Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination
Abstract
1. Introduction
2. Results and Discussion
2.1. XRD Analysis
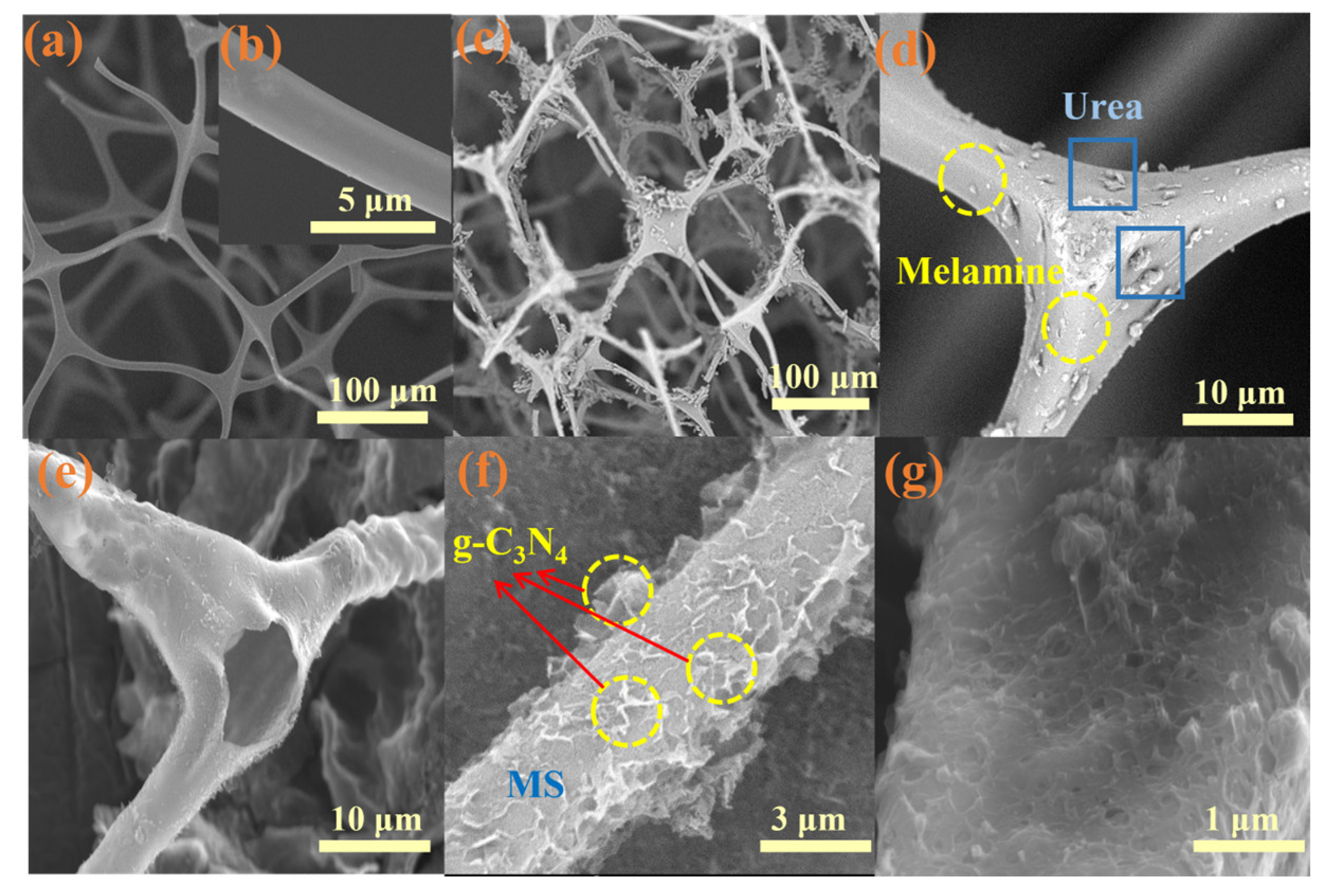
2.2. Morphology
2.3. XPS
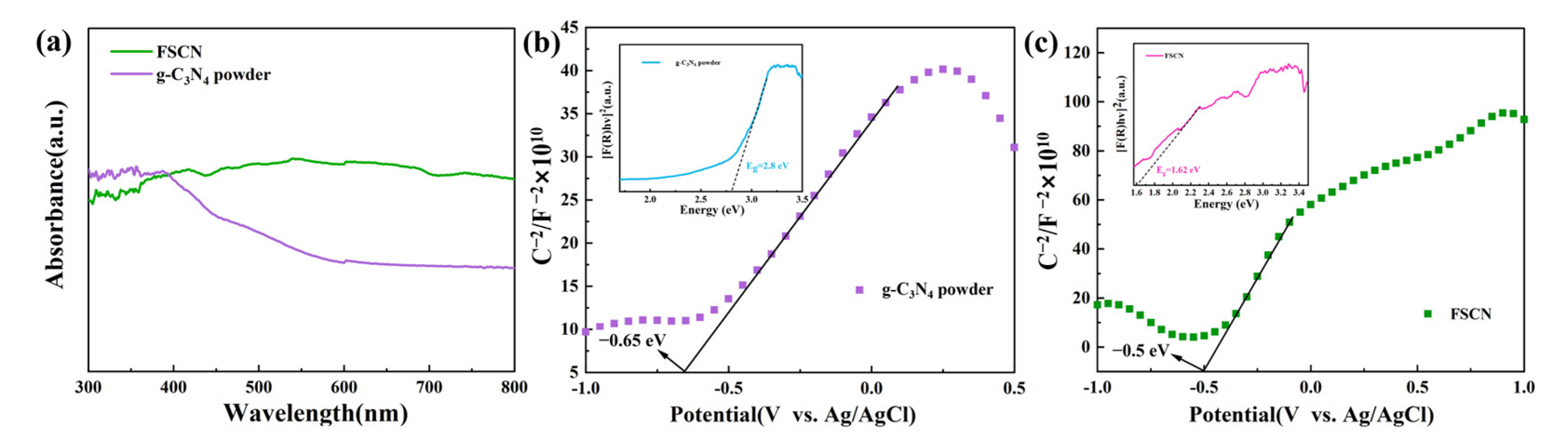
2.4. UV–Visible Spectroscopy
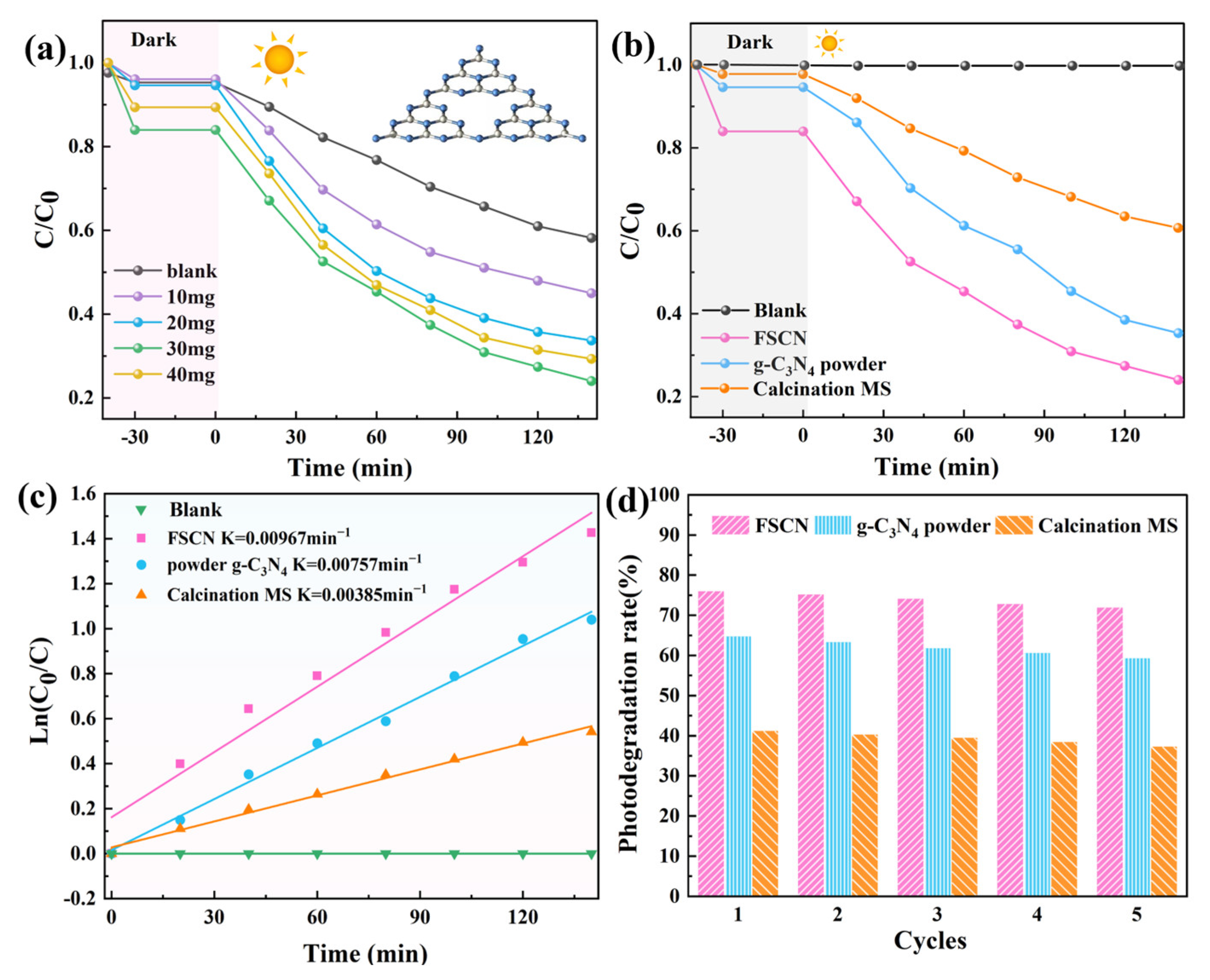
2.5. Photocatalytic Activities
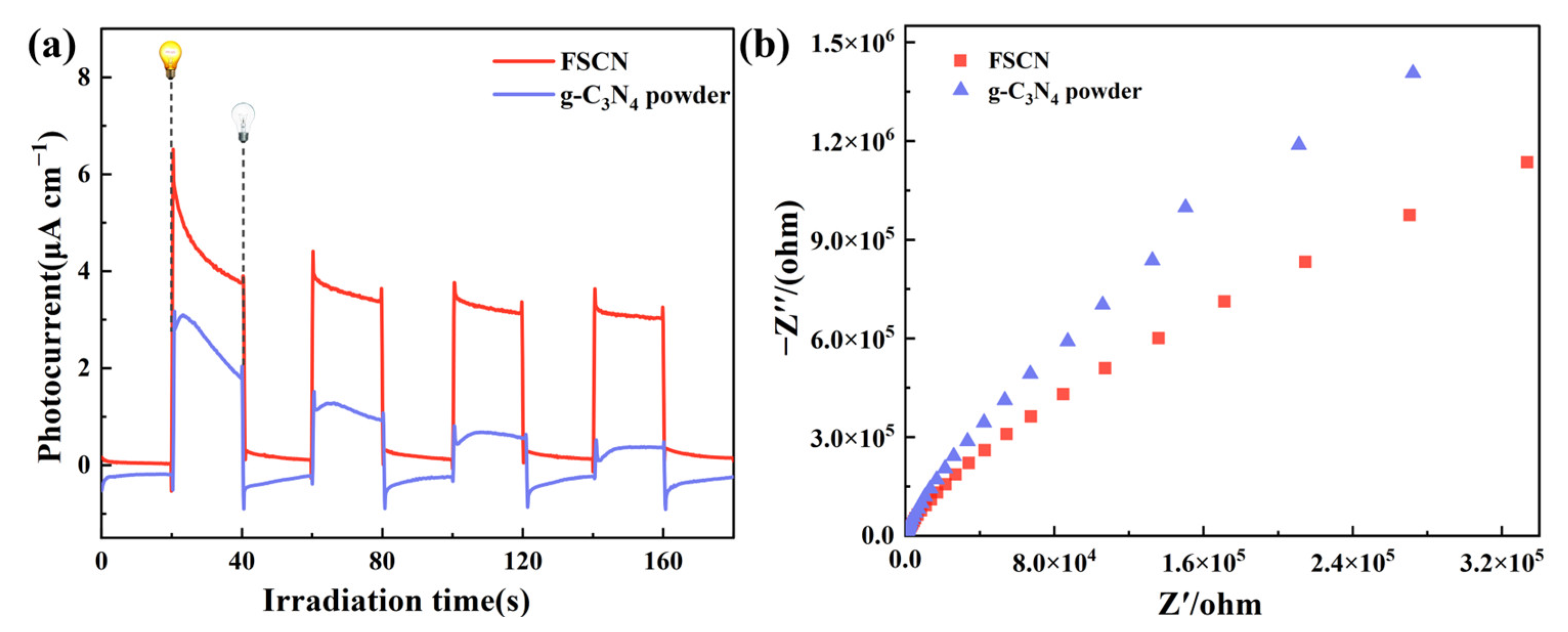
2.6. Electrochemical Test
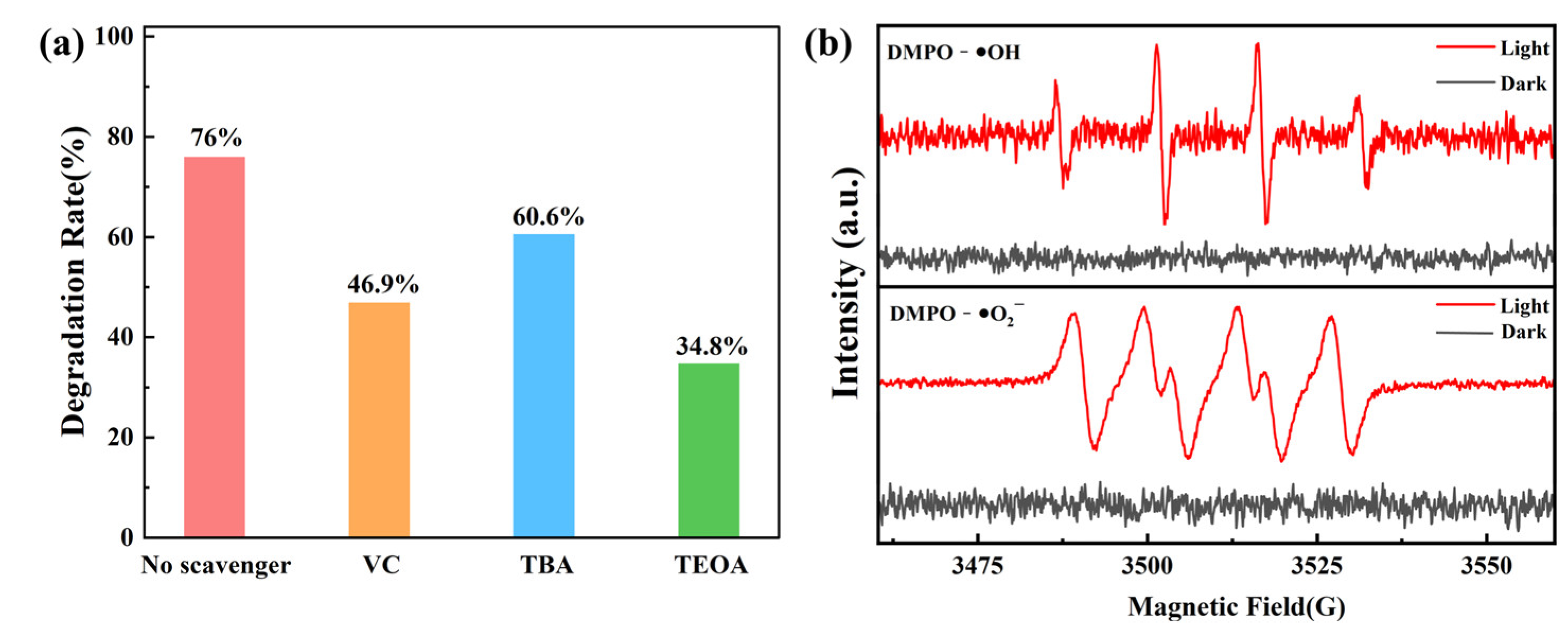
2.7. Radical Trapping and ESR
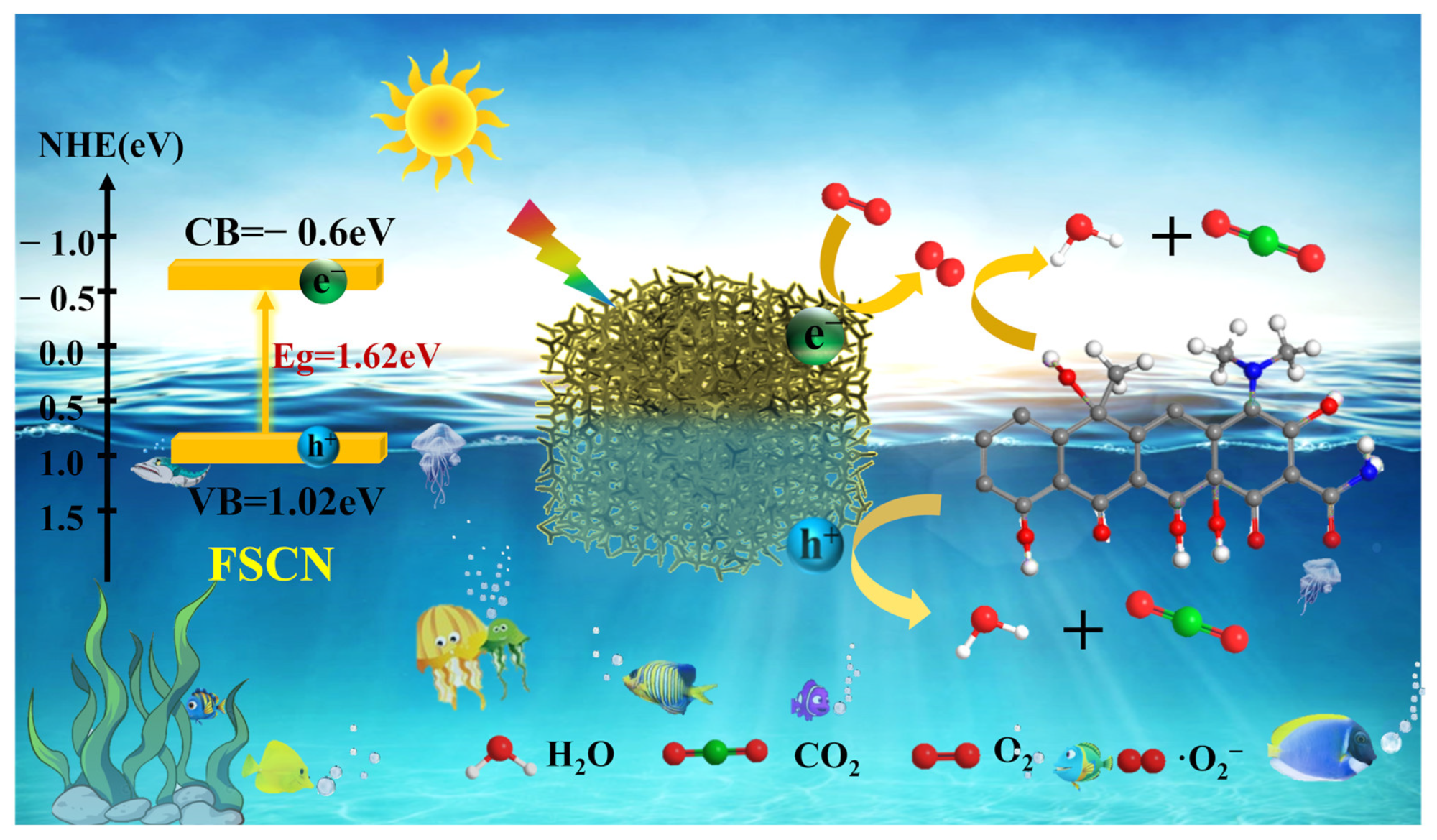
2.8. Photocatalytic Mechanism
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Characterization
3.3. Synthesis of g-C3N4 Powder and FSCN
3.4. Measurement of Photocatalytic Activity
3.4.1. Indoor Experiment
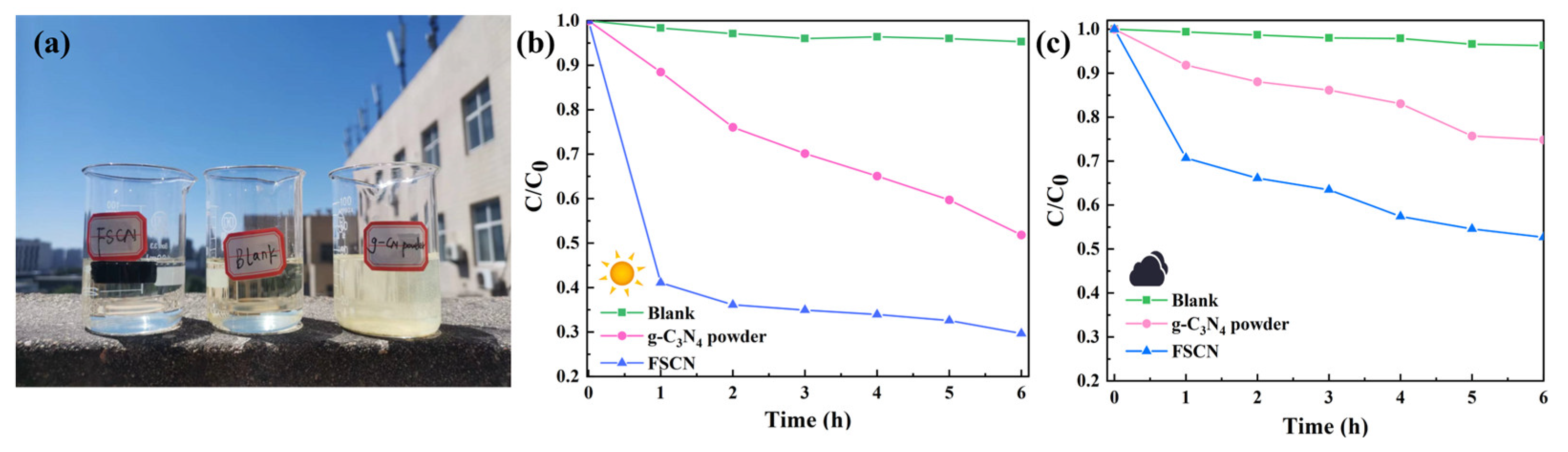
3.4.2. Outdoor Experiment
3.5. Reactive Radical Trapping Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xie, Z.J.; Feng, Y.P.; Wang, F.L.; Chen, D.N.; Zhang, Q.X.; Zeng, Y.Q.; Lv, W.Y.; Liu, G.G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Sun, H.R.; Guo, F.; Pan, J.J.; Huang, W.; Wang, K.; Shi, W.L. One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of sstetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem. Eng. J. 2021, 406, 126844. [Google Scholar] [CrossRef]
- Wang, D.B.; Jia, F.Y.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.B.; Zeng, G.M.; Li, X.M.; Yang, Q.; Yuan, X.Z. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, M.Y.; Renb, H.J.; Huang, X.L.; Hou, W.X.; Wang, C.; Shi, W.L.; Lu, C.Y. Fabrication of p-n CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl. Surf. Sci. 2019, 491, 88–94. [Google Scholar] [CrossRef]
- Guo, F.; Sun, H.R.; Huang, X.L.; Shi, W.L.; Yan, C. Fabrication of TiO2/high-crystalline g-C(3)N(4)composite with enhanced visible-light photocatalytic performance for tetracycline degradation. J. Chem. Technol. Biotechnol. 2020, 95, 2684–2693. [Google Scholar]
