Abstract
Seaweeds or algae are marine autotrophic organisms. They produce nutrients (e.g., proteins, carbohydrates, etc.) essential for the survival of living organisms as they participate in biochemical processes and non-nutritive molecules (such as dietary fibers and secondary metabolites), which can improve their physiological functions. Seaweed polysaccharides, fatty acids, peptides, terpenoids, pigments, and polyphenols have biological properties that can be used to develop food supplements and nutricosmetic products as they can act as antibacterial, antiviral, antioxidant, and anti-inflammatory compounds. This review examines the (primary and secondary) metabolites produced by algae, the most recent evidence of their effect on human health conditions, with particular attention to what concerns the skin and hair’s well-being. It also evaluates the industrial potential of recovering these metabolites from biomass produced by algae used to clean wastewater. The results demonstrate that algae can be considered a natural source of bioactive molecules for well-being formulations. The primary and secondary metabolites’ upcycling can be an exciting opportunity to safeguard the planet (promoting a circular economy) and, at the same time, obtain low-cost bioactive molecules for the food, cosmetic, and pharmaceutical industries from low-cost, raw, and renewable materials. Today’s lack of methodologies for recovering bioactive molecules in large-scale processes limits practical realization.
1. Introduction
The main goal of the Circular Economy is to reuse and recycle natural resources to minimize health, energy, and environmental impacts. The European citizen produces around 5 tonnes of waste, much of which finishes in incinerators or landfills, and a little is recycled [1]. Waste management policies have been investigated to avoid landfills and allow the recovery of renewable energy and recycled materials [2]. Organizations have developed circular waste management systems, promoting resource flow and enhancing product sustainability and processes [3]. Consumption of eco-friendly products and decreasing waste are crucial to achieving the European sustainable goals. Ten megatrends were recognized for 2022 by New Nutrition Business for food, nutrition, and health. Sustainability came fifth [4]. Representative population surveys indicate that many people (amongst them young consumers) wish to contribute to sustainable development [5,6,7,8,9,10]. Buying eco-friendly products is considered one way to intervene. In the European Union, 26% of consumers purchase eco-friendly products, and 54% rarely use such items [11]. The global market value of natural and organic skincare products will probably grow from 9.9 billion dollars in 2021 to 20.4 billion dollars by 2030 [12]. The organic segment (made from plant ingredients that have been grown in soil free of fungicides, pesticides, synthetic fertilizers, and herbicides, and genetically modified organisms) was valued at $28,323.2 million in 2021 and is expected to reach $74,058.5 million by (CAGR of 9.8%) [13]. This data supports the significant contribution of the cosmetics market worldwide to environmental sustainability. The seaweed waste (e.g., beach-casts) [14] and invasive species valorization [15], which are of no commercial value and must be disposed of in landfills, could represent an eco-friendly, attractive low-cost source for supplements and cosmetics formulations. Some scientific studies have shown the potential skincare properties of algae bioactive metabolites [16,17,18,19]. In seaweeds are found compounds with low allergen and cytotoxic profiles [20], such as peptides, polysaccharides, fatty acids, vitamins, carotenoids, phlorotannins, tocopherols, phycobilins, phycocyanins, and sterols [21,22,23,24] that can act as antioxidants, photoprotective, moisturizing, anti-inflammatory, antiallergic, anti-acne, anti-wrinkling, antiaging, antimicrobial, and whitening bioactive compounds [25,26,27,28]. The present review summarizes the algal functional and technological properties to highlight their use for the nutricosmetic market and provide reasons for reflection for subsequent studies. Bibliometric works published between 1991 and 2023 collected in two central citation databases (Scopus and Web of Science) were consulted for the work’s drafting.
2. Nutricosmetic Revolution
The term “nutricosmetic” indicates the association of food supplements and cosmeceuticals to improve skin care. Nutricosmetic formulations optimize the intake of nutritional macro and micro elements to meet the demands of the skin and appendages, improving their conditions and delaying aging [29,30,31]. A food supplement is a consumer product that aims to supplement the regular diet. Products based on vitamins, minerals, antioxidants, and extracts of vegetable origin, single and multi-compound, in pre-dosed forms with nutritional power or biological effect, fall into the vast category of food supplements [32]. Cosmetics represent a highly heterogeneous category of daily-use consumer products. In the European Union, Regulation (EC) no. 1223/2009 in Article 2 defines “cosmetic product” as “any substance or mixture intended to be applied on the external surfaces of the human body (epidermis, hair system, and hair, nails, lips, external genital organs) or the teeth and on the mucous membranes of the mouth for the sole or primary purpose of cleaning them, perfuming them, modifying their appearance, protecting them, keeping them in good condition or correcting body odors” [33]. A substance or mixture intended to be ingested, inhaled, injected, or implanted in the human body is not considered a cosmetic product. Nutricosmetic formulations combine the two previous formulations’ beneficial effects through an integrated “in and out” approach.
3. Algae (Seaweeds)
Algae are a group of photosynthetic organisms that differ in structure and size. They can grow in freshwater, marine water, deep oceans, and rocky shores. The bionetwork comprises 36,000 different kinds of algae. The seaweed macroalgae are multicellular organisms rich in lipids and proteins (40% and 71% of their dry weight) that can measure from a few centimeters to a meter, while the microalgae are microscopic unicellular carbohydrate-rich organisms [34]. Macroalgae are grouped in Chlorophyta (green algae), Phaeophyta (brown algae), and Rhodophyta (red algae) according to their pigment and chlorophyll profile (Figure 1).
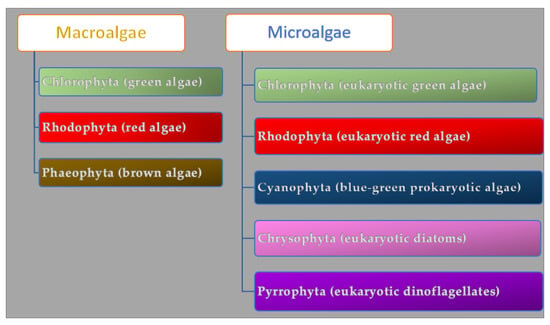
Figure 1.
Algae classification.
Microalgae are classified as prokaryotic and eukaryotic and, according to their color, subdivided into Cyanophyta (blue-green prokaryotic algae), Chlorophyta (eukaryotic green algae), Rhodophyta (eukaryotic red algae), Chrysophyta (golden eukaryotic diatoms), and Pyrrophyta (brown eukaryotic dinoflagellates) [35]. The chlorophyll responsible for the green color of the algae U. lactuca, C. vulgaris is employed as an antioxidant bioactive compound in cosmetics. Beta-carotene found in D. salina [36] and the red protein phycoerythrin found in red algae (e.g., Porphyra, Gracilaria, Irish moss) [37] are used as colorants in foods and cosmetics. The fucoxanthin in brown algae (Laminaria digitata, Isochrysis spp., Postelsia palmaeformis) prevents skin aging (by supporting collagen production and moisturizing skin) and has anti-inflammatory and tyrosinase inhibitory effects [38]. The algae metabolites’ composition is associated with internal factors (i.e., type and species), external factors (i.e., water temperature, water composition, salinity gradient, time of year, organism age), and cultivation conditions such as size and type of cultivation reactor [39]. During stress conditions, algae produce organic phenolic and phlorotannin and improve the uptake of inorganic ions to protect them from UV lights and desiccation. [40]. The wave exposure, environmental gradients, and algae reproductive cycles affect carbohydrate profile and content [41]. Chemicals (e.g., pH, carbon dioxide, salinity mixing/aeration), physical parameters (e.g., light, radiation, temperature), carbon sources (e.g., organic carbon like sugars and CO2), nitrogen, salts, phosphorous, and vitamins affect the algaes’ growth [42]. Microalgae can be grown autotrophically, heterotrophically, and mixotrophically. Cellular self-shading and low light availability negatively affect biomass production during autotrophic nutrition. Inorganic carbon sources can enhance biomass concentration and photosynthetic activities [42]. Organic substrates such as sugars, organic acids, etc. (heterotrophic nutrition), give rapid growth, low harvesting costs, and high biomass production [43]. The high cost of organic carbon sources, substrate inhibition, contamination, and the low number of microalgal species that can be grown in this way limit heterotrophic nutrition [44]. Mixotrophic algae can photosynthesize, assimilate, and metabolize organic carbon and are less dependent on light penetration for higher cell densities than autotrophy ones. During dark respiration, they manage biomass decrease, using lower organic substrate amounts than heterotrophic growth and enhancing the synthesis of the PUFA (polyunsaturated fatty acids) [44,45,46]. Algae can improve air quality by fixing CO2 [47] (they are responsible for 50% of the photosynthesis on earth) [48] and are an alternative source of bioenergy production since they produce biofuels [49]. Finally, they can reduce pollution [50] by converting water and CO2 into organic matter [51].
4. Algae Metabolites
4.1. Polysaccharides
Marine macroalgae are good carbohydrate sources (mainly polysaccharides and low concentration of disaccharides and monosaccharides) whose content is from 5 to 75% (w/w, DW) based on the age, period, species, and harvesting site [52,53]. Polysaccharides in seaweeds can be sulfated and non-sulfated [54]. They constitute the algae cell walls and are species-specific (Figure 2) [55,56]. They have some technological, rheological, and biological activities. They can have a prebiotic effect and improve gut human microbiota performance [57].
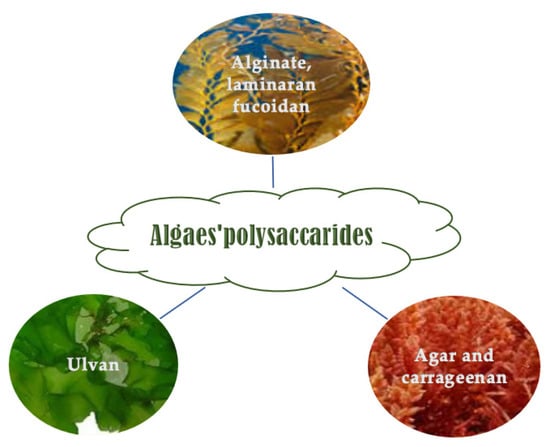
Figure 2.
Polysaccharides occurrence in the function of algae class.
4.1.1. Brown Algae Polysaccharides
Brown macroalgae are composed of sulfated and branched α-l-fucans containing predominantly sulfated l-Fucp (<90%), other monosaccharides (e.g., d-Manp, d-Galp, and d-Xylp), and uronic acids (d-GlcAp and d-GalAp). Brown algae polysaccharides have antioxidant, antiinflammatory, and antibacterial activity against E. coli, S. epidermidis, S. aureus, and B. licheniformis [58,59].
Ascophyllans (xylofucoglucuronanes) have a poly-(1→4)-β-d-glucuronan skeleton linked to l-Fucp and d-Xylp sulfated in position C-4 [60].
Sargassans (glucuronofucogalactans), identified in the genus Sargassum (e.g., Sargassum linifolium), have a poly-(1→4)-β-d-glucuronan skeleton linked with d-Manp residues [61].
