Microwave-Assisted Cu-Catalyzed Diaryletherification for Facile Synthesis of Bioactive Prenylated Diresorcinols
Abstract
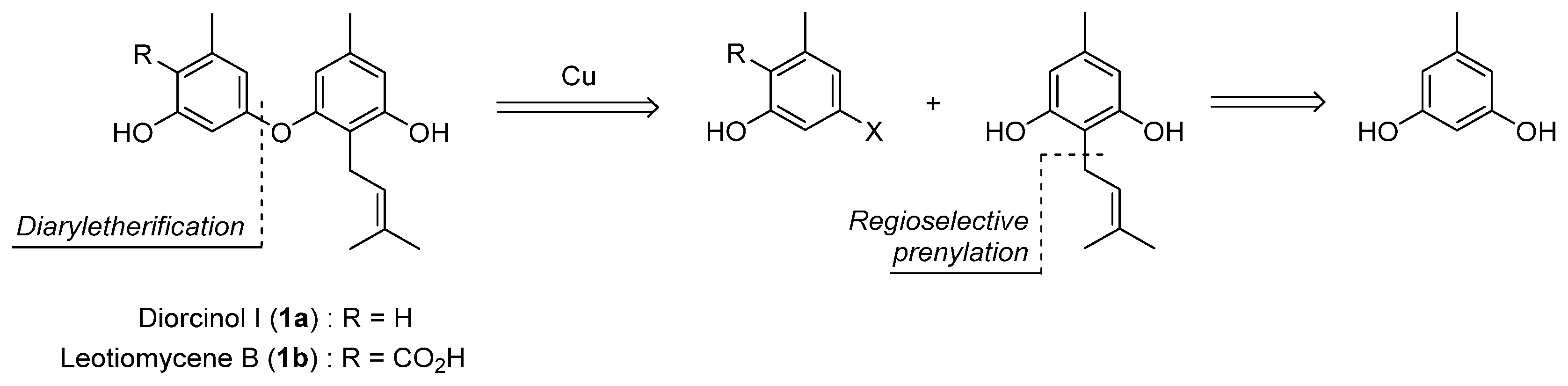
1. Introduction
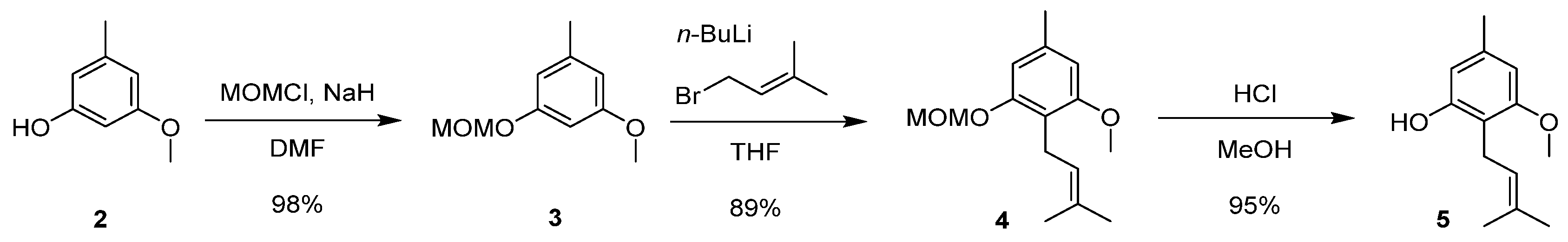
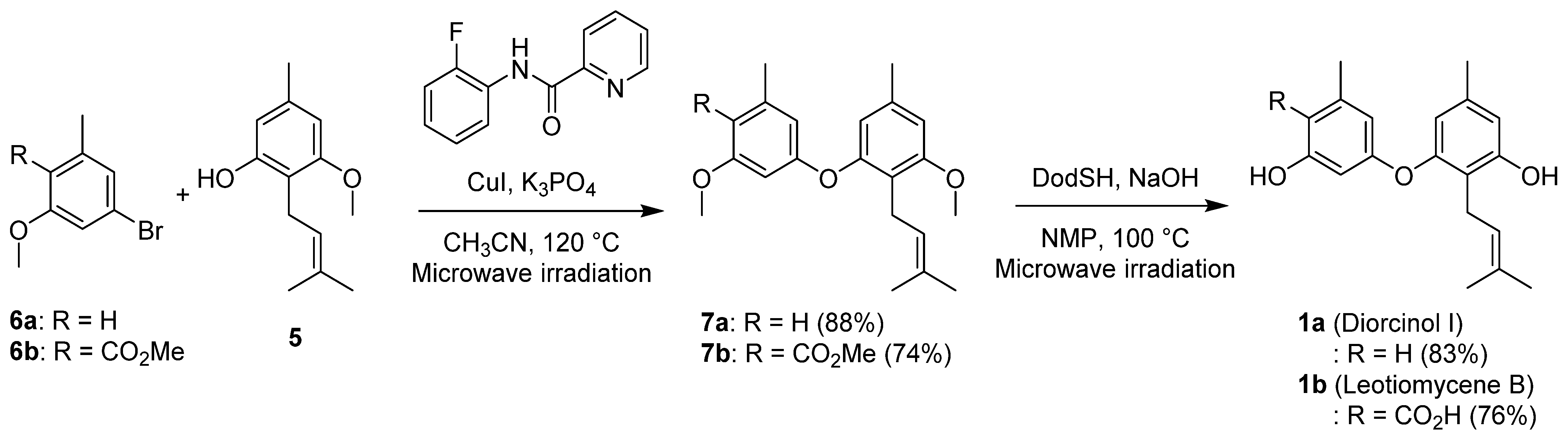
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Details
3.2. Preparation of 5
3.3. Synthesis of 7a and 7b
3.4. Synthesis of Diorcinol I (1a) and Leotiomycene (1b)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brezani, V.; Smejkal, K.; Hosek, J.; Tomasova, V. Anti-inflammatory natural prenylated phenolic compounds-potential lead substances. Curr. Med. Chem. 2018, 25, 1094–1159. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Jiang, Y.; Yang, B. An update of prenylated phenolics: Food sources, chemistry and health benefits. Trends Food Sci. Technol. 2021, 108, 197–213. [Google Scholar] [CrossRef]
- Li, X.B.; Zhou, Y.H.; Zhu, R.X.; Chang, W.Q.; Yuan, H.Q.; Gao, W.; Zhang, L.L.; Zhao, Z.T.; Lou, H.X. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem. Biodivers. 2015, 12, 575–592. [Google Scholar] [CrossRef]
- Boehlich, G.J.; de Vries, J.; Geismar, O.; Gudzuhn, M.; Streit, W.R.; Wicha, S.G.; Schützenmeister, N. Total Synthesis of Anti-MRSA Active Diorcinols and Analogues. Chem.–A Eur. J. 2020, 26, 9846–9850. [Google Scholar] [CrossRef]
- Paguigan, N.D.; Rivera-Chávez, J.; Stempin, J.J.; Augustinović, M.; Noras, A.I.; Raja, H.A.; Todd, D.A.; Triplett, K.D.; Day, C.; Figueroa, M. Prenylated diresorcinols inhibit bacterial quorum sensing. J. Nat. Prod. 2019, 82, 550–558. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, L.; Cai, S.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Diorcinols BE, new prenylated diphenyl ethers from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Antibiot. 2013, 66, 539–542. [Google Scholar] [CrossRef]
- Wang, X.; Mou, Y.; Hu, J.; Wang, N.; Zhao, L.; Liu, L.; Wang, S.; Meng, D. Cytotoxic Polyphenols from a Sponge-Associated Fungus Aspergillus versicolor Hmp-48. Chem. Biodivers. 2014, 11, 133–139. [Google Scholar] [CrossRef]
