HPLC Analysis and Molecular Docking Study of Myoporum serratum Seeds Extract with Its Bioactivity against Pathogenic Microorganisms and Cancer Cell Lines
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Constituents
2.2. Antimicrobial Activity of M. serratum Seeds Extract
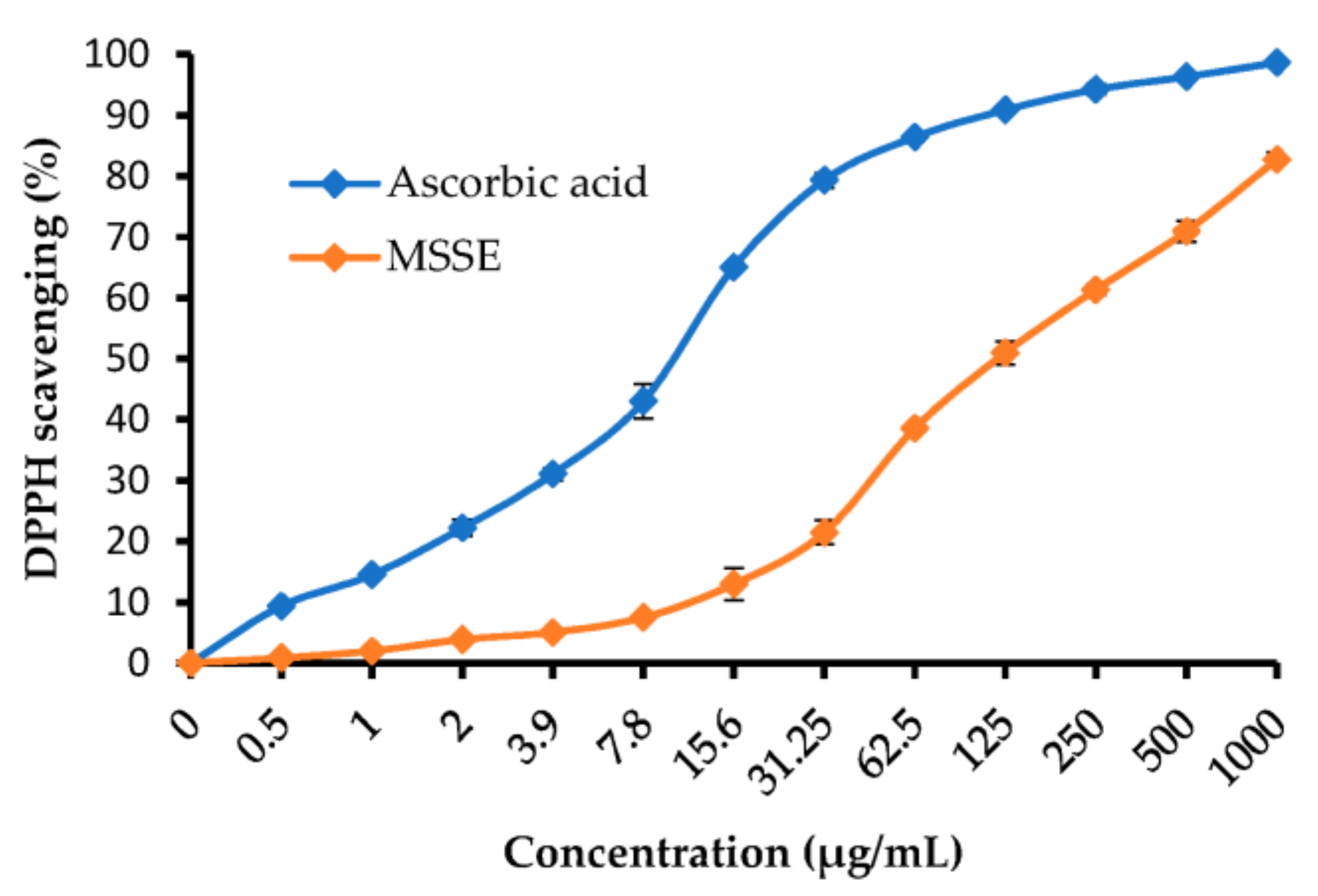
2.3. Antioxidant Activity of M. serratum Seeds Extract
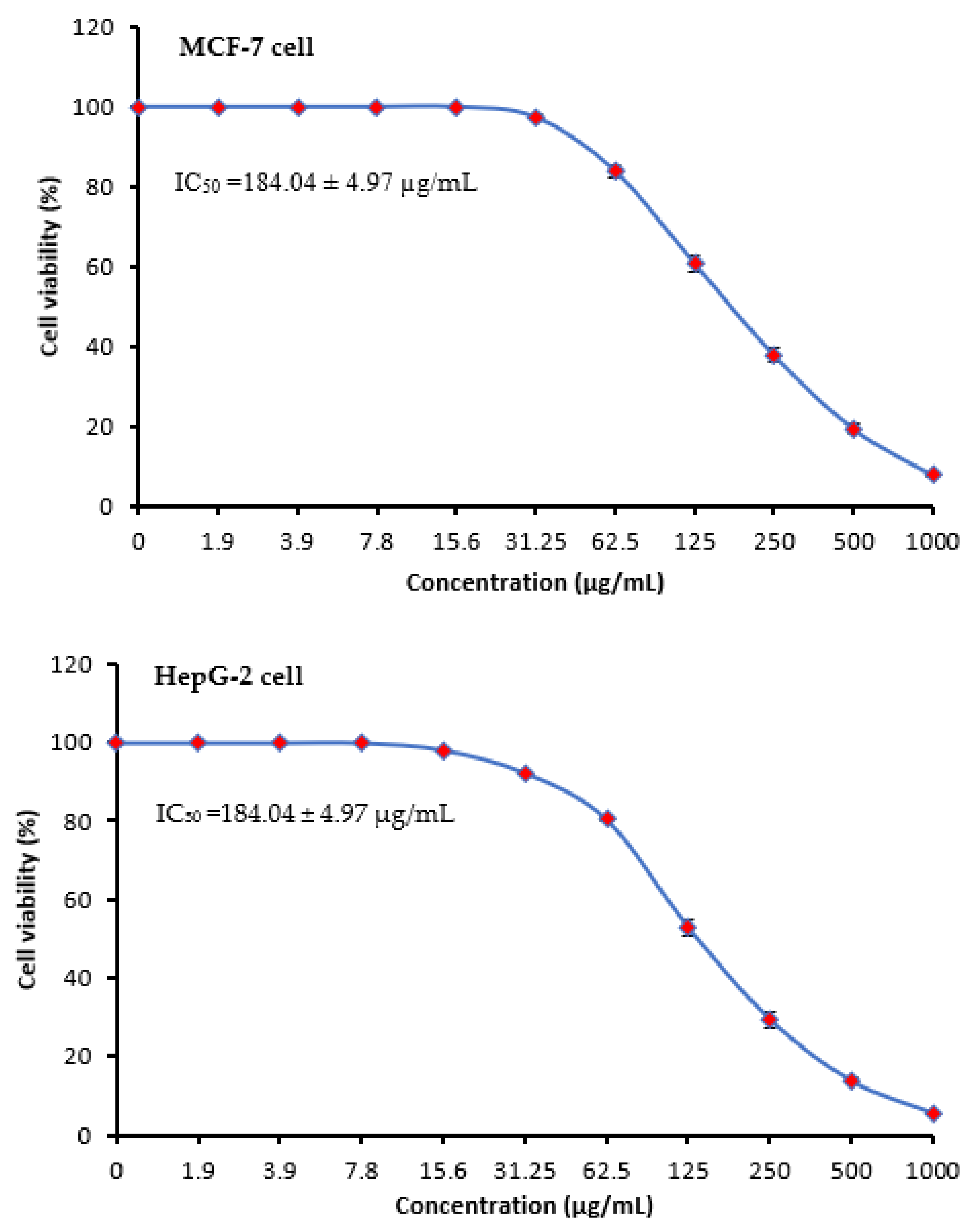
2.4. Anticancer Activity of M. serratum Seeds Extract
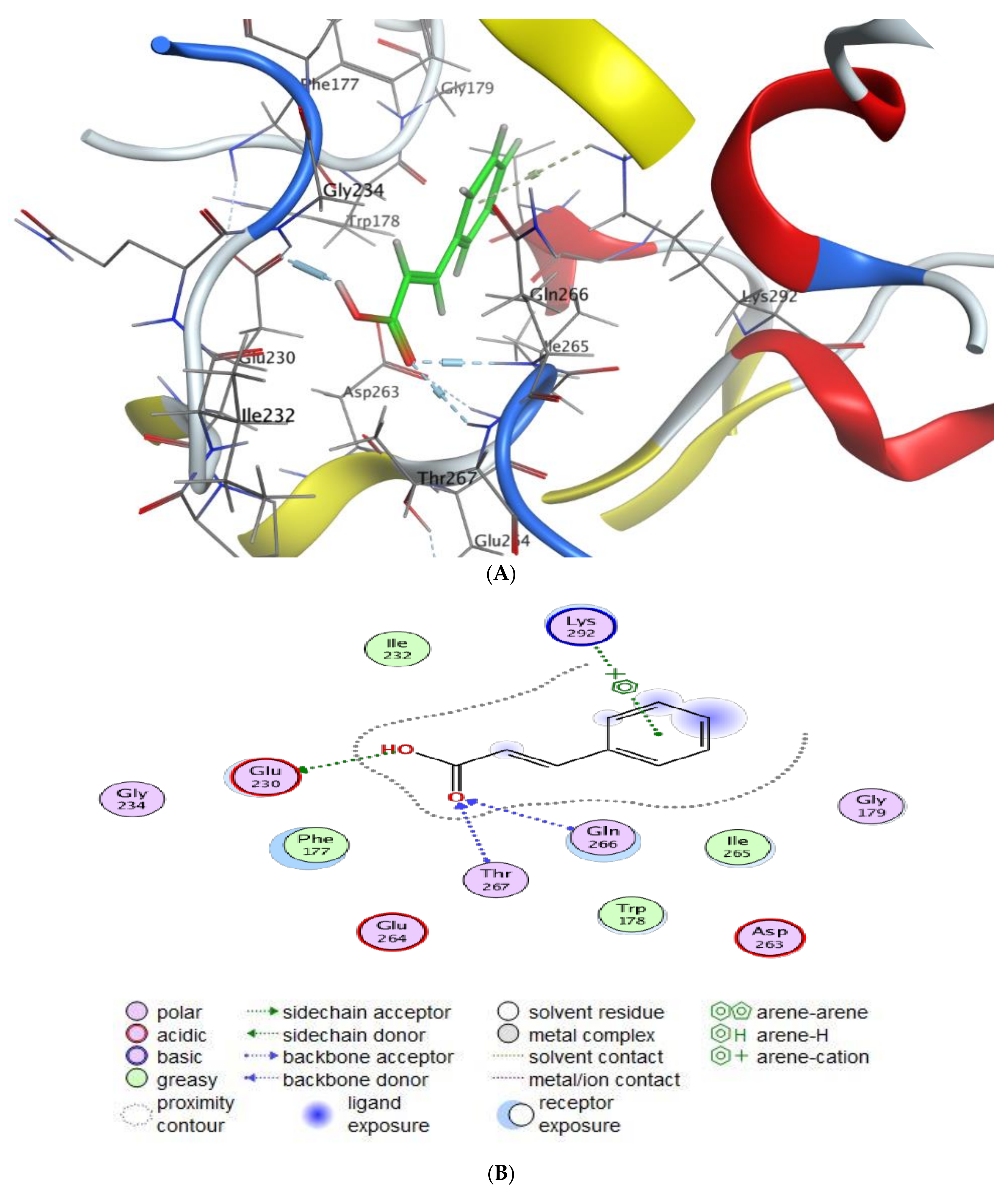
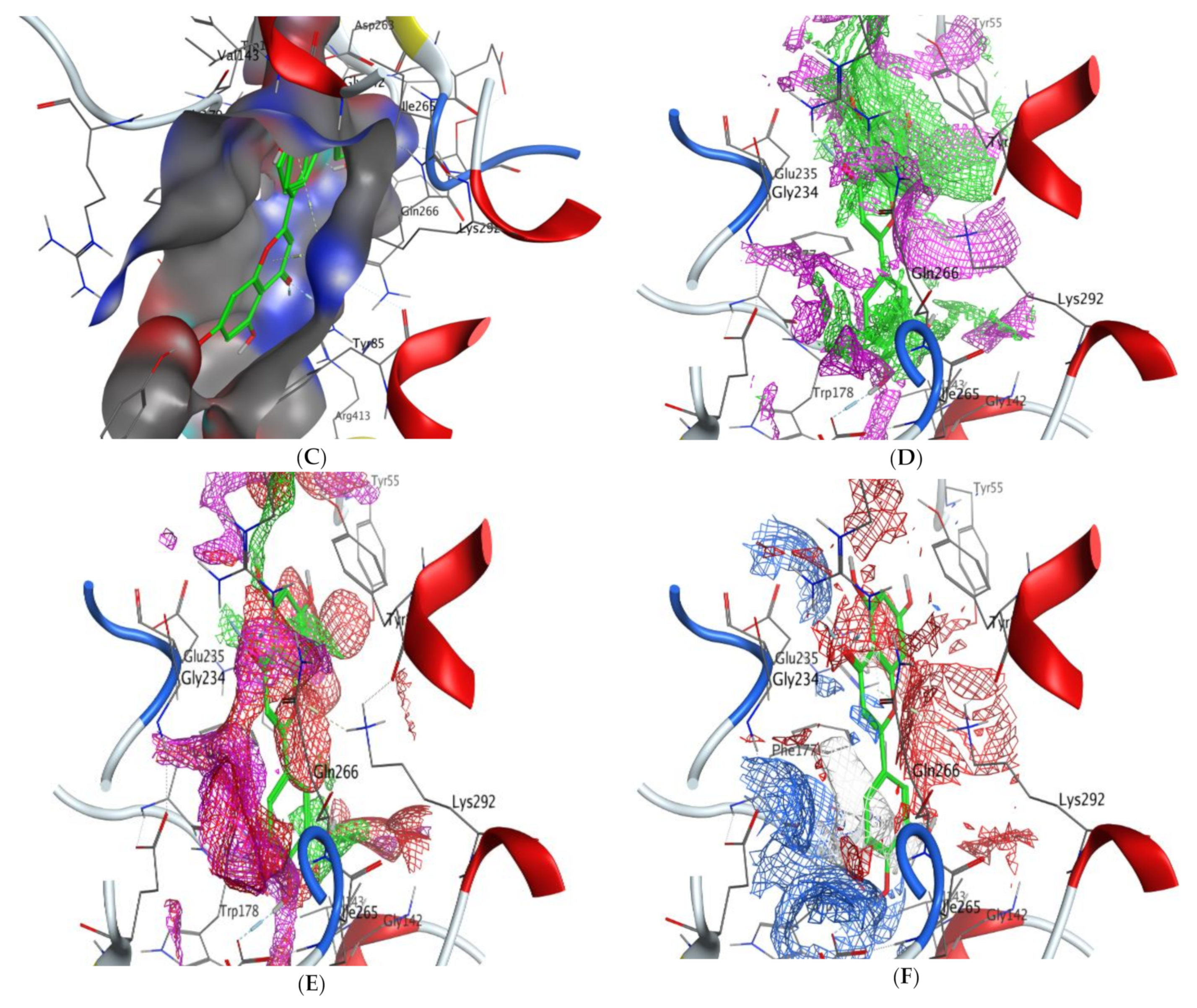
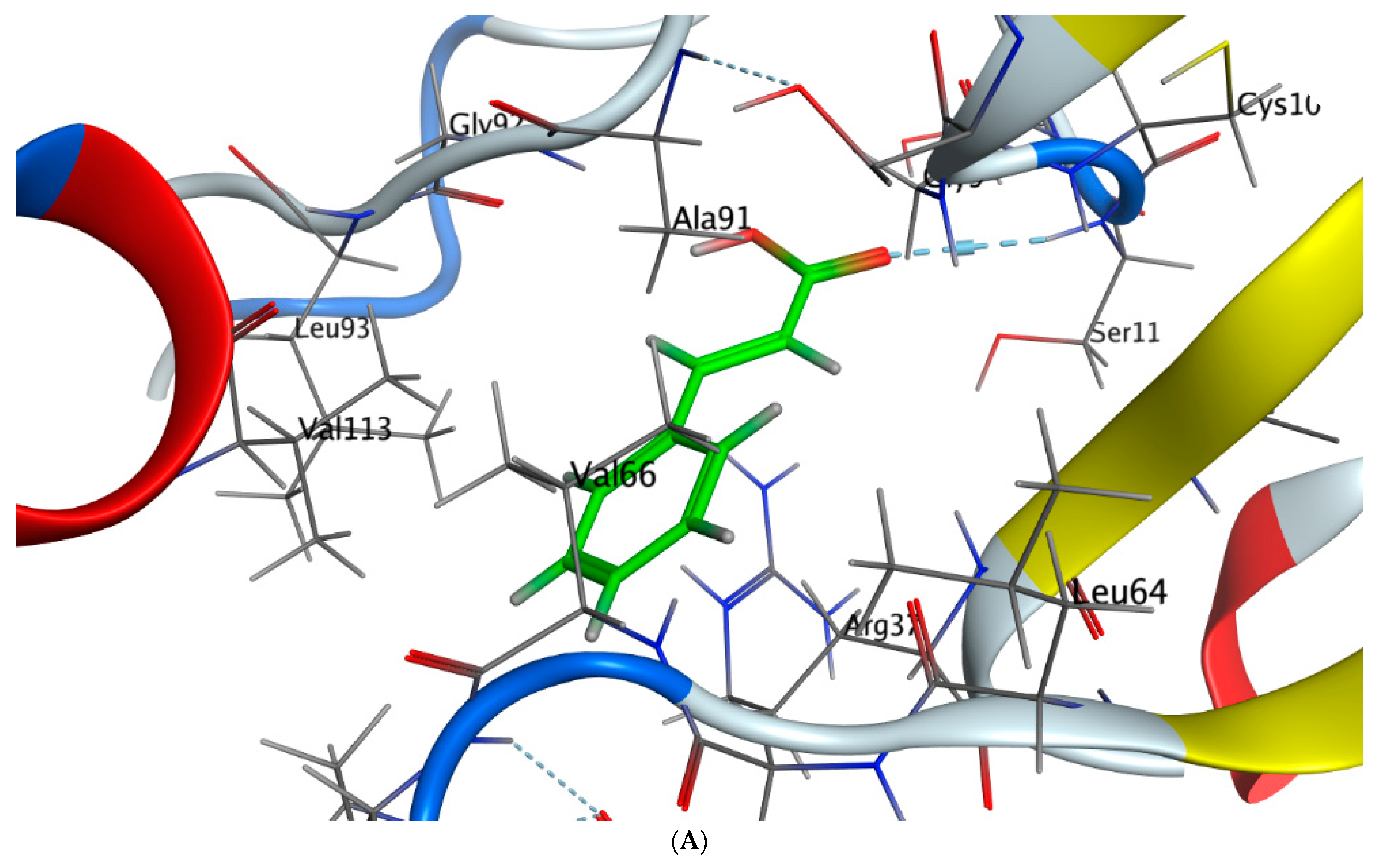
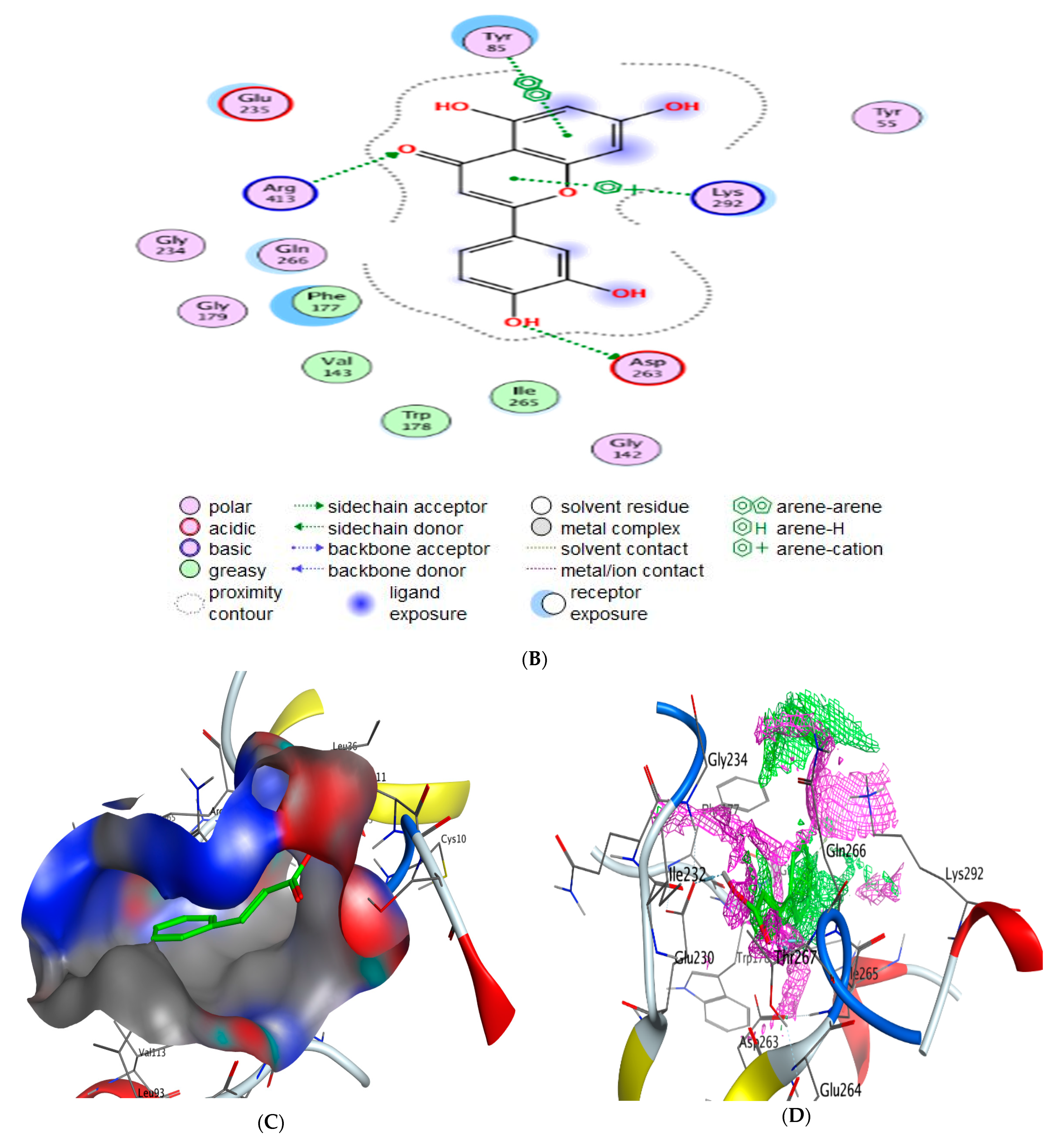
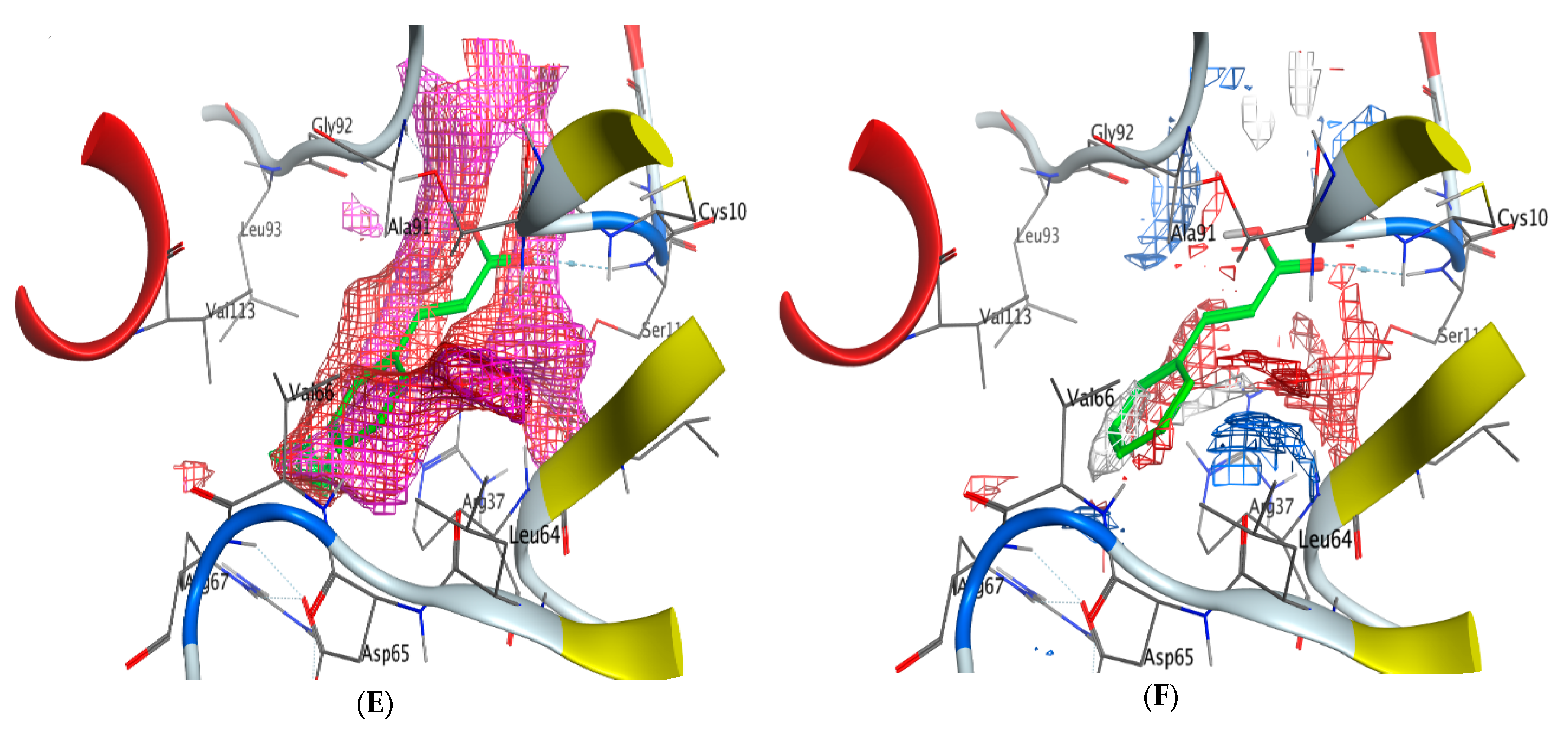
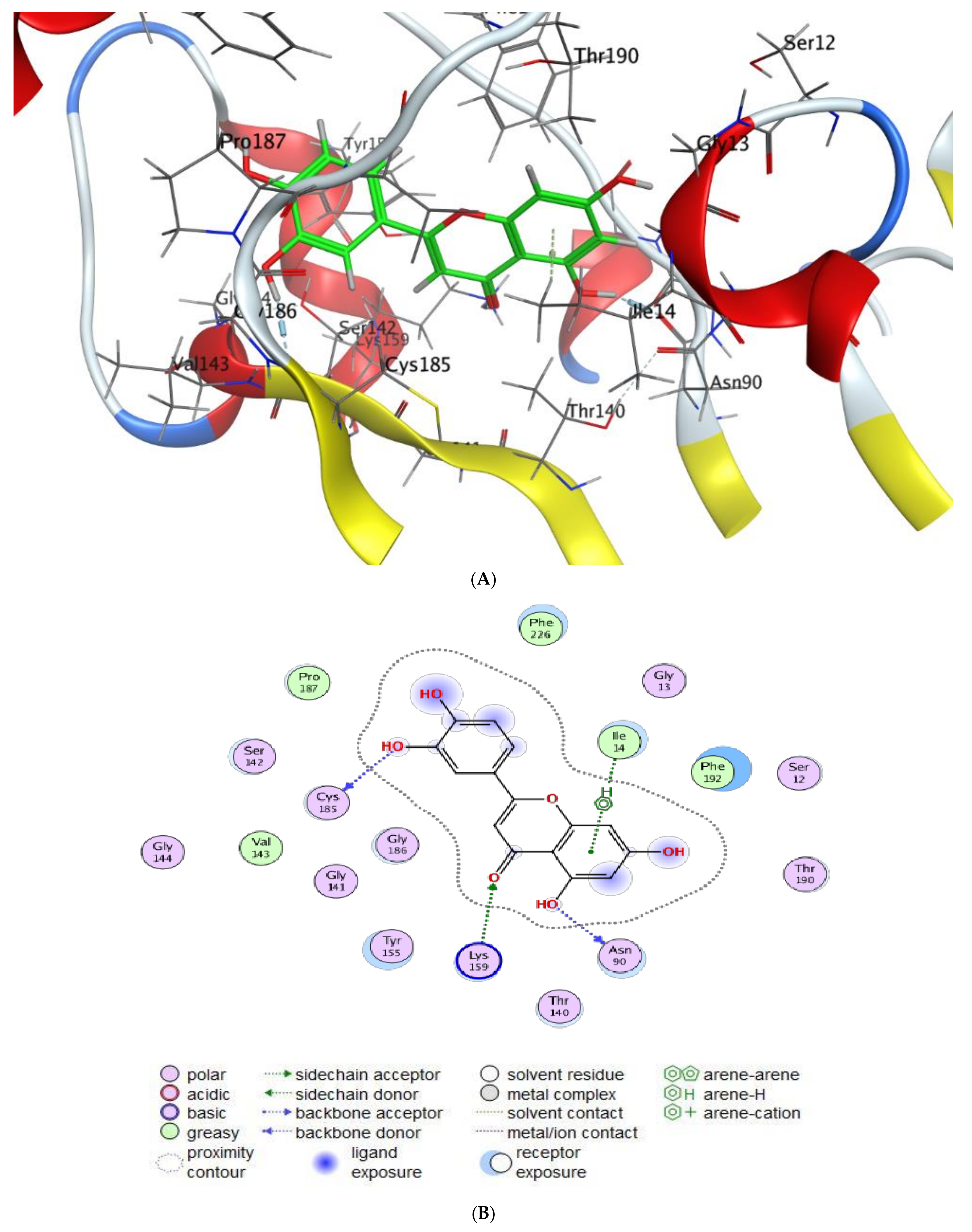
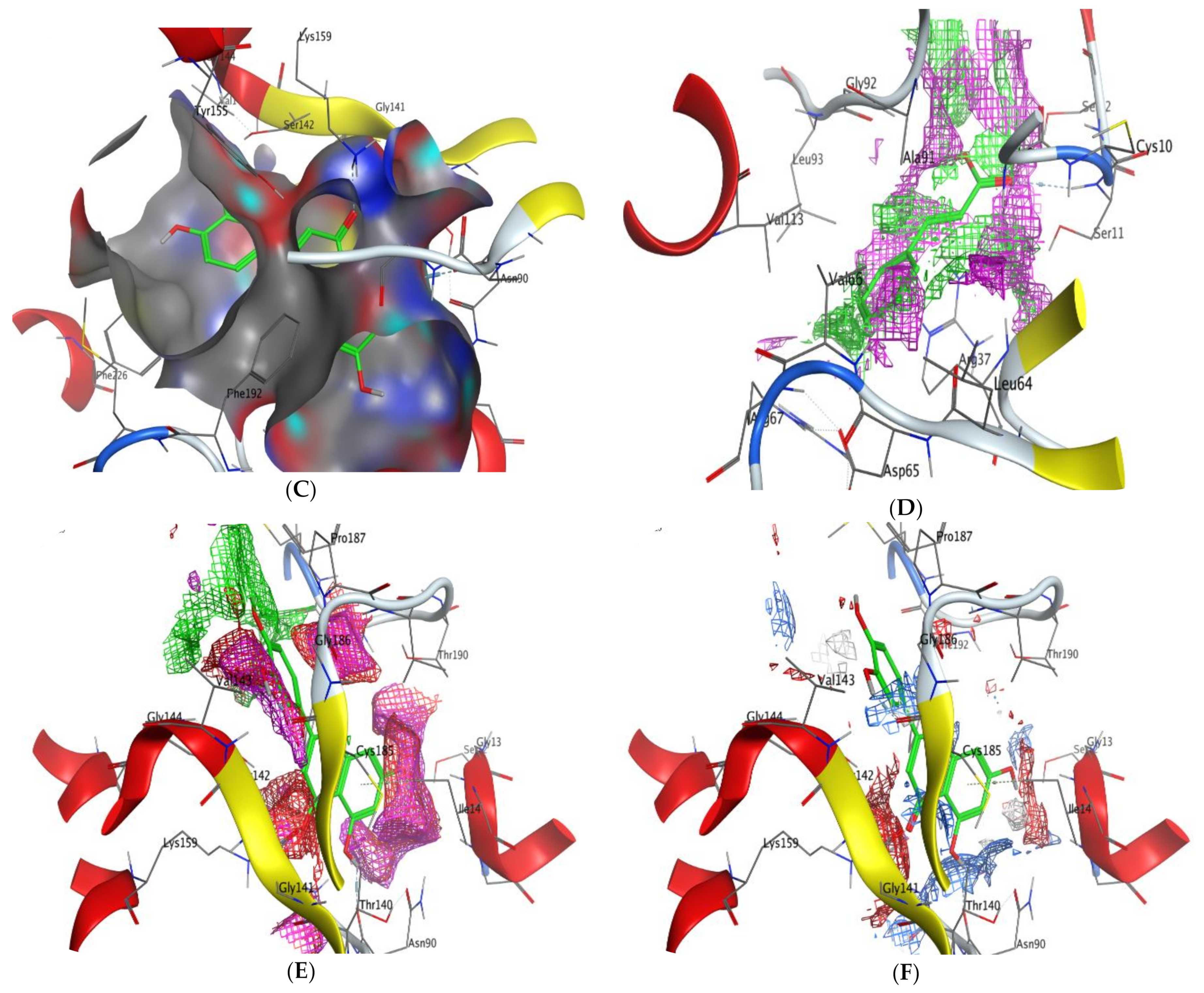
2.5. Molecular Docking
- Scoring energy values that were effective and validated came from −6.48903 to −6.13434 kcal/mol−1 range with Hepatocellular Carcinoma (7JX9) active site and from −6.11443 to −5.9643 kcal/mol−1 range with breast cancer cells (3HB5) residue.
- Numerous ligation sites for Luteolin interaction with (7JX9) are noted as O 28, O 27 6-ring through ASP 263, ARG 413, LYS 292, and TYR 85 amino acids receptors.
- ASN 90, CYS 185, LYS 159, and ILE 14 were the contributing amino acids in the interaction of Luteolin with breast cancer cells (3HB5).
- Cinnamic acid forms four hydrogen bonds with the Crystal Structure of Hepatocellular Carcinoma (7JX9) via GLU 230, GLN 266, THR 267, and LYS 292 to one acceptor hydrogen bond with breast cancer cells (3HB5) via the residue SER 11.
- The tested compounds showed favorable RMSD values and interaction results.
3. Materials and Methods
3.1. Reagents Used
3.2. Source of Cancer Cells
3.3. Tested Microorganisms
3.4. Plant Material Collection and Extraction
3.5. HPLC Analysis of the Extract Myoporum Serratum Seeds Extract (MSSE)
3.6. Antimicrobial Activities of MSSE Using Agar Well Diffusion Method
3.7. Broth Micro Dilution Method for MIC and MBC Detection
3.8. Microtitre Plate Assay for Biofilm Quantification
3.9. Antioxidant via DPPH Radical Scavenging Activity
3.10. Evaluation of Cytotoxic Effects of MSSE
3.11. Molecular Modeling Study
3.12. Statically Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dinda, B. Pharmacology and Applications of Naturally Occurring Iridoids; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Menut, C.; Cabalion, P.; Hnawia, E.; Agnaniet, H.; Waikedre, J.; Fruchier, A. Two new furanosesquiterpenes from Myoporum crassifolium from New Caledonia. Flav. Fragr. J. 2005, 20, 621–625. [Google Scholar] [CrossRef]
