Abstract
Iron is a trace element necessary for cell growth, development, and cellular homeostasis, but insufficient or excessive level of iron is toxic. Intracellularly, sufficient amounts of iron are required for mitochondria (the center of iron utilization) to maintain their normal physiologic function. Iron deficiency impairs mitochondrial metabolism and respiratory activity, while mitochondrial iron overload promotes ROS production during mitochondrial electron transport, thus promoting potential disease development. This review provides an overview of iron homeostasis, mitochondrial iron metabolism, and how mitochondrial iron imbalances-induced mitochondrial dysfunction contribute to diseases.
1. Introduction
Iron exerts an essential role in living organisms. On one hand, iron is a component of heme (e.g., myoglobin, hemoglobin, myeloperoxidase, cytochrome proteins, nitric oxide synthetases), iron-sulfur clusters (e.g., mitochondrial aconitase, coenzyme Q10, respiratory complexes I–III), or other functional groups (e.g., hypoxia inducible factor prolyl hydroxylases) incorporated into proteins as cofactors. These iron-containing proteins contribute to various biological processes, such as oxygen transport and energy metabolism [1]. On the other hand, iron is involved in oxidation-reduction reactions by readily shuttling between the oxidized ferric (Fe3+) and the reduced ferrous (Fe2+) forms. The reactions are required for a number of fundamental biologic processes. Notably, the cellular redox equilibrium can be easily disrupted by catalytic amounts of iron, thus resulting in the generation of toxic reactive oxygen species (ROS) and oxidative stress [2,3]. Under oxidative stress, mitochondria (the cellular energy centers) are impaired, leading to impaired energy state and potential disease development [4,5]. As such, iron has become a key target of interest in the progression and treatment of diseases related to dysfunction in mitochondria and energy metabolism. Preventing the dysfunctional role of iron in energy metabolism may help prevent or delay related metabolic diseases [6]. Therefore, this review emphasizes the importance of iron homeostasis in mitochondrial function and energy metabolism and discusses the diseases that are related to imbalances in iron homeostasis, mitochondrial dysfunction, and impaired energy metabolism.
2. Cellular Iron Absorption, Utilization, and Homeostasis
The molecular mechanism of cellular iron absorption and metabolism has been well characterized and shown in lots of reviews (Figure 1) [7,8]. Therefore, we only discuss it briefly in this review before discussing the role of iron in energy metabolism. The hemoglobin (at least 2.1 g in humans) of red blood cells and developing erythroid cells is the main place where body iron exists. In addition, body iron also exists in macrophages (up to 600 mg), the myoglobin of muscles (~300 mg), and the liver (~1 g). Notably, lower, but not negligible, quantities of iron also exist in other tissues. On the other hand, the main ways for iron excretion from the body are sloughing of mucosal and skin cells or during bleeding, but the underlying regulated mechanism remains unclear. In the presence of physiological pH and oxygen, dietary iron mainly exists in the form of highly insoluble iron Fe (III), while the iron transport system absorbs ferrous Fe (II) ions, which are very unstable and rapidly oxidized to trivalent iron [9,10]. On this basis, balance is maintained by the tight control of dietary absorption in the duodenum [11,12]. Dietary iron is absorbed in the following three forms: inorganic (mainly present in the oxidized form Fe3+), heme, and ferritin. Prior to intestinal uptake, dietary inorganic iron (Fe3+ form) must be reduced to the Fe2+ form by the cytochrome b on the duodenal enterocyte membrane [13,14]. Then, with the help of divalent metal transporter 1 (DMT1) on the membrane, the Fe2+ is further transported into intestinal epithelial cells [15]. Iron (Fe2+) taken up by enterocytes has the following four fates: (1) stored in ferritin in its Fe3+ form; (2) used directly as a cofactor by cytosolic proteins; (3) transported into mitochondria; and (4) transported out of the cell [7]. Unlike dietary inorganic iron, the mechanisms for uptake of dietary heme and ferritin are less well understood. However, after it is liberated, iron obtained from dietary heme and ferritin enters a common pathway similar to inorganic iron in the enterocyte [16].
