Research Progress of H2S Donors Conjugate Drugs Based on ADTOH
Abstract
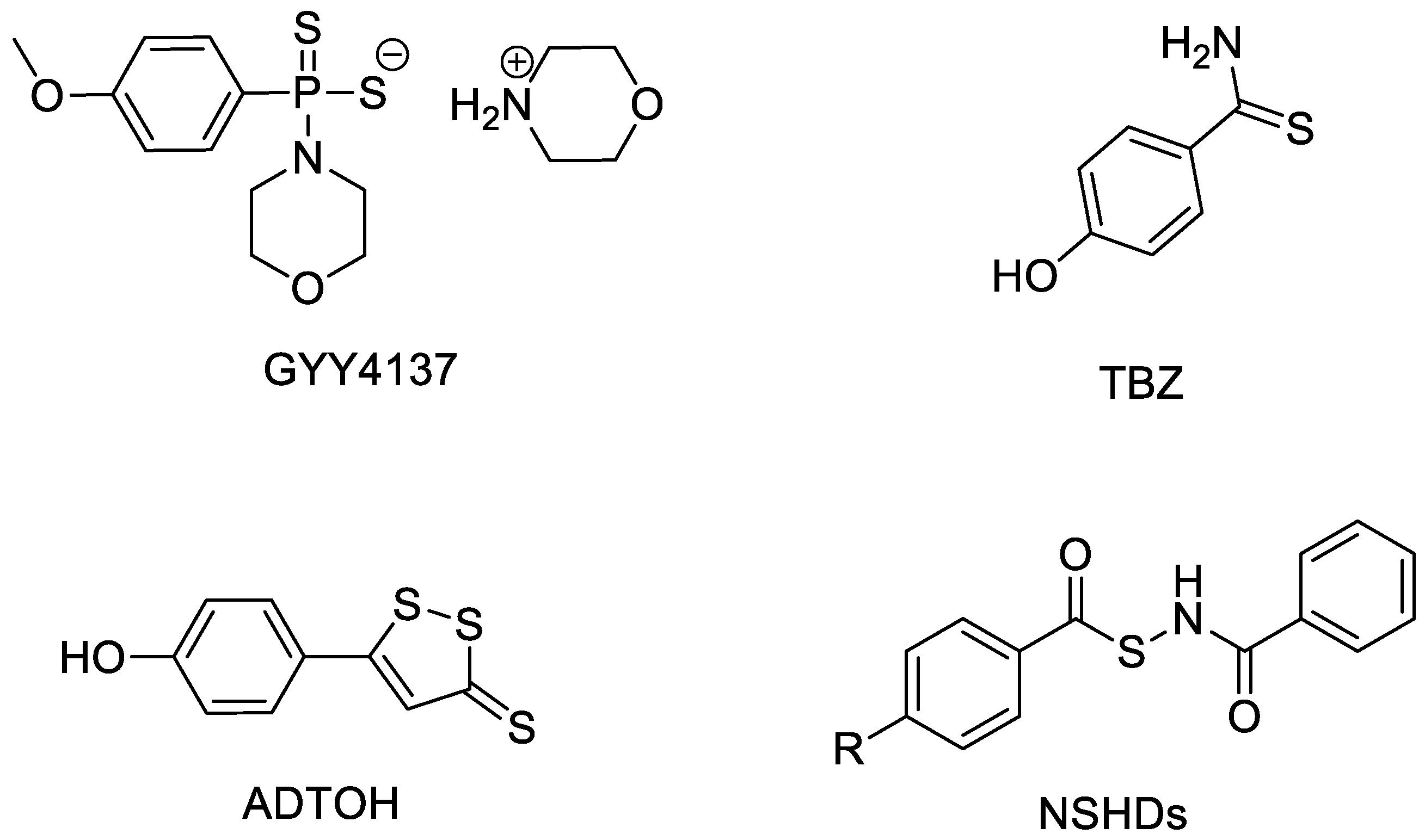
1. H2S and H2S Donors
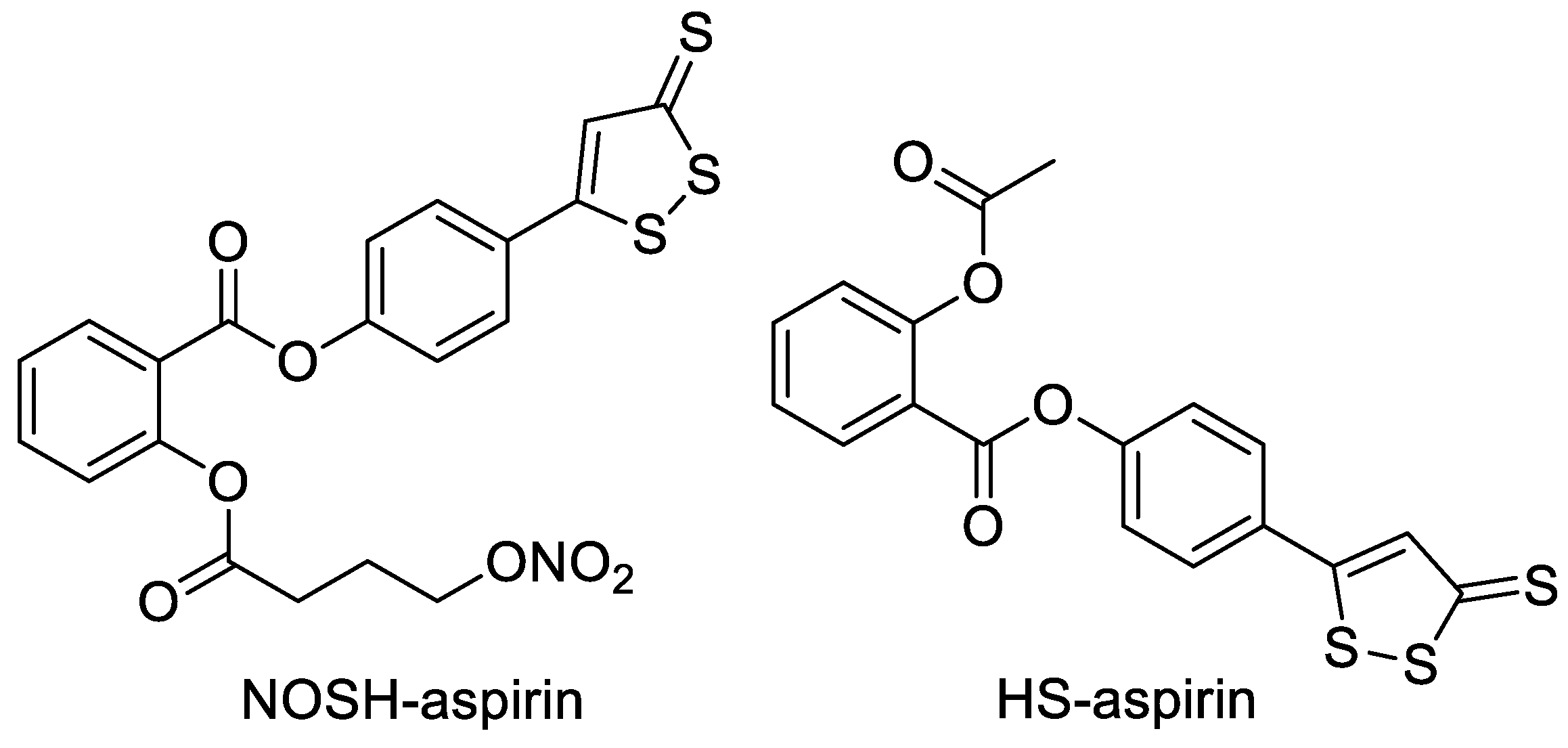
2. ADTOH and Its Conjugates
2.1. ADTOH–NSAID Conjugates
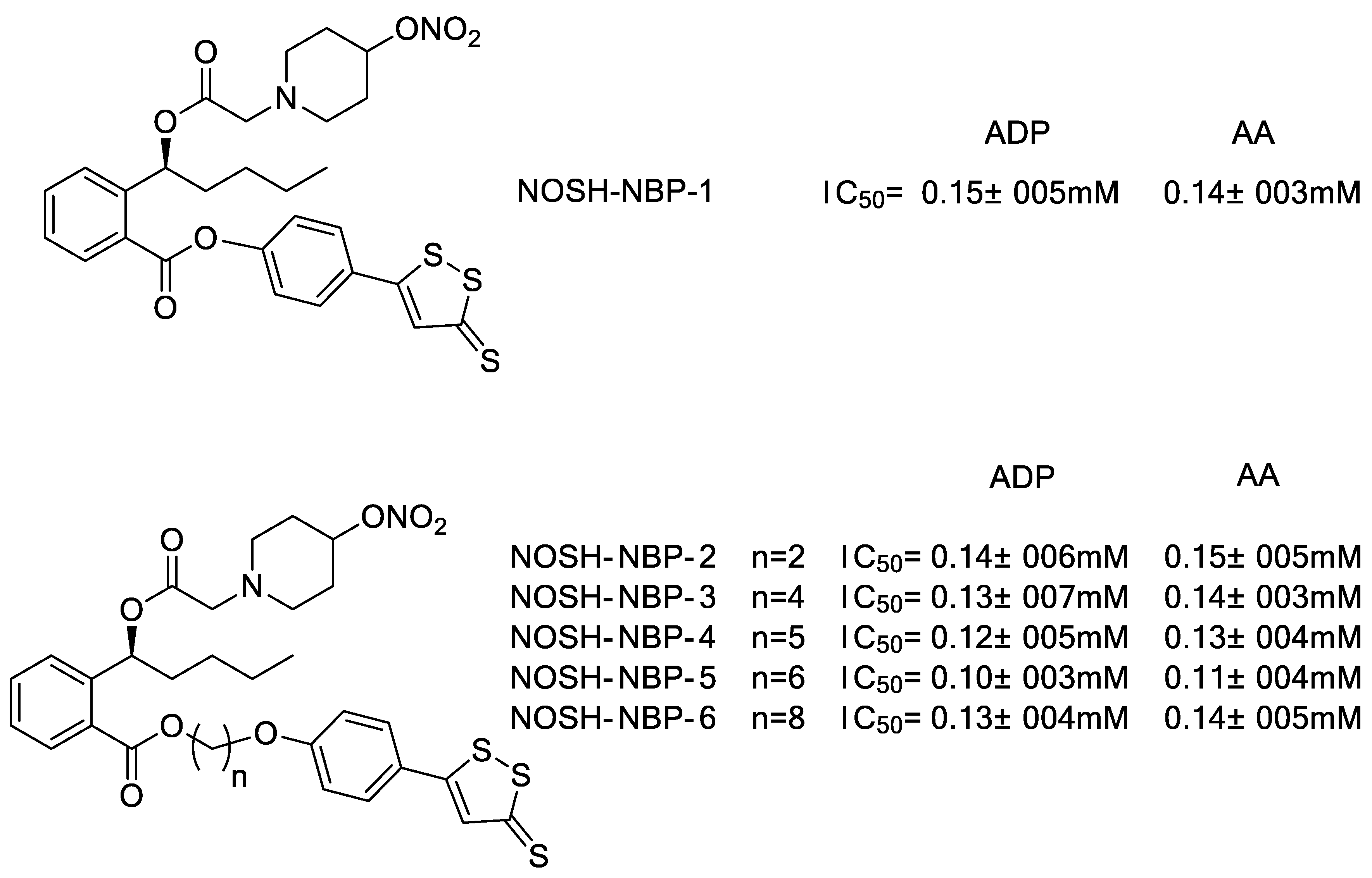
2.2. ADTOH–Butylphthalide Conjugates
2.3. ADTOH–Niacin Conjugates
2.4. ADTOH–Levodopa Conjugates
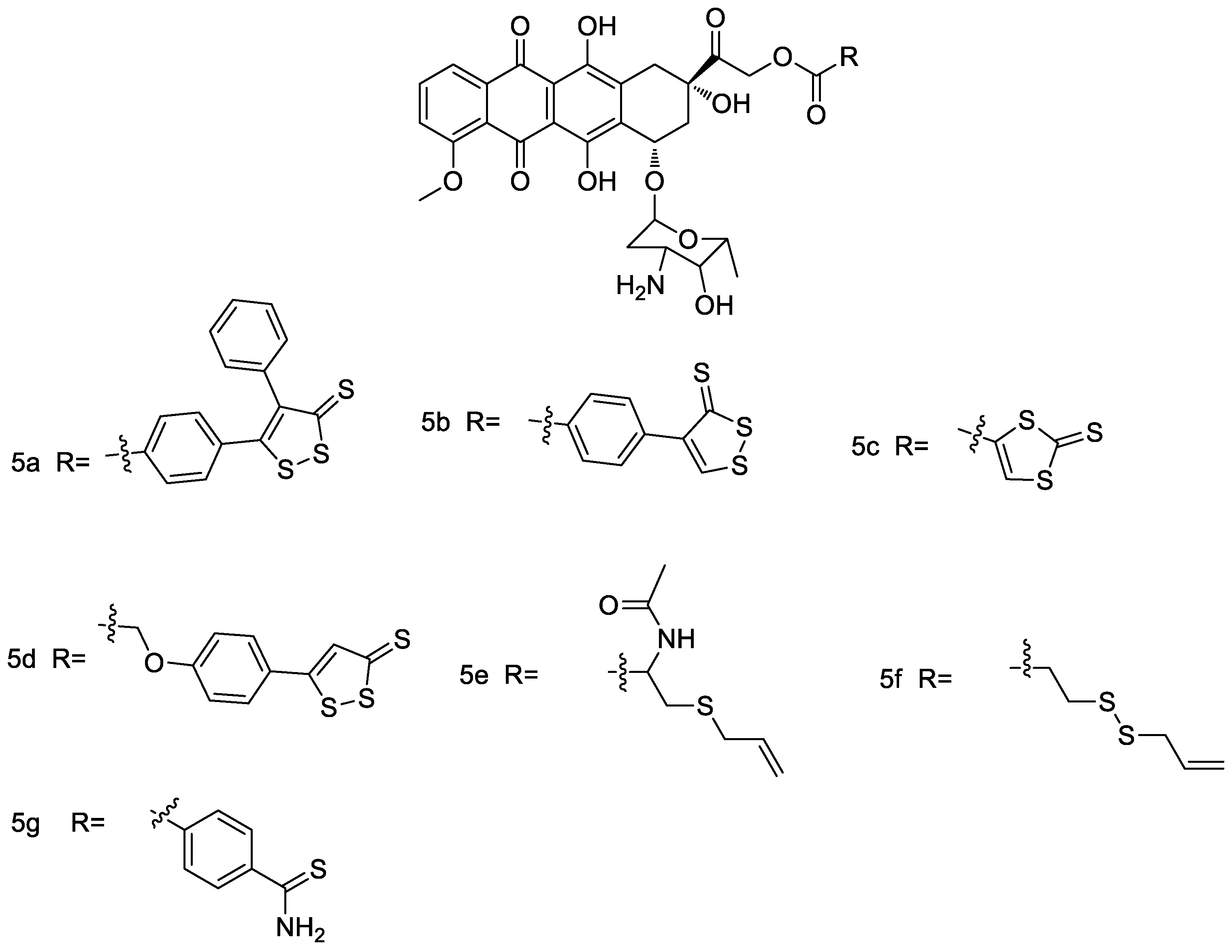
2.5. ADTOH–Doxorubicin Conjugates
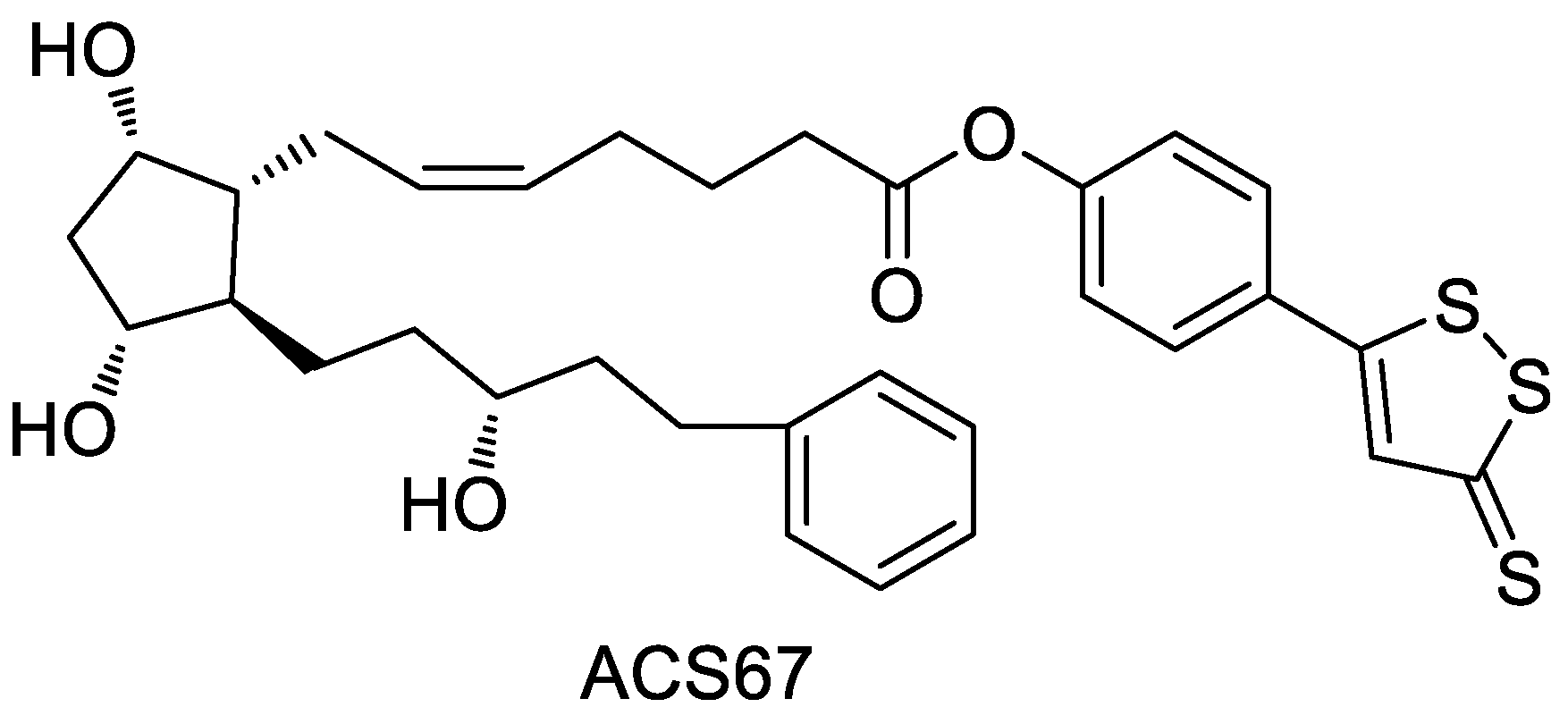
2.6. ADTOH–Latanoprost Conjugate
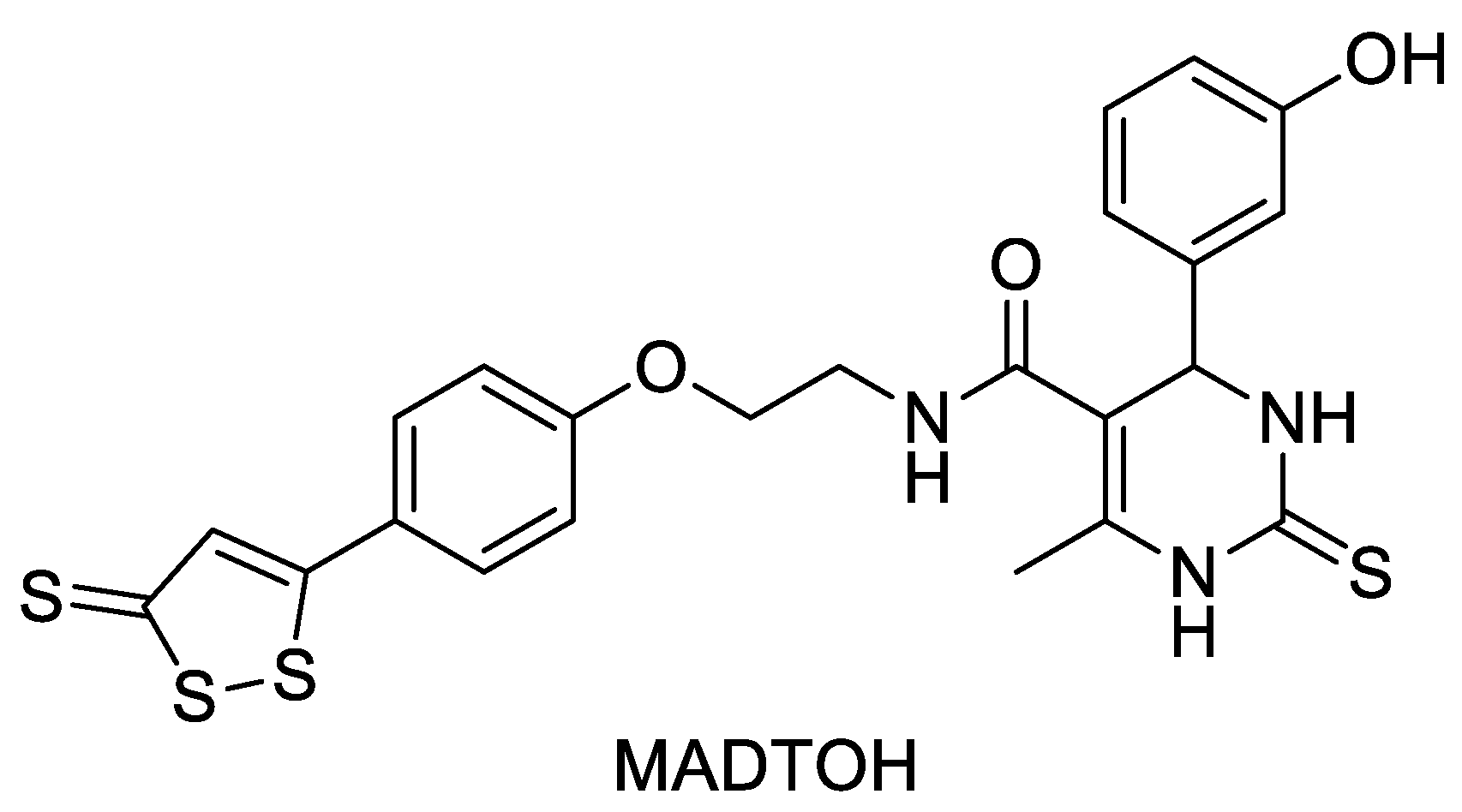
2.7. ADTOH–Monastrol Conjugate
2.8. ADTOH–Proglumide Conjugate
2.9. ADTOH–Sildenafil Conjugate
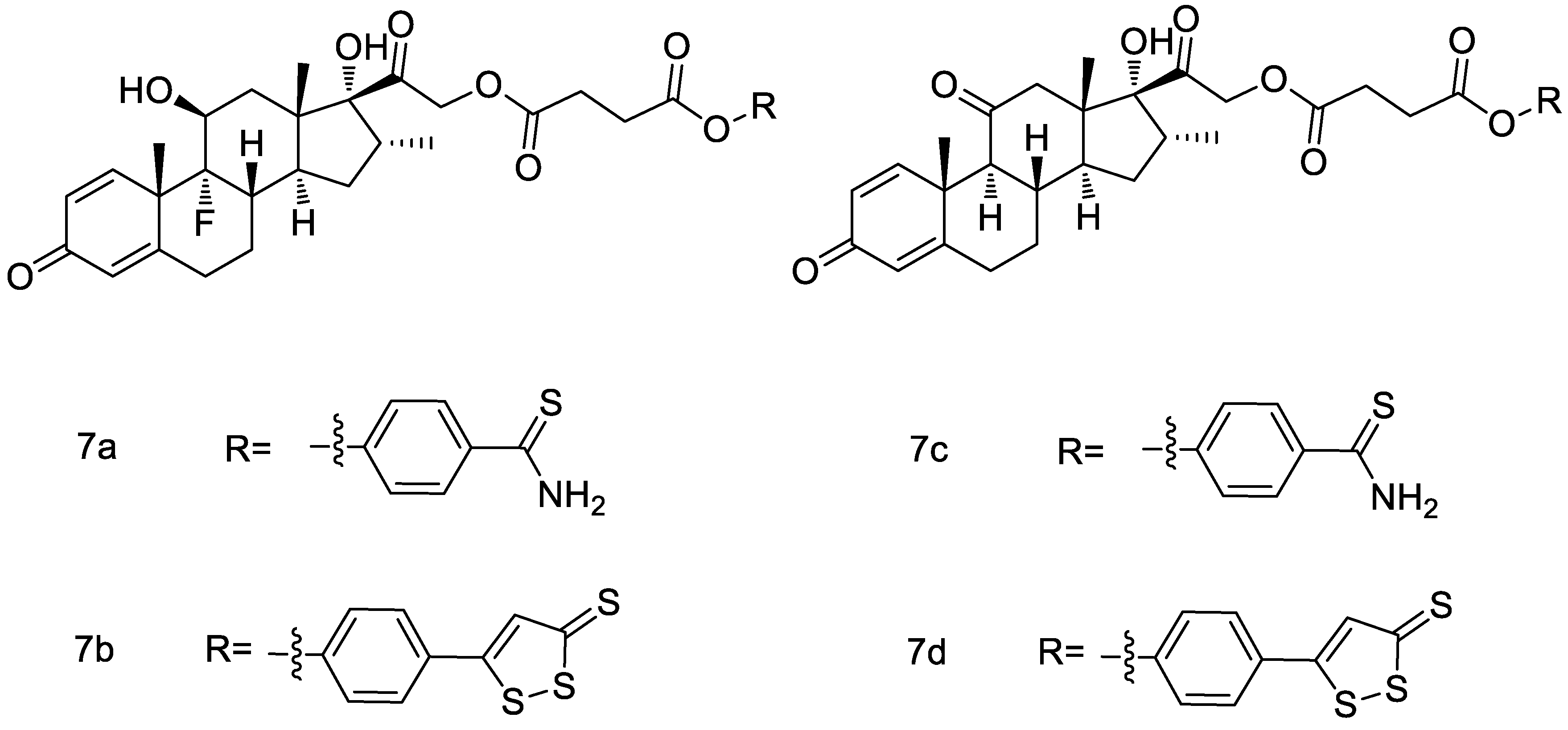
2.10. ADTOH–Glucocorticoids Conjugates
2.11. ADTOH–Atorvastatin Conjugates
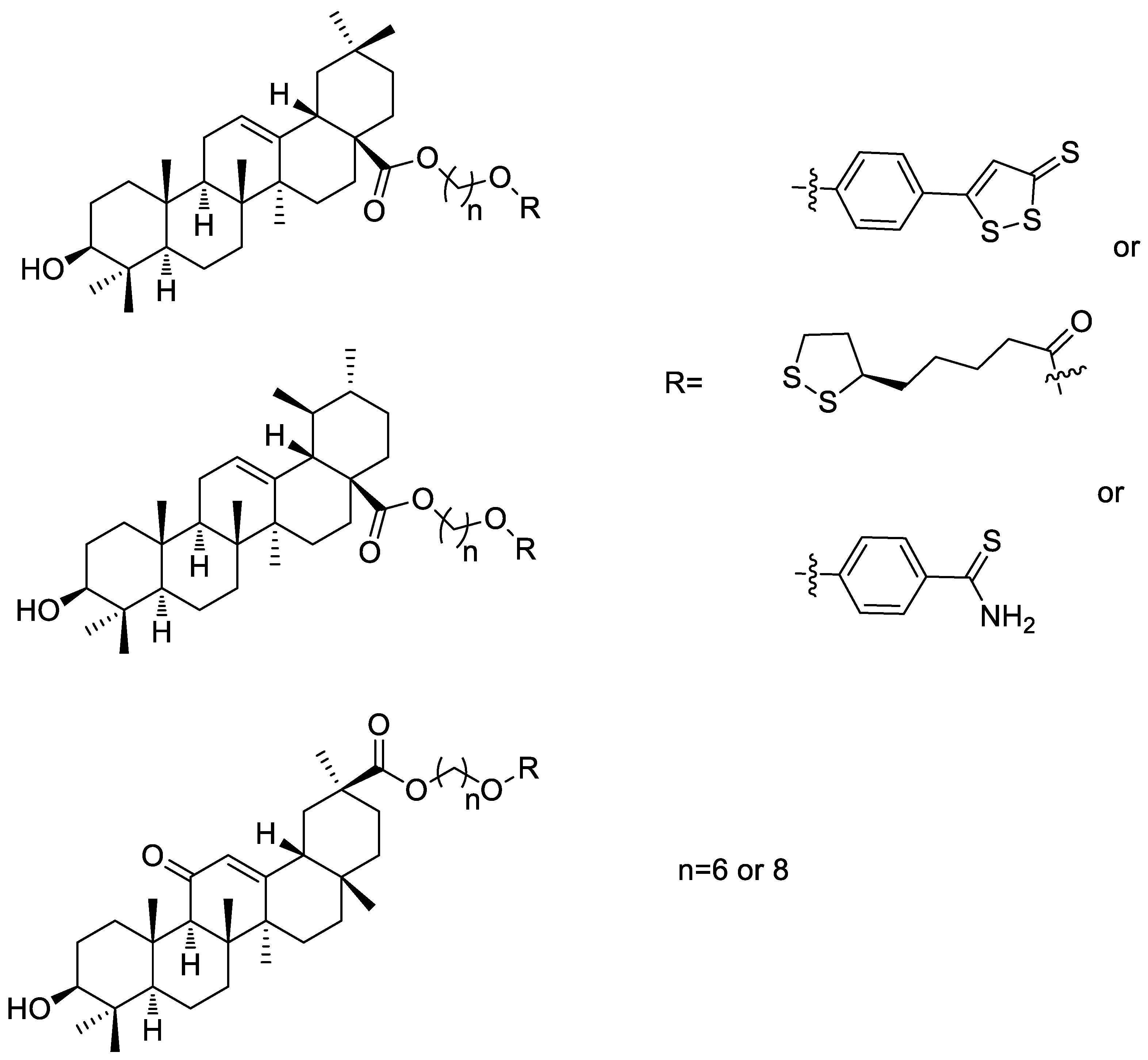
2.12. ADTOH–Pentacyclic Triterpene Conjugates
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandiver, M.; Snyder, S.H. Hydrogen sulfide: A gasotransmitter of clinical relevance. J. Mol. Med. 2012, 90, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Breschi, M.C.; Blandizzi, C.; Virdis, A.; Taddei, S.; Calderone, V. Hydrogen sulphide: Novel opportunity for drug discovery. Med. Res. Rev. 2012, 32, 1093–1130. [Google Scholar] [CrossRef]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef]
- Yonezawa, D.; Sekiguchi, F.; Miyamoto, M.; Taniguchi, E.; Honjo, M.; Masuko, T.; Nishikawa, H.; Kawabata, A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 2007, 241, 11–18. [Google Scholar] [CrossRef]