- Li, C.; Gong, B.; Cox, D.G.; Li, C.; Wang, J.; Ding, W. Dichlorodiaportinol A–A new chlorinecontaining isocoumarin from an endophytic fungus Trichoderma sp. 09 from Myoporum bontioides A. Gray and its cytotoxic activity. Pharmacog. Mag. 2014, 10, S153. [Google Scholar] [CrossRef] [PubMed]
- Yecheng, D.; Zhen, Y.; Yanzhen, Y.; Xiulian, B. Inhibitory activity against plant pathogenic fungi of extracts from Myoporum bontioides A. Gray and indentification of active ingredients. Pest Managem. Sci. 2008, 64, 203–207. [Google Scholar]
- Hu, L.; Guo, Y.; Gu, W. Flavonoids from the leaves of Myoporum bontioides. J. Trop. Subtrop. Bot. 2013, 21, 266–272. [Google Scholar]
- Dong, L.M.; Huang, L.L.; Dai, H.; Xu, Q.L.; Ouyang, J.K.; Jia, X.C.; Gu, W.X.; Tan, J.W. Anti MRSA sesquiterpenes from the semi-mangrove plant Myoporum bontioides A. Gray. Mar. Drugs 2018, 16, 438. [Google Scholar] [CrossRef]
- Zaleta-Pinet, D.; McCluskey, A.; Hall, S.; Brophy, J.; Ashhurst-Smith, C.; Sakoff, J.; Altena, I.V. The use of the toxic plant Myoporum montanum in a traditional Australian aboriginal medicine. Aust. J. Chem. 2016, 69, 161–168. [Google Scholar] [CrossRef]
- Weng, J.R.; Bai, L.Y.; Lin, W.Y.; Chiu, C.F.; Chen, Y.C.; Chao, S.W.; Chao, S.W.; Feng, C.H. A flavone constituent from Myoporum bontioides induces M-phase cell cycle arrest of MCF-7 breast cancer cells. Molecules 2017, 22, 472. [Google Scholar] [CrossRef]
- Arafa, M. Phytochemistry, Pharmacological Potency, and Potential Toxicity of Myoporum spp. Rec. Nat. Prod. 2021, 15, 168. [Google Scholar] [CrossRef]
- Sulaiman, M.; Nissapatorn, V.; Rahmatullah, M.; Paul, A.K.; Rajagopal, M.; Rusdi, N.A.; Seelan, J.S.S.; Suleiman, M.; Zakaria, Z.A.; Wiart, C. Antimicrobial Secondary Metabolites from the Mangrove Plants of Asia and the Pacific. Marine Drugs. 2022, 20, 643. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Sorensen, A.; Setzer, W.N. Volatile Constituents and Antimicrobial Activity of Naio (Myoporum Sandwicense, A. Gray), a Native Hawaiian Tree. Compounds 2023, 3, 142–152. [Google Scholar] [CrossRef]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, T.; Hakamy, O.M. Juniperus procera as food safe additive, their antioxidant, anticancer and antimicrobial activity against some food-borne bacteria. J. Biol. Chem. Res. 2014, 31, 668–677. [Google Scholar]