Fucoidans have low shear-thinning performance and low viscoelastic physical characteristics (they are affected by monovalent and divalent salts) [62]. They are biocompatible, non-toxic, biodegradable [63,64], and have antioxidant and antiradical properties [65,66,67,68]. Fucoidans can promote skin firmness, elasticity, brightness, hair growth, safety, cleanliness, rigidity, and gloss [69]. They prevent and treat skin photoaging, decreasing wrinkle-related enzymes (e.g., collagenase, gelatinase, elastase) [70,71,72], improving collagen synthesis [73], controlling matrix metalloproteinases and avoiding the extracellular matrix’s ruin [74,75,76,77].
Laminarins (also laminarans), identified in laminaria present in the North Atlantic, have a degree of polymerization of 15–40 and molecular weight (Mw of 2–10 kDa). They are β-(1→3)-d-glucans. The laminaribiosis are the diholosidic repeating unit consisting of β-(1→6)-d-Glcp [78]. Laminarins are biocompatible, have low cell toxicity, are biodegradable, and show some bioactivity, such as anti-inflammatory, antioxidant [79] anti-photoaging and regenerative abilities [80].
Alginate(s) are polysaccharides composed of α-l-guluronic acid (l-GulpA) (1C4 ring conformation) and (1→4)-β-d-mannuronic acid (d-ManpA) (4C1 ring conformation) [81] arranged in both homogeneous and heterogeneous blocks [81]. Alginates are used in the food, feed, cosmetic, and drug industries as gelifying and thickening agents, and bioactive molecules against allergy [82] and obesity [83,84].
4.1.2. Red Algae Polysaccharides
Red algae (Rhodophyta) contain water-soluble sulfated galactan (e.g., agarocolloids and carrageenans), constructed based on (1→4)-α-Galp and (1→3)-β-Galp units [53]. Carrageenans have gel and texture properties. They are the fourth principal hydrocolloids used by the food industry, after starch, gelatin, and pectin [85].
Sulfated dioside are linear polymers of carrabiosis that can contain 4-α-d-Galp and 3-β-d- Galp, other monosaccharides (Xylp, GlcAp, Glcp, and GalAp), methyl ether groups, and pyruvic acid ketals. They are extracted from Agardhiella, Eucheuma, Chondrus, Gigartina, Furcellaria, and Hypnea [53,86].
Agarans are sulfated galactan containing 4-α-l-Galp [87]. Agarans based on the percentages of 3-6-α-l-AnGalp residues and sulfate groups are defined agaroids that are weak gelling molecules (divided into funorans and porphyrans), and agars (high gelling molecules). Agaroids are extracted from Porphyra species, e.g., P. capensis, Porphyra haitanensis [88], or P. umbilicalis [89]. Agar has cosmetic and pharmaceutical applications as a thickener agent and an ingredient to carry and release drugs in capsules and tablets [89,90].
4.1.3. Green Seaweed Polysaccharides
Chlorophyceae contain sulfated polyholosides [91]. Polyholosides are distinct in sulfated xylorhamnoglycuronans, called ulvans [92,93,94,95], sulfated arabinoxylogalactans or xyloarabinogalactans (composed of Araf, d-Galp, l- and d-Xylp units) present in the orders of Cladophorales and Bryopsidales, and sulfated rhamnogalactogalacturonanes or glucuronoxylorhamnogalactans extracted from Ulvales [96]. Ulvans are used as gelling [97] and antiaging agents [98].
4.2. Lipids
Algae contain omega-3 and omega-6 polyunsaturated fatty acids (PUFA; usually under 5%). The γ-linolenic acid, eicosapentaenoic acid, arachidonic acid, and docosahexaenoic acid are the most abundant.
Phaeophyta algae have a C18-PUFAs profile next to green algae and a C20-PUFAs profile identical to red algae. Chlorophyta species have higher levels of C18-PUFAs than C20-PUFAs. In Rhodophyta happen the contrary. Green algae contain higher DHA (docosahexaenoic acid) levels (e.g., Chlorophyta algae genus Tetraselmis). Finally, red and brown algae have predominantly EPA (eicosapentaenoic acid), arachidonic acid [99,100], and phospholipids [101,102,103,104,105]. Polyunsaturated fatty acids can improve skin barrier protection [106,107] and regulate inflammatory responses [108]. Lipids in cosmetic formulations can act as moisturizing agents (forming a waterproof film on the skin to avoid water evaporation from the surface) [109], emollient [110], and softening agents (they make the corneocyte’s edges smoother) [36], surfactants [111], and emulsifiers (they decrease the surface tension) [112], texturizers (they improve the spreadability of gel-like products), and as color and fragrance carriers [113].
4.3. Proteins and Derivatives
Seaweeds are a rich source of proteins (in single or conjugate form) and protein derivatives (e.g., free amino acids and peptides) [23]. Red algae have the highest proteins and derivative contents (up to 47%), green algae have medium levels (between 9–26%), and brown algae contain the lowest concentrations (3–15%) [114]. Protein and bioactive peptides have high antioxidant, anti-inflammatory, skin proactive, and antiaging properties [115,116,117]. Pedoclimatic conditions affect the proteins, peptides, and amino acids contents in algae.
Taurine extracted from the thalli of Euthora cristata, Ahnfeltia plicata, and Ceramium virgatum has antioxidant and chelating abilities [118,119]. The peptides (PYP1-5, and Porphyra 334) from Porphyra yezoensis f. coreana increase collagen and elastin levels and reduce the expression of matrix metalloproteinases (MMP) MMP-1 and MMP-8 [120].
Mycosporine-like amino acids (MAAs) (Figure 3) are secondary metabolites with low molecular weight (<400 Da) synthesized for protection against solar radiation and found in the cell cytoplasm [121]. Mycosporine-like amino acids are made by cycloheximide or cyclohexenone conjugated to amino acid or an imino alcohol residue [122]. They are extracted mainly from Rhodophyceae (e.g., shinorine, asterina, porphyra, palythine, polyphenol, mycosporine-glycine, and palythene) [123,124] and from Asparagopsis armata, Mastocarpus stellatus, Chondrus crispus, Gelidium sp., Palmaria palmata, Gracilaria cornea, Grateloupia lanceola, Solieria chordalis, and Curdiea racovitzae. This compound class has shown antioxidant, photoprotective, anti-proliferative [125], anti-aging, and anti-inflammatory activities [126].
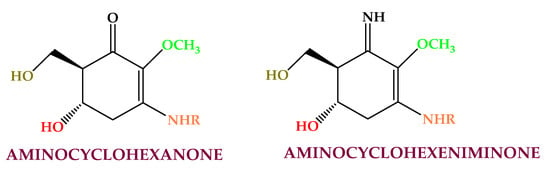
Figure 3.
Algaes’ mycosporine-like amino acids found in algae.
MAAs are employed as UV protectors, moisturizing, antiwrinkle, anti-roughness, and cell proliferation stimulators in personal care products and cosmetics [127,128,129].
4.4. Phenolics
Phenolic compounds are secondary plant metabolites with one or more aromatic rings with one or more -OH phenolic groups (e.g., phlorotannins, bromophenols, flavonoids, phenolic terpenoid, and mycosporine-like amino acids) [130]. They can defend algae from pedoclimatic injuries and parasite attacks [131,132]. The biological activities attributed to the algae’s phenolic compounds are summarized in Figure 4 [133].
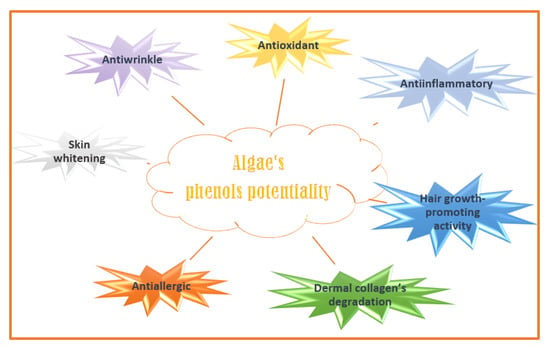
Figure 4.
Algaes’ phenols potentialities in nutricosmetic formulation.
Phlorotannins (Figure 5) are phloroglucinol (1,3,5-trihydroxybenzene) polymerized derivatives with ether, phenyl, or 1,4-dibenzodioxin linkages [134,135]. They are found only in brown algae [136]. Phlorotannins have antioxidant activity [137,138,139,140], reduce melanin synthesis, tyrosinase activity [141,142], damages caused by UV rays [143,144], and have anti-inflammatory [145,146], anti-proliferative [147,148,149,150,151], and anti-adipogenic activities [152]. Phlorotannins antioxidant power is 2 to 10 times higher than tocopherol and ascorbic acid [153,154]. Dieckol, eckol, dioxinodehydroeckol, phlorofucofuroeckol A, eckstolonol, and 7-phloroeckol, and decreasing tyrosinase and hyaluronidase activities can act as whiteners and antiwrinkle bioactive compounds in cosmetic formulations [155,156,157,158,159,160]. 7-derived phloroeckol promotes hair growth [161].
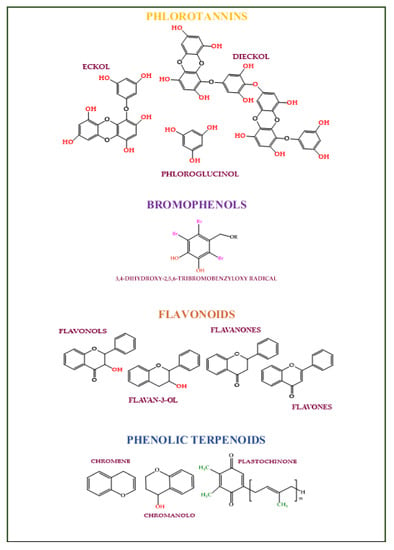
Figure 5.
The main class of phenolic compounds found in algae.
Phlorotannins from Ecklonia kurome (Phaeophyceae) act as antimicrobial agents against some methicillin-resistant food-borne pathogenic bacteria (Staphylococcus aureus strains, Campylobacter sp., and Streptococcus pyogenes) [162,163].
Dioxinodehydroeckol from Ecklonia cava and fucofuroeckol-A derived from the brown seaweed Ecklonia stolonifera Okamura can protect against UVB radiation [164,165].
Dieckol from Ecklonia stolonifera and other phlorotannins have antiallergic properties [166,167,168]. Phlorotannins also decrease the expression of the interstitial collagenase MMP-1 that regulates the dermal collagen’s degradation in the human skin aging process [169].
Bromophenols (B.P.s) (Figure 4) have one or several benzene rings with bromine and hydroxyl-substituents. They were isolated from red, green, and brown algae [170]. Bromophenols can act as antioxidants [171,172,173,174,175,176], antimicrobials (against Candida albicans [177,178], Pseudomonas fluorescence, and Staphylococcus aureus) [179], anti-inflammatories (decreasing the IgE-mediated responses, the interleukin-6, nuclear factor kappa-light-chain-enhancer, and activator of transcription1 pathways) [180], whitening (inhibiting the tyrosinase enzyme levels) [181], antiobesity, anticancer, and antiosteoporosis bioactive compounds (decreasing carbonic anhydrase [170,182], and glucose 6-phosphate dehydrogenase activities) [183,184].
