A Natural CHI3L1—Targeting Compound, Ebractenoid F, Inhibits Lung Cancer Cell Growth and Migration and Induces Apoptosis by Blocking CHI3L1/AKT Signals
Abstract
1. Introduction
2. Results
2.1. Ebractenoid F Was Selected as a Useful Substance Targeting CHI3L1
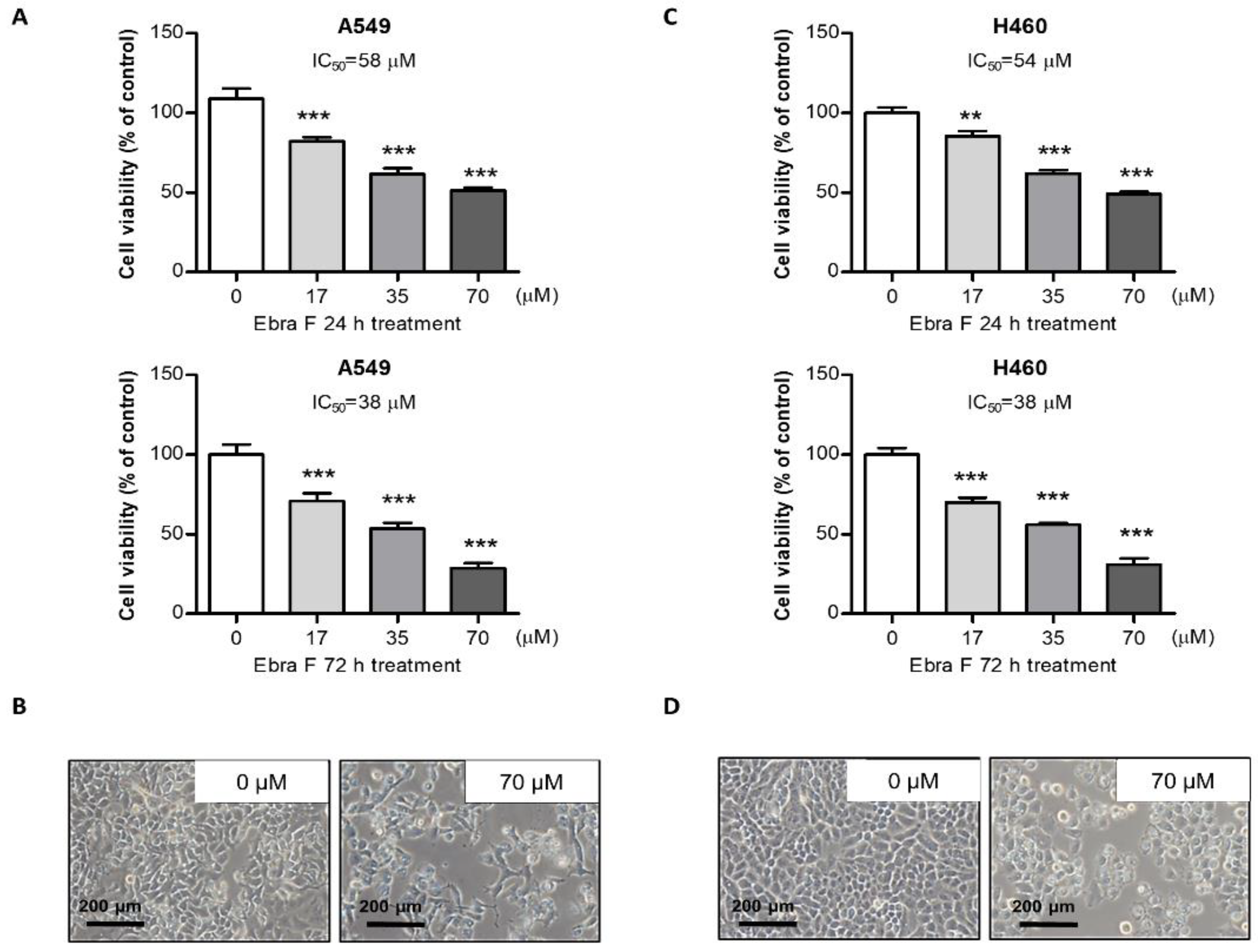
2.2. Ebractenoid F Inhibited Lung Cancer Cell Growth
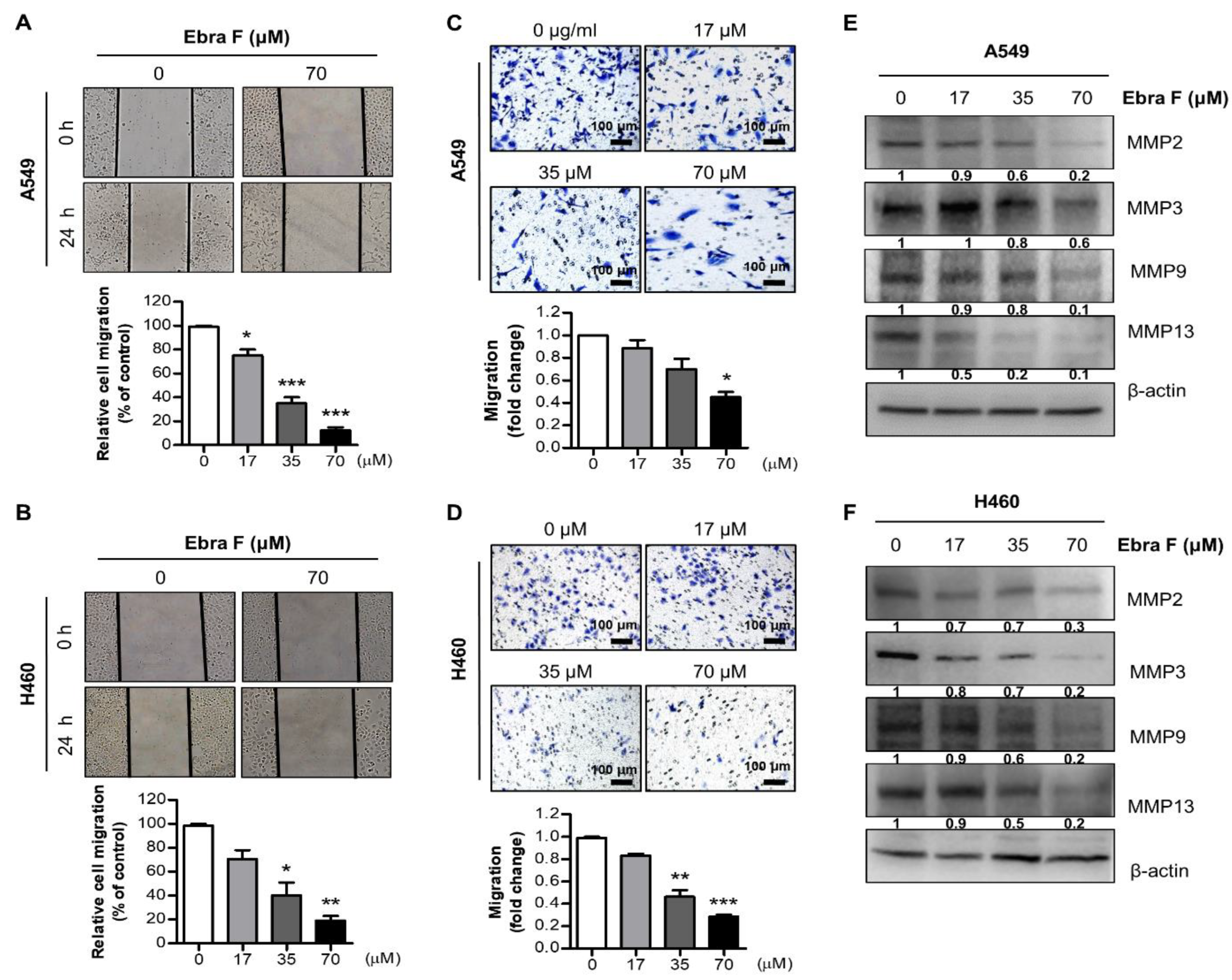
2.3. Ebractenoid F Suppressed the Migration of Lung Cancer Cells
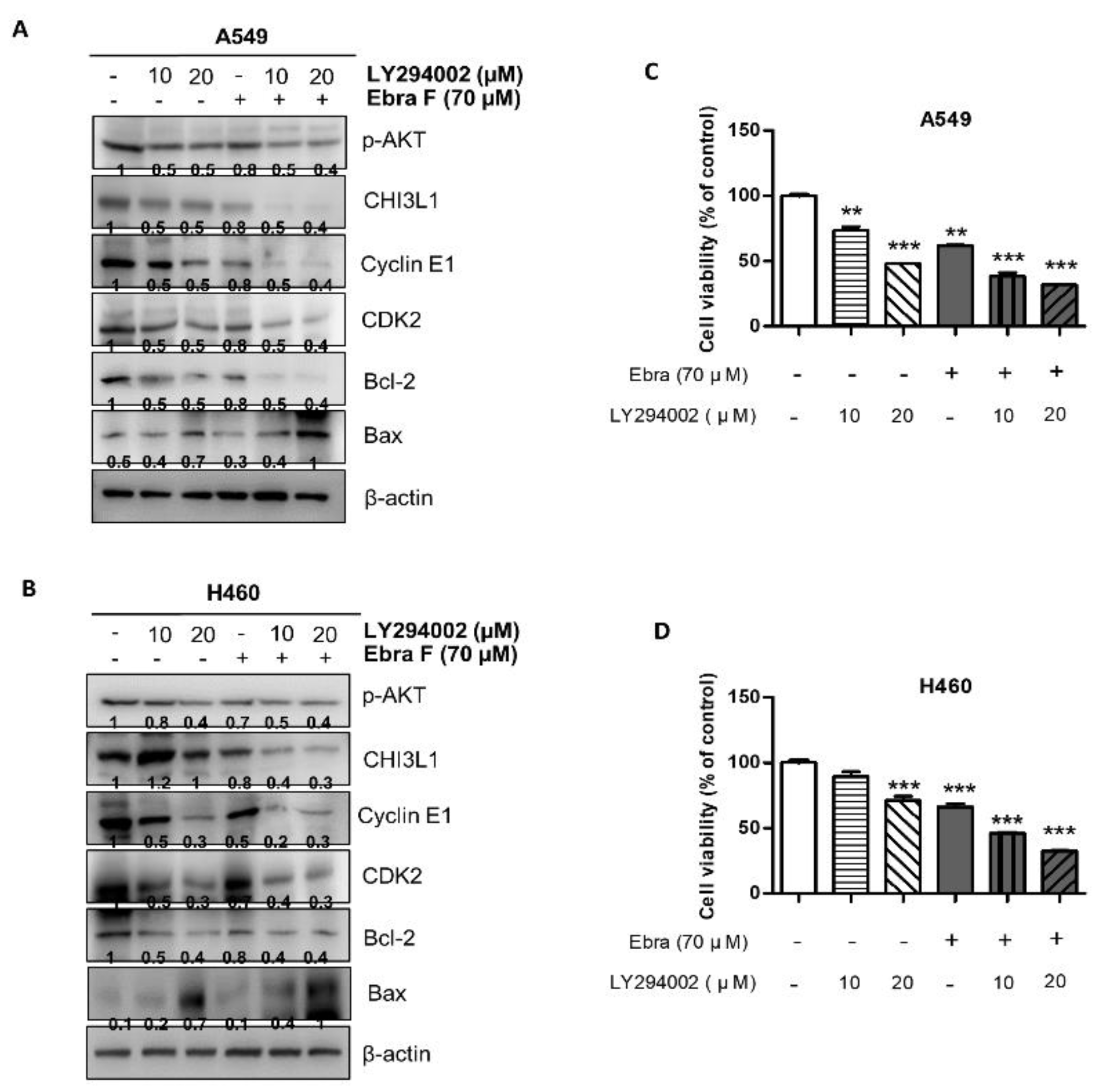
2.4. Ebractenoid F Arrested the Cell Cycle of Lung Cancer Cells
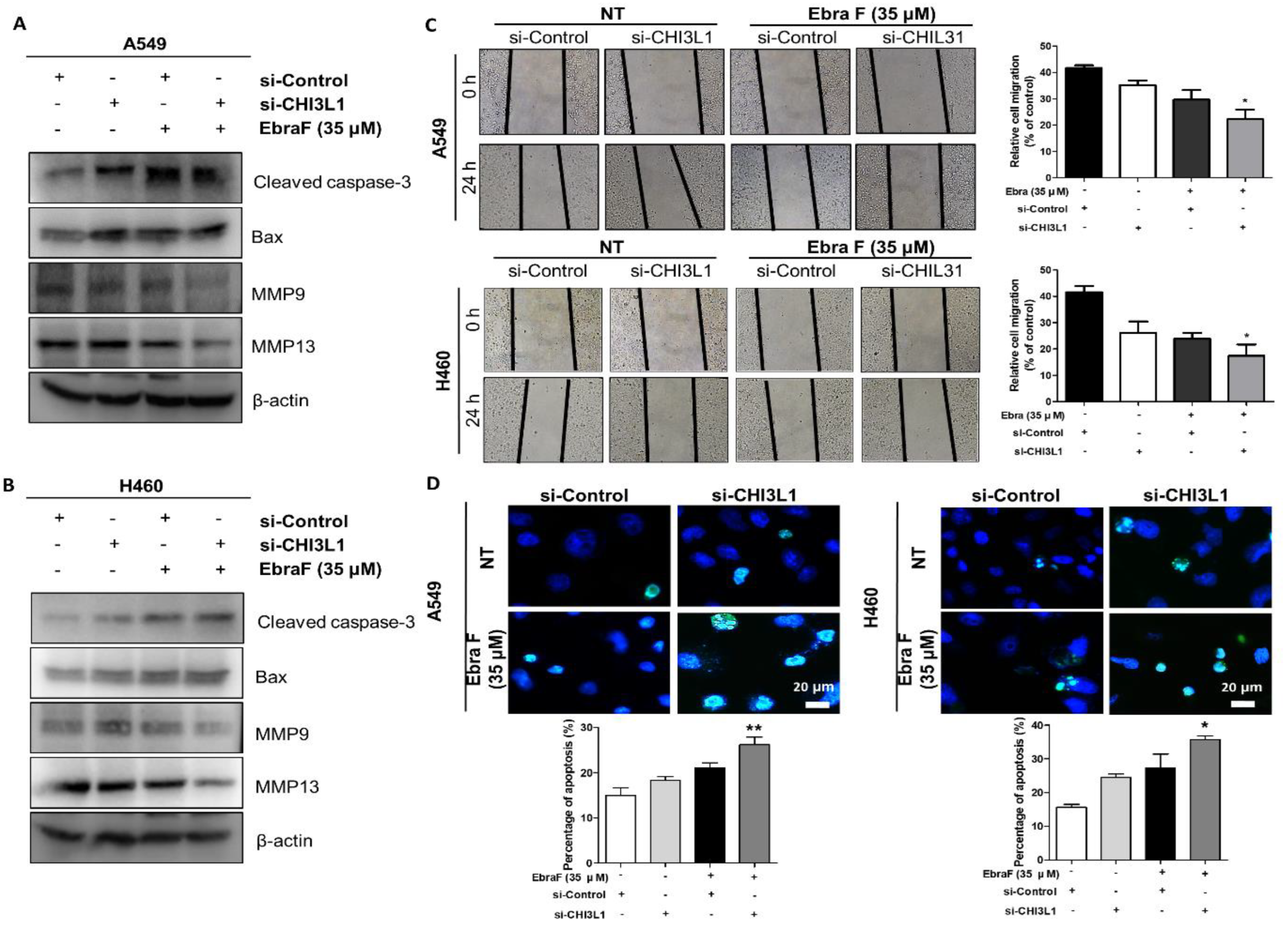
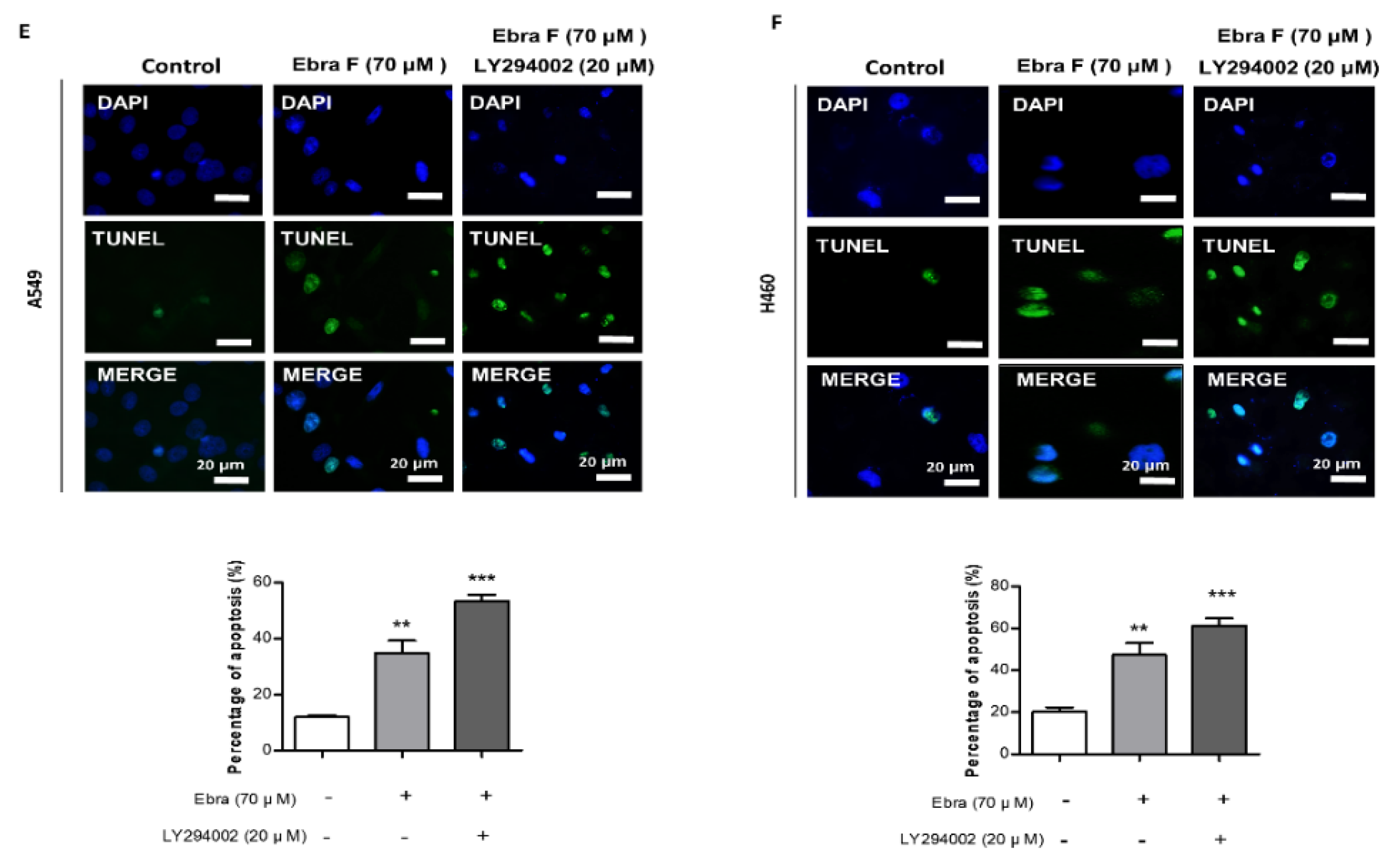
2.5. Ebractenoid F Induced Apoptosis in Lung Cancer Cells
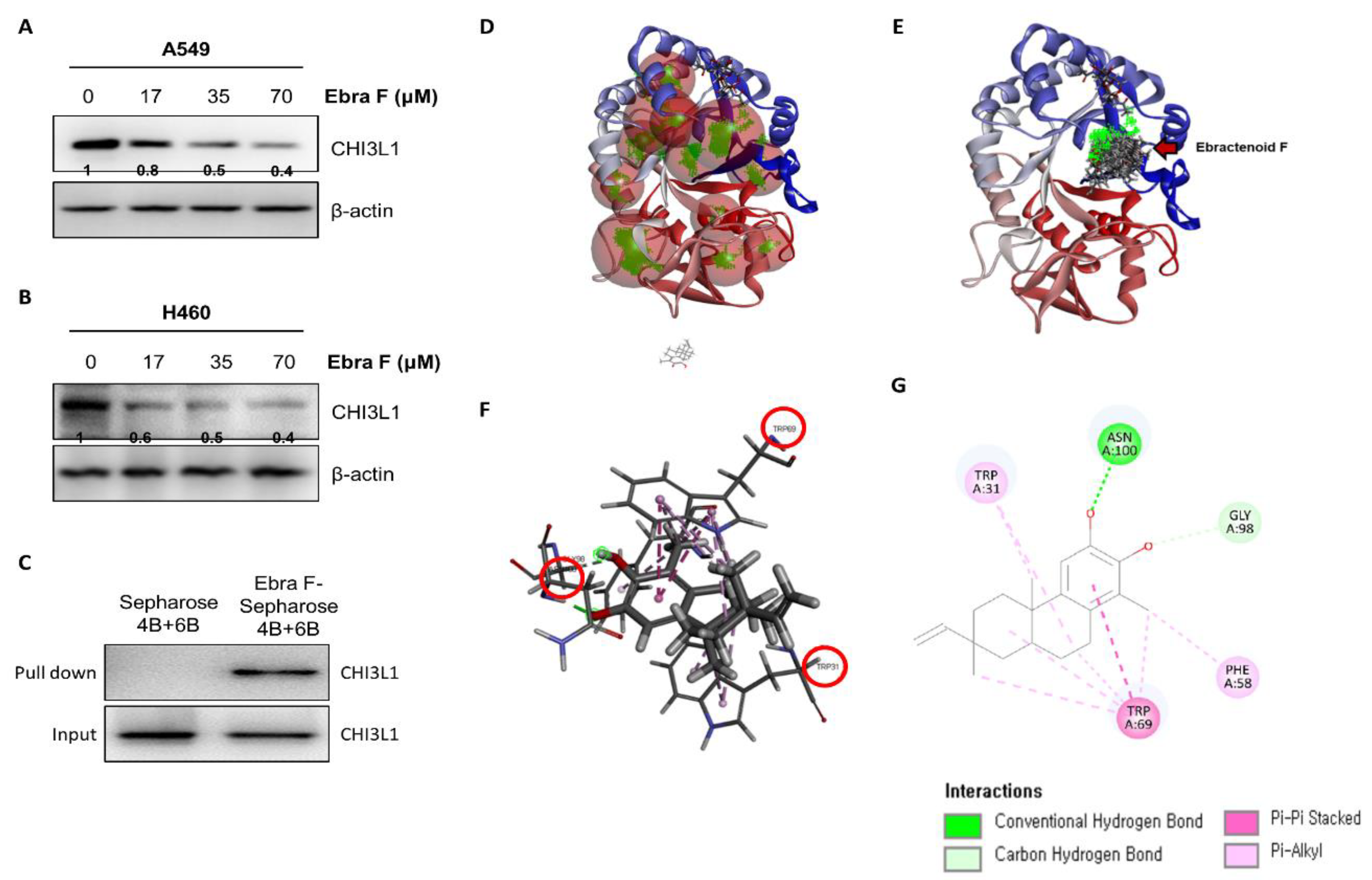
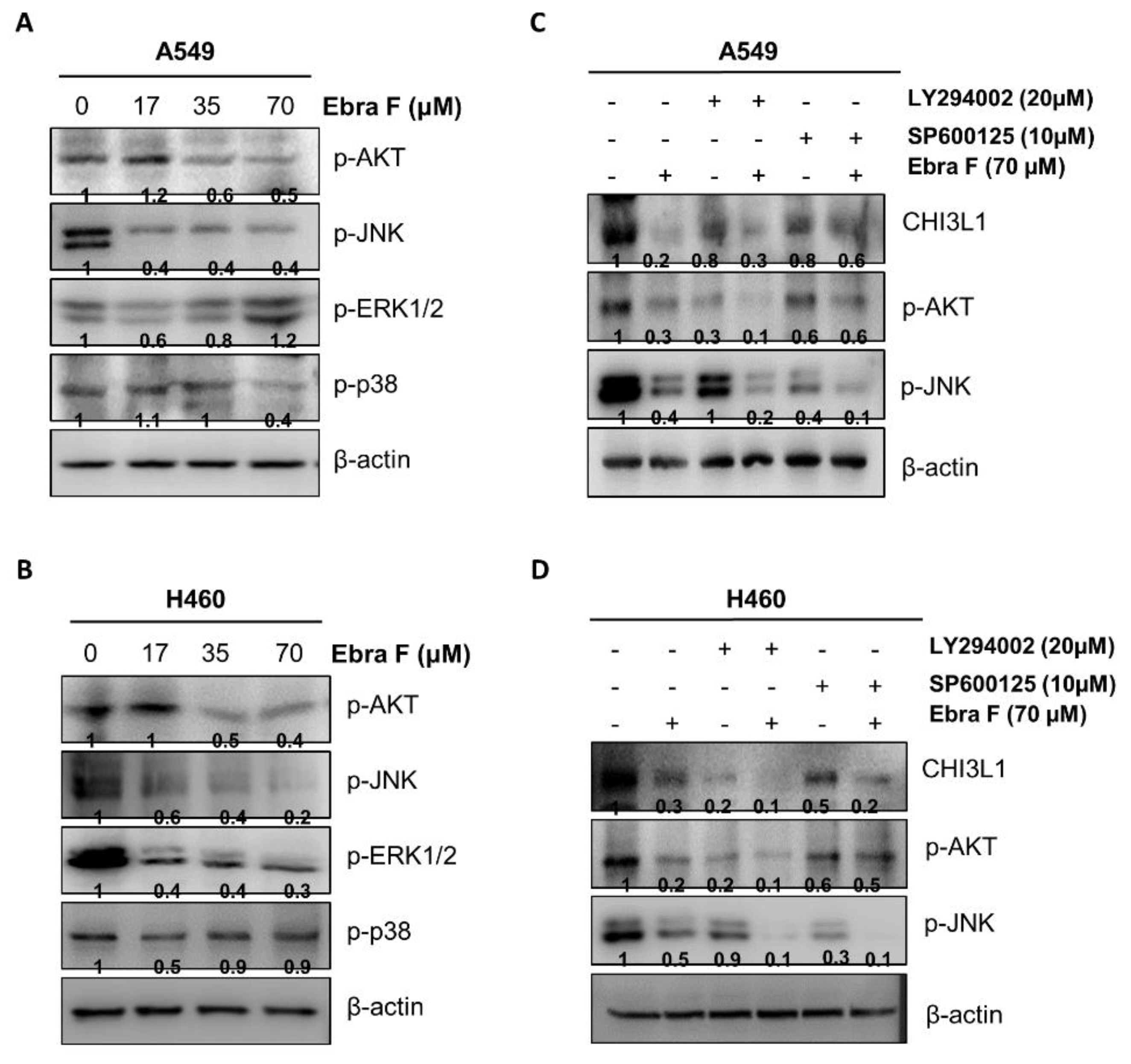
2.6. Ebractenoid F Interacted with CHI3L1
2.7. Combination Effects of Ebractenoid F and CHI3L1 siRNA on Lung Cancer Cell Growth
2.8. Ebractenoid F Downgraded the AKT Signaling Activation
2.9. Combination Treatment with Ebractenoid F and AKT Inhibitor on Cell Viability and Apoptosis
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Cell Culture and Transfection
4.3. Cell Viability Assay
4.4. Luciferase Activity Assay
4.5. Cell Migration Assay
4.6. Cell Cycle Assay
4.7. Evaluation of Apoptotic Cell Death
4.8. Docking Experiment
4.9. Pull-Down Assay
4.10. Cycloheximide Chase Assay
4.11. Western Blotting