- Laggner, H.; Hermann, M.; Esterbauer, H.; Muellner, M.K.; Exner, M.; Gmeiner, B.M.; Kapiotis, S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J. Hypertens. 2007, 25, 2100–2104. [Google Scholar] [CrossRef]
- Sivarajah, A.; Collino, M.; Yasin, M.; Benetti, E.; Gallicchio, M.; Mazzon, E.; Cuzzocrea, S.; Fantozzi, R.; Thiemermann, C. Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide in a rat model of regional myocardial I/R. Shock 2009, 31, 267–274. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Chen, S.; Tang, C.; Wang, G.; Du, J.; Jin, H. Hydrogen sulfide regulates insulin secretion and insulin resistance in diabetes mellitus, a new promising target for diabetes mellitus treatment? A review. J. Adv. Res. 2021, 27, 19–30. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliani, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Tazzari, V.; Giustarini, D.; Rossi, R.; Sparatore, A.; Del Soldato, P.; McGeer, E.; McGeer, P.L. Effects of hydrogen sulfide-releasing L-DOPA derivatives on glial activation: Potential for treating Parkinson disease. J. Biol. Chem. 2010, 285, 17318–17328. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Organ, C.; Li, Z.; Bhushan, S.; Otsuka, H.; Pacheco, A.; Kang, J.; Aguilar, H.C.; Lefer, D.J.; et al. Design, Synthesis, and Cardioprotective Effects of N-Mercapto-Based Hydrogen Sulfide Donors. J. Med. Chem. 2015, 58, 7501–7511. [Google Scholar] [CrossRef]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and biological effects of hydrogen sulfide (H2S): Development of H2S-releasing drugs as pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Schjerning, A.M.; McGettigan, P.; Gislason, G. Cardiovascular effects and safety of (non-aspirin) NSAIDs. Nat. Rev. Cardiol. 