Flavonoids are molecules derived from the phenylpropanoid metabolism and shikimate pathway. They have a high reduction potential and scavenging activity [185]. Flavones (e.g., luteolin, apigenin, chrysin, and baicalein) were isolated in the Ulva intestinalis and Cladophora vagabunda green seaweeds [186] and Phaeocystis globosa red alga [187]. Catechins (e.g., epicatechin and epigallocatechin) were detected in the U. pinnatifida brown seaweeds [188]. Flavonols (e.g., rutin, quercitin) in Chlorophyta, Rhodophyta, and Phaeophyceae species [136]. Isoflavones (e.g., daidzein or genistein) are present in red macroalgae (Chondrus crispus and Porphyra/Pyropia spp.) and brown seaweeds (Sargassum muticum and Sargassum vulgare) [189,190].
4.5. Terpenoids and Sterols
Mono- di- tri-and sesquiterpenoids were isolated from macro- and microalgae. Isoprenoid C5-subunits’ condensation forms terpenoids [191]. Terpenoids can act as antioxidants, antiaging (improving antioxidant enzymes such as catalase, superoxide dismutase, and glutathione peroxidase levels) [192], anti-inflammatory, skin-whitening (by inhibition of tyrosinase activity) [192], antibacterial (against gram-negative and gram-positive bacteria) [193], and anti-acne bioactive molecules (acting against Staphylococcus aureus, a gram-positive bacterium associated with acne vulgaris pathology) (Figure 6) [194,195].
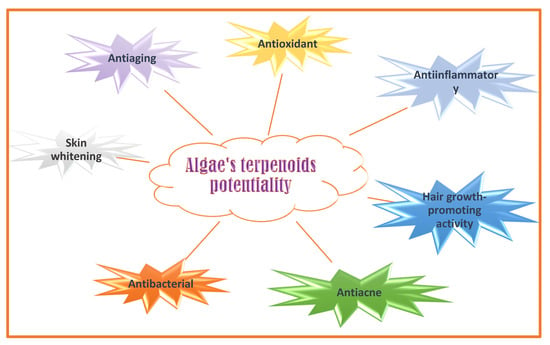
Figure 6.
Algaes’ terpenoids potentialities in nutricosmetic formulation.
The tetraprenyltoluquinol meroterpenoid from Sargassum muticum and meroterpenoid have antioxidant and anti-photoaging properties [196].
Loliolide monoterpenoid abundant in brown algae (Sargassum crassifolium and Padina tetrastromatica), and red algae (Corallina pilulifera), improve hair growth via AKT-mediated WNT(wingless-int) signaling activation [197].
The brown algae meroterpenoids determine skin-whitening [198]. The sesquiterpene 5β-Hydroxypalisadin B [(2R,5R,7S,9aS)-7-bromo-2-(bromomethyl)-3,6,6,9a-tetramethyl-2,5,5a,6,7,8,9,9a-octahydrobenzo[b]oxepin-5-ol] isolated from the red algae Laurencia snackeyi (Weber Bosse), and diterpenoid methyl 16(13→14)-abeo-7-labdebe(12-oxo)carboxylate from the red algae G. salicornia, have anti-inflammatory properties [199,200].
Phenolic terpenoids (Figure 5) are mero diterpenoids (chromanols, chromenes, plastoquinones) found in red and brown seaweeds [201]. Chromene-based molecule isolated from Gracilaria opuntia has shown antiinflammatory and antioxidant activity [202]. Tetraprenyltoluquinol meroterpenoids isolated from Halidrys siliquosa have shown antibacterial activity against Cobetia marina (ATTC 25374), Marinobacterium stanieri (ATCC 27130), Vibrio fischeri (ATCC 7744), and Pseudoalteromonas haloplanktis (ATCC 14393) [203,204].
Finally, seaweed contains sterols. Sterols are similar to cholesterol but have an alkyl substituent at C-24. In algae, they are present in free form or conjugated with fatty acids (e.g., oleate) or sugars (e.g., glucose) [205]. The brown algae contain principally fucosterol, red algae cholesterol, and green algae, a mixture of ergosterol, 28-isofucosterol, β-sitosterol, cholesterol, and poriferasterol [205]. Sterols can regulate membranes’ permeability and fluidity and have antioxidant, anti-inflammatory, and antiphotodamage [206,207,208,209].
4.6. Pigments
The algaes’ pigments can be brown (carotenes and xanthophylls), green (chlorophylls), and red (phycobilins).
Carotenoids are lipophilic isoprenoid molecules that can be used as natural color enhancers in food, cosmetic, and pharmaceutical formulations. They comprise carotenes and xanthophylls (e.g., β-carotene, zeaxanthin, astaxanthin, and fucoxanthin) with photoprotective, antioxidant, and antiaging properties [210]. β-Carotene acts as provitamin A and has antioxidant, anti-inflammatory, and antiaging properties [211,212]. Astaxanthin (xanthophyll compound) and fucoxanthin (in brown algae) have antioxidant and anti-macular degeneration properties [213,214,215]. Moreover, fucoxanthin can improve the fat-burning rate in adipose tissue [216]. The zeaxanthin (in red and green macroalgae) has whitening properties being able to control the tyrosinase’s activity (enzyme able to produce melanin) [217].
Chlorophylls are characterized for containing a porphyrin ring with a central magnesium ion. They protect algae against oxidative stress due to UV radiation [218].
Chlorophyll derivatives (pheophytin, pyropheophytin, and pheo-phorbide) also have antioxidant and antimutagenic abilities [83]. The chlorophyll level in the macroalgae is improved by overexposure to UV radiation [210].
Phycobiliproteins are mainly present in macroalgae and red macroalgae. They have antioxidant, antiaging, anti-inflammatory, and immune-modulator activities [218]. The phycobiliproteins remain stable in pH ranges between 5 and 9, allowing their use in cosmetics (e.g., eye shadows, creams, makeup, and lipsticks) [219].
The phycocyanin R-phycoerythrin and allophycocyanin are employed as colorants in cosmetic formulations [220].
5. Technological Properties of the Algae Metabolites
Algae metabolites can be used as technical ingredients to enhance cosmetics’ color, texture, and stability (Figure 7) [114,220]. Their efficiency and stability can be improved with carriers (e.g., nano/microparticles, liposomes, hydrogels, and emulsions) [221,222,223,224,225]. The cosmetic industry uses principally synthetic or mineral dyes, some of which can cause allergies. Algae pigments (e.g., chlorophylls, carotenoids, and phycobiliproteins) may be a valid alternative [226,227]. The FDA has authorized spirulina extracts (containing phycobiliproteins) as colorants in human foods [228,229].
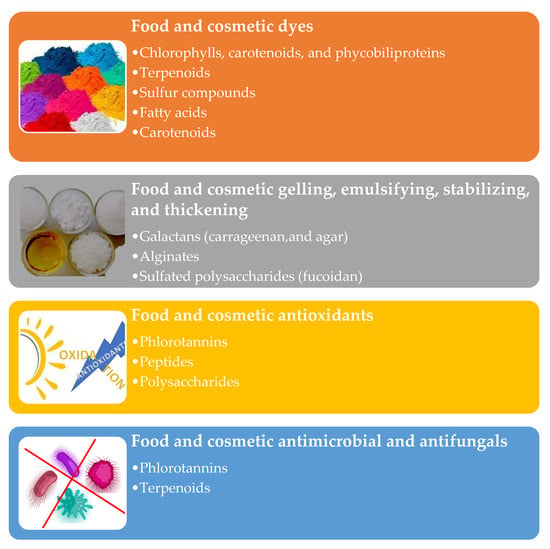
Figure 7.
Algae metabolites’ technological potentialities.
Algae terpenoids, sulfur compounds, fatty acids, and carotenoids can be employed as flavoring in cosmetic, food, and nutraceutical formulations [230,231].
Algae polysaccharides can be employed for their rheological behavior. Carrageenan, agar, and alginate can be used for gelling, emulsifying, stabilizing, and thickening since they form highly viscous solutions in water [232,233].
They are GRAS substances considered safe for human consumption by the European Food Safety Authority and the Food and Drug Administration [234]. The fucoidan (from U. pinnatifida and F. vesiculosus) was authorized by the European Commission (Regulation 2017/2470) in foods and food supplements [235]. The algal phlorotannins, peptides, and polysaccharides can protect the nutricosmetic formulation’s lipidic component from oxidative deterioration and maintain their original sensorial properties [236,237,238]. Finally, algae’s terpenoids and phlorotannins can be employed as preservative agents against bacteria and fungi [239].
6. Cosmetic Potenziality of Algae Metabolites
Algae’s metabolites in nutricosmetic products can be used as moisturizing, antiaging, skin whitening, anti-cellulite, and slimming care agents (Figure 8).
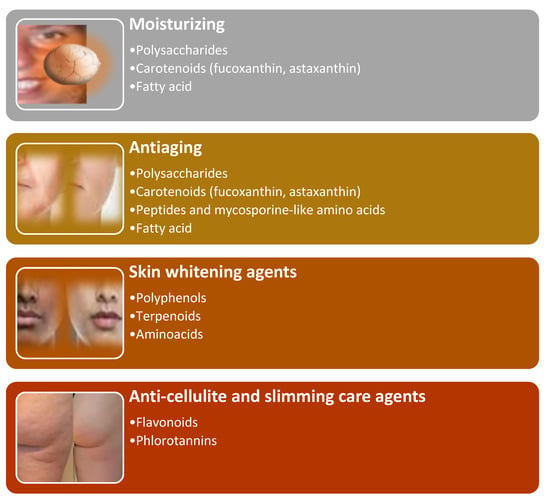
Figure 8.
Algae metabolites’ nutricosmetic potentialities.
6.1. Algae Metabolites in Moisturizing Formulations
The skin protects the body from the environment by maintaining an efficient epidermal barrier against injuries and preventing excessive water loss. The Natural Moisturizing Factors (NMF) present in the Stratum corneum, the epidermis’ outermost layer, contain lactic acid, pyrrolidone carboxylic acid urea, and amino acids (e.g., serine) able to uptake water [240]. The fat metabolism (in sebaceous glands) and conversion of phospholipids to free fatty acids produce glycerol [241] transported by the aquaporins through the epidermis via specific water/glycerol channels. Aquaporin expression is stimulated by retinoic acid [242].
Cosmetic products for dehydrated skin are based on ingredients with film-forming and occlusive properties (e.g., vegetable oils, fatty alcohols, hydrocarbons, waxes, silicones, and butter, etc.), or humectant agents, (which improve the Stratum corneum ability to capture water, e.g., glycerin or propylene glycol) [243] or moisturizers that penetrate the corneous layer permitting water to be retained [244].
