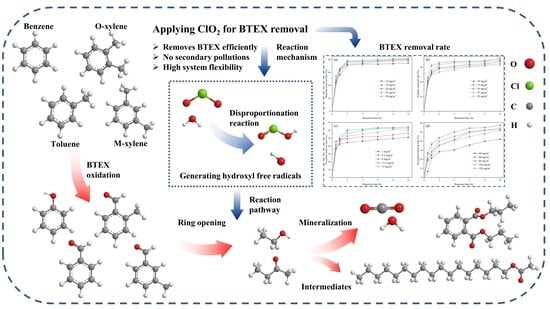
Performance and Mechanism of Chlorine Dioxide on BTEX Removal in Liquid and Indoor Air
Abstract
1. Introduction
2. Results and Discussion
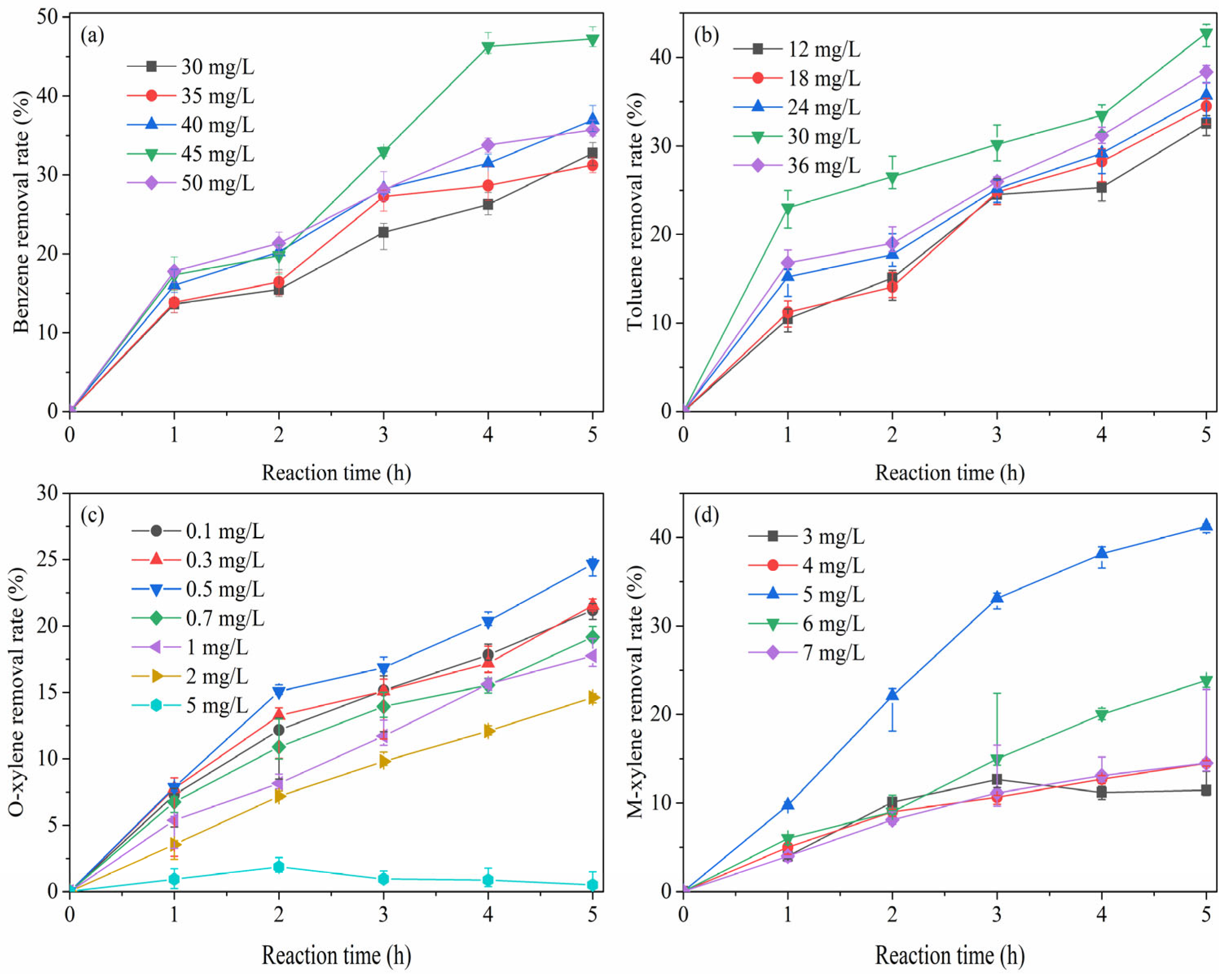
2.1. Removal of Liquid BTEX by ClO2
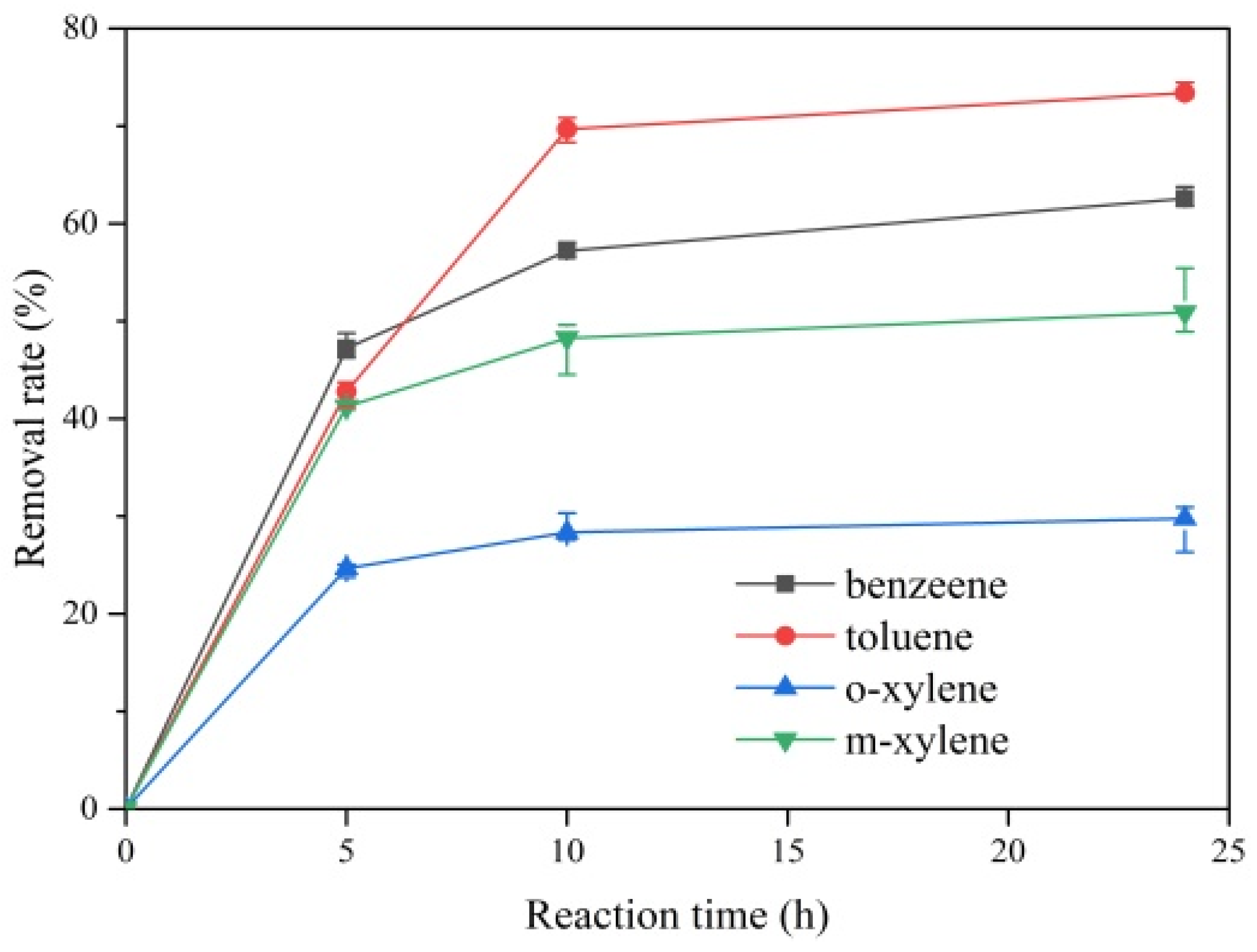
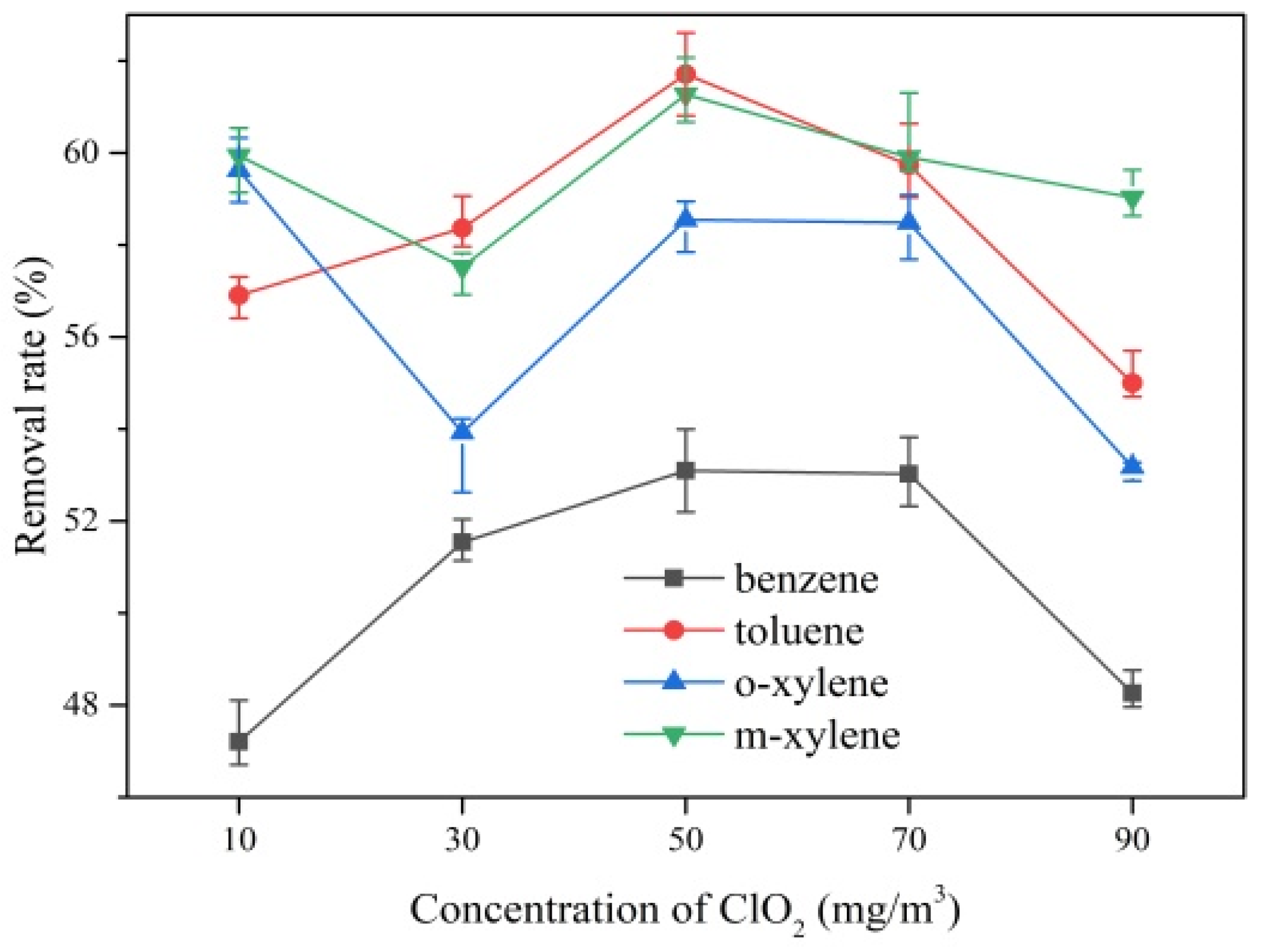
2.2. Removal of Gaseous BTEX by ClO2
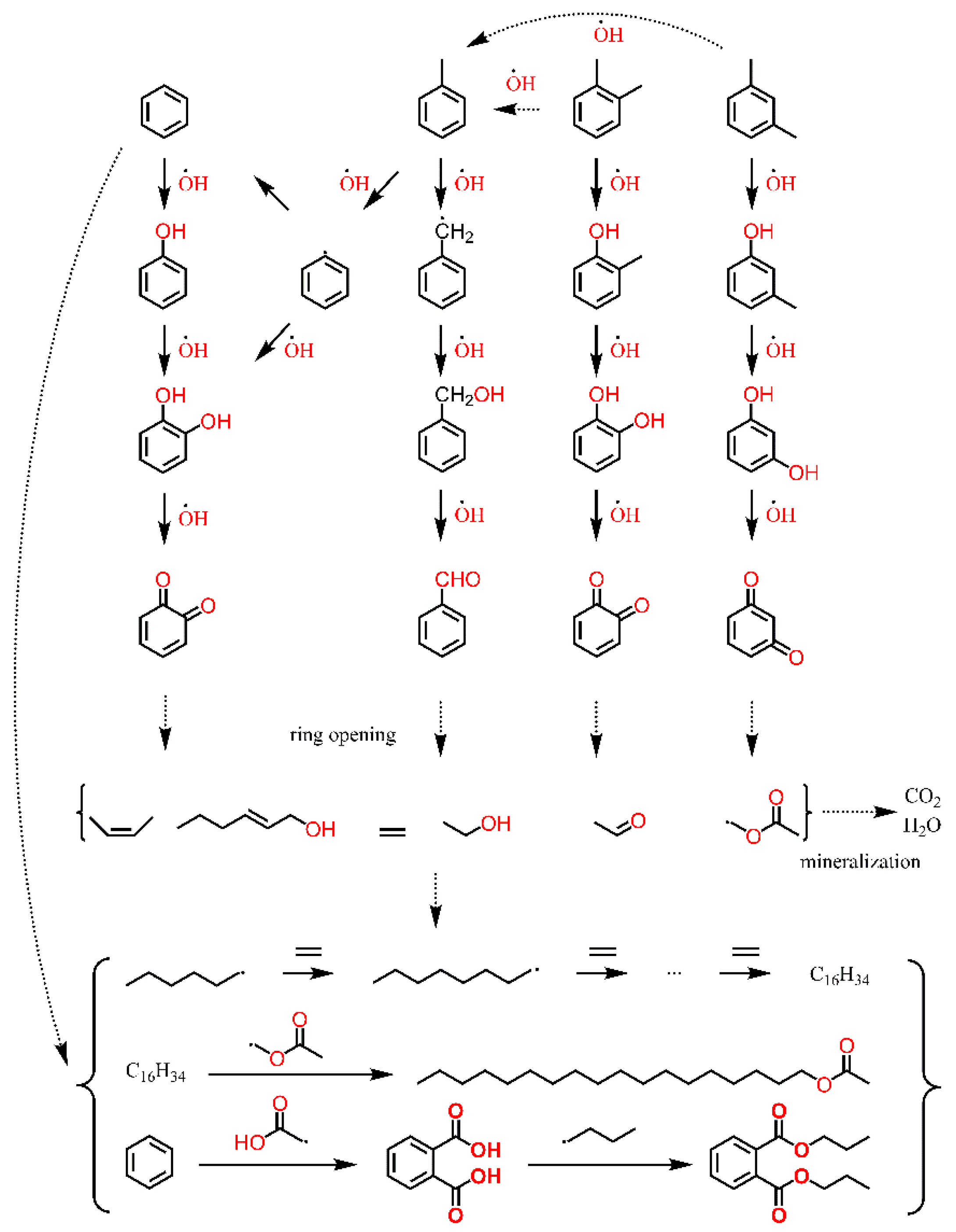
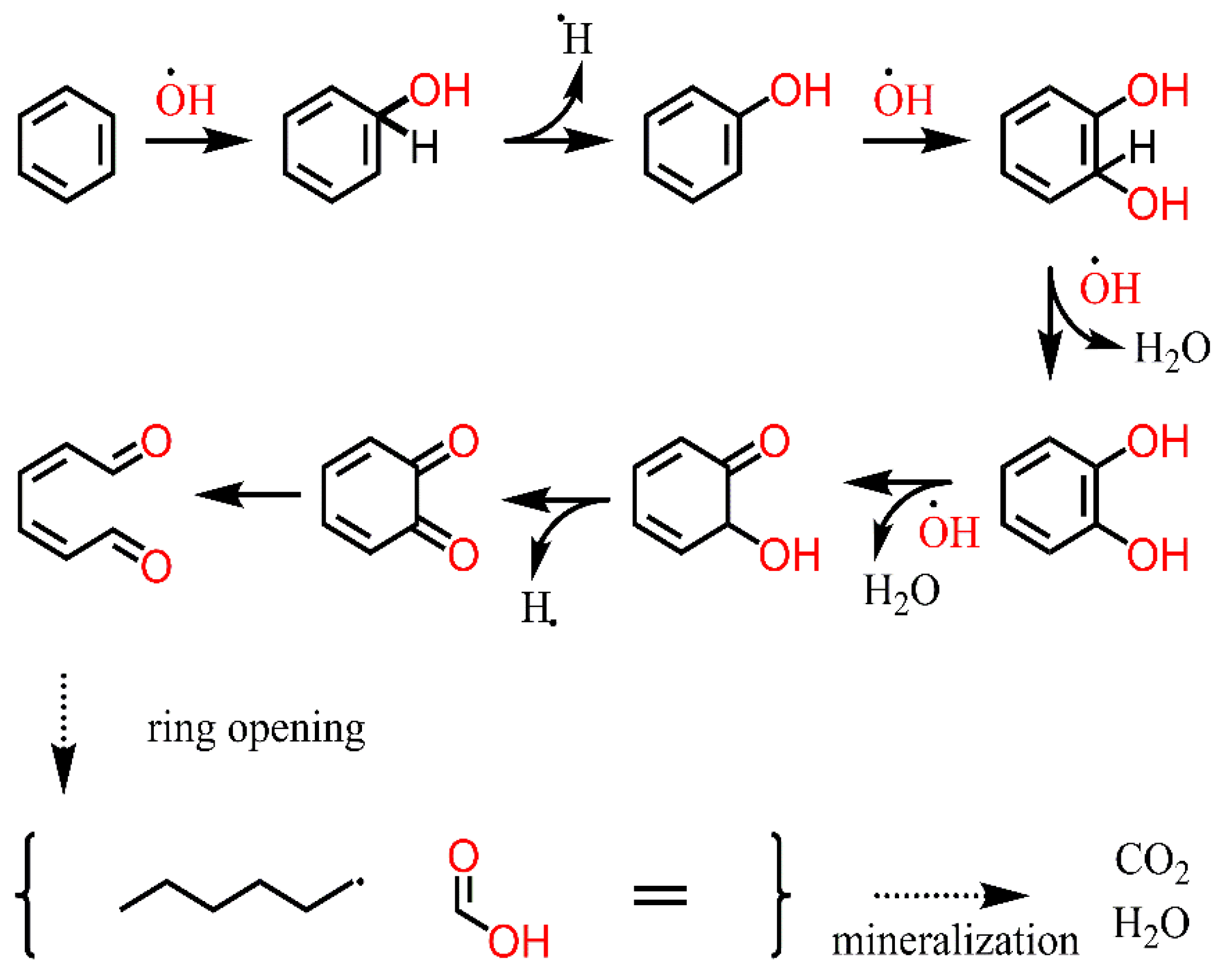
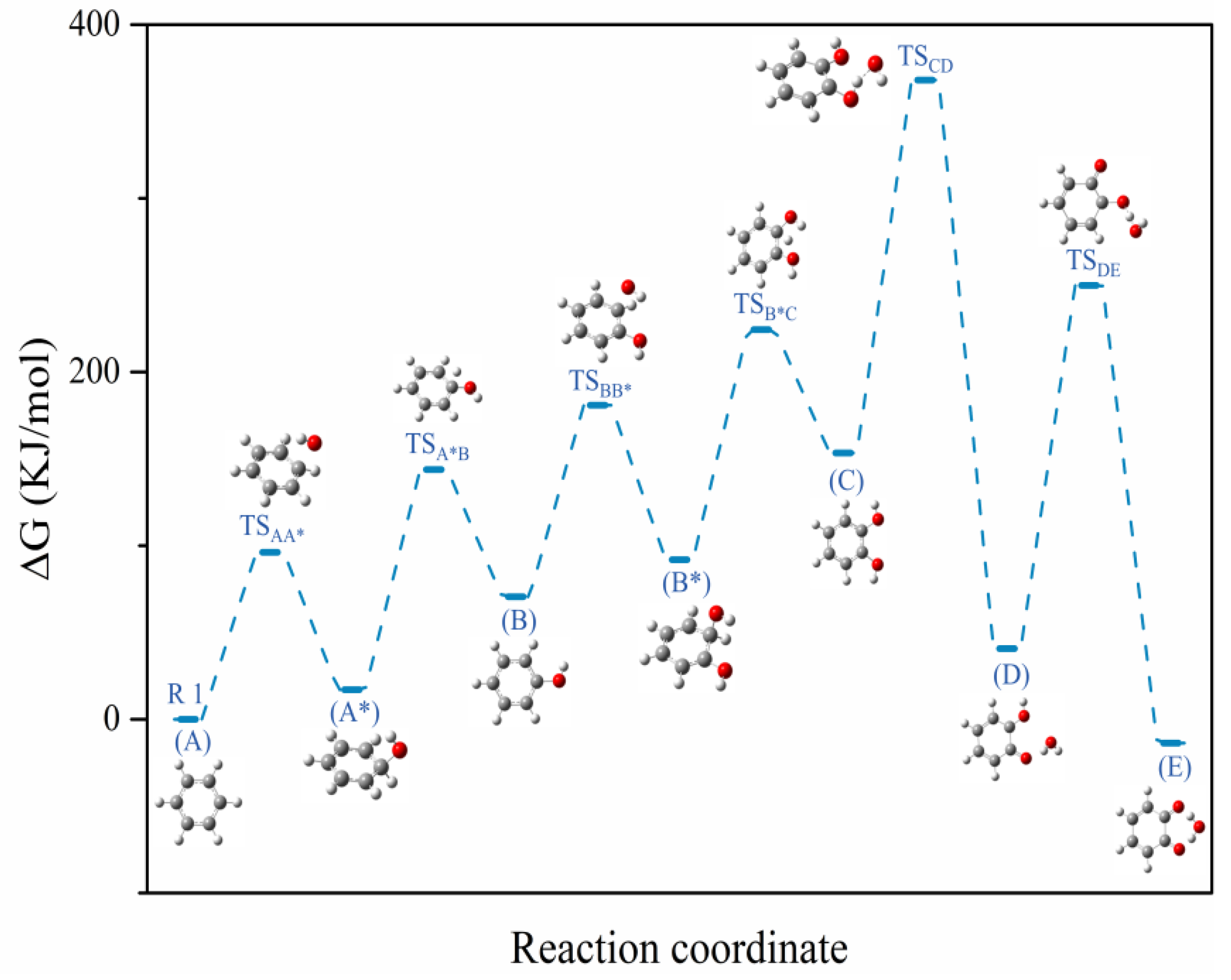
2.3. BTEX Removal Mechanism
3. Materials and Methods
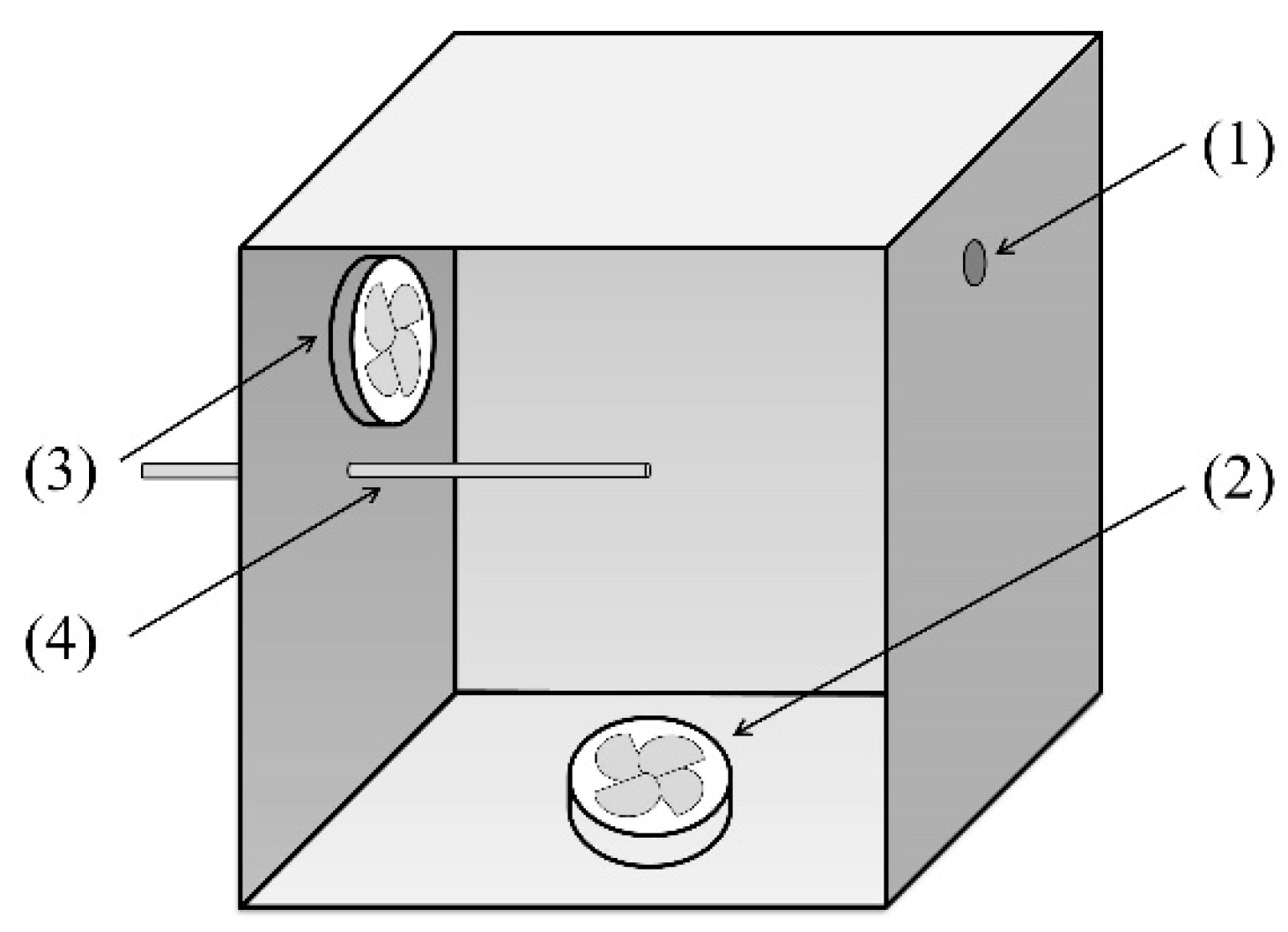
3.1. Experimental Materials and Apparatus
3.2. BTEX Removal Experiments
3.3. Analytical Methods
3.4. Calculation Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tian, C.; Wang, J.; Lu, J.; Shen, Z.; Huang, Y.; Sun, J.; Xu, H.; Wang, X.; Ren, D.; Cao, J. Evaluation of Indoor Air Pollution during Decorating Process and Inhalation Health Risks in Xi’an, China: A Case Study. Aerosol Air Qual. Res. 2018, 19, 854–864. [Google Scholar] [CrossRef]
- Fiebelkorn, S.; Meredith, C. Estimation of the Leukemia Risk in Human Populations Exposed to Benzene from Tobacco Smoke Using Epidemiological Data. Risk Anal. 2018, 38, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Zhang, L.; McHale, C.M.; Skibola, C.F.; Rappaport, S.M. Benzene, the exposome and future investigations of leukemia etiology. Chem. Biol. Interact. 2011, 192, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Tasbihi, M.; Călin, I.; Šuligoj, A.; Fanetti, M.; Lavrenčič Štangar, U. Photocatalytic degradation of gaseous toluene by using TiO2 nanoparticles immobilized on fiberglass cloth. J. Photochem. Photobiol. A Chem. 2017, 336, 89–97. [Google Scholar] [CrossRef]
- Basso, A.; Battisti, A.; Moreira, R.; José, H. Photocatalytic effect of addition of TiO2 to acrylic-based paint for passive toluene degradation. Environ. Technol. 2018, 41, 1568–1579. [Google Scholar] [CrossRef]
- Vlachokostas, C.; Michailidou, A.V.; Spyridi, D.; Moussiopoulos, N. Bridging the gap between traffic generated health stressors in urban areas: Predicting xylene levels in EU cities. Environ. Pollut. 2013, 180, 251–258. [Google Scholar] [CrossRef]
- Liu, F.F.; Escher, B.I.; Were, S.; Duffy, L.; Ng, J.C. Mixture effects of benzene, toluene, ethylbenzene, and xylenes (BTEX) on lung carcinoma cells via a hanging drop air exposure system. Chem. Res. Toxicol. 2014, 27, 952–959. [Google Scholar] [CrossRef]