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kzhyshkowska, J.; Yin, S.; Liu, T.; Riabov, V.; Mitrofanova, I. Role of chitinase-like proteins in cancer. Biol. Chem. 2016, 397, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Dela Cruz, C.S.; Ahangari, F.; Ma, B.; Kang, M.-J.; He, C.-H.; Takyar, S.; Elias, J.A. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu. Rev. Physiol. 2011, 73, 479–501. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Herzog, E.L.; Lee, C.G.; Peng, X.; Lee, C.M.; Chen, X.; Rockwell, S.; Koo, J.S.; Kluger, H.; Herbst, R.S.; et al. Role of chitinase 3-like-1 and semaphorin 7a in pulmonary melanoma metastasis. Cancer Res. 2015, 75, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Schimpl, M.; Rush, C.L.; Betou, M.; Eggleston, I.M.; Recklies, A.D.; Van Aalten, D.M. Human YKL-39 is a pseudo-chitinase with retained chitooligosaccharide-binding properties. Biochem. J. 2012, 446, 149–157. [Google Scholar] [CrossRef]
- Coffman, F.D. Chitinase 3-Like-1 (CHI3L1): A putative disease marker at the interface of proteomics and glycomics. Crit. Rev. Clin. Lab. Sci. 2008, 45, 531–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Dela Cruz, C.S.; Ma, B.; Ahangari, F.; Zhou, Y.; Halaban, R.; Sznol, M.; Elias, J.A. Chitinase-like proteins in lung injury, repair, and metastasis. Proc. Am. Thorac. Soc. 2012, 9, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sheng, Z.; Yang, W.; Cai, Y. Elevated serum concentration of chitinase 3-like 1 is an independent prognostic biomarker for poor survival in lung cancer patients. Cell. Physiol. Biochem. 