The algae’s polysaccharides (mainly made by green and brown algae), oligosaccharides, and fatty acids can be employed as moisturizing agents. The polysaccharides (mainly marine green algae) moisturize slower and retain more moisture than glycerin [245]. A moisturizing retention rate of over 94% was referred to the polysaccharides belonging to brown algae (e.g., Sargassum horneri [246], Sargassum vachellianum [247], Sargassum hemiphyllum [248]. When applied topically, the sulfated polysaccharides (from red algae Porphyra haitanensis) enhance dry facial skin features and moisturization, regulating the keratinized envelope’s maturation of the stratum corneum and dermal-epidermal junction [245]. Low molecular weight and sulfated group enhance the moisture-retention and absorption abilities [192]. The alginates (extracted from brown macroalgae) and agar (from red macroalgae) have hydrating properties linked to their ability to conserve water [249].
The lipids can maintain skin integrity and purity, restoring barrier permeability and preventing skin dehydration due to unsaturated fatty acid deficiency in the skin. The brown macroalgae Laminaria ochroleuca produces numerous unsaturated fatty acids (e.g., oleic acid, linoleic acid, linolenic acid, and palmitoleic acid) with moisturizing properties widely used in oil/water emulsions to maintain water loss in the skin [250]. Oral or topical administration of astaxanthin (carotenoid) can improve skin moisture by improving the aquaporin levels (substances that regulate skin moisture and function) [251]. The green microalga Cladophora glomerata contains unsaturated fatty acids C16:1 (n-7) and C18:1 (n-3) and saturated fatty acids (palmitic acid C16:0) that can be used as emollients and to reduce water loss, and sulfated polysaccharides that have moisturizing properties [252].
6.2. Algae Metabolites in Antiaging Formulations
During the aging process, the dermis change. The matrix metalloproteinases (MMPs) activity increases, and collagen (one of the significant components of the extracellular matrix) levels decline [253]. Intrinsic (natural skin degradation) and extrinsic (ROS generated by UV radiation, pollution, etc.) factors can cause dryness, thinning, laxity, enlarged pores, fragility, wrinkles, and fine lines. The bioactive molecules that inhibit metalloproteinases help constrain aging. Sulfated polysaccharides (found in Phaeophyceae, Rhodophyceae, and Chlorophyceae), and polyphenols, derived from phloroglucinol, downregulate the metalloproteinases activity [254,255]. Fucoidan can regulate fibroblasts and restore skin tissue function [256]. Carrageenans act as thickening, water-binding [257] antioxidant, and antiphotoaging bioactive molecules [258]. Galactan of P. haitanensis decreases the cell’s aging process regulating the p53-p21 signaling pathway [259]. Astaxanthin (a carotenoid) protect against photo-oxidation [215]. Fucoxanthin upregulates the fibroblasts’ procollagen synthesis and decreases the expression of matrix metalloproteinases in wrinkle care cosmetics [260]. Amino acids and peptides from macroalgae stimulate collagen production in the skin [219]. Mycosporine-like amino acids act as antioxidants, antiinflammatories, UV-absorbing agents, and down-regulate the protein-glycation and collagenase activity [126]. Ascorbyl palmitate antioxidant effect is used in anti-aging and anti-wrinkle formulations [261,262].
6.3. Algae Metabolites in Skin Whitening Formulations
The pigmentation process controls the color of mammalians’ hair, skin, and eyes [263].
Tyrosinase enzyme regulates the conversion of l-tyrosine and l-3,4-dihydroxyphenylalanine (L-DOPA) in pheomelanin (red-orange pigment) and eumelanins (dark brown pigments) [264,265]. When tyrosinase is upregulated, hyperpigmentation determines freckles, age spots, irregular dark patches, and nevi. On the contrary, when tyrosinase is downregulated, melanin synthesis is reduced, and white patches (e.g., vitiligo) are observed [266]. Some algae’s phenols, terpenoids, amino acids, sugars, and amines, used as skin-whitening agents, are tyrosinase inhibitors [192,267]. Red algae, the richest sources of mycosporine-like amino acids, are a helpful source of whitening bioactive molecules for the cosmeceutical industry [268].
6.4. Algae Metabolites in Anticellulite and Slimming Care Formulations
In cosmetology, the term “slimming product” is preferred to “anti-cellulite” since cellulite is a disorder produced by a deep dermis and subcutaneous tissue change and, therefore, a term linked to the medical world [269]. Cellulite has a multifactorial etiology [270]. Estrogens and microcirculation disorders (decreasing blood flow in the capillaries), the nervous system (downregulating the lipolysis process), and genetic factors can be involved. The slimming product objectives include correcting the” orange peel” appearance and “mattress symptom” characterized by roughness, skin surface collapse, and yellow-gray skin tone.
The iodine-rich algae (e.g., Laminaria Japonica) can be used to constrain cellulite since iodine regulates the thyroid hormones’ synthesis, which boosts lipolysis by facilitating the penetration of fatty acids into the mitochondria [192,271,272].
Examples of patents claiming the use of algae and algae metabolites in cosmetic formulations are reported in Table 1.

Table 1.
Examples of algaes’ use in the cosmetic field.
7. Macroalgae Biomass in a Circular Economy Perspective
Recent studies have considered algae a sustainable and environmentally friendly way to eliminate contamination from wastewater since they use low energy and pollutants to grow [282] and to produce biomass [283]. The dry biomass or wet paste of microalgae can be employed to extract bioactive metabolites. Selling prices improve from biomass to secondary metabolites [284]. The “chemicals and materials” and bio-energy market use whole biomass. The “food, pharmaceuticals and personal care” markets employ primary and secondary metabolites in the feed, food, supplement, nutraceutical, and cosmeceutical preparations. Raw biomass can enhance the soil organic matter and water capacity in agriculture. The defatted biomass from biodiesel extraction, mixed with water, can produce biogas after anaerobic digestion and can be used to extract metabolites. For example, the residual lipids can be upcycled as supplements in animal feed [285]. Glycerol, a byproduct of the microalgal lipids’ transesterification to biodiesel, can be converted to solvents, polymers, and aliphatic polyesters, to generate electricity directly in biofuels cells or to prepare foods, cosmetics, and drugs [286]. The digestate resulting from biogas production can be employed as fertilizer and conditioner. Microalgae biomass can be employed as a food supplement, feed additive, and feed in the aquaculture of crustaceans, fishes, and mollusks [287]. Proteins, lipids (e.g., phospholipids and glycolipids), starches, and sugars can be used in food, nutraceutical and personal care, and drug products. Chlorophylls and carotenoids can be used as food and cosmetic dyes [288]. Sterols can be used as anti-inflammatory and cholesterol-lowering bioactive molecules in foods and supplements [289]. PUFA and oxylipins can be used as nutricosmetics, food supplements, and feeds [290,291]. The cost, microbial and chemical contaminants’ accumulation, and the lack of technology viable for large-scale applications give a setback to algal wastewater treatments [292]. Different is the speech of the potential use of the beach-cast macroalgae. Tonnes of marine algae are removed per year and dumped in landfills. Very few registers of abundance and composition of beach-cast marine algae worldwide exist. These algae should be less rich in toxic products than algal wastewater and probably do not need detoxification processes [293]. Thus, it would be enough to imagine strategies for large-scale extraction of bioactive molecules to take advantage of this natural and eco-sustainable source of raw materials for industry.
8. Conclusions
Algae are rich sources of bioactive molecules (amino acids, carbohydrates, lipids, phenols, and terpenoids), helpful for improving the functional, stability, and sensorial characteristics of nutricosmetic products. The vast array of bioactive molecules makes algae an attractive and versatile resource to obtain safe bio-based products. Algae extract and their purified metabolites are gaining increasing commercial importance. Many patents concerning algae extracts or metabolites application in nutricosmetic products have been registered recently. Unfortunately, many do not report the mechanisms responsible for cosmetic performance. It would be helpful that more works evaluate the algae extract profiles to identify functional properties, stability, compatibility, and toxicology aspects to facilitate the development of new nutricosmetic. Concerning the use of algae to eliminate pollution from wastewater and produce biomass from which obtain bioactive molecules, the cost, non-sterile conditions, and lack of technology viable for large-scale applications limit their application. Better potential can be seen for the recycling of beach-cast macroalgae.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eurostat Statistics Explained: Waste Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics (accessed on 1 January 2023).
- Colasante, A.; D’Adamo, I.; Morone, P.; Rosa, P. Assessing the Circularity Performance in a European Cross-Country Comparison. Environ. Impact Assess. Rev. 2022, 93, 106730. [Google Scholar] [CrossRef]
- Luo, Y.; Song, K.; Ding, X.; Wu, X. Environmental sustainability of textiles and apparel: A review of evaluation methods. Environ. Impact Assess. Rev. 2021, 86, 106497. [Google Scholar] [CrossRef]
- New Nutrition. 10 Key Trends in Food, Nutrition & Health. 2022. Available online: New-nutrition.com (accessed on 22 July 2021).
- Eurostat (European Commission). Sustainable Development in the European Union: 2015 Monitoring Report of the E.U. Sustainable Development Strategy: 2015 Edition; Publications Office of European Union: Luxembourg, 2015. [CrossRef]
- Eurostat (European Commission). Sustainable Development in the European Union: Monitoring Report on Progress Towards the S.D.G.s in an E.U. Context: 2017 Edition; Publications Office of European Union: Luxembourg, 2018. [CrossRef]
- European Commission. Directorate-General for Communication. Towards a Sustainable Europe by 2030: Reflection Paper; Publications Office of European Union: Luxembourg, 2019. [CrossRef]
- Cho, Y.-N.; Soster, R.L.; Burton, S. Enhancing Environmentally Conscious Consumption through Standardized Sustainability Information. J. Consum. Aff. 2017, 52, 393–414. [Google Scholar] [CrossRef]
- Yarimoglu, E.; Binboga, G. Understanding sustainable consumption in an emerging country: The antecedents and consequences of the ecologically conscious consumer behavior model. Bus. Strat. Environ. 2018, 28, 642–651. [Google Scholar] [CrossRef]
- Lendvai, B.M.; Kovács, I.; Lisányi, J.B. Helyi élelmiszer termékekkel kapcsolatos észlelései. Generation Z’s perceptions of local food products. In Proceedings of the Georgikon Conference, Keszthely, Hungary, 7 October 2021. [Google Scholar]
- Eurobarometer. Attitudes of Europeans towards Building the Single Market for Green Products. Flash Eurobarometer 367. 2013. Available online: http://ec.europa.eu/public_opinion/flash/fl_367_en.pdf (accessed on 22 January 2014).
- Statista. Value of the Natural and Organic Skin Care Products Market from 2021 to 2030 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/1116674/global-market-value-for-natural-organic-skin-care/ (accessed on 1 March 2021).
- Sustainable Personal Care Market by Nature (Organic, Natural and Green), by Type (Skin Care, Hair Care, Oral Care, Hygiene Products, Others), by Sales Channel (Hypermarkets and Supermarkets, Specialty Stores, Online Retail, Others): Global Opportunity Analysis and Industry Forecast, 2021–2031. Available online: https://www.alliedmarketresearch.com/sustainable-personal-care-market-A16262 (accessed on 1 May 2022).