- Majumdar, D.; Mukherjee, A.K.; Mukhopadhaya, K.; Sen, S. Variability of BTEX in Residential Indoor Air of Kolkata Metropolitan City. Indoor Built Environ. 2012, 21, 374–380. [Google Scholar] [CrossRef]
- Hadei, M.; Hopke, P.K.; Rafiee, M.; Rastkari, N.; Yarahmadi, M.; Kermani, M.; Shahsavani, A. Indoor and outdoor concentrations of BTEX and formaldehyde in Tehran, Iran: Effects of building characteristics and health risk assessment. Environ. Sci. Pollut. Res. Int. 2018, 25, 27423–27437. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Yoshino, H.; Lu, B.N.; Jiang, A.X.; Li, F. Use of Biotechnology Coupled with Bake-Out Exhaust to Remove Indoor VOCs. Indoor Built Environ. 2012, 21, 741–748. [Google Scholar] [CrossRef]
- Yue, X.; Ma, N.L.; Sonne, C.; Guan, R.; Lam, S.S.; Van Le, Q.; Chen, X.; Yang, Y.; Gu, H.; Rinklebe, J.; et al. Mitigation of indoor air pollution: A review of recent advances in adsorption materials and catalytic oxidation. J. Hazard. Mater. 2021, 405, 124138. [Google Scholar] [CrossRef]
- Isinkaralar, K. A Study on the Gaseous Benzene Removal Based on Adsorption onto the Cost-Effective and Environmentally Friendly Adsorbent. Molecules 2023, 28, 3453. [Google Scholar] [CrossRef]
- Shim, I.-K.; Kim, J.; Lee, J.K.; Oh, J.-M.; Park, J.K. Surface-Modified Wrinkled Mesoporous Nanosilica as an Effective Adsorbent for Benzene, Toluene, Ethylbenzene, and Xylene in Indoor Air. ACS Appl. Nano Mater. 2022, 5, 18138–18148. [Google Scholar] [CrossRef]
- Sui, H.; An, P.; Li, X.G.; Cong, S.; He, L. Removal and recovery of o-xylene by silica gel using vacuum swing adsorption. Chem. Eng. J. 2017, 316, 232–242. [Google Scholar] [CrossRef]
- Yang, C.T.; Miao, G.; Pi, Y.H.; Xia, Q.B.; Wu, J.L.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, C.; Hojo, H.; Einaga, H. Insights into the effect of cobalt substitution into copper-manganese oxides on enhanced benzene oxidation activity. Appl. Catal. B Environ. 2023, 323, 122099. [Google Scholar] [CrossRef]
- Xiao, M.; Han, D.; Yang, X.; Tsona Tchinda, N.; Du, L.; Guo, Y.; Wei, Y.; Yu, X.; Ge, M. Ni-doping-induced oxygen vacancy in Pt-CeO2 catalyst for toluene oxidation: Enhanced catalytic activity, water-resistance, and SO2-tolerance. Appl. Catal. B Environ. 2023, 323, 122173. [Google Scholar] [CrossRef]