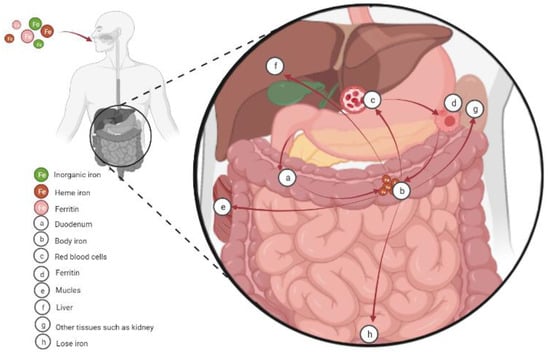
Figure 1.
Cellular iron absorption, utilization, and homeostasis. Dietary iron is absorbed in the duodenum in the following three forms: inorganic (mainly present in the oxidized form Fe3+), heme, and ferritin. Then the iron is transported to the body. Body iron is mainly present in the hemoglobin of red blood cells and developing erythroid cells (at least 2.1 g in humans). In addition, macrophages (up to 600 mg) and the myoglobin of muscles (~300 mg) also contain significant amounts of iron, and the liver stores the excess body iron (~1 g). Other tissues also contain lower, but not negligible, quantities of iron. Finally, mammals lose iron from sloughing of mucosal and skin cells or during bleeding.
With the help of ferroportin1 (FPN1), a known iron transmembrane efflux protein in vertebrate cells, intracellular iron is exported out of the cell [17,18,19]. Another important way to remove intracellular iron is by extracellular vesicles (specifically, by exosomes), hence protecting cells from ferroptotic cell death [20]. To guard dissociative iron against oxidative damage to cells, excess cellular iron is stored in ferritin [21]. Exported iron is scavenged by transferrin, which maintains Fe3+ in a redox-insert state and delivers it into tissues by the ubiquitously expressed transferrin receptor 1 (TFR1) [22]. Under normal conditions, iron exists in the bloodstream mainly in the form of transferrin-bound iron, which is not redox active and does not produce extrahepatic iron overload. Once plasma iron exceeds the carrying capacity of transferrin, iron and transferrin are not tightly bound to form non-transferrin-bound iron (NTBI), which is taken up by tissues (such as the heart, pancreas, and liver) through endocytosis [23], thus giving rise to tissue damage [24].
The level of body iron needs close regulation since imbalances between the two oxidation states of iron produce ROS [25]. The maintenance of iron homeostasis is largely modulated by the iron regulatory protein (IRP)-iron response element (IRE) system, which is a relatively simple and ubiquitous post-transcriptional regulatory loop. In response to alterations in the levels of intracellular iron, this system can regulate the expression of post-translational ferritin and transferrin receptors and alter the synthesis of pivotal iron metabolic proteins [26,27]. That is, when cellular iron levels are low, IRP rescues cellular iron deficiency by the following two mechanisms: (1) binding of IRP to the 5′UTR of mRNA blocks mRNA translation of key proteins associated with iron storage and export; (2) binding of IRP to the 3′UTR of mRNA elevates mRNA translation of key proteins related to iron uptake. The opposite effect occurs when cellular iron levels are high [28]. Thus, when iron supply exceeds cell demand, the IRE-IRP switch minimizes further iron uptake via TfR1 and facilitates the storage of excess iron in newly synthesized ferritin to reach cellular iron homeostasis.