- Abdelghany, T.M.; Ganash, M.; Alawlaqi, M.M.; Al-Rajhi, A.M. Antioxidant, Antitumor, Antimicrobial Activities Evaluation of Musa paradisiaca L. Pseudostem Exudate Cultivated in Saudi Arabia. BioNanoScience 2019, 9, 172–178. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Mashraqi, A.; Al Abboud, M.A.; Shater, A.-R.M.; Al Jaouni, S.K.; Selim, S.; Abdelghany, T.M. Screening of bioactive compounds from endophytic marine-derived fungi in Saudi Arabia: Antimicrobial and anticancer potential. Life 2022, 12, 1182. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Hassan, M.M.; El-Naggar, M.A.; Abd El-Mongy, M. GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol. Rep. 2020, 27, e00496. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Abdel Ghany, T.M. Nanoemulsions of some edible oils and their antimicrobial, antioxidant, and anti-hemolytic activities. BioResources 2023, 18, 1465–1481. [Google Scholar] [CrossRef]
- Yahya, R.; Al-Rajhi, A.M.H.; Alzaid, S.Z.; Al Abboud, M.A.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Ismail, K.S.; Abdelghany, T.M. Molecular Docking and Efficacy of Aloe vera Gel Based on Chitosan Nanoparticles against Helicobacter pylori and Its Antioxidant and Anti-Inflammatory Activities. Polymers 2022, 14, 2994. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Tm, A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Abdelghany, T.M. Safe food additives: A review. J. Biol. Chem. Res. 2015, 32, 402–437. [Google Scholar]
- Lopes, C.M.; Dourado, A.; Oliveira, R. Phytotherapy and nutritional supplements on breast cancer. BioMed Res. Int. 2017, 7207983. [Google Scholar] [CrossRef] [PubMed]
- Niero, E.L.d.O.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 31. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Shi, Y.; Wang, J.; Shen, X.; Zhang, J. Anticancer agents derived from natural cinnamic acids. Anticancer Agents Med. Chem. 2015, 15, 980–987. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Manju, V.; Nalini, N. Protective role of luteolin in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Cell Biochem. Funct. 2007, 25, 189–194. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anticancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, M. Antibacterial activity and mechanism of luteolin on Staphylococcus aureus. Wei Sheng Wu Xue Bao 2010, 50, 1180–1184. [Google Scholar]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, F.; Feng, W.; Wang, Q.; Liu, F.; Xia, P.; Qiu, X. Luteolin Inhibits the Biofilm Formation and Cytotoxicity of Methicillin-Resistant Staphylococcus aureus via Decreasing Bacterial Toxin Synthesis. Evid. Based Complement. Altern. Med. 2022, 2022, 4476339. [Google Scholar] [CrossRef] [PubMed]
- Zardi-Bergaoui, A.; Jelizi, S.; Flamini, G.; Ascrizzi, R.; Jannet, H.B. Comparative study of the chemical composition and bioactivities of essential oils of fresh and dry seeds from Myoporum insulare R. Br. Ind. Crops Prod. 2018, 111, 232–237. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107. [Google Scholar] [CrossRef]
- Iwashina, T.; Kokubugata, G. Flavonoids in the leaves and flowers of Myoporum bontioides native to northernmost region in the Myoporaceae. Bull. Natl. Sci. Mus. Ser. B 2010, 36, 117–125. [Google Scholar]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Gospodinova, Z.I.; Zupkó, I.; Bózsity, N.; Manova, V.I.; Georgieva, M.S.; Todinova, S.J.; Taneva, S.G.; Ocsovszki, I.; Krasteva, M.E. Cotinus coggygria Scop. induces cell cycle arrest, apoptosis, genotoxic effects, thermodynamic and epigenetic events in MCF7 breast cancer cells. Für Nat. C 2020, 76, 129–140. [Google Scholar] [CrossRef]
- Feng, L.S.; Cheng, J.B.; Su, W.Q.; Li, H.Z.; Xiao, T.; Chen, D.A.; Zhang, Z.L. Cinnamic acid hybrids as anticancer agents: A mini-review. Arch. Der Pharm. 2022, 355, 2200052. [Google Scholar] [CrossRef]
- Pal, A.; Tapadar, P.; Pal, R. Exploring the Molecular Mechanism of Cinnamic Acid-Mediated Cytotoxicity in Triple Negative MDA-MB-231 Breast Cancer Cells. Anticancer Agents Med. Chem. 2021, 21, 1141–1150. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Xu, L.; Zhou, H.; Yuan, M.; Xu, H. Recent Progress in Understanding the Action of Natural Compounds at Novel Therapeutic Drug Targets for the Treatment of Liver Cancer. Front. Oncol. 2022, 11, 795548. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Mansor, M.A.; Rad, M.A.; Khoo, A.S.B.; Ahmad, M.; Marzuki, M. The Effects of Thymus Plant Extracts on Single Breast Cancer Cell Morphology in the Microfluidic Channel. In Proceedings of the 2018 IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; pp. 647–651. [Google Scholar] [CrossRef]