- He, L.; Yu, Y.; Zhang, C.; He, H. Complete catalytic oxidation of o-xylene over CeO2 nanocubes. J. Environ. Sci. 2011, 23, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Gao, R.; Dong, T.; Hou, Z.; Jing, L.; Duan, E.; Deng, J.; Dai, H. N-doped carbon-modified palladium catalysts with superior water resistant performance for the oxidative removal of toxic aromatics. J. Hazard. Mater. 2022, 437, 129358. [Google Scholar] [CrossRef]
- Giang, H.M.; Huyen Nga, N.T.; Rene, E.R.; Ha, H.N.; Varjani, S. Performance and neural modeling of a compost-based biofilter treating a gas-phase mixture of benzene and xylene. Environ. Res. 2023, 217, 114788. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, A.A.; Sorial, G.A.; Wendell, D. Simultaneous acidic air biofiltration of toluene and styrene mixture in the presence of rhamnolipids: Performance evaluation and neural model analysis. Biochem. Eng. J. 2022, 187, 108637. [Google Scholar] [CrossRef]
- Li, Y.; Feng, K.; Wu, C.; Mei, J.; Zhang, S.; Ye, J.; Chen, J.; Zhao, J.; Chen, J. Mass transfer and reaction simultaneously enhanced airlift microbial electrolytic cell system with high gaseous o-xylene removal capacity. Chemosphere 2022, 291, 132888. [Google Scholar] [CrossRef]
- Zou, W.; Gao, B.; Ok, Y.S.; Dong, L. Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review. Chemosphere 2019, 218, 845–859. [Google Scholar] [CrossRef]
- Chmielewski, A.G.; Sun, Y.; Bułka, S.; Zimek, Z. Review on gaseous chlorinated organic pollutants electron beam treatment. Radiat. Phys. Chem. 2007, 76, 1795–1801. [Google Scholar] [CrossRef]
- Son, Y.-S.; Kim, T.-H.; Choi, C.Y.; Park, J.-H.; Ahn, J.-W.; Dinh, T.-V. Treatment of toluene and its by-products using an electron beam/ultra-fine bubble hybrid system. Radiat. Phys. Chem. 2018, 144, 367–372. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, J.; Son, Y.S.; Chung, S.G.; Kim, J.C. Advanced oxidation of aromatic VOCs using a pilot system with electron beam-catalyst coupling. Radiat. Phys. Chem. 2012, 81, 561–565. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, X. Kinetic studies on using photocatalytic coatings for removal of indoor volatile organic compounds. Indoor Built Environ. 2019, 29, 689–700. [Google Scholar] [CrossRef]
- Lin, W.J.; Xie, X.F.; Wang, X.; Wang, Y.; Segets, D.; Sun, J. Efficient adsorption and sustainable degradation of gaseous acetaldehyde and o-xylene using rGO-TiO2 photocatalyst. Chem. Eng. J. 2018, 349, 708–718. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, H.; Wang, C.; Sun, Y.; Han, H.; Kang, J.; Dong, Y.; Wang, L. Surface Acidification of BiOI/TiO2 Composite Enhanced Efficient Photocatalytic Degradation of Benzene. Separations 2022, 9, 315. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Yang, Y.; Xue, T.; Wang, H.; Lei, B.; Sheng, J.; Dong, F.; Sun, Y. Amorphous SnO(2) decorated ZnSn(OH)(6) promotes interfacial hydroxyl polarization for deep photocatalytic toluene mineralization. J. Hazard. Mater. 2023, 444, 130436. [Google Scholar] [CrossRef]
- Wang, Z.; Mahmood, A.; Xie, X.; Wang, X.; Qiu, H.; Sun, J. Surface adsorption configurations of H3PO4 modified TiO2 and its influence on the photodegradation intermediates of gaseous o-xylene. Chem. Eng. J. 2020, 393, 124723. [Google Scholar] [CrossRef]
- Zhang, J.; Vikrant, K.; Kim, K.-H.; Dong, F.; Won Chung, M.; Weon, S. Unveiling the collective effects of moisture and oxygen on the photocatalytic degradation of m-Xylene using a titanium dioxide supported platinum catalyst. Chem. Eng. J. 2022, 439, 135747. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Shi, J.; Ji, Y. Recent Advances in the Heterogeneous Photocatalytic Hydroxylation of Benzene to Phenol. Molecules 2022, 27, 5457. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B.; Kim, C.N. Transient Characteristics of VOCs Removal by an Air Cleaner in Association with a Humidifier Combined with Different Ventilation Strategies in an Office. Indoor Built Environ. 2011, 21, 71–78. [Google Scholar] [CrossRef]
- Tian, F.X.; Xu, B.; Zhang, T.Y.; Gao, N.Y. Degradation of phenylurea herbicides by chlorine dioxide and formation of disinfection by-products during subsequent chlor(am)ination. Chem. Eng. J. 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Han, G.D.; Kwon, H.; Kim, B.H.; Kum, H.J.; Kwon, K.; Kim, W. Effect of gaseous chlorine dioxide treatment on the quality of rice and wheat grain. J. Stored Prod. Res. 2018, 76, 66–70. [Google Scholar] [CrossRef]
- Chen, T.L.; Chen, Y.H.; Zhao, Y.L.; Chiang, P.C. Application of Gaseous ClO2 on Disinfection and Air Pollution Control: A Mini Review. Aerosol Air Qual. Res. 2020, 20, 2289–2298. [Google Scholar] [CrossRef]
- Wu, M.S.; Xu, X.Y.; Xu, X.; Wang, X.T.; Zhao, X.T.; Pei, M.; Ma, X.H.; Wang, M.Z.; Wang, F.; Li, J.X. Comparison of Removal Efficiency of Formaldehyde by Chlorine Dioxide, Photocatalyst and Active Carbon. Appl. Mech. Mater. 2015, 723, 648–651. [Google Scholar] [CrossRef]
- Ganiev, I.M.; Timergazin, Q.K.; Kabalnova, N.N.; Shereshovets, V.V.; Tolstikov, G.A. Reactions of Chlorine Dioxide with Organic Compounds. Eurasian Chem. Technol. J. 2016, 7, 1–31. [Google Scholar] [CrossRef]