- Lu, C.Y.; Wang, J.; Cao, D.L.; Guo, F.; Hao, X.L.; Li, D.; Shi, W.L. Synthesis of magnetically recyclable g-C3N4/NiFe2O4 S-scheme heterojunction photocatalyst with promoted visible-light-response photo-Fenton degradation of tetracycline. Mater. Res. Bull. 2023, 158, 112064. [Google Scholar] [CrossRef]
- Lu, C.Y.; Wang, L.T.; Yang, D.Q.; Jin, Z.H.; Wang, X.; Xu, J.M.; Li, Z.D.; Shi, W.L.; Guan, W.S.; Huang, W. Boosted tetracycline and Cr(VI) simultaneous cleanup over Z-Scheme BiPO4/CuBi2O4 p-n heterojunction with 0D/1D trepang-like structure under simulated sunlight irradiation. J. Alloys Compd. 2022, 919, 165849. [Google Scholar] [CrossRef]
- Zhao, D.M.; Wang, Y.Q.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.H.; Guo, L.J. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- He, F.; Meng, A.Y.; Cheng, B.; Ho, W.K.; Yu, J.G. Enhanced photocatalytic H-2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Lu, C.Y.; Yang, D.Q.; Wang, L.T.; Wen, S.J.; Cao, D.L.; Tu, C.Q.; Gao, L.N.; Li, Y.L.; Zhou, Y.H.; Huang, W. Facile construction of CoO/Bi2WO6 p-n heterojunction with following Z-Scheme pathways for simultaneous elimination of tetracycline and Cr(VI) under visible light irradiation. J. Alloys Compd. 2022, 904, 164046. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, J.L.; Hu, S.W.; Wang, H.L.; Jiang, W.; Chen, X.B. Facile construction of novel Bi2WO6/Ta3N5 Z-scheme heterojunction nanofibers for efficient degradation of harmful pharmaceutical pollutants. Chem. Eng. J. 2020, 402, 126165. [Google Scholar] [CrossRef]
- Lu, C.Y.; Guo, F.; Yan, Q.Z.; Zhang, Z.J.; Li, D.; Wang, L.P.; Zhou, Y.H. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Chen, J.; Wallace, G.G.; Yang, J.P. Engineering electrocatalytic fiber architectures. Prog. Mater. Sci. 2023, 133, 101069. [Google Scholar] [CrossRef]
- Liang, S.J.; Zhou, Z.M.; Wu, X.W.; Zhu, S.Y.; Bi, J.H.; Zhou, L.M.; Liu, M.H.; Wu, L. Constructing a MoS2 QDs/CdS Core/Shell Flower-like Nanosphere Hierarchical Heterostructure for the Enhanced Stability and Photocatalytic Activity. Molecules 2016, 21, 213. [Google Scholar] [CrossRef]