3. Iron and Energy Metabolism
Mitochondrial function is traditionally associated with energy supply for all cell compartments [29]. However, fresh insights into the relationship between mitochondrial energy metabolism and mitochondrial iron levels necessitates an expansion of the concept. The mitochondrion requires sufficient amounts of iron to maintain its normal physiologic function, since iron is the most prevalent metal inside the mitochondrial matrix and serves to facilitate the complex redox chemistry of the electron transport chain [7,30]. Once imported into mitochondria, iron is stored in the mitochondrial ferritin, or used for the biosynthesis of heme [31] and the biogenesis of iron-sulfur cluster (ISC or Fe-S) [32,33]. Both of them facilitate oxidation-reduction reactions and are essential components of enzymes involved in electron transport [34,35]. Specifically, mitochondrial iron-containing proteins that are implicated in the electron-transport chain include heme-containing proteins (succinate dehydrogenase, cytochrome c, cytochrome c oxidase, and cytochrome bc1), the ISC-containing proteins (nicotinamide adenine dinucleotide (NADH) ubiquinone oxidoreductase, Rieske iron-sulfur protein, subunits of succinate dehydrogenase, biotin synthase, lipoic acid synthase, and aconitase), and iron-ion cofactor-containing proteins (iron monooxy-genases and dioxygenases) [36]. Notably, the unique redox properties of iron allow for efficient electron transfer, accompanied by the generation of ROS. Accordingly, insufficient or excessive levels of mitochondrial iron can impair the synthesis of Fe-S cluster and heme, induce mitochondrial dysfunction, and cause oxidative stress, consequently affecting mitochondrial ATP production via the tricarboxylic acid (TCA) and/or glycolysis [37,38,39].
4. Mitochondrial Iron and Diseases
All mammalian cells possess mitochondria, and mitochondrial function is required for normal cell physiological processes. Consequently, these cells are vulnerable to diseases related to failure of mitochondrial iron homeostasis and consequent mitochondrial dysfunction [37], as shown in Table 1. These diseases are discussed in detail in the following sections.

Table 1.
Classification of diseases based on major consequences of changes in iron levels.
4.1. Cardiovascular Disease
Heart failure is a pressing public health problem with no curative treatment currently available. According to the report, heart failure is caused by the changes in mitochondrial iron homeostasis and mitochondrial function [50,51,52]. Mitochondrial iron is involved in the energy metabolism of the heart and is a fundamental element of cardiomyocyte viability and contractility [40,53]. On the one hand, systemic iron deficiency decreases mitochondrial function, leading to iron deficiency in cardiomyocytes even without anemia [54,55,56]. Recent work in mice [57] and patients with heart failure [40] has been clarified that mitochondrial function is reduced when intracellular iron is deficient, which leads to severe heart failure and is associated with cardiomyocyte injury [41]. The myocardium cannot provide sufficient blood flow. On the other hand, increased heme iron intake and body iron stores have been reported to be strongly associated with cardiovascular risk [58,59,60]. Excess iron can lead to impaired vascular function, aggravating atherosclerosis, arrhythmia, and heart failure [61]. ROS production is also catalyzed by excess iron, which causes lipid peroxidation and organelle damage [62]. This leads to cardiomyocyte death and fibrosis, ultimately leading to impaired systolic and diastolic function. Support for this theory derives from observations of increased mitochondrial iron levels in patients with heart failure [42]. The best documented example has been clearly shown in a human genetic disease, namely, cardiomyopathy of Friedreich’s ataxia (FRDA) [42]. Recent work in patients [63] and in mouse models [64,65] provides evidence that this disease is characterized by significant accumulation of iron inside the mitochondria, extensive mitochondrial dysfunction, and oxidative damage [42,66]. Luckily reducing mitochondrial iron is able to protect the heart by inhibiting oxidative stress [67]. Interestingly, the cardiac phenotype observed in FRDA is partially ameliorated in response to combined therapy with the mitochondria-permeable iron chelator deferiprone and an antioxidant [68,69], supporting the role of mitochondrial iron in the pathophysiology of cardiac dysfunction. Further confirmation for a relationship between mitochondrial iron accumulation and heart failure comes from the findings that deletion of mitochondrial ATP binding cassette transporter B8 in the heart inhibits iron export from this organelle and results in mitochondrial iron overload and subsequently increased oxidative stress [70].