2020, 17, 574–584. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Kodela, R.; Nath, N.; Barsegian, A.; Boring, D.; Kashfi, K. Hydrogen sulfide-releasing aspirin suppresses NF-kappaB signaling in estrogen receptor negative breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 2012, 83, 723–732. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Nath, N.; Kodela, R.; Sobocki, T.; Metkar, S.; Gan, Z.Y.; Kashfi, K. Hydrogen sulfide-releasing aspirin inhibits the growth of leukemic Jurkat cells and modulates beta-catenin expression. Leuk Res. 2013, 37, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; Sediari, L.; Mencarelli, A.; Renga, B.; Orlandi, S.; Russo, G.; Caliendo, G.; Santagada, V.; Cirino, G.; Wallace, J.L.; et al. 5-Amino-2-hydroxybenzoic acid 4-(5-thioxo-5H-[1,2]dithiol-3yl)-phenyl ester (ATB-429), a hydrogen sulfide-releasing derivative of mesalamine, exerts antinociceptive effects in a model of postinflammatory hypersensitivity. J. Pharmacol. Exp. Ther. 2006, 319, 447–458. [Google Scholar] [CrossRef]
- Wang, C.; Xia, G.; Liu, X.; Zhang, R.; Chai, Y.; Zhang, J.; Li, X.; Yang, Y.; Wang, J.; Liu, M. Synthesis and antitumor activity of ATB-429 derivatives containing a nitric oxide-releasing moiety. Bioorg. Med. Chem. Lett. 2016, 26, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Rossoni, G.; Sparatore, A.; Tazzari, V.; Manfredi, B.; Del Soldato, P.; Berti, F. The hydrogen sulphide-releasing derivative of diclofenac protects against ischaemia-reperfusion injury in the isolated rabbit heart. Br. J. Pharmacol. 2008, 153, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Gemici, B.; Elsheikh, W.; Feitosa, K.B.; Costa, S.K.; Muscara, M.N.; Wallace, J.L. H2S-releasing drugs: Anti-inflammatory, cytoprotective and chemopreventative potential. Nitric Oxide 2015, 46, 25–31. [Google Scholar] [CrossRef]