- Bai, J.X.; Shen, R.C.; Jiang, Z.M.; Zhang, P.; Li, Y.J.; Li, X. Integration of 2D layered CdS/WO3 S-scheme heterojunctions and metallic Ti3C2 MXene-based Ohmic junctions for effective photocatalytic H-2 generation. Chin. J. Catal. 2022, 43, 359–369. [Google Scholar] [CrossRef]
- Wu, X.F.; Fang, S.; Zheng, Y.; Sun, J.; Lv, K.L. Thiourea-Modified TiO2 Nanorods with Enhanced Photocatalytic Activity. Molecules 2016, 21, 181. [Google Scholar] [CrossRef]
- Bose, R.; Manna, G.; Jana, S.; Pradhan, N. Ag2S-AgInS2: P-n junction heteronanostructures with quasi type-II band alignment. Chem. Commun. 2014, 50, 3074–3077. [Google Scholar] [CrossRef]
- Nishad, K.K.; Joseph, J.; Tiwari, N.; Kurchania, R.; Pandey, R.K. Investigation on Size Dependent Elemental Binding Energies and Structural Properties of ZnO Nanoparticles and Their Correlation with Observed Photo-Luminescence Behavior. Sci. Adv. Mater. 2015, 7, 1368–1378. [Google Scholar] [CrossRef]
- Elshypany, R.; Selim, H.; Zakaria, K.; Moustafa, A.H.; Sadeek, S.A.; Sharaa, S.I.; Raynaud, P.; Nada, A.A. Magnetic ZnO Crystal Nanoparticle Growth on Reduced Graphene Oxide for Enhanced Photocatalytic Performance under Visible Light Irradiation. Molecules 2021, 26, 2269. [Google Scholar] [CrossRef]
- Tantraviwat, D.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced photoactivity and selectivity over BiOI-decorated Bi2WO6 microflower for selective oxidation of benzylamine: Role of BiOI and mechanism. J. Colloid Interface Sci. 2023, 629, 854–863. [Google Scholar] [CrossRef]
- Guo, F.; Huang, X.L.; Chen, Z.H.; Cao, L.W.; Cheng, X.F.; Chen, L.Z.; Shi, W.L. Construction of Cu3P-ZnSnO3-g-C3N4 p-n-n heterojunction with multiple built-in electric fields for effectively boosting visible-light photocatalytic degradation of broad-spectrum antibiotics. Sep. Purif. Technol. 2021, 265, 118477. [Google Scholar] [CrossRef]