- Lv, J.; Wang, Y.; Li, N. Oxidation of Citalopram with Sodium Hypochlorite and Chlorine Dioxide: Influencing Factors and NDMA Formation Kinetics. Molecules 2019, 24, 3065. [Google Scholar] [CrossRef] [PubMed]
- Epping, R.; Koch, M. On-Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ma, S.; Yang, B.; Cui, R.; Wang, J. NO Removal from Flue Gas by Using Chlorine Dioxide Solution. Energy Fuels 2019, 33, 10004–10010. [Google Scholar] [CrossRef]
- Marcon, J.; Mortha, G.; Marlin, N.; Molton, F.; Duboc, C.; Burnet, A. New insights into the decomposition mechanism of chlorine dioxide at alkaline pH. Holzforschung 2017, 71, 599–610. [Google Scholar] [CrossRef]
- Hao, R.L.; Mao, X.Z.; Wang, Z.; Zhao, Y.; Wang, T.H.; Sun, Z.H.; Yuan, B.; Li, Y.K. A novel method of ultraviolet/NaClO2-NH4OH for NO removal: Mechanism and kinetics. J. Hazard. Mater. 2019, 368, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lehtimaa, T.; Kuitunen, S.; Tarvo, V.; Vuorinen, T. Reactions of aldehydes with chlorous acid and chlorite in chlorine dioxide bleaching. Holzforschung 2010, 64, 555–561. [Google Scholar] [CrossRef]
- Fan, M.; Yang, X.; Kong, Q.; Lei, Y.; Zhang, X.; Aghdam, E.; Yin, R.; Shang, C. Sequential ClO2-UV/chlorine process for micropollutant removal and disinfection byproduct control. Sci. Total Environ. 2022, 806, 150354. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Z.; Wei, X.; Huang, X.; Bai, S.; Chen, J. Surface enrichment promotes the decomposition of benzene from air. Catal. Sci. Technol. 2022, 12, 2340–2345. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Niu, S.; Zhang, F. New theoretical insights into the reaction kinetics of toluene and hydroxyl radicals. Phys. Chem. Chem. Phys. 2020, 22, 22279–22288. [Google Scholar] [CrossRef]
- Nicovich, J.M.; Thompson, R.L.; Ravishankara, A.R. Kinetics of the reactions of the hydroxyl radical with xylenes. J. Phys. Chem. 1981, 85, 2913–2916. [Google Scholar] [CrossRef]
- Gery, M.W.; Fox, D.L.; Kamens, R.M.; Stockburger, L. Investigation of hydroxyl radical reactions with o-xylene and m-xylene in a continuous stirred tank reactor. Environ. Sci. Technol. 1987, 21, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Noda, J.; Volkamer, R.; Molina, M.J. Dealkylation of alkylbenzenes: A significant pathway in the toluene, o-, m-, p-xylene + OH reaction. J. Phys. Chem. A 2009, 113, 9658–9666. [Google Scholar] [CrossRef] [PubMed]
