Semi-Synthesis of Different Pyranoflavonoid Backbones and the Neurogenic Potential
Abstract
1. Introduction
2. Results and Discussion

2.1. Synthesis
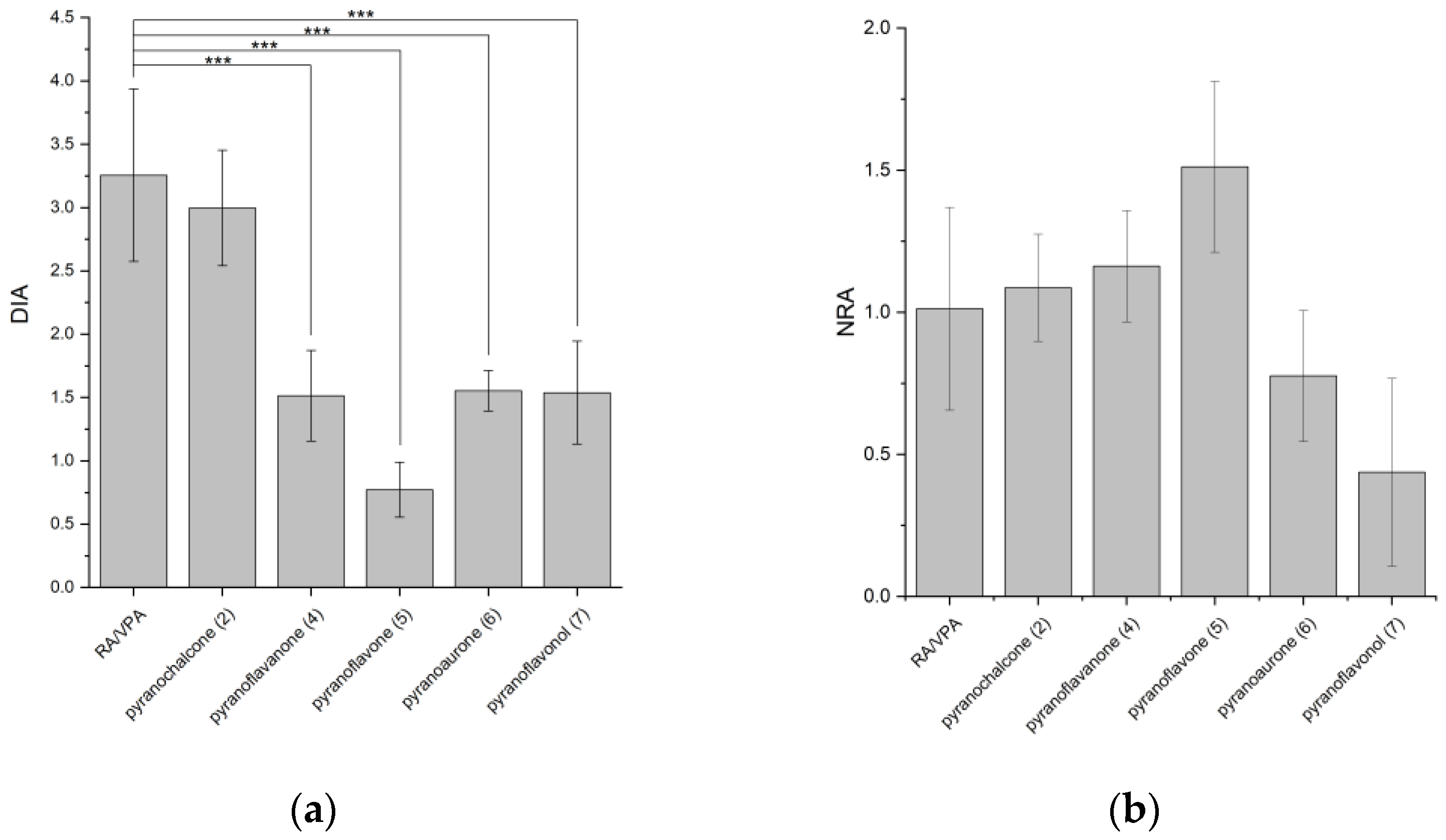
2.2. Neurogenic Potential
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Xanthohumol
3.3. Ring Closure of Prenyl Group to Pyran Ring
3.4. Synthesis of Pyranoflavone
3.5. Synthesis of Pyranoaurones
3.6. Synthesis of Flavonols
3.7. Reporter Assay
3.8. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Elias, D.; Beazely, M.; Kandepu, N. Bioactivities of chalcones. Curr. Med. Chem. 1999, 6, 1125. [Google Scholar]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic Chemistry Follows Where Nature Leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clement, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sanchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef]
- Guan, L.P.; Liu, B.Y. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016, 121, 47–57. [Google Scholar] [CrossRef]
- Hritcu, L.; Ionita, R.; Postu, P.A.; Gupta, G.K.; Turkez, H.; Lima, T.C.; Carvalho, C.U.S.; de Sousa, D.P. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxid. Med. Cell. Longev. 2017, 2017, 5762172. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.; Jeon, S.J.; Son, K.H.; Lee, S.; Yoon, B.H.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. Tanshinone I enhances learning and memory, and ameliorates memory impairment in mice via the extracellular signal-regulated kinase signalling pathway. Br. J. Pharmacol. 2009, 158, 1131–1142. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Chen, X.; Xiao, J. Inhibition of flavonoids on acetylcholine esterase: Binding and structure-activity relationship. Food Funct. 2014, 5, 2582–2589. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity-An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Urmann, C.; Bieler, L.; Priglinger, E.; Aigner, L.; Couillard-Despres, S.; Riepl, H.M. Neuroregenerative Potential of Prenyl- and Pyranochalcones: A Structure-Activity Study. J. Nat. Prod. 2021, 84, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Bieler, L.; Vogl, M.; Kirchinger, M.; Urmann, C.; Riepl, H.; Bandtlow, C.; Klimaschewski, L.; Aigner, L.; Couillard-Despres, S. The Prenylflavonoid ENDF1 Overrules Central Nervous System Growth Inhibitors and Facilitates Regeneration of DRG Neurons. Front. Cell. Neurosci. 2019, 13, 332. [Google Scholar] [CrossRef]
- Oberbauer, E.; Urmann, C.; Steffenhagen, C.; Bieler, L.; Brunner, D.; Furtner, T.; Humpel, C.; Baumer, B.; Bandtlow, C.; Couillard-Despres, S.; et al. Chroman-like cyclic prenylflavonoids promote neuronal differentiation and neurite outgrowth and are neuroprotective. J. Nutr. Biochem. 2013, 24, 1953–1962. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef]