- Bavani, T.; Madhavan, J.; Preeyanghaa, M.; Neppolian, B.; Murugesan, S. Construction of direct Z-scheme g-C3N4/Bi2WO6 heterojunction photocatalyst with enhanced visible light activity towards the degradation of methylene blue. Environ. Sci. Pollut. Res. 2023, 30, 10179–10190. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.S.; Naushad, M.; Vo, D.V.N.; Iqbal, J.; Stadler, F.J. Construction of dual Z-scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 heterojunction for visible and solar powered coupled photocatalytic antibiotic degradation and hydrogen production: Boosting via I−/I3− and Bi3+/Bi5+ redox mediators. Appl. Catal. B Environ. 2021, 284, 119808. [Google Scholar] [CrossRef]
- Guo, F.; Shi, W.L.; Zhu, C.; Li, H.; Kang, Z.H. CoO and g-C3N4 complement each other for highly efficient overall water splitting under visible light. Appl. Catal. B Environ. 2018, 226, 412–420. [Google Scholar] [CrossRef]
- Wei, X.Q.; Wang, X.; Pu, Y.; Liu, A.N.; Chen, C.; Zou, W.X.; Zheng, Y.L.; Huang, J.S.; Zhang, Y.; Yang, Y.C.; et al. Facile ball-milling synthesis of CeO2/g-C3N4 Z-scheme heterojunction for synergistic adsorption and photodegradation of methylene blue: Characteristics, kinetics, models, and mechanisms. Chem. Eng. J. 2021, 420, 127719. [Google Scholar] [CrossRef]
- Szyman, J. Multiple Steady States in the Photocatalytic Reactor for Colored Compounds Degradation. Molecules 2021, 26, 3804. [Google Scholar] [CrossRef]
- Duan, S.F.; Tao, C.L.; Geng, Y.Y.; Yao, X.Q.; Kang, X.W.; Su, J.Z.; Rodriguez-Gutierrez, I.; Kan, M.; Romero, M.; Sun, Y.; et al. Phosphorus-doped Isotype g-C3N4/g-C3N4: An Efficient Charge Transfer System for Photoelectrochemical Water Oxidation. ChemCatChem 2019, 11, 729–736. [Google Scholar] [CrossRef]
- Ho, W.C.J.; Tay, Q.L.; Qi, H.; Huang, Z.H.; Li, J.; Chen, Z. Photocatalytic and Adsorption Performances of Faceted Cuprous Oxide (Cu2O) Particles for the Removal of Methyl Orange (MO) from Aqueous Media. Molecules 2017, 22, 677. [Google Scholar] [CrossRef]