- Wang, J.; Lai, X.; Yuan, D.; Liu, Y.; Wang, J.; Liang, Y. Effects of Ferulic acid, a Major Component of Rice Bran, on Proliferation, Apoptosis, and Autophagy of HepG2 Cells. Food Res. Int. 2022, 161, 111816. [Google Scholar] [CrossRef]
- Sundarraj, S.; Thangam, R.; Sreevani, V.; Kaveri, K.; Gunasekaran, P.; Achiraman, S.; Kannan, S. γ-Sitosterol from Acacia nilotica L. Induces G2/M Cell Cycle Arrest and Apoptosis Through c-Myc Suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012, 141, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Alotaibi, K.; Aldarhami, A.; Bazaid, A.S.; Ganash, M.; Saeedi, N.H.; Ghany, T.A. Effectiveness of oil-based nanoemulsions with molecular docking of its antimicrobial potential. BioResources 2023, 18, 1554–1576. [Google Scholar] [CrossRef]
- Al-Rajhi, A.M.H.; Qanash, H.; Bazaid, A.S.; Binsaleh, N.K.; Abdelghany, T.M. Pharmacological Evaluation of Acacia nilotica Flower Extract against Helicobacter pylori and Human Hepatocellular Carcinoma In Vitro and In Silico. J. Funct. Biomater. 2023, 14, 237. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana Seeds Extract for Microbial Pathogens and Cancer Control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
- Abdelghany, T.M. Stachybotrys chartarum: A novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indones. J. Biotechnol. 2013, 18, 75–82. [Google Scholar]
- Antunes, A.L.S.; Trentin, D.S.; Bonfanti, J.W.; Pinto, C.C.F.; Perez, L.R.R.; Macedo, A.J.; Barth, A.L. Application of a feasible method for determination of biofilm antimicrobial susceptibility in staphylococci. Apmis 2010, 118, 873–877. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Yahya, R.; Bakri, M.M.; Al Abboud, M.A.; Yahya, R.; Qanash, H.; Bazaid, A.S. Phytofabrication of zinc oxide nanoparticles with advanced characterization and its antioxidant, anticancer, and antimicrobial activity against pathogenic microorganisms. Biomass Conv. Bioref. 2023, 13, 417–430. [Google Scholar] [CrossRef]



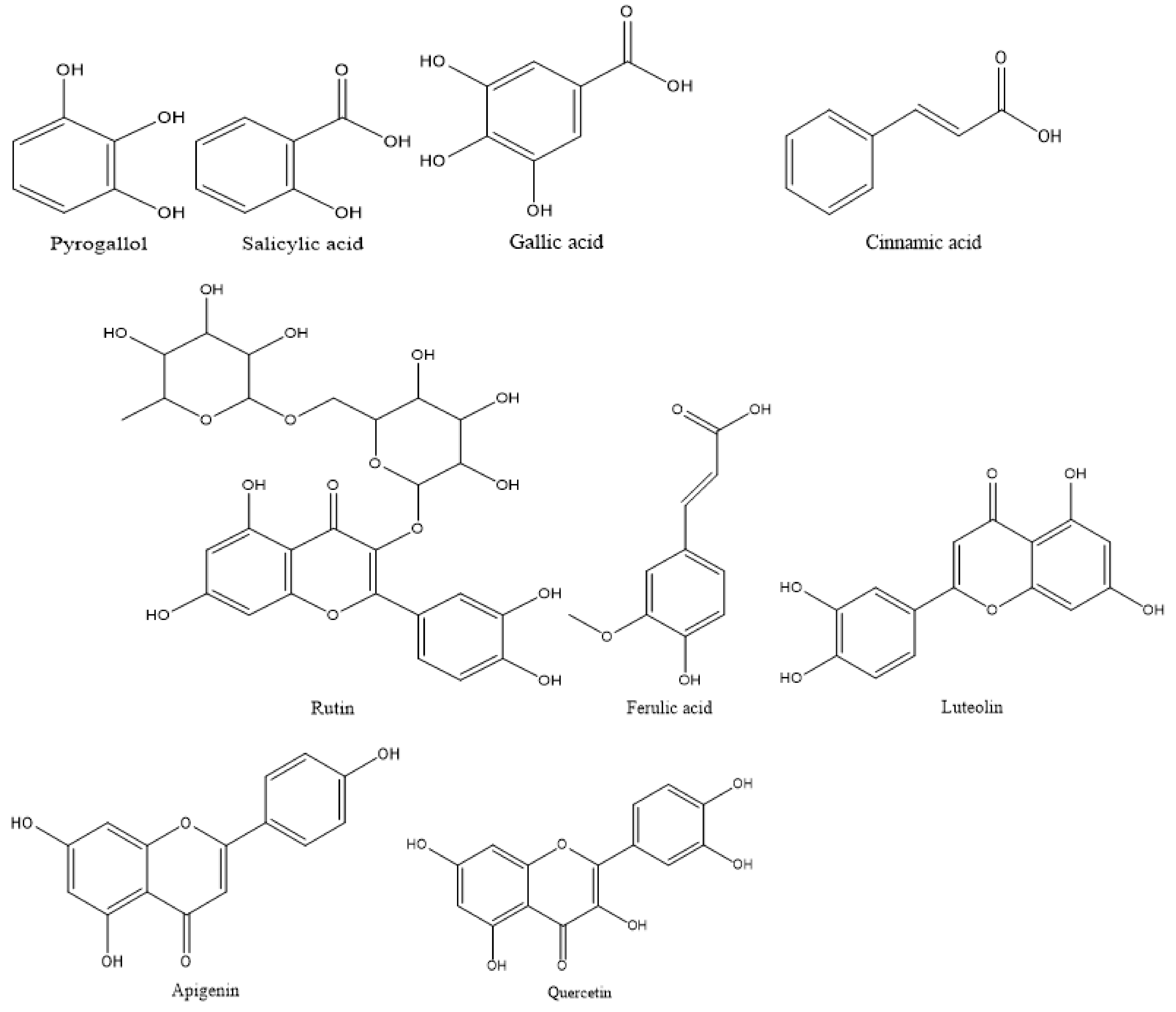


| Phenolic | Flavonoid | ||||
|---|---|---|---|---|---|
| RT | Compound | Concentration µg/mL | RT | Compound | Concentration µg/mL |
| 6.8 | Cinnamic acid | 12.75 | 5.2 | Rutin | 5.14 |
| 8.8 | Pyrogallol | 4.55 | 7 | Quercetin | 5.36 |
| 10 | Gallic acid | 3.42 | 9 | Luteolin | 10.74 |
| 12 | Salicylic acid | 7.14 | 10 | Apegenin | 8.87 |