2016, 38, 461–468. [Google Scholar] [CrossRef]
- Xu, C.-H.; Yu, L.-K.; Hao, K.-K. Serum YKL-40 level is associated with the chemotherapy response and prognosis of patients with small cell lung cancer. PLoS ONE 2014, 9, e96384. [Google Scholar] [CrossRef]
- Kim, K.C.; Yun, J.; Son, D.J.; Kim, J.Y.; Jung, J.-K.; Choi, J.S.; Kim, Y.R.; Song, J.K.; Kim, S.Y.; Kang, S.K. Suppression of metastasis through inhibition of chitinase 3-like 1 expression by miR-125a-3p-mediated up-regulation of USF1. Theranostics 2018, 8, 4409. [Google Scholar] [CrossRef]
- He, C.H.; Lee, C.G.; Cruz, C.S.D.; Lee, C.-M.; Zhou, Y.; Ahangari, F.; Ma, B.; Herzog, E.L.; Rosenberg, S.A.; Li, Y. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013, 4, 830–841. [Google Scholar] [CrossRef]
- Lee, C.M.; He, C.H.; Nour, A.M.; Zhou, Y.; Ma, B.; Park, J.W.; Kim, K.H.; Dela Cruz, C.; Sharma, L.; Nasr, M.L.; et al. IL-13Ralpha2 uses TMEM219 in chitinase 3-like-1-induced signalling and effector responses. Nat. Commun. 2016, 7, 12752. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Yu, J.E.; Kim, K.C.; Lee, D.H.; Son, D.J.; Lee, H.P.; Jung, J.K.; Kim, N.D.; Ham, Y.W.; Yun, J.; et al. A small molecule targeting CHI3L1 inhibits lung metastasis by blocking IL-13Ralpha2-mediated JNK-AP-1 signals. Mol. Oncol. 