- Liu, L.; Liu, R.; Basnet, B.B.; Bao, L.; Han, J.; Wang, L.; Liu, H. New phenolic bisabolane sesquiterpenoid derivatives with cytotoxicity from Aspergillus tennesseensis. J. Antibiot. 2018, 71, 538–542. [Google Scholar] [CrossRef]
- Hu, S.-s.; Jiang, N.; Wang, X.-l.; Chen, C.-j.; Fan, J.-y.; Wurin, G.; Ge, H.-m.; Tan, R.-x.; Jiao, R.-H. Prenylated diphenyl ethers from the mantis-associated fungus Aspergillus versicolor GH-2. Tetrahedron Lett. 2015, 56, 3894–3897. [Google Scholar] [CrossRef]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Diorcinol D exerts fungicidal action against Candida albicans through cytoplasm membrane destruction and ROS accumulation. PLoS ONE 2015, 10, e0128693. [Google Scholar] [CrossRef]
- Li, Y.; Chang, W.; Zhang, M.; Li, X.; Jiao, Y.; Lou, H. Synergistic and drug-resistant reversing effects of diorcinol D combined with fluconazole against Candida albicans. FEMS Yeast Res. 2015, 15, fov001. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.-Y.; Li, L.; Yang, C.-G.; Luo, D.-Q.; Zheng, Z.-H.; Lu, X.-H.; Shi, B.-Z. A novel oxybis cresol verticilatin with highly varying degrees of biological activities from the insect pathogenic fungus Paecilomyces verticillatus. J. Asian Nat. Prod. Res. 2014, 16, 1153–1157. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-M.; Shang, Z.; Li, C.-S.; Ji, N.-Y.; Wang, B.-G. Meroterpenoid and diphenyl ether derivatives from Penicillium sp. MA-37, a fungus isolated from marine mangrove rhizospheric soil. J. Nat. Prod. 2012, 75, 1888–1895. [Google Scholar] [CrossRef]
- Gao, X.-M.; Yu, T.; Lai, F.S.F.; Zhou, Y.; Liu, X.; Qiao, C.-F.; Song, J.-Z.; Chen, S.-L.; Luo, K.Q.; Xu, H.-X. Identification and evaluation of apoptotic compounds from Garcinia paucinervis. Bioorg. Med. Chem. 2010, 18, 4957–4964. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Xue, J.; Li, X.; Li, D.; Li, Z.; Hua, H. New depsidone and dichromone from the stems of Garcinia paucinervis with antiproliferative activity. J. Nat. Med. 2019, 73, 278–282. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Xu, H.; Yin, L.; Chen, Z.; Shen, H. Diphenyl ethers from a marine-derived isolate of Aspergillus sp. CUGB-F046. Nat. Prod. Res. 2018, 32, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Permana, D.; Lajis, N.H.; Shaari, K.; Ali, A.M.; Mackeen, M.M.; Kitajima, M.; Takayama, H.; Aimi, N. A new prenylated hydroquinone from the roots of Garcinia atroviridis Griff ex T. Anders (Guttiferae). Z. Nat. B 2003, 58, 332–335. [Google Scholar] [CrossRef]
- Permana, D.; Lajis, N.H.; Mackeen, M.M.; Ali, A.M.; Aimi, N.; Kitajima, M.; Takayama, H. Isolation and Bioactivities of Constitutents of the Roots of Garcinia a troviridis. J. Nat. Prod. 2001, 64, 976–979. [Google Scholar] [CrossRef]
- Syahida, A.; Israf, D.A.; Permana, D.; Lajis, N.; Khozirah, S.; Afiza, A.; Khaizurin, T.; Somchit, M.; Sulaiman, M.; Nasaruddin, A. Atrovirinone inhibits pro-inflammatory mediator release from murine macrophages and human whole blood. Immunol. Cell Biol. 2006, 84, 250–258. [Google Scholar] [CrossRef]
- Gudzuhn, M.; Alio, I.; Moll, R.; de Vries, J.; Boehlich, J.; Assmann, M.; Janneschütz, J.; Schützenmeister, N.; Himmelbach, A.; Poehlein, A. Molecular Insight into Gene Response of Diorcinol-and Rubrolide-Treated Biofilms of the Emerging Pathogen Stenotrophomonas maltophilia. Microbiol. Spectr. 2022, e02582-21. [Google Scholar] [CrossRef]
- Enthaler, S. Palladium-catalysed hydroxylation and alkoxylation. Chem. Soc. Rev. 2011, 40, 4912–4924. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Pawar, G.G.; Kumar, S.V.; Jiang, Y.; Ma, D. Selected Copper-Based Reactions for C−N, C−O, C−S, and C−C Bond Formation. Angew. Chem. Int. Ed. 2017, 56, 16136–16179. [Google Scholar] [CrossRef] [PubMed]
- Fui, C.J.; Sarjadi, M.S.; Sarkar, S.M.; Rahman, M.L. Recent advancement of ullmann condensation coupling reaction in the formation of aryl-oxygen (CO) bonding by copper-mediated catalyst. Catalysts 2020, 10, 1103. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F.; Irfan, M.; Bokhari, T.H. Recent green synthetic approaches toward Ullmann reaction: A review. Chem. Pap. 2022, 1–19. [Google Scholar] [CrossRef]