- Kodela, R.; Chattopadhyay, M.; Kashfi, K. Synthesis and biological activity of NOSH-naproxen (AVT-219) and NOSH-sulindac (AVT-18A) as potent anti-inflammatory agents with chemotherapeutic potential. Med. Chem. Comm. 2013, 4, 1472–1481. [Google Scholar] [CrossRef]
- Kashfi, K.; Chattopadhyay, M.; Kodela, R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015, 6, 287–296. [Google Scholar] [CrossRef]
- Tao, T.; Liu, M.; Chen, M.; Luo, Y.; Wang, C.; Xu, T.; Jiang, Y.; Guo, Y.; Zhang, J.H. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol. Ther. 2020, 216, 107695. [Google Scholar] [CrossRef]
- Jia, J.; Li, J.; Cheng, J. H(2)S-based therapies for ischaemic stroke: Opportunities and challenges. Stroke Vasc. Neurol. 2019, 4, 63–66. [Google Scholar] [CrossRef]
- Chen, X.Q.; Qiu, K.; Liu, H.; He, Q.; Bai, J.H.; Lu, W. Application and prospects of butylphthalide for the treatment of neurologic diseases. Chin. Med. J. 2019, 132, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, F.; Huang, L.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. Dl-3-n-Butylphthalide (NBP): A Promising Therapeutic Agent for Ischemic Stroke. CNS Neurol. Disord. Drug Targets 2018, 17, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Sheng, X.; Huang, Z.; Li, T.; Zhang, M.; Xu, J.; Ji, H.; Yin, J.; Zhang, Y. Design, synthesis and biological evaluation of hydrogen sulfide releasing derivatives of 3-n-butylphthalide as potential antiplatelet and antithrombotic agents. Org. Biomol. Chem. 2014, 12, 5995–6004. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Wang, L.; Ji, H.; Zhang, Y.; Yin, J. Design, synthesis and evaluation of hydrogen sulfide-releasing derivatives of ring opening 3-n-butylphthalide as novel platelet aggregation inhibitors. J. China Pharm. Univ. 2016, 47, 158–162. [Google Scholar]
- Yin, W.; Lan, L.; Huang, Z.; Ji, J.; Fang, J.; Wang, X.; Ji, H.; Peng, S.; Xu, J.; Zhang, Y. Discovery of a ring-opened derivative of 3-n-butylphthalide bearing NO/H2S-donating moieties as a potential anti-ischemic stroke agent. Eur. J. Med. Chem. 2016, 115, 369–380. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wang, Z.-Y.; Ling, J.-J.; Zhang, Y.-H.; Yin, J. Synthesis and biological evaluation of nitric oxide (NO)-hydrogen sulfide (H2S) releasing derivatives of (S)-3- n -butylphthalide as potential antiplatelet agents. Chin. J. Nat. Med. 2016, 14, 946–953. [Google Scholar] [CrossRef]
- Kothawade, P.B.; Thomas, A.B.; Chitlange, S.S. Novel Niacin Receptor Agonists: A Promising Strategy for the Treatment of Dyslipidemia. Mini Rev. Med. Chem. 2021, 21, 2481–2496. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Mitha, A.P.; Ogilvy, C.S.; Maynard, K.I. Post-treatment with nicotinamide (vitamin B(3)) reduces the infarct volume following permanent focal cerebral ischemia in female Sprague-Dawley and Wistar rats. Neurosci. Lett. 2000, 281, 111–114. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y.; Li, Y.; Cheng, J.; Chen, S.; Xiao, Y.; Ao, G. Synthesis and biological evaluation of novel hydrogen sulfide releasing nicotinic acid derivatives. Bioorg. Med. Chem. 2016, 24, 5368–5373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Sparatore, A.; Del Soldato, P.; Bian, J.S. ACS84, a novel hydrogen sulfide-releasing compound, protects against amyloid beta-induced cell cytotoxicity. Neurochem. Int. 2011, 58, 591–598. [Google Scholar] [CrossRef]
- Chegaev, K.; Rolando, B.; Cortese, D.; Gazzano, E.; Buondonno, I.; Lazzarato, L.; Fanelli, M.; Hattinger, C.M.; Serra, M.; Riganti, C.; et al. H2S-Donating Doxorubicins May Overcome Cardiotoxicity and Multidrug Resistance. J. Med. Chem. 2016, 59, 4881–4889. [Google Scholar] [CrossRef] [PubMed]
- Perrino, E.; Uliva, C.; Lanzi, C.; Soldato, P.D.; Masini, E.; Sparatore, A. New prostaglandin derivative for glaucoma treatment. Bioorg. Med. Chem. Lett. 2009, 19, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.N.; Ji, D.; Abdul Majid, A.S.; Fawcett, R.J.; Sparatore, A.; Del Soldato, P. ACS67, a hydrogen sulfide-releasing derivative of latanoprost acid, attenuates retinal ischemia and oxidative stress to RGC-5 cells in culture. Investig. Ophthalmol. Vis. Sci. 2010, 51, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, L.; Yang, G.; Wu, L.; Wang, R. Hydrogen sulfide-induced inhibition of L-type Ca2+ channels and insulin secretion in mouse pancreatic beta cells. Diabetologia 2013, 56, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.C.; de Jesus, I.C.G.; Soares, K.V.; Guatimosim, S.; da Silva Neto, L.; da-Silva, C.J.; Modolo, L.V.; Menezes Filho, J.E.R.; Rhana, P.; Cruz, J.S.; et al. A novel H2S releasing-monastrol hybrid (MADTOH) inhibits L-type calcium channels. New J. Chem. 2021, 45, 671–678. [Google Scholar] [CrossRef]