- Zárate, R.; Portillo, E.; Teixidó, S.; de Carvalho, M.A.A.P.; Nunes, N.; Ferraz, S.; Seca, A.M.L.; Rosa, G.P.; Barreto, M.C. Pharmacological and cosmeceutical potential of Seaweed Beach-casts of Macaronesia. Appl. Sci. 2020, 10, 5831. [Google Scholar] [CrossRef]
- Félix, R.; Carmona, A.M.; Félix, C.; Novais, S.C.; Lemos, M.F.L. Industry-friendly hydroethanolic extraction protocols for Grateloupia turuturu UV-shielding and antioxidant compounds. Appl. Sci. 2020, 10, 5304. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vazquez, J.A.; Perez-Martin, R.I. Sustainable sources from aquatic organisms for cosmeceuticals ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Wahyuni, T. The Potential and Application of Eucheuma sp. For Solid Soap: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 750, 012048. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Lafarga, T.; Acién-Fernándeza, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal. Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Structures and Antiallergic Activities of Natural Products from Marine Organisms. Mar. Drugs 2023, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Nieri, P.; Carpi, S.; Esposito, R.; Costantini, M.; Zupo, V. Bioactive Molecules from Marine Diatoms and Their Value for the Nutraceutical Industry. Nutrients 2023, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Sukhikh, S.; Larina, V.; Kalashnikova, O.; Kashirskikh, E.; Prosekov, A.; Noskova, S.; Ivanova, S.; Fendri, I.; Smaoui, S.; et al. Algae: Study of Edible and Biologically Active Fractions, Their Properties and Applications. Plants 2022, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Kharkwal, H.; Joshi, D.; Panthari, P.; Pant, M.K.; Kharkwal, A.C. Algae as future drugs. Asian J. Pharm. Clin. Res. 2012, 5, 1–4. [Google Scholar]
- Senevirathne, W.S.M.; Kim, S.K. Cosmeceuticals from Algae. In Functional Ingredients from Algae for Foods and Nutraceuticals, 1st ed.; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 694–713. [Google Scholar]
- Vo, T.; Ngo, D.; Kang, K.; Jung, W.; Kim, S. The beneficial properties of marine polysaccharides in alleviation of allergic responses. Mol. Nutr. Food Res. 2015, 59, 129–138. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV Photoprotection, Cytotoxicity and Immunology Capacity of Red Algae Extracts. Molecules 2019, 24, 341. [Google Scholar] [CrossRef]
- Dini, I. Bio Discarded from Waste to Resource. Foods 2021, 10, 2652. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytot. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef] [PubMed]
- Dini, I. The commercial importance to develop validated analytical methods to define phytochemical levels in herbal medicinal products. Phytot. Res. 2022, 36, 3675–3677. [Google Scholar] [CrossRef] [PubMed]
- Regulation (E.C.) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223 (accessed on 31 July 2022).
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Freitas, F.; Torres, C.A.V.; Araújo, D.; Farinha, I.; Pereira, J.R.; Concórdio-Reis, P.; Reis, M.A.M. Advanced Microbial Polysaccharides. In Biopolymers for Biomedical and Biotechnological Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 19–62. [Google Scholar]
- Joshi, S.; Kumari, R.; Upasani, V.N. Applications of algae in cosmetics: An overview. Int. J. Innov. Sci. Eng. Technol. 2018, 7, 1269–1278. [Google Scholar]
- Brindhadevi, K.; Mathimani, T.; Rene, E.R.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 284. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- del Río, P.G.; Gomes-Dias, J.S.; Rocha, C.M.R.; Romaní, A.; Garrote, G.; Domingues, L. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour. Technol. 2019, 299, 122613. [Google Scholar] [CrossRef] [PubMed]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Jayashree, S.; Kumar, G.; Sharmili, S.A.; Gopal, M.; Dharmaraj, S.; Chen, W.-H.; Kothari, R.; Manasa, I.; Park, J.H.; et al. Recent developments in biorefining of macroalgae metabolites and their industrial applications—A circular economy approach. Bioresour. Technol. 2022, 359, 127235. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2021, 806, 151387. [Google Scholar] [CrossRef] [PubMed]
- Zili, F.; Bouzidi, N.; Ammar, J.; Zakhama, W.; Ghoul, M.; Sayadi, S.; Ben Ouada, H. Mixotrophic cultivation promotes growth, lipid productivity, and P.U.F.A. production of a thermophilic Chlorophyta strain related to the genus Graesiella. J. Appl. Phycol. 2017, 29, 35–43. [Google Scholar] [CrossRef]
- Jareonsin, S.; Pumas, C. Advantages of Heterotrophic microalgae as a host for phytochemicals production. Front. Bioeng. Biotechnol. 2021, 9, 628597. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of polyunsaturated fatty acids and lipids from autotrophic, mixotrophic and heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Joun, J.; Hong, M.E.; Patel, A.K. Split mixotrophy: A novel cultivation strategy to enhance the mixotrophic biomass and lipid yields of Chlorella protothecoides. Bioresour. Technol. 2019, 291, 121820. [Google Scholar] [CrossRef]
- Varvoutis, G.; Lampropoulos, A.; Mandela, E.; Konsolakis, M.; Marnellos, G.E. Recent Advances on CO2 Mitigation Technologies: On the Role of Hydrogenation Route via Green H2. Energies 2022, 15, 4790. [Google Scholar] [CrossRef]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and Micro Algae in Pollution Control and Biofuel Production—A Review. ChemBioEng Rev. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: The applications of high-rate algal ponds (H.R.A.P.s) and algal turf scrubber (A.T.S.). J. Environ. Manag. 2021, 296, 113193. [Google Scholar] [CrossRef]
- da Rosa, M.D.H.; Alves, C.J.; dos Santos, F.N.; de Souza, A.O.; Zavareze, E.d.R.; Pinto, E.; Noseda, M.D.; Ramos, D.; de Pereira, C.M.P. Macroalgae and Microalgae Biomass as Feedstock for Products Applied to Bioenergy and Food Industry: A Brief Review. Energies 2023, 16, 1820. [Google Scholar] [CrossRef]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Zheng, L.X.; Chen, X.Q.; Cheong, K.L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Delattre, C.; Gardarin, C.; Desbrières, J.; Le Cerf, D.; Rihouey, C.; Michaud, P.; Abdelkafi, S.; Pierre, G. Structural features and rheological properties of a sulfated xylogalactan-rich fraction isolated from tunisian red seaweed. Jania adhaerens. Appl. Sci. 2020, 10, 1655. [Google Scholar] [CrossRef]
- Aumeerun, S.; Soulange-Govinden, J.; Driver, M.F.; Rao, A.R.; Ravishankar, G.A.; Neetoo, H. Macroalgae and Microalgae. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; p. 207. [Google Scholar]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Kraan, S. Algal Polysaccharides, Novel applications and outlook. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; InTech: Rijeka, Croatia, 2012; Chapter 22; pp. 489–524. [Google Scholar]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Percival, E. The polysaccharides of green, red and brown seaweeds: Their basic structure, biosynthesis and function. Br. Phycol. J. 1979, 14, 103–117. [Google Scholar] [CrossRef]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial Properties of Fucoidans from the Brown Algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Hentati, F.; Pierre, G.; Ursu, A.V.; Vial, C.; Delattre, C.; Abdelkafi, S.; Michaud, P. Rheological investigations of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Food Hydrocoll. 2020, 103, 105631. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.-L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an antiwrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds compounds: An ecosustainable source of cosmetic ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.-G.; Jeon, Y.-J. The potential of sulfated polysaccharides isolated from the brown seaweed Ecklonia maxima in cosmetics: Antioxidant, anti-melanogenesis, and photoprotective activities. Antioxidants 2020, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-H.; Fang, Y.; Lin, H.; Chen, L.; Li, Z.-J.; Deng, D.; Lu, C.-X. Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. J. Appl. Phycol. 2001, 13, 67–70. [Google Scholar] [CrossRef]
- Usov, A.I.; Zelinsky, N.D. Chemical Structures of Algal Polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 23–86. ISBN 978-0-85709-512-1. [Google Scholar]
- Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Anti-photoaging and potential skin health benefits of seaweeds. Mar. Drugs 2021, 19, 172. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseca, T.N.; Ermakova, S.P.; Lee, Y.P. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef]
- Su, W.; Wang, L.; Fu, X.; Ni, L.; Duan, D.; Xu, J.; Gao, X. Protective effect of a fucose-rich fucoidan isolated from Saccharina japonica against ultraviolet B-induced photodamage in vitro in human keratinocytes and in vivo in Zebrafish. Mar. Drugs 2020, 18, 316. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Kim, Y.-S.; Lee, H.-G.; Lee, J.-S.; Jeon, Y.-J. Anti-Photoaging and Anti-Melanogenesis Effects of Fucoidan Isolated from Hizikia fusiforme and Its Underlying Mechanisms. Mar. Drugs 2020, 18, 427. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.-E.; Kim, K.H.; Kang, N.J. Beneficial effects of marine algae-derived carbohydrates for skin health. Mar. Drugs 2018, 16, 459. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.-Y.; Lee, W.; Jeon, Y.-J. Fucoidan isolated from Hizikia fusiforme suppresses ultraviolet B-induced photodamage by down-regulating the expressions of matrix metalloproteinases and pro-inflammatory cytokines via inhibiting NF-κB, AP-1, and M.A.P.K. signaling pathways. Int. J. Biol. Macromol. 2021, 166, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Ali Karami, M.; Sharif Makhmalzadeh, B.; Pooranian, M.; Rezai, A. Preparation and optimization of silibinin-loaded chitosan–fucoidan hydrogel: An in vivo evaluation of skin protection against UVB. Pharm. Dev. Technol. 2021, 26, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Stiger-Pouvreau, V.; Bourgougnon, N.; Deslandes, E. Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention; Academic Press: London, UK, 2016; pp. 223–247. [Google Scholar]
- Sellimi, S.; Maalej, H.; Rekik, D.M.; Benslima, A.; Ksouda, G.; Hamdi, M.; Sahnoun, Z.; Li, S.; Nasri, M.; Hajji, M. Antioxidant, antibacterial and in vivo wound healing properties of laminaran purified from Cystoseira barbata seaweed. Int. J. Biol. Macromol. 2018, 119, 633–644. [Google Scholar] [CrossRef]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical applications of laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Ursu, A.V.; Pierre, G.; Delattre, C.; Bogdan, T.; Abdelkafi, S.; Gholamereza, D.; Tanase, D.; Michaud, P. Production, extraction and characterization of alginates from seaweeds. In Handbook of Algal Technologies and Phytochemicals; Ravishankar, G.A., Ambati, R.R., Eds.; CRC Press (Taylor & Francis Group, Royaume-Uni): Boca Raton, FL, USA, 2019; pp. 33–42. [Google Scholar]