- Gould, E.; Beylin, A.; Tanapat, P.; Reeves, A.; Shors, T.J. Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 1999, 2, 260–265. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Wuertinger, C.; Kandasamy, M.; Caioni, M.; Stadler, K.; Aigner, R.; Bogdahn, U.; Aigner, L. Ageing abolishes the effects of fluoxetine on neurogenesis. Mol. Psychiatry 2009, 14, 856–864. [Google Scholar] [CrossRef]
- Urmann, C.; Riepl, H. Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules 2020, 25, 4007. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, R.C.; Sarpal, P.D. Synthesis of (+/−) Lupinifolin, Di-O-Methyl Xanthohumol and Isoxanthohumol and Related Compounds. Tetrahedron 1978, 34, 3563–3567. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. 2006, 5, 51–63. [Google Scholar]
- Tronina, T.; Strugala, P.; Poplonski, J.; Wloch, A.; Sordon, S.; Bartmanska, A.; Huszcza, E. The Influence of Glycosylation of Natural and Synthetic Prenylated Flavonoids on Binding to Human Serum Albumin and Inhibition of Cyclooxygenases COX-1 and COX-2. Molecules 2017, 22, 1230. [Google Scholar] [CrossRef]
- Venkatesan, P.; Maruthavanan, T. Synthesis of substituted flavone derivatives as potent antimicrobial agents. Bull. Chem. Soc. Ethiop. 2011, 25, 419–425. [Google Scholar] [CrossRef]
- Imafuku, K.; Honda, M.; McOmie, J.F.W. ChemInform Abstract: Cyclodehydrogenation of 2′-Hydroxychalcones (I) with DDQ: A Simple Route for Flavones (III) and Aurones (IV). ChemInform 1987, 18, 224. [Google Scholar] [CrossRef]
- Banerji, A.; Luthria, D.L.; Prabhu, B.R. Prenylated compounds from Atalantia racemosa: Isolation and synthesis of two pyranoflavones. Phytochemistry 1988, 27, 3637–3640. [Google Scholar] [CrossRef]
- Banerji, A.; Luthria, D.L. Benzene Induced1H NMR Shifts of Chromeno-Compounds: An Aid to Differentiate Linear and Angular Chromenoflavones. Spectrosc. Lett. 1990, 23, 1–12. [Google Scholar] [CrossRef]
- Banerji, A.; Luthria, D.L. Structural Elucidation of Isomeric Pyranoflavones by 2D-Noesy. Spectrosc. Lett. 2006, 24, 471–483. [Google Scholar] [CrossRef]
- Kurosawa, K. Manganic Acetate Oxidation of 2′-Hydroxychalcones. Bull. Chem. Soc. Jpn. 1969, 42, 1456–1458. [Google Scholar] [CrossRef]
- Thakkar, K.; Cushman, M. A Novel Oxidative Cyclization of 2′-Hydroxychalcones to 4,5-Dialkoxyaurones by Thallium(III) Nitrate. J. Org. Chem. 1995, 60, 6499–6510. [Google Scholar] [CrossRef]
- Sekizaki, H. Synthesis of 2-Benzylidene-3(2H)-benzofuran-3-ones (Aurones) by Oxidation of 2′-Hydroxychalcones with Mercury(II) Acetate. Bull. Chem. Soc. Jpn. 1988, 61, 1407–1409. [Google Scholar] [CrossRef]
- Roussaki, M.; Costa Lima, S.; Kypreou, A.M.; Kefalas, P.; Cordeiro da Silva, A.; Detsi, A. Aurones: A promising heterocyclic scaffold for the development of potent antileishmanial agents. Int. J. Med. Chem. 2012, 2012, 196921. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.; Kim, H.; Choi, H.; Kim, J.; Jeon, W.H.; Lee, P.H.; Lee, K. Synthesis of Aurones through Silver-catalyzed Intramolecular Cyclization from o-Alkynonylphenols. Bull. Korean Chem. Soc. 2018, 39, 397–400. [Google Scholar] [CrossRef]