Concerning the mechanisms (Figure 2), the elevation in mitochondrial iron levels that results in heart failure is likely mediated by potential disruption of Fe-S cluster biogenesis and by an ROS-dependent mechanism [71]. Iron is specially involved in the formation of atherosclerosis by catalyzing the generation of free radicals, promoting the peroxidation of the lipid and protein parts of lipoproteins, and forming oxidized low-density lipoprotein (LDL). ROS causes mitochondrial damage by attacking mitochondrial DNA and mitochondrial proteins and impairing mitochondrial aerobic metabolism, and mitochondrial dysfunction will also increase the production of ROS, thereby forming a vicious circle, which ultimately manifests as cardiovascular disease and its complications [72,73]. Meanwhile, oxidized-LDL can induce macrophages to form foam cells and promote the development of atherosclerosis [74,75,76]. Then, to make matters worse, mitochondrial antioxidant enzymes are significantly reduced in patients with heart failure compared with normal subjects [77].
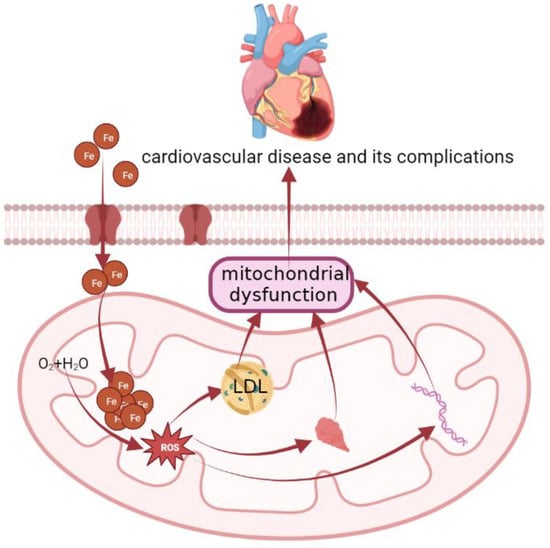
Figure 2.
The molecular mechanism of cardiovascular disease. Concerning the mechanisms, the elevation in mitochondrial iron levels that results in heart failure is likely mediated by potential disruption of Fe-S cluster biogenesis and by an ROS-dependent mechanism. Iron is specially involved in the formation of atherosclerosis by catalyzing the generation of ROS, promoting the peroxidation of the lipid and protein parts of lipoproteins, and forming oxidized low-density lipoprotein (LDL). ROS causes mitochondrial damage by attacking mitochondrial DNA and mitochondrial proteins, impairing mitochondrial aerobic metabolism, and mitochondrial dysfunction will also increase the production of ROS, thereby forming a vicious circle, which ultimately manifests as cardiovascular disease and its complications. Meanwhile, oxidized-LDL can induce macrophages to form foam cells and promote the development of atherosclerosis.
Similar to iron overload, iron deficiency can also be detrimental to the heart, an organ with high energy demands. There is validation that iron deficiency is present in approximately 30%~50% of patients with chronic heart failure [78]. Heart failure symptoms in the patient population can be improved by intravenous iron supplementation, which has been a recommended treatment for patients with heart failure with iron deficiency [79,80].
4.2. Liver Disease
The liver, the main site for iron storage, is the main target organ of iron overload-induced injury. When the iron storage and antioxidant capacity of the liver is exceeded, iron overload can lead to liver fibrosis, cirrhosis, and even hepatoma, as seen in β-thalassemia and hereditary hemochromatosis [43,81]. In addition, other chronic liver diseases such as viral hepatitis, nonalcoholic fatty liver disease, and alcoholic liver disease, are also related to liver iron overload [43]. Liver iron overload-induced oxidative stress may be a contributing mechanism for the progression of these diseases [43,82]. The liver is susceptible to oxidative damage by its intermediate metabolites during the process of metabolic detoxification. Excessive pro-oxidative forms of iron in the parenchymal cells of the liver promote oxidative damage, triggering lipid peroxidation [83]. Iron-driven injury of hepatocytes can lead to paracrine induction of hepatic stellate cells and portal myofibroblasts through lipid peroxidation byproducts, leading to increased collagen deposition, fibrosis and long-term micronodular cirrhosis and hepatocellular carcinoma [84]. In explanation of the reason for iron accumulation in the liver, recent studies have suggested that pathogenic factors related to the underlying liver disease may contribute the iron overload by directly affecting the expression of hepcidin (for autocrine downregulation of FPN expression to reduce iron export) [85].