- Yu, B.; Bi, D.; Yao, L.; Li, T.; Gu, L.; Xu, H.; Li, X.; Li, H.; Hu, Z.; Xu, X. The inhibitory activity of alginate against allergic reactions in an ovalbumin-induced mouse model. Food Funct. 2020, 11, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Valado, A.; Pereira, M.; Amaral, M.; Cotas, J.; Pereira, L. Bioactivity of Carrageenans in Metabolic Syndrome and Cardiovascular Diseases. Nutraceuticals 2022, 2, 441–454. [Google Scholar] [CrossRef]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and its applications. In Polysaccharides; Springer: Cham, Switzerland, 2014; pp. 1–37. [Google Scholar]
- Knutsen, S.; Myslabodski, D.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–169. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef]
- Chen, X.; Fu, X.; Huang, L.; Xu, J.; Gao, X. Agar oligosaccharides: A review of preparation, structures, bioactivities and application. Carbohydr. Polym. 2021, 265, 118076. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydary, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.-H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Altern. Complement. Med. 2021, 17, 20190203. [Google Scholar] [CrossRef] [PubMed]
- Miladi, R.; Manghisi, A.; Minicante, S.A.; Genovese, G.; Abdelkafi, S.; Morabito, M. A DNA barcoding survey of Ulva (Chlorophyta) in Tunisia and Italy reveals the presence of the overlooked alien U. ohnoi. Cryptogam. Algol. 2018, 39, 85–107. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Luong, D.V.; Bui, M.L.; Van Tran, T.T. Structure and cytotoxic activity of ulvan extracted from green seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Briand, X.; Cluzet, S.; Dumas, B.; Esquerre-Tugaye, M.T.; Salamagne, S. Use of Ulvans as Activators of Plant Defence and Resistance Reactions against Biotic and Abiotic Stresses. U.S. Patent 0232494 A1, 13 October 2005. [Google Scholar]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales, Chlorophyta). Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313318. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Carnachan, S.M.; Magnusson, M.; Lawton, R.J.; Sims, I.M.; Hinkley, S.F.R.; de Nys, R.; Glasson, C.R.K. Are all ulvans equal? A comparative assessment of the chemical and gelling properties of ulvan from blade and filamentous Ulva. Carbohyd. Polym. 2021, 264, 118010. [Google Scholar] [CrossRef]
- Fournière, M.; Bedoux, G.; Lebonvallet, N.; Lescchiera, R.; Goff-Pain, C.L.; Bourgougnon, N.; Latire, T. Poly-and oligosaccharide ulva sp. Fractions from enzyme-assisted extraction modulate the metabolism of extracellular matrix in human skin fibroblasts: Potential in antiaging dermo-cosmetic applications. Mar. Drugs 2021, 19, 156. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry-An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Moser, G.A.O.; Barrera-Alba, J.J.; Ortega, M.J.; Alves-de-Souza, C.; Bartual, A. Comparative characterization of three Tetraselmis chui (Chlorophyta) strains as sources of nutraceuticals. J. Appl. Phycol. 2022, 34, 821–835. [Google Scholar] [CrossRef]
- Neto, R.T.; Marçal, C.; Queirós, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs 2020, 18, 142. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, T.R.; Santos, G.S.; Turatti, I.C.C.; Paziani, M.H.; von Zeska Kress, M.R.; Colepicolo, P.; Debonsi, H.M. Characterization of the lipid profile of Antarctic brown seaweeds and their endophytic fungi by gas chromatography–mass spectrometry (G.C.–M.S.). Polar Biol. 2019, 42, 1431–1444. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Trepiana, J.; Eseberri, I.; Fernández-Quintela, A.; Milton-Laskibar, I.; Aguirre, L.; González, M.; Portillo, M.P. Anti-Obesity Effects of Macroalgae. Nutrients 2020, 12, 2378. [Google Scholar] [CrossRef]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Moisturizers. J. Cosmet. Dermatol. 2018, 17, 138–144. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Law, S.Q.; Mettu, S.; AshokKumar, M.; Scales, P.J.; Martin, G.J. Emulsifying properties of ruptured microalgae cells: Barriers to lipid extraction or promising biosurfactants? Colloids Surf. B Biointerfaces 2018, 170, 438–446. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.E.T.; Leal, M.A.; de Oliveira Resende, M.; Martins, M.A.; dos Reis Coimbra, J.S. Scenedesmus Obliquus Protein Concentrate: A Sustainable Alternative Emulsifier for the Food Industry. Algal Res. 2021, 59, 102468. [Google Scholar] [CrossRef]
- Hernandez, E.M. Pharmaceutical and Cosmetic Use of Lipids. In Bailey’s Industrial Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2020; pp. 1–28. [Google Scholar]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Abdalbasit, M.; Gasmalla, A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; De Biasi, M.-G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Toy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. [Google Scholar]
- Yanshin, N.; Kushnareva, A.; Lemesheva, V.; Birkemeyer, C.; Tarakhovskaya, E. Chemical composition and potential practical application of 15 red algal species from the White Sea Coast (the Arctic Ocean). Molecules 2021, 26, 2489. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Medic. 2014, 34, 796–803. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (M.A.A.s): Biology, Chemistry and Identification Features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Karsten, U.; Sawall, T.; Wiencke, C. A survey of the distribution of UV-absorbing substances in tropical macroalgae. Phycol. Res. 2006, 46, 271–279. [Google Scholar]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.U.V. Photoprotectants from Algae—Synthesis and Bio-Functionalities. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. [Google Scholar]
- Suh, S.S.; Oh, S.K.; Lee, S.G.; Kim, I.C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Antiinflammatory, and Antiaging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Maia Campos, P.M.B.G. Efficacy evaluation of a multifunctional cosmetic formulation: The benefits of a combination of active antioxidant substances. Molecules 2014, 19, 18268–18282. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Gager, L.; Lalegerie, F.; Connan, S.; Stiger-Pouvreau, V. Marine Algal Derived Phenolic Compounds and their Biological Activities for Medicinal and Cosmetic Applications. In Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 278–334. [Google Scholar]
- Dini, I.; Laneri, S. Spices, Condiments, extra virgin olive oil and aromas as not only flavorings, but precious allies for our wellbeing. Antioxidants 2021, 10, 868. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.; Ahn, G. Marine algal flavonoids and phlorotannins; an intriguing frontier of biofunctional secondary metabolites. Crit. Rev. Biotechnol. 2021, 42, 23–45. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Pradhan, R.; Ghotekar, S.; Dahikar, S.; Marasini, B.P. Phytochemical Analysis and Anti-Microbial Activity of Desmostachya Bipinnata: A review. J. Med. Chem. Sci. 2021, 4, 36–41. [Google Scholar]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Franco, C.; Su, P.; Zhang, W. Improved antioxidant activities of brown seaweed Ecklonia radiata extracts prepared by microwave-assisted enzymatic extraction. J. Appl. Phycol. 2015, 27, 2049–2058. [Google Scholar] [CrossRef]
- Chang, M.Y.; Byon, S.H.; Shin, H.C.; Han, S.E.; Kim, J.Y.; Byun, J.Y.; Lee, J.D.; Park, M.K. Protective effects of the seaweed phlorotannin polyphenolic compound dieckol on gentamicin-induced damage in auditory hair cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 31–36. [Google Scholar] [CrossRef]
- Kirke, D.A.; Smyth, T.J.; Rai, D.K.; Kenny, O.; Stengel, D.B. The chemical and antioxidant stability of isolated low molecular weight phlorotannins. Food Chem. 2017, 221, 1104–1112. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Park, S.; Park, J.S.; Kim, Y.H.; Yang, S.Y. Slow-Binding Inhibition of Tyrosinase by Ecklonia cava Phlorotannins. Mar. Drugs 2019, 17, 359. [Google Scholar] [CrossRef]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the Dermatological Potential of the Brown Seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135. [Google Scholar] [CrossRef]
- Piao, M.J.; Hewage, S.R.; Han, X.; Kang, K.A.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Protective Effect of Diphlorethohydroxycarmalol against Ultraviolet B Radiation-Induced D.N.A. Damage by Inducing the Nucleotide Excision Repair System in HaCaT Human Keratinocytes. Mar. Drugs 2015, 13, 5629–5641. [Google Scholar] [CrossRef]
- Kang, S.M.; Heo, S.J.; Kim, K.N.; Lee, S.H.; Yang, H.M.; Kim, A.D.; Jeon, Y.J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Lopes, G.; Andrade, P.-B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown algae phlorotannins: A marine alternative to break the oxidative stress, inflammation and cancer network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Kim, E.K.; Tang, Y.; Kim, Y.S.; Hwang, J.W.; Choi, E.J.; Lee, J.H.; Lee, S.H.; Jeon, Y.J.; Park, P.J. First evidence that Ecklonia cava-derived dieckol attenuates MCF-7 human breast carcinoma cell migration. Mar. Drugs 2015, 13, 1785–1797. [Google Scholar] [CrossRef]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, J.H.; Park, S.A.; Joo, N.R.; Lee, B.H.; Lee, K.B.; Oh, S.M. Dieckol or phlorofucofuroeckol extracted from Ecklonia cava suppresses lipopolysaccharide-mediated human breast cancer cell migration and invasion. J. Appl. Phycol. 2020, 32, 631–640. [Google Scholar] [CrossRef]
- Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Cytotoxic activities of phlorethol and fucophlorethol derivatives isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. In Nineteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 2007; pp. 255–261. [Google Scholar]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef]
- Kumar, L.R.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins–bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Nurrochmad, A.; Wirasti, W.; Dirman, A.; Lukitaningsih, E.; Rahmawati, A.; Fakhrudin, N. Effects of Antioxidant, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase of The Extract and Fraction From Turbinaria decurrens Bory. Indones. J. Pharm. 2018, 29, 188. [Google Scholar] [CrossRef]
- Bak, S.S.; Sung, Y.K.; Kim, S.K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 789–793. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef]
- Ryu, B.; Ahn, B.-N.; Kang, K.-H.; Kim, Y.-S.; Li, Y.-X.; Kong, C.-S.; Kim, S.-K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B 2015, 153, 352–357. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.H.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N.; Ngo, D.N. The suppressive activity of fucofuroeckol-a derived from brown algal Ecklonia stolonifera okamura on UVB-induced mast cell degranulation. Mar. Drugs 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Joe, M.; Kim, S.; Choi, H.; Shin, W.; Park, G.; Kang, D.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739. [Google Scholar] [CrossRef]