- Patil, V.C. Synthesis and in vitro antiplaque activity of chalcone, flavonol and flavanol derivatives. Int. J. Pharm. Sci. Res. 2012, 3, 5006. [Google Scholar]
- Reisch, J.; Hussain, R.; Szendrei, K.; Adesina, S. Extractives from Evodia hupehensis fruit hull, peduncle, twig and leaf. CIV: Natural product chemistry. Pharmazie 1985, 40, 812–813. [Google Scholar]
- Karl, C.; Couillard-Despres, S.; Prang, P.; Munding, M.; Kilb, W.; Brigadski, T.; Plotz, S.; Mages, W.; Luhmann, H.; Winkler, J.; et al. Neuronal precursor-specific activity of a human doublecortin regulatory sequence. J. Neurochem. 2005, 92, 264–282. [Google Scholar] [CrossRef]
- Auld, D.S.; Lovell, S.; Thorne, N.; Lea, W.A.; Maloney, D.J.; Shen, M.; Rai, G.; Battaile, K.P.; Thomas, C.J.; Simeonov, A.; et al. Molecular basis for the high-affinity binding and stabilization of firefly luciferase by PTC124. Proc. Natl. Acad. Sci. USA 2010, 107, 4878–4883. [Google Scholar] [CrossRef]
- Taupin, P. Apigenin and related compounds stimulate adult neurogenesis. Mars, Inc., the Salk Institute for Biological Studies: WO2008147483. Expert Opin. Ther. Pat. 2009, 19, 523–527. [Google Scholar] [CrossRef]
- Namsi, A.; Nury, T.; Hamdouni, H.; Yammine, A.; Vejux, A.; Vervandier-Fasseur, D.; Latruffe, N.; Masmoudi-Kouki, O.; Lizard, G. Induction of Neuronal Differentiation of Murine N2a Cells by Two Polyphenols Present in the Mediterranean Diet Mimicking Neurotrophins Activities: Resveratrol and Apigenin. Diseases 2018, 6, 67. [Google Scholar] [CrossRef]
- Kuntz, S.; Wenzel, U.; Daniel, H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999, 38, 133–142. [Google Scholar] [CrossRef]
- Gerhauser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Ivancic, M.; Hsu, V.L.; Deinzer, M.L. Prenylflavonoids from Humulus lupulus. Phytochemistry 1997, 44, 1575–1585. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urmann, C.; Bieler, L.; Hackl, M.; Chia-Leeson, O.; Couillard-Despres, S.; Riepl, H. Semi-Synthesis of Different Pyranoflavonoid Backbones and the Neurogenic Potential. Molecules 2023, 28, 4023. https://doi.org/10.3390/molecules28104023
Urmann C, Bieler L, Hackl M, Chia-Leeson O, Couillard-Despres S, Riepl H. Semi-Synthesis of Different Pyranoflavonoid Backbones and the Neurogenic Potential. Molecules. 2023; 28(10):4023. https://doi.org/10.3390/molecules28104023
Chicago/Turabian StyleUrmann, Corinna, Lara Bieler, Michael Hackl, Olivia Chia-Leeson, Sebastien Couillard-Despres, and Herbert Riepl. 2023. "Semi-Synthesis of Different Pyranoflavonoid Backbones and the Neurogenic Potential" Molecules 28, no. 10: 4023. https://doi.org/10.3390/molecules28104023
APA StyleUrmann, C., Bieler, L., Hackl, M., Chia-Leeson, O., Couillard-Despres, S., & Riepl, H. (2023). Semi-Synthesis of Different Pyranoflavonoid Backbones and the Neurogenic Potential. Molecules, 28(10), 4023. https://doi.org/10.3390/molecules28104023