- Bharagav, U.; Reddy, N.R.; Rao, V.N.K.; Ravi, P.; Sathish, M.; Rangappa, D.; Prathap, K.; Chakra, C.S.; Shankar, M.V.; Appels, L.; et al. Bifunctional g-C3N4/carbon nanotubes/WO3 ternary nanohybrids for photocatalytic energy and environmental applications. Chemosphere 2023, 311, 137030. [Google Scholar] [CrossRef]
- Kavil, J.; Anjana, P.M.; Joshy, D.; Babu, A.; Raj, G.; Periyat, P.; Rakhi, R.B. g-C3N4/CuO and g-C3N4/Co3O4 nanohybrid structures as efficient electrode materials in symmetric supercapacitors. RSC Adv. 2019, 9, 38430–38437. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, C.H.; Chen, J.H.; Chen, L.Y. Influence of B, Zn, and B-Zn doping on electronic structure and optical properties of g-C3N4 photocatalyst: A first-principles study. Results Phys. 2021, 26, 104338. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Ahmadzadeh, H.; Goharshadi, E.K. Porous perovskite-lanthanum cobaltite as an efficient cocatalyst in photoelectrocatalytic water oxidation by bismuth doped g-C3N4. Sol. Energy 2021, 227, 426–437. [Google Scholar] [CrossRef]
- Tang, J.L.; Wang, J.J.; Tang, L.; Feng, C.Y.; Zhu, X.; Yi, Y.Y.; Feng, H.P.; Yu, J.F.; Ren, X.Y. Preparation of floating porous g-C3N4 photocatalyst via a facile one-pot method for efficient photocatalytic elimination of tetracycline under visible light irradiation. Chem. Eng. J. 2022, 430, 132669. [Google Scholar] [CrossRef]
- Meng, X.F.; Zhuang, Y.; Tang, H.; Lu, C.H. Hierarchical structured ZnFe2O4@SiO2@TiO2 composite for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2018, 761, 15–23. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhu, Y.F.; Zhu, G.X.; Zhang, Z.J.; Chen, X.J.; Yao, W.Q. Three-dimensional photocatalysts with a network structure. J. Mater. Chem. A 2017, 5, 5661–5679. [Google Scholar] [CrossRef]
- Sevastaki, M.; Suchea, M.P.; Kenanakis, G. 3D Printed Fully Recycled TiO2-Polystyrene Nanocomposite Photocatalysts for Use against Drug Residues. Nanomaterials 2020, 10, 2144. [Google Scholar] [CrossRef]