- Ryu, M.; Kim, M.; Jeong, M.; Jang, J.; Lee, M.; Jin, H.-E.; Jung, J.-W. Studies on the alkylation of phenolate in an organofluorine solvent and its application to the synthesis of myrsinoic acids A and E. Synth. Commun. 2017, 47, 818–824. [Google Scholar] [CrossRef]
- Simas, A.B.; Coelho, A.L.; Costa, P.R. Regioselective lithiation of resorcinol derivatives: Synthesis of mono O-MOM-and O-benzylresorcinols prenylated at C-2 or C-4 positions. Synthesis 1999, 1999, 1017–1021. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Min, X.-T.; Ji, D.-W.; Chen, Q.-A. Catalytic prenylation and reverse prenylation of aromatics. Trends Chem. 2022, 4, 658–675. [Google Scholar] [CrossRef]
- Shen, L.; Simmons, C.J.; Sun, D. Microwave-assisted synthesis of macrocycles via intramolecular and/or bimolecular Ullmann coupling. Tetrahedron Lett. 2012, 53, 4173–4178. [Google Scholar] [CrossRef]
- Maiti, D.; Buchwald, S.L. Cu-catalyzed arylation of phenols: Synthesis of sterically hindered and heteroaryl diaryl ethers. J. Org. Chem. 2010, 75, 1791–1794. [Google Scholar] [CrossRef]
- He, H.; Wu, Y.-J. Synthesis of diaryl ethers through the copper-catalyzed arylation of phenols with aryl halides using microwave heating. Tetrahedron Lett. 2003, 44, 3445–3446. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Unger, J.B.; Taft, B.R. Copper-in-charcoal (Cu/C) promoted diaryl ether formation. Org. Lett. 2007, 9, 1089–1092. [Google Scholar] [CrossRef]
- Collins, J.C.; Farley, K.A.; Limberakis, C.; Liras, S.; Price, D.; James, K. Macrocyclizations for medicinal chemistry: Synthesis of druglike macrocycles by high-concentration Ullmann coupling. J. Org. Chem. 2012, 77, 11079–11090. [Google Scholar] [CrossRef]
- Sambiagio, C.; Munday, R.H.; Marsden, S.P.; Blacker, A.J.; McGowan, P.C. Picolinamides as Effective Ligands for Copper-Catalysed Aryl Ether Formation: Structure–Activity Relationships, Substrate Scope and Mechanistic Investigations. Chem.–A Eur. J. 2014, 20, 17606–17615. [Google Scholar] [CrossRef]
- Chae, J. Practical demethylation of aryl methyl ethers using an odorless thiol reagent. Arch. Pharmacal Res. 2008, 31, 305–309. [Google Scholar] [CrossRef]