- D’Hennezel, O.; Pichat, P.; Ollis, D.F. Benzene and toluene gas-phase photocatalytic degradation over H2O and HCL pretreated TiO2: By-products and mechanisms. J. Photochem. Photobiol. A Chem. 1998, 118, 197–204. [Google Scholar] [CrossRef]
- Raupp, G.B.; Junio, C.T. Photocatalytic oxidation of oxygenated air toxics. Appl. Surf. Sci. 1993, 72, 321–327. [Google Scholar] [CrossRef]
- Jacoby, W.A.; Blake, D.M.; Penned, J.A.; Boulter, J.E.; Vargo, L.M.; George, M.C.; Dolberg, S.K. Heterogeneous Photocatalysis for Control of Volatile Organic Compounds in Indoor Air. J. Air Waste Manag. Assoc. 1996, 46, 891–898. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Li, T.; Li, G. Solvothermal synthesis of mesoporous TiO2: The effect of morphology, size and calcination progress on photocatalytic activity in the degradation of gaseous benzene. Chem. Eng. J. 2014, 237, 312–321. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, J.; Tao, L.; Gong, M.; Zhimin, L.; Chen, Y. Photocatalytic degradation of gaseous benzene over TiO2/Sr2CeO4: Kinetic model and degradation mechanisms. J. Hazard. Mater. 2007, 139, 323–331. [Google Scholar] [CrossRef]
- Yang, J.; Yang, L.; Fang, M.; Li, L.; Fu, F.; Xu, H.; Li, M.; Fan, X. A compact Z-scheme heterojunction of BiOCl/Bi(2)WO(6) for efficiently photocatalytic degradation of gaseous toluene. J. Colloid Interface Sci. 2023, 631, 44–54. [Google Scholar] [CrossRef]
- Dhada, I.; Sharma, M.; Nagar, P.K. Quantification and human health risk assessment of by-products of photo catalytic oxidation of ethylbenzene, xylene and toluene in indoor air of analytical laboratories. J. Hazard. Mater. 2016, 316, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Guo, X.; Zhang, R.M.; Zhang, H.; Zhang, X.; Xu, X. Pressure-dependent kinetics of the o-xylene reaction with OH radicals. Phys. Chem. Chem. Phys. 2022, 24, 8672–8682. [Google Scholar] [CrossRef]
- Gail, S.; Dagaut, P. Oxidation of m-xylene in a JSR: Experimental study and detailed chemical kinetic modeling. Combust. Sci. Technol. 2007, 179, 813–844. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wang, X.; Rao, Z.; Sun, J.; Chen, A.; Xie, X. Rare-earth single atoms decorated 2D-TiO(2) nanosheets for the photodegradation of gaseous O-xylene. J. Colloid Interface Sci. 2022, 605, 674–684. [Google Scholar] [CrossRef]
- Rao, Z.; Lu, G.; Chen, L.; Mahmood, A.; Shi, G.; Tang, Z.; Xie, X.; Sun, J. Photocatalytic oxidation mechanism of Gas-Phase VOCs: Unveiling the role of holes, ·OH and·O2−. Chem. Eng. J. 2022, 430, 132766. [Google Scholar] [CrossRef]