2022, 16, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Li, Z.-L.; Bai, J.; Meng, D.-L.; Li, N.; Pei, Y.-H.; Zhao, F.; Hua, H.-M. Anti-inflammatory diterpenoids from the roots of Euphorbia ebracteolata. J. Nat. Prod. 2014, 77, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.Z.; Jiang, C.R.; Huang, T.; Hao, X.J. Two New Rosane—Type Diterpenoids from Euphorbia ebracteolata Hayata. Helv. Chim. Acta 2013, 96, 2299–2303. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein- ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.-H.; Qasim, M.; Kim, J.-H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022. [Google Scholar] [CrossRef]
- Rappaport, N.; Nativ, N.; Stelzer, G.; Twik, M.; Guan-Golan, Y.; Stein, T.I.; Bahir, I.; Belinky, F.; Morrey, C.P.; Safran, M.; et al. MalaCards: An integrated compendium for diseases and their annotation. Database 2013, 2013, bat018. [Google Scholar] [CrossRef]
- Liu, C.-C.; Tseng, Y.-T.; Li, W.; Wu, C.-Y.; Mayzus, I.; Rzhetsky, A.; Sun, F.; Waterman, M.; Chen, J.J.W.; Chaudhary, P.M.; et al. DiseaseConnect: A comprehensive web server for mechanism-based disease-disease connections. Nucleic Acids Res. 2014, 42, W137–W146. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, H.; Sun, H.; Peng, X.; Tang, C.; Gan, Y.; Chen, X.; Mathur, A.; Hu, B.; Slade, M.D. Chitinase 3–like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Sci. Transl. Med. 2014, 6, 240ra76. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Son, D.J.; Yun, J.; Han, S.B.; Hong, J.T. Anti-Chi3L1 antibody suppresses lung tumor growth and metastasis through inhibition of M2 polarization. Mol. Oncol. 2022, 16, 2214–2234. [Google Scholar] [CrossRef] [PubMed]
- Libreros, S.; Garcia-Areas, R.; Keating, P.; Carrio, R.; Iragavarapu-Charyulu, V.L. Exploring the role of CHI3L1 in “pre-metastatic” lungs of mammary tumor-bearing mice. Front. Physiol. 2013, 4, 392. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Akosman, B.; Kamle, S.; Lee, C.-M.; He, C.H.; Koo, J.S.; Lee, C.G.; Elias, J.A. CHI3L1 regulates PD-L1 and anti–CHI3L1–PD-1 antibody elicits synergistic antitumor responses. J. Clin. Investig. 2021, 131, e137750. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Karachaliou, N. Optimizing lung cancer treatment approaches. Nat. Rev. Clin. Oncol. 2015, 12, 75–76. [Google Scholar] [CrossRef]
- Wu, L.; Leng, D.; Cun, D.; Foged, C.; Yang, M. Advances in combination therapy of lung cancer: Rationales, delivery technologies and dosage regimens. J. Control Release 2017, 260, 78–91. [Google Scholar] [CrossRef]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, K.C.; Hwang, C.J.; Park, K.R.; Jung, Y.S.; Kim, S.Y.; Kim, J.Y.; Song, J.K.; Song, M.J.; Choi, M.K. Decreased lung tumor development in SwAPP mice through the downregulation of CHI3L1 and STAT 3 activity via the upregulation of miRNA342-3p. Mol. Ther. Nucleic Acids 2019, 16, 63–72. [Google Scholar] [CrossRef]