- Li, X.B.; Xiong, J.; Gao, X.M.; Huang, J.T.; Feng, Z.J.; Chen, Z.; Zhu, Y.F. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Compd. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Kim, D.; Kim, D.W.; Buyukcakir, O.; Kim, M.K.; Polychronopoulou, K.; Coskun, A. Highly Hydrophobic ZIF-8/Carbon Nitride Foam with Hierarchical Porosity for Oil Capture and Chemical Fixation of CO2. Adv. Funct. Mater. 2017, 27, 1700706. [Google Scholar] [CrossRef]
- Zhang, M.X.; Du, H.X.; Ji, J.; Li, F.F.; Lin, Y.C.; Qin, C.W.; Zhang, Z.; Shen, Y. Highly Efficient Ag3PO4/g-C3N4 Z-Scheme Photocatalyst for Its Enhanced Photocatalytic Performance in Degradation of Rhodamine B and Phenol. Molecules 2021, 26, 2062. [Google Scholar] [CrossRef]
- Kumaresan, N.; Sinthiya, M.M.A.; Kumar, M.P.; Ravichandran, S.; Babu, R.R.; Sethurman, K.; Ramamurthi, K. Investigation on the g-C3N4 encapsulated ZnO nanorods heterojunction coupled with GO for effective photocatalytic activity under visible light irradiation. Arabian J. Chem. 2020, 13, 2826–2843. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Fu, X.L.; Peng, Z.J. One-Step Calcination to Gain Exfoliated g-C3N4/MoO2 Composites for High-Performance Photocatalytic Hydrogen Evolution. Molecules 2022, 27, 7178. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, X.J.; Sun, Q.N.; Yuan, M.; Sun, Z.H.; Xia, S.Q.; Zhao, J.F. Enhanced visible light photo-Fenton-like degradation of tetracyclines by expanded perlite supported FeMo3Ox/g-C3N4 floating Z-scheme catalyst. J. Hazard. Mater. 2022, 424, 127387. [Google Scholar] [CrossRef]
- Yousefi, M.; Eshghi, H.; Karimi-Nazarabad, M.; Farhadipour, A. P5W30/g-C3N4 heterojunction thin film with improved photoelectrochemical performance for solar water splitting. New J. Chem. 2020, 44, 20470–20478. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.H.; Ye, X.J.; Guo, F.S.; Wang, X.C. Helical Graphitic Carbon Nitrides with Photocatalytic and Optical Activities. Angew. Chem. Int. Ed. 2014, 53, 11926–11930. [Google Scholar] [CrossRef]