- Ou, X.; Xia, T.; Yang, C.; Yu, C.; Zhang, S.; Huang, R.; Chen, C.; Zhou, C. Novel H2S donor proglumide-ADT-OH protects HUVECs from ox-LDL-induced injury through NF-kappaB and JAK/SATA pathway. Open Med. (Wars) 2021, 16, 1318–1327. [Google Scholar] [CrossRef]
- Shukla, N.; Rossoni, G.; Hotston, M.; Sparatore, A.; Del Soldato, P.; Tazzari, V.; Persad, R.; Angelini, G.D.; Jeremy, J.Y. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009, 103, 1522–1529. [Google Scholar] [CrossRef] [PubMed]
- d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Maffia, P.; Mirone, V.; Imbimbo, C.; Fusco, F.; De Palma, R.; Ignarro, L.J.; Cirino, G. Hydrogen sulfide as a mediator of human corpus cavernosum smooth-muscle relaxation. Proc. Natl. Acad. Sci. USA 2009, 106, 4513–4518. [Google Scholar] [CrossRef]
- Tang, X.Q.; Chen, R.Q.; Dong, L.; Ren, Y.K.; Del Soldato, P.; Sparatore, A.; Liao, D.F. Role of Paraoxonase-1 in the Protection of Hydrogen Sulfide-Donating Sildenafil (ACS6) Against Homocysteine-Induced Neurotoxicity. J. Mol. Neurosci. 2013, 50, 70–77. [Google Scholar] [CrossRef]
- Giordano, F.; Corvino, A.; Scognamiglio, A.; Citi, V.; Gorica, E.; Fattorusso, C.; Persico, M.; Caliendo, G.; Fiorino, F.; Magli, E.; et al. Hybrids between H2S-donors and betamethasone 17-valerate or triamcinolone acetonide inhibit mast cell degranulation and promote hyperpolarization of bronchial smooth muscle cells. Eur. J. Med. Chem. 2021, 221, 113517. [Google Scholar] [CrossRef]