- Le, Q.; Li, Y.; Qian, Z.; Kim, M.; Kim, S. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Sugiura, Y.; Kinoshita, Y.; Misumi, S.; Yamatani, H.; Katsuzaki, H.; Hayashi, Y.; Murase, N. Correlation between the seasonal variations in phlorotannin content and the antiallergic effects of the brown alga Ecklonia cava subsp. stolonifera. Algal Res. 2021, 58, 102398. [Google Scholar] [CrossRef]
- Handajani, F.; Prabowo, S. Sargassum duplicatum extract reduced artritis severity score and periarticular tissue matrix metalloproteinase-1 (MMP-1) expression in ajuvan artritis exposed to cold stressor. Syst. Rev. Pharm. 2020, 11, 302–307. [Google Scholar]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, L.; Guo, M.; Stagos, D.; Giakountis, A.; Trachana, V.; Lin, X.; Liu, Y.; Liu, M. Antioxidant and Anticancer Activities of Synthesized Methylated and Acetylated Derivatives of Natural Bromophenols. Antioxidants 2022, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Fernando, P.D.S.M.; Kang, K.A.; Piao, M.J.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Hyun, J.W. Marine compound 3-bromo-4,5-dihydroxybenzaldehyde protects skin cells against oxidative damage via the Nrf2/HO-1 pathway. Mar. Drugs 2019, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Heo, S.J.; Yang, H.W.; Ko, E.Y.; Jung, M.S.; Cha, S.H.; Ahn, G.; Jeon, Y.J.; Kim, K.N. Protective effect of 3-bromo-4,5-dihydroxybenzaldehyde from Polysiphonia morrowii harvey against hydrogen peroxide-induced oxidative stress in vitro and in vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef]
- Hyun, Y.J.; Piao, M.J.; Zhang, R.; Choi, Y.H.; Chae, S.; Hyun, J.W. Photo-protection by 3-bromo-4, 5-dihydroxybenzaldehyde against ultraviolet B-induced oxidative stress in human keratinocytes. Ecotoxicol. Environ. Saf. 2012, 83, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hyun, Y.; Hewage, S.R.; Piao, M.; Kang, K.; Kang, H.; Koh, Y.; Ahn, M.; Hyun, J. 3-bromo-4,5-dihydroxybenzaldehyde enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2017, 15, 291. [Google Scholar] [CrossRef]
- Xu, X.L.; Yin, L.Y.; Gao, J.H.; Gao, L.J.; Song, F.H. Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem. Biodivers. 2015, 11, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Yin, L.Y.; Wang, Y.H.; Wang, S.Y.; Song, F.H. A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat. Prod. Res. 2012, 27, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Rajasulochana, P.; Krishnamoorthy, P.; Dhamotharan, R. Isolation, identification of bromophenol compound and antibacterial activity of Kappaphycus sp. Int. J. Pharm. Biol. Sci. 2012, 3, 173–186. [Google Scholar]
- Kang, N.J.; Han, S.C.; Kang, H.J.; Ko, G.; Yoo, E.S. Antiinflammatory effect of 3-bromo-4,5-dihydroxybenzaldehyde, a component of Polysiphonia morrowii, in vivo and in vitro. Toxicol. Res. 2017, 33, 325–332. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzyme. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef]
- Mikami, D.; Kurihara, H.; Kim, S.M.; Takahashi, K. Red algal bromophenols as glucose 6-phosphate dehydrogenase inhibitors. Mar. Drugs 2013, 11, 4050–4057. [Google Scholar] [CrossRef]
- Mikami, D.; Kurihara, H.; Ono, M.; Kim, S.M.; Takahashi, K. Inhibition of algal bromophenols and their related phenols against glucose 6-phosphate dehydrogenase. Fitoterapia 2016, 108, 20–25. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Sava, C.; Sibru, R. Analytical study of the determination of flavonoids in Black Sea algae. Ovidius Univ. Ann. Chem. 2010, 21, 29–34. [Google Scholar]
- Xiao, X.; Li, C.; Huang, H.; Lee, Y.P. Inhibition effect of natural flavonoids on red tide alga Phaeocystis globosa and its quantitative structure-activity relationship. Environ. Sci. Pollut. Res. 2019, 26, 23763–23776. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory effects of edible seaweeds, polyphenolics and alginates on the activities of porcine pancreatic α-amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štĕrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva-Reis, R.; Chouh, A.; Silva, S.; Braga, S.S.; Silva, A.M.S.; Cardoso, S.M. Applications of Antioxidant Secondary Metabolites of Sargassum spp. Mar. Drugs 2023, 21, 172. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, U.T.; Yusof, Z.N.B. Algal terpenoids: A potential source of antioxidants for cancer therapy. In Terpenes and Terpenoids—Recent Advances; Perveen, S., Al-Taweel, A.M., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Michalak, I.; Dmytryk, A.; Chojnacka, K. Algae Cosmetics. In Encyclopedia of Marine Biotechnology; John Wiley and Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Dini, I. Use of Essential Oils in Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Januário, A.P.; Félix, R.; Félix, C.; Reboleira, J.; Valentão, P.; Lemos, M.F.L. Red Seaweed-Derived Compounds as a Potential New Approach for Acne Vulgaris Care. Pharmaceutics 2021, 13, 1930. [Google Scholar] [CrossRef]
- Balboa, E.; Li, Y.-X.; Ahn, B.-N.; Eom, S.-H.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B Biol. 2015, 148, 51–58. [Google Scholar] [CrossRef]
- Lee, Y.R.; Bae, S.; Kim, J.Y.; Lee, J.; Cho, D.H.; Kim, H.S.; An, I.S.; An, S. Monoterpenoid Loliolide Regulates Hair Follicle Inductivity of Human Dermal Papilla Cells by Activating the Akt/beta-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2019, 29, 1830–1840. [Google Scholar] [CrossRef]
- Azam, M.S.; Choi, J.; Lee, M.-S.; Kim, H.-R. Hypopigmenting Effects of Brown Algae-Derived Phytochemicals: A Review on Molecular Mechanisms. Mar. Drugs 2017, 15, 297. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.; Kim, E.-A.; Kang, M.-C.; Lee, W.-W.; Lee, H.-S.; Vairappan, C.S.; Jeon, Y.-J. Assessment of antiinflammatory effect of 5β-hydroxypalisadin B isolated from red seaweed Laurencia snackeyi in zebrafish embryo in vivo model. Environ. Toxicol. Pharmacol. 2014, 37, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Antony, T.; Chakraborty, K. First report of antioxidant abeo-labdane type diterpenoid from intertidal red seaweed Gracilaria salicornia with 5-lipoxygenase inhibitory potential. Nat. Prod. Res. 2018, 34, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Pereira, L. Dermo-Cosmetic Benefits of Marine Macroalgae-Derived Phenolic Compounds. Appl. Sci. 2022, 12, 11954. [Google Scholar] [CrossRef]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2018, 32, 2756–2765. [Google Scholar] [CrossRef]
- Gerald Culioli, A.O.M.; Valls, R.; Hellio, C.; Clare, A.S.; Piovetti, L. Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. J. Nat. Prod. 2008, 71, 1121–1126. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed Secondary Metabolites in Vitro and in Vivo Anticancer Activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Sun, Z.-W.; Shin, H.-S.; Lee, D.-G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-Irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Antioxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological aspects of microalgae pigments for cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G. Micro-Macroalgae Properties and Applications. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 9780128035818. [Google Scholar]
- Wijesinghe, W.; Jeon, Y. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae Metabolites in Cosmeceutical: An Overview of Current Applications and Challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Gammone, M.A.; D’Orazio, N. Antiobesity activity of the marine carotenoid fucoxanthin. Mar. Drugs 2015, 13, 2196–2214. [Google Scholar] [CrossRef]
- Morone, J.; Lopes, G.; Morais, J.; Neves, J.; Vasconcelos, V.; Martins, R. Cosmetic Application of Cyanobacteria Extracts with a Sustainable Vision to Skincare: Role in the Antioxidant and Antiaging Process. Mar. Drugs 2022, 20, 761. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. In Natural Bioactive Compounds: Technological Advancements; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: London, UK, 2021; pp. 57–81. [Google Scholar]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Resende, D.I.S.P.; Ferreira, M.; Magalhães, C. Trends in the use of marine ingredients in antiaging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromol. 2019, 122, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Prima, N.R.; Andriyono, S. Techniques of additional Kappaphycus alvarezii on seaweed face mask production. IOP Conf. Ser. Earth Environ. Sci. 2021, 679, 012021. [Google Scholar] [CrossRef]
- Dini, I. Contribution of Nanoscience Research in Antioxidants Delivery Used in Nutricosmetic Sector. Antioxidants 2022, 11, 563. [Google Scholar] [CrossRef]
- Li, D.; Wu, Z.; Martini, N.; Wen, J. Advanced carrier systems in cosmetics and cosmeceuticals: A review. J. Cosmet. Sci. 2011, 62, 549–563. [Google Scholar]
- Koutsaviti, A.; Ioannou, E.; Roussis, V. Bioactive seaweed substances. In Bioactive Seaweeds for Food Applications, 1st ed.; Quin, Y., Ed.; Academic Press: London, UK, 2018; pp. 25–52. [Google Scholar]
- Sari, D.S.P.; Saputra, E.; Alamsjah, M.A. Potential of fucoxanthin content in Sargassum sp. On sunscreen cream preparation. Int. J. Recent Technol. Eng. 2019, 7, 448–451. [Google Scholar]
- Fransiska, D.; Darmawan, M.; Sinurat, E.; Sedayu, B.B.; WArdana, Y.W.; Herdiana, Y.; Setiana, G.P. Characteristics of Oil in Water (o/w) Type Lotions Incorporated with Kappa/Iota Carrageenan. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012050. [Google Scholar] [CrossRef]
- Galetović, A.; Seura, F.; Gallardo, V.; Graves, R.; Cortés, J.; Valdivia, C.; Núñez, J.; Tapia, C.; Neira, I.; Sanzana, S.; et al. Use of Phycobiliproteins from Atacama Cyanobacteria as Food Colorants in a Dairy Beverage Prototype. Foods 2020, 9, 244. [Google Scholar] [CrossRef]
- Marmion, D.M. Handbook of U.S. Colorants: Foods, Drugs, Cosmetics, and Medical Devices, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 1991. [Google Scholar]
- Dini, I. Chapter 14—Use of Essential Oils in Food Packaging. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 139–147. [Google Scholar]
- Cunha, S.C.; Fernandes, J.O.; Vallecillos, L.; Cano-Sancho, G.; Domingo, J.L.; Pocurull, E.; Borrull, F.; Mauvault, A.L.; Ferrari, E.; Fernández-Tejedor, M.; et al. Co-occurrence of musk fragrances and UV-filters in seafood and macroalgae collected in European hotspots. Environ. Res. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Qin, Y. Seaweed Hydrocolloids as Thickening, Gelling, and Emulsifying Agents in Functional Food Products. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: San Diego, CA, USA, 2018; pp. 135–152. [Google Scholar]