| 15 | Ferulic acid | 0.97 | |||
| Tested Microorganisms | Inhibition Zone (mm) | MIC of MSSE | MBC of MSSE | MBC/MIC Index MSSE | |
|---|---|---|---|---|---|
| MSSE | Control | ||||
| S.aureus | 24.33 ± 0.58 | 21.83 ± 0.29 | 73.33 ± 1.15 | 133.33 ± 1.52 | 1.82 |
| B. subtilis | 26.33 ± 1.15 | 25.33 ± 0.58 | 42.08 ± 0.07 | 73.33 ± 0.29 | 1.74 |
| E. coli | 12.67 ± 2.08 | 28.67 ± 1.53 | 135.33 ± 0.29 | 545.33 ± 6.77 | 4.03 |
| P. vulgaris | 20.67 ± 1.15 | 25.33 ± 0.58 | 26.58 ± 0.58 | 73.60 ± 0.17 | 2.77 |
| C. albicans | 18.33 ± 0.58 | 20.17 ± 0.29 | 136.33 ± 0.07 | 257.67 ± 5.77 | 1.91 |
| A. fumigatus | NA | 17.50 ± 1.80 | - | - | - |
| Treatment | Anti-Biofilm % | ||||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P.vulgaris | C. albicans | |
| 25% of MBC | 62.40 ± 1.73 | 62.94 ± 1.15 | 31.65 ± 1.15 | 59.33 ± 1.15 | 44.00 ± 0.78 |
| 50% of MBC | 63.67 ± 0.29 | 67.30 ± 0.58 | 46.83 ± 1.58 | 70.17 ± 0.29 | 47.00 ± 1.00 |
| 75% of MBC | 81.25 ± 0.22 | 70.71 ± 0.62 | 50.45 ± 2.31 | 72.17 ± 1.04 | 57.33 ± 0.58 |
| Concentration (µg/mL) | MSSE | Ascorbic Acid | ||
|---|---|---|---|---|
| DPPH Scavenging % | ±SD | DPPH Scavenging % | ±SD | |
| 1000 | 82.74 | 1.28 | 98.65 | 0.10 |
| 500 | 70.95 | 1.71 | 96.34 | 0.65 |
| 250 | 61.34 | 0.92 | 94.25 | 0.92 |
| 125 | 50.97 | 1.89 | 90.89 | 0.17 |
| 62.5 | 38.56 | 0.78 | 86.42 | 0.71 |
| 31.25 | 21.43 | 1.95 | 79.35 | 1.22 |
| 15.6 | 12.96 | 2.62 | 65.05 | 0.21 |
| 7.8 | 7.48 | 0.46 | 43.01 | 2.87 |
| 3.9 | 5.03 | 0.31 | 31.04 | 1.04 |
| 2 | 3.87 | 0.25 | 22.18 | 1.37 |
| 1 | 1.95 | 0.11 | 14.48 | 0.62 |
| 0.5 | 0.84 | 0.18 | 9.34 | 0.46 |
| 0 | 0 | 0 | 0 | 0 |
| IC50 | 120.11 ± 3.69 µg/mL | 10.21 ± 0.77 µg/mL | ||
| Concentration (µg/mL) | MSSE | Vinblastine Sulfate | ||||
|---|---|---|---|---|---|---|
| MCF-7 | HepG-2 Cells | HepG-2 Cells | ||||
| Viability % | Inhibitory % | Viability % | Inhibitory % | Viability % | Inhibitory % | |
| 1000 | 7.83 | 92.17 ± 0.42 | 5.41 | 94.59 ± 0.35 | 3.27 | 96.73 ± 1.48 |
| 500 | 19.47 | 80.53 ± 1.09 | 13.68 | 86.32 ± 0.74 | 5.89 | 94.11 ± 1.30 |
| 250 | 37.89 | 62.11 ± 1.73 | 29.43 | 70.57 ± 1.98 | 10.92 | 89.08 ± 1.25 |
| 125 | 60.84 | 39.16 ± 2.18 | 52.97 | 47.03 ± 2.11 | 14.36 | 85.64 ± 0.31 |
| 62.5 | 83.95 | 16.05 ± 1.39 | 80.46 | 19.54 ± 0.68 | 19.24 | 80.76 ± 0.48 |
| 31.25 | 97.41 | 2.59 ± 0.75 | 92.35 | 7.65 ± 0.41 | 26.85 | 73.15 ± 1.25 |