- Rao, Z.P.; Shi, G.S.; Wang, Z.; Mahmood, A.; Xie, X.F.; Sun, J. Photocatalytic degradation of gaseous VOCs over Tm3+-TiO2: Revealing the activity enhancement mechanism and different reaction paths. Chem. Eng. J. 2020, 395, 125078. [Google Scholar] [CrossRef]
- Pan, S.-S.; Wang, L.-M. The Atmospheric Oxidation Mechanism of o-Xylene Initiated by Hydroxyl Radicals. Acta Phys. Chim. Sin. 2015, 31, 2259–2268. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, P.; Li, L.; Wang, K.; Yang, H.; Jin, J.; Chen, Y.; Zeng, Q.; Du, J.; Liu, Y.; et al. VUV photoionization and dissociative photoionization of o-xylene: Experimental and theoretical insights. Eur. Phys. J. D 2021, 75, 128. [Google Scholar] [CrossRef]
- Morales-Roque, J.; Carrillo-Cárdenas, M.; Jayanthi, N.; Cruz, J.; Pandiyan, T. Theoretical and experimental interpretations of phenol oxidation by the hydroxyl radical. J. Mol. Struct. THEOCHEM 2009, 910, 74–79. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Ben Amor, H.; Laat, J.D.; Dore, M. Mode d’action du bioxyde de chlore sur les composes organiques en milieu aqueux: Consommations de bioxyde de chlore et reactions sur les composes phenoliques. Water Res. 1984, 18, 1545–1560. [Google Scholar] [CrossRef]
- Tian, L.; Li, H.; Zhu, R.; Li, T.; Sun, S.; Hong, L.; Zhu, Z.; Hu, G.; Zhou, X.; Te, J.; et al. Hygienic Requirements for Chlorine Dioxide Disinfectant GBT26366-2021; State Administration for Market Regulation: Beijing, China, 2021. [Google Scholar]
- Dalian, E.M.C. Ambient Air—Determination of Benzene and Its Analogies by Activated Charcoal Adsorption Carbon Disulfide Desorption and Gas Chromatography HJ 584—2010; China Environmental Science Press: Beijing, China, 2010. [Google Scholar]
- Czaplicka, M.; Klejnowski, K. Determination of volatile organic compounds in ambient air. Comparison of methods. J. Chromatogr. A 2002, 976, 369–376. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09W, Revision A. 02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction Path Following in Mass-Weighted Internal Coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Shermo: A general code for calculating molecular thermochemistry properties. Comput. Theor. Chem. 2021, 1200, 113249. [Google Scholar] [CrossRef]



| Main Product | Retention Time/min | m/z | Structure Formula |
|---|---|---|---|