- Rioux, L.; Turgeon, S.L. Seaweed carbohydrates. In Seaweed Sustainability: Food and Non-Food Applications; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: London, UK, 2015; pp. 141–192. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (A.N.S.); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of carrageenan (E 407) and processed Eucheuma seaweed (E 407a) as food additives. EFSA J. 2018, 16, e05238. [Google Scholar]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.; Almetida-Lima, J.; Oliveira, R.M.; Alburquerque, I.R.; et al. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.A.D.O.; Castro, A.J.G.; Nascimento, M.S.; Will, L.S.E.P.; Santos, N.D.; Araújo, R.M.; Xavier, C.A.C.; Rocha, F.A.; Leite, E.L. Antioxidant and antiinflammatory effect of polysaccharides from Lobophora variegata on zymosan-induced arthritis in rats. Int. Immunopharmacol. 2011, 11, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Nursid, M.; Khatulistiani, T.S.; Noviendri, D.; Hapsari, F.; Hardiyati, T. Total phenolic content, antioxidant activity and tyrosinase inhibitor from marine red algae extract collected from Kupang, East Nusa Tenggara. IOP Conf. Ser. Earth Environ. Sci. 2020, 493, 012013. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Marine algae as efficacious bioresources housing antimicrobial compounds for preserving foods—A review. Int. J. Food Microbiol. 2021, 358, 109416. [Google Scholar] [CrossRef]
- Maeno, K. Direct quantification of natural moisturizing factors in stratum corneum using direct analysis in real time mass spectrometry with inkjet-printing technique. Sci. Rep. 2019, 9, 17789. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.; O’Boyle, N.M. Skin Lipids in Health and Disease: A Review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Xing, F.; Liao, W.; Jiang, P.; Xu, W.; Jin, X. Effect of retinoic acid on aquaporin 3 expression in keratinocytes. Genet. Mol. Res. 2016, 15, 15016951. [Google Scholar] [CrossRef]
- Couteau, C.; Coiffard, L. Phycocosmetics and Other Marine Cosmetics, Specific Cosmetics Formulated Using Marine Resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef]
- Idalgo Vasques, L.; Ricci Leonardi, G. User Experience in Cosmetics: Perception Analysis Regarding the Use of an Anti-Aging Moisturizer. Cosmetics 2023, 10, 33. [Google Scholar] [CrossRef]
- Zhang, T.; Guo, Q.; Xin, Y.; Liu, Y. Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus. Arab. J. Chem. 2022, 15, 104163. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.X.; Sun, P.L. Improvement of antioxidant and moisture-preserving activities of Sargassum horneri polysaccharide enzymatic hydrolyzates. Int. J. Biol. Macromol. 2015, 74, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, W.; Hou, Y.; Niu, X.; Zhang, H.; Zhang, Q. Chemical composition and moisture-absorption/retention ability of polysaccharides extracted from five algae. Int. J. Biol. Macromol. 2013, 57, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Podkorytova, A.V.; Vafina, L.H.; Kovaleva, E.A.; Mikhailov, V.I. Production of algal gels from the brown alga, Laminaria japonica Aresch., and their biotechnological applications. J. Appl. Phycol. 2007, 19, 827–830. [Google Scholar] [CrossRef]
- Otero, P.; López-Martínez, M.I.; García-Risco, M.R. Application of pressurized liquid extraction (P.L.E.) to obtain bioactive fatty acids and phenols from Laminaria ochroleuca collected in Galicia (NW Spain). J. Pharm. Biomed. 2019, 164, 86–92. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Nagoya, C.; Ishikura, A.; Sugiyama, Y.; Takahashi, J.; Sugiyama, K. Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an in-Vitro Study. Life 2020, 10, 193. [Google Scholar] [CrossRef]
- Messyasz, B.; Michalak, I.; Łęska, B. Valuable natural products from marine and freshwater macroalgae obtained from supercritical fluid extracts. J. Appl. Phycol. 2018, 30, 591–603. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Im, A.R.; Nam, K.W.; Hyun, J.W.; Chae, S. Phloroglucinol Reduces Photodamage in Hairless Mice via Matrix Metalloproteinase Activity through M.A.P.K. Pathway. Photochem. Photobiol. 2016, 92, 173–179. [Google Scholar] [CrossRef]
- Fayad, S.; Tannoury, M.; Morin, P.; Nehmé, R. Simultaneous elastase-, hyaluronidase- and collagenase-capillary electrophoresis based assay. Application to evaluate the bioactivity of the red alga Jania rubens. Anal. Chim. Acta 2018, 1020, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Oh, W.-S.; Song, P.H.; Yun, S.; Kwon, Y.-S.; Lee, Y.J.; Ku, S.-K.; Song, C.-H.; Oh, T.-H. Anti-Photoaging Effects of Low Molecular-Weight Fucoidan on Ultraviolet B-Irradiated Mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef]
- Guo, Z.; Wei, Y.; Zhang, Y.; Xu, Y.; Zheng, L.; Zhu, B.; Yao, Z. Carrageenan oligosaccharides: A comprehensive review of preparation, isolation, purification, structure, biological activities and applications. Algal Res. 2022, 61, 102593. [Google Scholar] [CrossRef]
- Shafie, M.H.; Kamal, M.L.; Zulkiflee, F.F.; Hasan, S.; Uyop, N.H.; Abdullah, S.; Hussin, N.A.M.; Tan, Y.C.; Zafarina, Z. Application of Carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. J. Indian Chem. Soc. 2022, 99, 100613. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Su, H.L.; Pan, Y.L.; Han, J.F.; Zhang, T.S.; Mao, G.X. Effect of sulfated galactan from Porphyra haitanensis on H2O2-induced premature senescence in WI-38 cells. Int. J. Biol. Macromol. 2018, 106, 1235–1239. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kang, H.; Lee, J.E.; Jo, C.S.; Moon, C.B.; Ha, J.; Hwang, J.S.; Choi, J. Antiaging Potential of Fucoxanthin Concentrate Derived from Phaeodactylum tricornutum. J. Cosmet. Sci. 2020, 71, 53–64. [Google Scholar] [PubMed]
- Fabrowska, J.; Łȩska, B.; Schroeder, G. Freshwater Cladophora glomerata as a new potential cosmetic raw material. Chemik 2015, 69, 491–497. [Google Scholar]
- Yarnpakdee, S.; Benjakul, S.; Senphan, T. Antioxidant Activity of the Extracts from Freshwater Macroalgae (Cladophora glomerata) Grown in Northern Thailand and Its Preventive Effect against Lipid Oxidation of Refrigerated Eastern Little Tuna Slice. Turk. J. Fish. Aquat. Sci. 2018, 19, 209–219. [Google Scholar]
- Vachtenheim, J.; Borovanský, J. Transcription physiology of pigment formation in melanocytes: Central role of M.I.T.F. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrzesniok, D. From tyrosine to melanin: Signaling pathways and factors regulating melanogenesis. Postep. Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin Lightening & Management of Hyperpigmentation. Pharma Sci. Anal. Res. J. 2019, 2, 180020. [Google Scholar]
- David, S.R.; Baharulnizam, N.B.; Rajabalaya, R. A review on biological assays of red algae marine compounds: An insight into skin whitening activities. J. Herb. Med. 2022, 35, 100585. [Google Scholar] [CrossRef]
- Kasanah, N.; Ulfah, M.; Imania, O.; Hanifah, A.N.; Marjan, M.I.D. Rhodophyta as Potential Sources of Photoprotectants, Antiphotoaging Compounds, and Hydrogels for Cosmeceutical Application. Molecules 2022, 27, 7788. [Google Scholar] [CrossRef]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Czerwińska-Ledwig, O. Effect of Three-Week Vibrotherapy on Selected Skin Parameters of Thighs and Buttocks in Women with Cellulite. Cosmetics 2022, 9, 16. [Google Scholar] [CrossRef]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and Microbial Infections. Curr. Probl. Dermatol. 2018, 54, 87–94. [Google Scholar]
- Miyaji, A.; Sugimori, K.; Hayashi, N. Short- and long-term effects of using a facial massage roller on facial skin blood flow and vascular reactivity. Complement. Ther. Med. 2018, 41, 271–276. [Google Scholar] [CrossRef]
- Seaweed-Derived Cosmetic Compositions. Available online: https://patents.google.com/patent/US20140242130A1/en (accessed on 28 August 2014).
- Microalgae Derived Compositions for Improving the Health and Appearance of Skin. Available online: https://patents.google.com/patent/US10493007B2/en?oq=US10493007B2 (accessed on 3 December 2019).
- Seaweed Extracts, Isolated Compounds and Methods of Treatment. Available online: https://patents.google.com/patent/US20210161980A1/en?oq=US20210161980A1 (accessed on 3 June 2021).
- Marine Extracts and Biofermentations for Use in Cosmetics. Available online: https://patents.google.com/patent/US9717932B2/en?oq=US9717932B2 (accessed on 1 August 2017).
- Preparation of Algal Polysaccharide and Application of Algal Polysaccharide in Cosmetics. Available online: https://patents.google.com/patent/CN105777933A/en?oq=CN105777933A (accessed on 29 May 2018).
- Method for Using Green Algae Extract to Retard Aging of Skin Cells and Cosmetic Composition Containing Green Algae Extract. Available online: https://patents.google.com/patent/TW200914061A/en?oq=TW200914061A (accessed on 1 April 2009).
- Cosmetic Composition Containing Gulfweed Extract Sea Staghorn Extract and Brown Seaweed Extract. Available online: https://patents.google.com/patent/WO2012070835A2/en?oq=PCT%2fKR2011%2f008910 (accessed on 27 September 2012).
- Laminaria saccharina Extract and Vitamin B3 as Whitening Agents. WIPO (P.C.T.). Available online: https://patents.google.com/patent/WO2012011907A1/en?oq=WO2012011907A1 (accessed on 26 January 2012).
- Use of Algal Proteins in Cosmetics. Available online: https://patents.google.com/patent/EP1433463B1/de?oq=EP1433463B1 (accessed on 22 September 2010).
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Rodríguez-Rángel, H.; Arias, D.M.; Morales-Rosales, L.A.; Gonzalez-Huitron, V.; Valenzuela Partida, M.; García, J. Machine Learning Methods Modeling Carbohydrate-Enriched Cyanobacteria Biomass Production in Wastewater Treatment Systems. Energies 2022, 15, 2500. [Google Scholar] [CrossRef]
- Mishra, S.; Pang, S.; Zhang, W.; Lin, Z.; Bhatt, P.; Chen, S. Insights into the microbial degradation and biochemical mechanisms of carbamates. Chemosphere 2021, 279, 130500. [Google Scholar] [CrossRef] [PubMed]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Xinyi, E.; Crofcheck, C.; Crocker, M. Application of Recycled Media and Algae-Based Anaerobic Digestate in Scenedesmus Cultivation. J. Renew. Sust. Energy 2016, 8, 013116. [Google Scholar]
- Kaur, J.; Sarma, A.K.; Jha, M.K.; Gera, P. Valorisation of crude glycerol to value-added products: Perspectives of process technology, economics and environmental issues. Biotechnol. Rep. 2020, 27, e00487. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Ranglová, K.; Masojídek, J.; Drosg, B.; Meixner, K. Digestate as Sustainable Nutrient Source for Microalgae—Challenges and Prospects. Appl. Sci. 2021, 11, 1056. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Randhir, A.; Laird, D.W.; Maker, G.; Trengove, R.; Moheimani, N.R. Microalgae: A potential sustainable commercial source of sterols. Algal Res. 2020, 46, 101772. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Linares-Maurizi, A.; Reversat, G.; Awad, R.; Bultel-Poncé, V.; Oger, C.; Galano, J.-M.; Balas, L.; Durbec, A.; Bertrand-Michel, J.; Durand, T.; et al. Bioactive Oxylipins Profile in Marine Microalgae. Mar. Drugs 2023, 21, 136. [Google Scholar] [CrossRef]
- Hashmi, Z.; Bilad, M.R.; Fahrurrozi; Zaini, J.; Lim, J.W.; Wibisono, Y. Recent Progress in Microalgae-Based Technologies for Industrial Wastewater Treatment. Fermentation 2023, 9, 311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).