| 15.6 | 100 | 0 | 98.17 | 1.83 ± 0.28 | 34.19 | 65.81 ± 0.50 |
| 7.8 | 100 | 0 | 100 | 0 | 45.06 | 54.94 ± 1.33 |
| 3.9 | 100 | 0 | 100 | 0 | 54.28 | 45.72 ± 1.42 |
| 1.9 | 100 | 0 | 100 | 0 | 60.94 | 39.06 ± 0.45 |
| 0.0 | 100 | 0 | 100 | 0 | 100 | 0.0 |
| IC50 | 184.04 ± 4.97 µg/mL | 140.77 ± 3.86 µg/mL | 2.93 ± 0.33 µg/mL | |||
| Mol | rseq | mseq | S | rmsd_Refine | E_Conf | E_Place | E_Score1 | E_Refine | E_Score2 |
|---|---|---|---|---|---|---|---|---|---|
| Cinnamic acid | 1 | 1 | −5.25012 | 0.96225 | −37.5672 | −72.1759 | −9.03118 | −23.385 | −5.25012 |
| Cinnamic acid | 1 | 1 | −5.14903 | 2.133763 | −36.5926 | −64.9391 | −9.42912 | −24.0258 | −5.14903 |
| Cinnamic acid | 1 | 1 | −5.13294 | 0.851975 | −39.5336 | −60.0528 | −9.17796 | −24.4316 | −5.13294 |
| Cinnamic acid | 1 | 1 | −5.10296 | 1.69946 | −37.5272 | −63.3036 | −9.82104 | −24.0133 | −5.10296 |
| Cinnamic acid | 1 | 1 | −5.08605 | 0.644272 | −37.956 | −51.767 | −10.2895 | −20.4546 | −5.08605 |
| Luteolin | 1 | 2 | −6.48903 | 1.592102 | −27.0053 | −86.8895 | −11.4305 | −40.1925 | −6.48903 |
| Luteolin | 1 | 2 | −6.40791 | 1.900243 | −27.0096 | −90.0438 | −11.7922 | −33.0823 | −6.40791 |
| Luteolin | 1 | 2 | −6.25776 | 1.593486 | −30.0341 | −102.073 | −12.1727 | −37.6265 | −6.25776 |
| Luteolin | 1 | 2 | −6.23779 | 1.485299 | −24.4523 | −110.889 | −11.7833 | −38.4676 | −6.23779 |
| Luteolin | 1 | 2 | −6.13434 | 0.917851 | −27.2434 | −84.5921 | −12.2857 | −35.6893 | −6.13434 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| Cinnamic acid | O 17 | OE1 GLU 230 (A) | H-donor | 2.80 | −4.1 |
| O 19 | N GLN 266 (A) | H-acceptor | 3.04 | −2.0 | |
| O 19 | N THR 267 (A) | H-acceptor | 3.30 | −0.6 | |
| 6-ring | NZ LYS 292 (A) | Pi- Cation | 4.65 | −0.6 | |
| Luteolin | O 28 | OD2 ASP 263 (A) | H-donor | 3.10 | −3.8 |
| O 27 | NH1 ARG 413 (A) | H-acceptor | 2.97 | −4.8 | |
| O 27 | NH2 ARG 413 (A) | H-acceptor | 3.02 | −1.7 | |
| 6-ring | NZ LYS 292 (A) | Pi-Cation | 3.97 | −2.8 | |
| 6-ring | 6-ring TYR 85 (A) | Pi-Pi | 3.98 | −0.0 |
| Mol | rseq | mseq | S | rmsd_Refine | E_Conf | E_Place | E_Score1 | E_Refine | E_Score2 |
|---|---|---|---|---|---|---|---|---|---|
| Cinnamic acid | 1 | 1 | −4.78831 | 0.554468 | −38.1018 | −53.3074 | −8.50818 | −12.759 | −4.78831 |
| Cinnamic acid | 1 | 1 | −4.67368 | 1.864181 | −37.6401 | −58.6463 | −8.51607 | −19.7046 | −4.67368 |