- Mao, Z.Y.; Chen, J.J.; Yang, Y.F.; Wang, D.J.; Bie, L.J.; Fahlman, B.D. Novel g-C3N4/CoO Nanocomposites with Significantly Enhanced Visible-Light Photocatalytic Activity for H-2 Evolution. ACS Appl. Mater. Interface 2017, 9, 12427–12435. [Google Scholar] [CrossRef]
- Tan, S.Y.; Xing, Z.P.; Zhang, J.Q.; Li, Z.Z.; Wu, X.Y.; Cui, J.Y.; Kuang, J.Y.; Yin, J.W.; Zhou, W. Meso-g-C3N4/g-C3N4 nanosheets laminated homojunctions as efficient visible-light-driven photocatalysts. Int. J. Hydrogen Energy 2017, 42, 25969–25979. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, W.X.; He, D.L.; Yue, S.T.; Huang, H. Construction of Heterostructured g-C3N4/Ag/TiO2 Microspheres with Enhanced Photocatalysis Performance under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Sun, J.W.; Yang, S.R.; Liang, Z.Q.; Liu, X.; Qiu, P.Y.; Cui, H.Z.; Tian, J. Two-dimensional/one-dimensional molybdenum sulfide (MoS2) nanoflake/graphitic carbon nitride (g-C3N4) hollow nanotube photocatalyst for enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2020, 567, 300–307. [Google Scholar] [CrossRef]
- Qian, X.F.; Wu, Y.W.; Kan, M.; Fang, M.Y.; Yue, D.T.; Zeng, J.; Zhao, Y.X. FeOOH quantum dots coupled g-C3N4 for visible light driving photo- Fenton degradation of organic pollutants. Appl. Catal. B Environ. 2018, 237, 513–520. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.L.; Zeng, G.M.; Huang, J.H.; Lai, C.; Zhou, C.Y.; Wang, W.J.; Guo, H.; Xue, W.J.; et al. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2019, 245, 87–99. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g-C3N4)-based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Fu, J.W.; Xu, Q.L.; Low, J.X.; Jiang, C.J.; Yu, J.G. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H-2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Kianipour, S.; Razavi, F.S.; Hajizadeh-Oghaz, M.; Abdulsahib, W.K.; Mahdi, M.A.; Jasim, L.S.; Salavati-Niasari, M. The synthesis of the P/N-type NdCoO3/g-C3N4 nano-heterojunction as a high-performance photocatalyst for the enhanced photocatalytic degradation of pollutants under visible-light irradiation. Arabian J. Chem. 2022, 15, 103840. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, C.C.; Cai, M.J.; Yang, F.; Liu, Y.P.; Chen, J.L.; Zhang, P.; Li, X.; Chen, X.B. Facile fabrication of TaON/Bi2MoO6 core-shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- Huang, L.Y.; Zhang, R.X.; Sun, X.J.; Cheng, X.N. Synthesis and Characterization of g-C3N4/Alpha-Fe2O3 Composites with Enhanced Photocatalytic Activity. In Proceedings of the 2nd International Congress on Advanced Materials (ICAM), Zhenjiang, China, 16–19 May 2013; Jiangsu University: Zhenjiang, China, 2014; pp. 225–228. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, D.; Wang, X.; Zhang, H.; Yang, D.; Yin, Z.; Liu, Z.; Lu, C.; Guo, F. Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination. Molecules 2023, 28, 3989. https://doi.org/10.3390/molecules28103989
Cao D, Wang X, Zhang H, Yang D, Yin Z, Liu Z, Lu C, Guo F. Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination. Molecules. 2023; 28(10):3989. https://doi.org/10.3390/molecules28103989
Chicago/Turabian StyleCao, Delu, Xueying Wang, Hefan Zhang, Daiqiong Yang, Ze Yin, Zhuo Liu, Changyu Lu, and Feng Guo. 2023. "Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination" Molecules 28, no. 10: 3989. https://doi.org/10.3390/molecules28103989
APA StyleCao, D., Wang, X., Zhang, H., Yang, D., Yin, Z., Liu, Z., Lu, C., & Guo, F. (2023). Rational Design of Monolithic g-C3N4 with Floating Network Porous-like Sponge Monolithic Structure for Boosting Photocatalytic Degradation of Tetracycline under Simulated and Natural Sunlight Illumination. Molecules, 28(10), 3989. https://doi.org/10.3390/molecules28103989