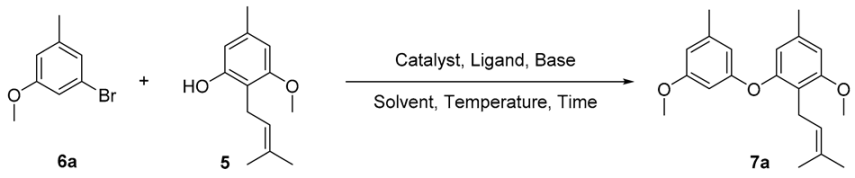 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Ligand (mol%) | Base (eq) | Solvent | Temperature (°C) | Time (h) | Yield a |
| 1 [28] | CuO (250) | - | K2CO3 (1) | Pyridine | 200 b | 1 | ND c |
| 2 [29] | CuI (10) | Picolinic acid (20) | K3PO4 (2) | DMSO | 90 | 30 | ND |
| 3 | CuI (10) | Picolinic acid (20) | K3PO4 (2) | DMSO | 120 b | 0.5 | 27% |
| 4 | CuI (10) | Picolinic acid (20) | K3PO4 (2) | DMSO | 150 b | 0.5 | 50% |
| 5 | CuI (10) | Picolinic acid (20) | K3PO4 (2) | DMSO | 200 b | 0.5 | 33% |
| 6 | CuI (10) | Pyrrole-2-carboxylic acid (20) | K3PO4 (2) | DMSO | 150 b | 0.5 | ND |
| 7 [33] | Cul (10) | N-(2-fluorophenyl) picolinamide (10) | Cs2CO3 (2) | CH3CN | 90 | 24 | 29% |
| 8 | Cul (10) | N-(2-fluorophenyl) picolinamide (10) | Cs2CO3 (2) | CH3CN | 90 b | 0.5 | 40% |
| 9 | Cul (10) | N-(2-fluorophenyl) picolinamide (10) | Cs2CO3 (2) | CH3CN | 120 b | 0.5 | 45% |
| 10 | Cul (10) | N-(2-fluorophenyl) picolinamide (10) | K3PO4 (2) | CH3CN | 120 b | 0.5 | 88% |
| 11 | Cul (10) | N-(2-fluorophenyl) picolinamide (10) | K3PO4 (2) | CH3CN | 150 b | 0.5 | 46% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, S.; Kang, B.; Jung, J.-W. Microwave-Assisted Cu-Catalyzed Diaryletherification for Facile Synthesis of Bioactive Prenylated Diresorcinols. Molecules 2023, 28, 62. https://doi.org/10.3390/molecules28010062
Jo S, Kang B, Jung J-W. Microwave-Assisted Cu-Catalyzed Diaryletherification for Facile Synthesis of Bioactive Prenylated Diresorcinols. Molecules. 2023; 28(1):62. https://doi.org/10.3390/molecules28010062
Chicago/Turabian StyleJo, Seoyoung, Bohun Kang, and Jong-Wha Jung. 2023. "Microwave-Assisted Cu-Catalyzed Diaryletherification for Facile Synthesis of Bioactive Prenylated Diresorcinols" Molecules 28, no. 1: 62. https://doi.org/10.3390/molecules28010062
APA StyleJo, S., Kang, B., & Jung, J.-W. (2023). Microwave-Assisted Cu-Catalyzed Diaryletherification for Facile Synthesis of Bioactive Prenylated Diresorcinols. Molecules, 28(1), 62. https://doi.org/10.3390/molecules28010062








