolved spectra of sonoluminescence. Phys. Rev. Lett. 1998, 80, 1090–1093. [Google Scholar] [CrossRef]
- Chen, W.S.; Shih, Y.C. Mineralization of aniline in aqueous solution by sono-activated peroxydisulfate enhanced with PbO semiconductor. Chemosphere 2020, 239, 124686. [Google Scholar] [CrossRef]
- Chen, W.S.; Chang, T.Y. Decomposition of nitrobenzene in wastewater using persulfate combined with Ag/PbO under ultrasonic irradiation. Desalination Water Treat. 2021, 226, 125–136. [Google Scholar] [CrossRef]
- Nami, M.; Sheibani, S.; Rashchi, F. Photocatalytic performance of coupled semiconductor ZnO-CuO nanocomposite coating prepared by a facile brass anodization process. Mater. Sci. Semicond. Process. 2021, 135, 106083. [Google Scholar] [CrossRef]
- Du, Q.; Lu, G. The roles of various Ni species over SnO2 in enhancing the photocatalytic properties for hydrogen generation under visible light irradiation. Appl. Surf. Sci. 2014, 305, 235–241. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, L.; Wu, Y.; Singh, M.; Zhu, B. The composite electrolyte with an insulation Sm2O3 and semiconductor NiO for advanced fuel cells. Int. J. Hydrogen Energy 2018, 43, 12739–12747. [Google Scholar] [CrossRef]
- Sambasivam, S.; Kim, S.B.; Jeong, J.H.; Choi, B.C.; Lim, K.T.; Kim, S.S.; Song, T.K. Effect of Er3+ doping in SnO2 semiconductor nanoparticles synthesized by solgel technique. Curr. Appl. Phys. 2010, 10, 1383–1386. [Google Scholar] [CrossRef]
- Gondal, M.A.; Sayeed, M.N.; Alarfaj, A. Activity comparison of Fe2O3, NiO, WO3, TiO2 semiconductor catalysts in phenol degradation by laser enhanced photo-catalytic process. Chem. Phys. Lett. 2007, 445, 325–330. [Google Scholar] [CrossRef]
- Xiong, L.; Huang, S.; Yang, X.; Qiu, M.; Chen, Z.; Yu, Y. P-type and n-type Cu2O semiconductor thin films: Controllable preparation by simple solvothermal method and photoelectrochemical properties. Electrochim. Acta 2011, 56, 2735–2739. [Google Scholar] [CrossRef]
- Alwis, D.D.D.H.; Chandrika, U.G.; Jayaweera, P.M. Photostability of apocarotenoids on surface of TiO2 semiconductor nanoparticles. J. Photochem. Photobiol. A 2021, 407, 113061. [Google Scholar] [CrossRef]
- Pavlov, D. Semiconductor mechanism of the processes during electrochemical oxidation of PbO to PbO2. J. Electroanal. Chem. 1981, 118, 167–185. [Google Scholar] [CrossRef]
- Terpstra, H.J.; De Groot, R.A.; Haas, C. The electronic structure of the mixed valence compound Pb3O4. J. Phys. Chem. Solids 1997, 58, 561–566. [Google Scholar] [CrossRef]
- Jyothi, K.P.; Yesodharan, S.; Yesodharan, E.P. Ultrasound (US), Ultraviolet light (UV) and combination (US + UV) assisted semiconductor catalyzed degradation of organic pollutants in water: Oscillation in the concentration of hydrogen peroxide formed in situ. Ultrason. Sonochem. 2014, 21, 1787–1796. [Google Scholar] [CrossRef]
- Sakthivel, Y.; Venugopal, G.; Durairaj, A.; Vasanthkumar, S.; Huang, X. Utilization of the internal electric field in semiconductor photocatalysis: A short review. J. Ind. Eng. Chem. 2019, 72, 18–30. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Hamd, A.; Amin, R.R.; Khalek, A.A. Photocatalytic removal of Congo red dye using MCM-48/Ni2O3 composite synthesized based on silica gel extracted from rice husk ash; fabrication and application. J. Environ. Manag. 2017, 204, 189–199. [Google Scholar] [CrossRef]
- Karunakaran, C.; Dhanalakshmi, R.; Gomathisankar, P. Phenol-photodegradation on ZrO2. Enhancement by semiconductors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 92, 201–206. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. Appl. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Bera, A.; Hajra, P.; Shyamal, S.; Mandal, H.; Sariket, D.; Kundu, S.; Mandal, S.; Bhattacharya, C. Solvent effects on the photoelectrochemical water oxidation behaviour of TiO2 semiconductors. Mater. Today Proc. 2018, 5, 10161–10168. [Google Scholar] [CrossRef]