- Chen, C.-C.; Llado, V.; Eurich, K.; Tran, H.T.; Mizoguchi, E. Carbohydrate-binding motif in chitinase 3-like 1 (CHI3L1/YKL-40) specifically activates Akt signaling pathway in colonic epithelial cells. Clin. Immunol. 2011, 140, 268–275. [Google Scholar] [CrossRef]
- Bi, J.; Lau, S.-H.; Lv, Z.-L.; Xie, D.; Li, W.; Lai, Y.-R.; Zhong, J.-M.; Wu, H.-q.; Su, Q.; He, Y.-l. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum. Pathol. 2009, 40, 1790–1797. [Google Scholar] [CrossRef]
- Li, L.; Wei, K.; Ding, Y.; Ahati, P.; Xu, H.; Fang, H.; Wang, H. M2a Macrophage-Secreted CHI3L1 promotes extracellular matrix metabolic imbalances via activation of IL-13Rα2/MAPK pathway in rat intervertebral disc degeneration. Front. Immunol. 2021, 12, 666361. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Pan, J.; Zhao, T.; Ji, J.; Zhang, C.; Che, Y.; Yang, J.; Shi, H.; Li, J.; Zhou, H.; et al. Chitinase 3-like 1-CD44 interaction promotes metastasis and epithelial-to-mesenchymal transition through beta-catenin/Erk/Akt signaling in gastric cancer. J. Exp. Clin. Cancer Res. 2018, 37, 208. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, C.; Jin, Q.; Jang, H.; Lee, D.; Lee, H.J.; Shin, J.W.; Han, S.B.; Hong, J.T.; Kim, Y.; et al. Diterpenoids from the Roots of Euphorbia fischeriana with Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2016, 79, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt-Ferreira, G.; de Azevedo, W.F. Docking with SwissDock. In Docking Screens for Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 189–202. [Google Scholar] [CrossRef]
- Liu, C.; Gen, Y.; Tanimoto, K.; Muramatsu, T.; Inoue, J.; Inazawa, J. Concurrent targeting of MAP3K3 and BRD4 by miR-3140-3p overcomes acquired resistance to BET inhibitors in neuroblastoma cells. Mol. Ther. Nucleic Acids 2021, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Activity Value |
|---|---|
| LogP | 5.82/Very lipophilic |
| M.W | 286.40/Good |
| H-bond donors | 2/Good |
| H-bond acceptors | 2/Good |
| Number of rotatable bonds | 1/Good |
| Number of rings | 3/Good |
| Lipinski | Moderate (1) |
| Lead-like | Moderate (1) |
| Solubility | 6.27/Highly insoluble |
| P-gp substrates | Non-substrate undefined |
| CYP1A2 Inhibitor | 0.44/Undefined |
| CYP2C9 Inhibitor | 0.48/Undefined |
| CYP2C19 Inhibitor | 0.49/Undefined |
| CYP2D6 Inhibitor | 0.44/Undefined |
| CYP3A4 Inhibitor | 0.47/Undefined |
| Ames | Negative, Class 5 |
| hERG | 0.03/Non-Inhibitor |
| Caco-2 | Highly permeability |
| PPB | 94.72/Extensive bound |
| CNS | Penetrant |
| HIA | 100/Highly absorbed |