| I | 2.07 | 52, 78, 117 | 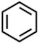 |
| II | 7.73 | 43, 57, 71 |  |
| III | 9.12 | 43, 57, 71 |  |
| IV | 11.64 | 43, 74, 87 | 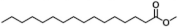 |
| V | 12.22 | 149, 150, 223 | 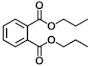 |
| VI | 13.68 | 43, 57, 83 | 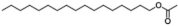 |
| VII | 3.60 | 39, 65, 91 | 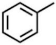 |
| VIII | 7.75 | 43, 57, 71 |  |
| IX | 9.13 | 43, 57, 71 |  |
| X | 12.19 | 41, 149, 150 | 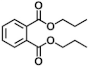 |
| XI | 13.67 | 43, 69, 83 | 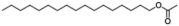 |
| XII | 5.79 | 77, 91, 106 | 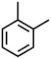 |
| XIII | 5.30 | 32, 91, 106 |  |
| Reaction | Species | ∆Hr (kJ/mol) | ∆Gr (kJ/mol) | ∆Sr(J/mol·K) |
|---|---|---|---|---|
| Reaction 1 | A-·OH | 0.00 | 0.00 | 0 |
| TSAA* | 62.11 | 96.24 | −114.50 | |
| A* | −18.62 | 16.20 | −119.44 | |
| TSA*B | 105.42 | 143.83 | −128.81 | |
| B-·H | 63.80 | 70.53 | −22.55 | |
| Reaction 2 | B-·OH | 0.00 | 0.00 | 0 |
| TSBB* | 68.90 | 110.32 | −138.91 | |
| B* | −21.38 | 21.34 | −143.23 | |
| TSB*C | 109.51 | 153.92 | −148.97 | |
| C-·H | 71.63 | 82.83 | −37.56 | |
| Reaction 3 | C-·OH | 0.00 | 0.00 | 0 |
| TSCD | 168.11 | 214.75 | −156.42 | |
| D-H2O | −111.98 | −112.63 | 2.18 | |
| Reaction 4 | D-·OH | 0.00 | 0.00 | 0 |
| TSDE | 162.80 | 209.07 | −155.18 | |
| E-H2O | −52.32 | −54.43 | 7.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Qiao, Y.; Zhang, Y.; Jin, R.; Liu, J.; He, Z.; Jia, M.; Gao, J.; Guo, C. Performance and Mechanism of Chlorine Dioxide on BTEX Removal in Liquid and Indoor Air. Molecules 2023, 28, 4342. https://doi.org/10.3390/molecules28114342
Wang A, Qiao Y, Zhang Y, Jin R, Liu J, He Z, Jia M, Gao J, Guo C. Performance and Mechanism of Chlorine Dioxide on BTEX Removal in Liquid and Indoor Air. Molecules. 2023; 28(11):4342. https://doi.org/10.3390/molecules28114342
Chicago/Turabian StyleWang, Anlong, Yina Qiao, Yufan Zhang, Riya Jin, Jiaoqin Liu, Zengdi He, Mengye Jia, Jingshuai Gao, and Chengjie Guo. 2023. "Performance and Mechanism of Chlorine Dioxide on BTEX Removal in Liquid and Indoor Air" Molecules 28, no. 11: 4342. https://doi.org/10.3390/molecules28114342
APA StyleWang, A., Qiao, Y., Zhang, Y., Jin, R., Liu, J., He, Z., Jia, M., Gao, J., & Guo, C. (2023). Performance and Mechanism of Chlorine Dioxide on BTEX Removal in Liquid and Indoor Air. Molecules, 28(11), 4342. https://doi.org/10.3390/molecules28114342