- Sharon, M.; Kumar, S.; Jawalekar, S.R. Characterization of Pb3O4 films by electrochemical techniques. Bull. Mater. Sci. 1986, 8, 415–418. [Google Scholar] [CrossRef]
- Sakthivel, S.; Geissen, S.-U.; Bahnemann, D.W.; Murugesan, V.; Vogelpohl, A. Enhancement of photocatalytic activity by semiconductor heterojunctions: α-Fe2O3, WO3 and CdS deposited on ZnO. J. Photochem. Photobiol. A 2002, 148, 283–293. [Google Scholar] [CrossRef]
- Muthukumaran, M.; Niranjani, S.; Samuel Barnabas, K.; Narayanan, V.; Raju, T.; Venkatachalam, K. Green route synthesis and characterization of cuprous oxide (Cu2O): Visible light irradiation photocatalytic activity of MB dye. Mater. Today Proc. 2019, 14, 563–568. [Google Scholar] [CrossRef]
- Droessler, L.M.; Assender, H.E.; Watt, A.A.R. Thermally deposited lead oxides for thin film photovoltaics. Mater. Lett. 2012, 71, 51–53. [Google Scholar] [CrossRef]
- Alahmad, A.; Al-Zereini, W.A.; Hijazin, T.J.; Al-Madanat, O.Y.; Alghoraibi, I.; Al-Qaralleh, O.; Al-Qaraleh, S.; Feldhoff, A.; Walter, J.G.; Scheper, T. Green synthesis of silver nanoparticles using Hypericum perforatum L. aqueous extract with the evaluation of its antibacterial activity against clinical and food pathogens. Pharmaceutics 2022, 14, 1104. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Fujimoto, T.; Yamanaka, S. Characterization of submicro-sized Ag/ZnO particles generated using the spray pyrolysis method. Adv. Powder Technol. 2022, 33, 103525. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Hou, F.; Li, H.; Yang, Y.; Zhang, X.; Yang, Y.; Wang, Y. Effects of Ag loading on structural and photocatalytic properties of flower-like ZnO microspheres. Appl. Surf. Sci. 2017, 391, 476–483. [Google Scholar] [CrossRef]
- Han, Z.; Ren, L.; Cui, Z.; Chen, C.; Pan, H.; Chen, J. Ag/ZnO flower heterostructures as a visible-light driven photocatalyst via surface plasmon resonance. Appl. Catal. B Environ. 2012, 126, 298–305. [Google Scholar] [CrossRef]
- Chen, W.S.; Liu, Y.C. Photocatalytic degradation of nitrobenzene in wastewater by persulfate integrated with Ag/Pb3O4 semiconductor under visible light irradiation. Heliyon 2021, 7, e06984. [Google Scholar] [CrossRef]
- Maji, S.K.; Mukherjee, N.; Dutta, A.K.; Srivastava, D.N.; Paul, P.; Karmakar, B.; Mondal, A.; Adhikary, B. Deposition of nanocrystalline CuS thin film from a single precursor: Structural, optical and electrical properties. Mater. Chem. Phys. 2011, 130, 392–397. [Google Scholar] [CrossRef]
- Štengl, V.; Grygar, T.M. The simplest way to Iodine-doped anatase for photocatalysts activated by visible light. Int. J. Photoenergy 2011, 2011, 685935–685948. [Google Scholar] [CrossRef]
- Kamaraj, E.; Somasundaram, S.; Balasubramani, K.; Eswaran, M.P.; Muthuramalingam, R.; Park, S. Facile fabrication of CuO-Pb2O3 nanophotocatalysts for efficient degradation of Rose Bengal dye under visible light irradiation. Appl. Surf. Sci. 2018, 433, 206–212. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Dillert, R.; Bahnemann, D.W. Photocatalytic H2 production from naphthalene by various TiO2 photocatalysts: Impact of Pt loading and formation of intermediates. Catalysts 2021, 11, 107. [Google Scholar] [CrossRef]
- Pandey, S.K.; Tripathi, M.K.; Ramanathan, V.; Mishra, P.K. Highly facile Ag/NiO nanocomposite synthesized by sol-gel method for mineralization of rhodamine B. J. Phys. Chem. Solids 2021, 159, 110287. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Y.; Qiu, X.; Yin, J.; Su, C.; Pu, X. Use of synergistic effects of the co-catalyst, p-n heterojunction, and porous structure for improvement of visible-light photocatalytic H2 evolution in porous Ni2O3/Mn0.2Cd0.8S/Cu3P@Cu2S. J. Alloys. Compd. 2020, 845, 155569. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Sivakumar, M.; Pandit, A.B. Ultrasound enhanced degradation of Rhodamine B: Optimization with power density. Ultrason. Sonochem. 2001, 8, 233–240. [Google Scholar] [CrossRef]