| Metabolic stability | Undefined |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.E.; Yu, J.E.; Lee, J.W.; Son, D.J.; Lee, H.P.; Kim, Y.; Chang, J.Y.; Lee, D.W.; Lee, W.K.; Yun, J.; et al. A Natural CHI3L1—Targeting Compound, Ebractenoid F, Inhibits Lung Cancer Cell Growth and Migration and Induces Apoptosis by Blocking CHI3L1/AKT Signals. Molecules 2023, 28, 329. https://doi.org/10.3390/molecules28010329
Hong DE, Yu JE, Lee JW, Son DJ, Lee HP, Kim Y, Chang JY, Lee DW, Lee WK, Yun J, et al. A Natural CHI3L1—Targeting Compound, Ebractenoid F, Inhibits Lung Cancer Cell Growth and Migration and Induces Apoptosis by Blocking CHI3L1/AKT Signals. Molecules. 2023; 28(1):329. https://doi.org/10.3390/molecules28010329
Chicago/Turabian StyleHong, Da Eun, Ji Eun Yu, Jin Woo Lee, Dong Ju Son, Hee Pom Lee, Yuri Kim, Ju Young Chang, Dong Won Lee, Won Kyu Lee, Jaesuk Yun, and et al. 2023. "A Natural CHI3L1—Targeting Compound, Ebractenoid F, Inhibits Lung Cancer Cell Growth and Migration and Induces Apoptosis by Blocking CHI3L1/AKT Signals" Molecules 28, no. 1: 329. https://doi.org/10.3390/molecules28010329
APA StyleHong, D. E., Yu, J. E., Lee, J. W., Son, D. J., Lee, H. P., Kim, Y., Chang, J. Y., Lee, D. W., Lee, W. K., Yun, J., Han, S. B., Hwang, B. Y., & Hong, J. T. (2023). A Natural CHI3L1—Targeting Compound, Ebractenoid F, Inhibits Lung Cancer Cell Growth and Migration and Induces Apoptosis by Blocking CHI3L1/AKT Signals. Molecules, 28(1), 329. https://doi.org/10.3390/molecules28010329