- Corvino, A.; Citi, V.; Fiorino, F.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Calderone, V.; Martelli, A.; Gorica, E.; et al. H2S donating corticosteroids: Design, synthesis and biological evaluation in a murine model of asthma. J. Adv. Res. 2022, 35, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Del Soldato, P.; Santus, G. Ophthalmic Pharmaceutical Compositions. WO2009109501A2 [P], 22 May 2009. [Google Scholar]
- Tong, J.; Shen, X.; Ju, C. Atorvastatin Derivative, Pharmaceutical Composition and Pharmaceutical Applications Thereof. CN103113360A [P], 19 February 2013. [Google Scholar]
- Sheng, L.-X.; Huang, J.-Y.; Liu, C.-M.; Zhang, J.-Z.; Cheng, K.-G. Synthesis of oleanolic acid/ursolic acid/glycyrrhetinic acid-hydrogen sulfide donor hybrids and their antitumor activity. Med. Chem. Res. 2019, 28, 1212–1222. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Cao, C.; Ge, J.; Yang, W.; Wang, Y.; Mou, Y. Research Progress of H2S Donors Conjugate Drugs Based on ADTOH. Molecules 2023, 28, 331. https://doi.org/10.3390/molecules28010331
Wen S, Cao C, Ge J, Yang W, Wang Y, Mou Y. Research Progress of H2S Donors Conjugate Drugs Based on ADTOH. Molecules. 2023; 28(1):331. https://doi.org/10.3390/molecules28010331
Chicago/Turabian StyleWen, Shuai, Changchang Cao, Jianwen Ge, Wenzhe Yang, Yan Wang, and Yi Mou. 2023. "Research Progress of H2S Donors Conjugate Drugs Based on ADTOH" Molecules 28, no. 1: 331. https://doi.org/10.3390/molecules28010331
APA StyleWen, S., Cao, C., Ge, J., Yang, W., Wang, Y., & Mou, Y. (2023). Research Progress of H2S Donors Conjugate Drugs Based on ADTOH. Molecules, 28(1), 331. https://doi.org/10.3390/molecules28010331