- Sivakumar, M.; Tatake, P.A.; Pandit, A.B. Kinetics of p-nitrophenol degradation: Effect of reaction conditions and cavitational parameters for a multiple frequency system. Chem. Eng. J. 2002, 85, 327–338. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhang, H. Degradation of bisphenol A in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep. Purif. Technol. 2013, 117, 18–23. [Google Scholar] [CrossRef]
- Hou, L.W.; Zhang, H.; Xue, X.F. Ultrasound enhanced heterogeneous activation of peroxydisulfate by magnetite catalyst for the degradation of tetracycline in water. Sep. Purif. Technol. 2012, 84, 147–152. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Berberidou, C.; Poulios, I.; Xekoukoulotakis, N.P.; Mantzavinos, D. Sonolytic, photocatalytic and sonophotocatalytic degradation of malachite green in aqueous solutions. Appl. Catal. B: Environ. 2007, 74, 63–72. [Google Scholar] [CrossRef]
- Larson, R.A.; Miller, P.L.; Crowley, T.O. Borohydride photoreduction of nitroaromatic compounds related to military ordance constituents. Environ. Sci. Technol. 1996, 30, 1192–1197. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chlorine ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, J.; Xiao, D.; Guo, Y.; Fang, C.; Lou, X.; Wang, Z.; Liu, J. Transformations of chloro and nitro groups during the peroxymonosulfate-based oxidation of 4-chloro-2-nitrophenol. Chem. Eng. J. 2015, 134, 446–451. [Google Scholar] [CrossRef]
- Chen, W.S.; Huang, C.P. Mineralization of aniline in aqueous solution by electro-activated persulfate oxidation enhanced with ultrasound. Chem. Eng. J. 2015, 266, 279–288. [Google Scholar] [CrossRef]




| Semiconductor | Ag (wt%) | Ni (wt%) | O (wt%) | Band Gap Energy (eV) |
|---|---|---|---|---|
| NiO | 0.00 | 86.48 | 13.52 | 3.45 |
| Ag (1 wt%)/NiO | 0.97 | 81.37 | 17.66 | 3.15 |
| Ag (2 wt%)/NiO | 1.43 | 81.11 | 17.46 | 2.87 |
| Ag (3 wt%)/NiO | 2.56 | 81.04 | 16.40 | 2.77 |
| Ag (4 wt%)/NiO | 3.90 | 78.08 | 18.02 | 2.53 |
| Component | m/z (Relative Abundance, %) |
|---|---|
| Feedstock | |
| 2,4-Dinitrotoluene Degradation intermediate | 51 (13.2), 63 (35.7), 78 (16.4), 89 (60.8), 90 (26.1), 119 (25.5), 165 (100), 166 (13.9) |
| o-Mononitrotoluene | 39 (28.2), 63 (27.7), 65 (82.9), 77 (30.4), 89 (30.8), 91 (61.3), 92 (62.3), 120 (100) |
| p-Mononitrotoluene | 39 (24.9), 63 (25.4), 65 (71.0), 77 (26.8), 89 (20.9), 91 (100), 107 (34.5), 137 (86.9) |
| Nitrobenzene | 50 (15.6), 51 (37.6), 65 (13.5), 74 (8.9), 77 (100), 78 (7.4), 93 (16.9), 123 (70.0) |
| 2-Nitrophenol | 39 (15.6), 53 (9.5), 63 (20.1), 64 (13.9), 65 (25.4), 81 (19.5), 109 (18.0), 139 (100) |
| 3-Nitrophenol | 39 (35.8), 53 (10.6), 63 (14.7), 64 (7.9), 65 (63.7), 81 (15.8), 93 (51.3), 139 (100) |
| 4-Nitrophenol | 39 (44.2), 53 (23.2), 63 (287.1), 65 (79.9), 81 (32.9), 93 (27.0), 109 (67.0), 139 (100) |
| Phenol | 38 (5.2), 39 (12.5), 40 (6.9), 55 (6.3), 63 (6.5), 65 (20.8), 66 (27.2), 94 (100), 95 (7.6) |
| Hydroquinone | 39 (6.9), 53 (14.3), 54 (12.8), 55 (10.5), 81 (25.4), 82 (12.2), 110 (100), 143 (9.6) |
| p-Benzoquinone | 26 (18.0), 52 (17.7), 53 (17.2), 54 (63.2), 80 (28.3), 82 (36.0), 108 (100), 110 (12.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Hsu, M.-C. Ultrasound-Assisted Mineralization of 2,4-Dinitrotoluene in Industrial Wastewater Using Persulfate Coupled with Semiconductors. Molecules 2023, 28, 4351. https://doi.org/10.3390/molecules28114351
Chen W-S, Hsu M-C. Ultrasound-Assisted Mineralization of 2,4-Dinitrotoluene in Industrial Wastewater Using Persulfate Coupled with Semiconductors. Molecules. 2023; 28(11):4351. https://doi.org/10.3390/molecules28114351
Chicago/Turabian StyleChen, Wen-Shing, and Min-Chih Hsu. 2023. "Ultrasound-Assisted Mineralization of 2,4-Dinitrotoluene in Industrial Wastewater Using Persulfate Coupled with Semiconductors" Molecules 28, no. 11: 4351. https://doi.org/10.3390/molecules28114351
APA StyleChen, W.-S., & Hsu, M.-C. (2023). Ultrasound-Assisted Mineralization of 2,4-Dinitrotoluene in Industrial Wastewater Using Persulfate Coupled with Semiconductors. Molecules, 28(11), 4351. https://doi.org/10.3390/molecules28114351