| Cinnamic acid | 1 | 1 | −4.56755 | 1.101168 | −39.3437 | −54.2494 | −9.44964 | −20.8978 | −4.56755 |
| Cinnamic acid | 1 | 1 | −4.54845 | 1.782263 | −39.8343 | −56.9743 | −8.96893 | −18.9359 | −4.54845 |
| Cinnamic acid | 1 | 1 | −4.53663 | 0.688159 | −37.7271 | −66.7538 | −8.70658 | −16.5558 | −4.53663 |
| Luteolin | 1 | 2 | −6.11443 | 4.067323 | −26.4148 | −79.6864 | −12.3615 | −35.4336 | −6.11443 |
| Luteolin | 1 | 2 | −6.05433 | 0.856852 | −30.4067 | −107.456 | −13.9814 | −31.4485 | −6.05433 |
| Luteolin | 1 | 2 | −6.04351 | 1.407485 | −25.4523 | −117.441 | −12.3122 | −36.4046 | −6.04351 |
| Luteolin | 1 | 2 | −5.96586 | 0.519985 | −26.7221 | −108.051 | −13.2917 | −30.9524 | −5.96586 |
| Luteolin | 1 | 2 | −5.9643 | 2.209804 | −27.7025 | −82.7211 | −12.102 | −33.9415 | −5.9643 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| Cinnamic acid | O 19 | N SER 11 (X) | H-acceptor | 3.51 | −0.8 |
| Luteolin | O 23 | O ASN 90 (X) | H-donor | 2.81 | −2.7 |
| O 30 | O CYS 185 (X) | H-donor | 2.90 | −2.3 | |
| O 27 | NZ LYS 159 (X) | H-acceptor | 3.18 | −3.9 | |
| 6-ring | CD1 ILE 14 (X) | Pi-H | 3.76 | −0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashraqi, A.; Modafer, Y.; Al Abboud, M.A.; Salama, H.M.; Abada, E. HPLC Analysis and Molecular Docking Study of Myoporum serratum Seeds Extract with Its Bioactivity against Pathogenic Microorganisms and Cancer Cell Lines. Molecules 2023, 28, 4041. https://doi.org/10.3390/molecules28104041
Mashraqi A, Modafer Y, Al Abboud MA, Salama HM, Abada E. HPLC Analysis and Molecular Docking Study of Myoporum serratum Seeds Extract with Its Bioactivity against Pathogenic Microorganisms and Cancer Cell Lines. Molecules. 2023; 28(10):4041. https://doi.org/10.3390/molecules28104041
Chicago/Turabian StyleMashraqi, Abdullah, Yosra Modafer, Mohamed A. Al Abboud, Hanaa M. Salama, and Emad Abada. 2023. "HPLC Analysis and Molecular Docking Study of Myoporum serratum Seeds Extract with Its Bioactivity against Pathogenic Microorganisms and Cancer Cell Lines" Molecules 28, no. 10: 4041. https://doi.org/10.3390/molecules28104041
APA StyleMashraqi, A., Modafer, Y., Al Abboud, M. A., Salama, H. M., & Abada, E. (2023). HPLC Analysis and Molecular Docking Study of Myoporum serratum Seeds Extract with Its Bioactivity against Pathogenic Microorganisms and Cancer Cell Lines. Molecules, 28(10), 4041. https://doi.org/10.3390/molecules28104041