4.3. Muscle Atrophy
Muscle atrophy, also called sarcopenia, is characterized by loss of skeletal muscle mass [86] and can be induced by aging [87] and various chronic diseases [88,89]. Recent evidence points to a strong relationship between mitochondrial iron accumulation and muscle atrophy [90], possibly manifested as a decrease in type II muscle fiber content [91,92]. Previous work on aged rats shows that, due to alterations in iron metabolism, increased iron accumulation and decreased muscle mass occur in parallel [44,93,94,95]. The adaptive downregulation in IRP2 results in a decreased expression of TFR1 (an iron transporter) and an increased expression of ferritin (an iron storage protein), which constitutes a proposed mechanism that may explain the accelerated iron accumulation in skeletal muscle of aged rats [44]. In line with these findings, recent studies showed that ablation of TFR1 in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis [96]. Further support for this mechanism drives from observations of higher ferritin levels in women with sarcopenia or sarcopenic obese people [97,98,99]. Another potential mechanism for the iron accumulation is related to the lower expression of FPN and the upregulation of genes related to iron uptake (such as DMT1 and Zip14) [100]. In addition, an animal model of disuse atrophy was used to further our understanding of the underlying mechanisms for the iron accumulation. The researchers found that iron accumulation induced by acute muscle atrophy was related to extensive oxidative stress after reloading in skeletal muscles of aged rats [100]. Oxidative stress induced by excessive iron causes muscle damage [101,102]. In support, sarcopenia and oxidative stress in skeletal muscles of mice were induced in response to iron administration [103]. Despite these interesting findings, our understanding of the precise molecular mechanism of iron-induced muscle atrophy is incomplete. Upon further investigation, the E3 ubiquitin ligase mediated by the reduction of Akt-forkhead box O3a signaling by oxidative stress is a contributing mechanism for the iron-induced skeletal muscle atrophy [86]. It is manifested in the promotion of protein degradation and inhibition of protein synthesis [86,103,104]. In 2019, it was revealed for the first time that the iron metabolism regulatory molecule Hemojuvelin (HJV or HFE2) is a protective gene that inhibits the occurrence of Duchenne muscular dystrophy and senile muscle atrophy. The molecular regulation mechanism of HJV dependent on TGFb/SMAD2/3 pathway was elucidated, and the important physiological role of HJV in protecting muscle and resisting muscle fiber aging was further explored [105]. This achievement provides a new target for the prevention and treatment of muscle atrophy diseases.
4.4. Obesity and Diabetes
Obesity and diabetes are becoming one of the most pressing health issues facing society. Several studies provide strong evidence for the correlation between dysregulated iron homeostasis and obesity as well as diabetes [29,106,107]. The liver and adipose tissues of obese participants had higher iron concentrations [108,109,110,111]. Iron accumulation and the related oxidative stress contribute to the pathophysiology of obesity and its related metabolic disturbances, such as type 2 diabetes mellitus [108]. Iron accumulation increases ROS through Fenton reaction, leading to mitochondrial dysfunction in adipocytes. This toxic effect on β cells leads to defects in insulin synthesis and secretion [45,112,113]. Hyperglycemia exacerbates iron accumulation, promotes oxidative stress and the development of type 2 diabetes [114]. In support, adipogenesis and mitochondrial biosynthesis are greatly inhibited when transferrin is knocked down or iron is chelated by using deferoxamine (DFO) [115,116], thereby inhibiting the development of obesity in diabetic states [107]. Consistently, adiposity can be ameliorated in response to DFO (100 mg/kg body weight), accompanied by increased insulin sensitivity in ob/ob mice [107]. On the contrary, lipolysis is promoted when adipocytes are treated with either iron or transferrin [117]. The status and development of obesity and diabetes can be ameliorated when body iron content is reduced to an appropriate level [106]. However, contradictory results are reported by other studies, which indicate that iron deficiency increases the risk of developing diabetes in obese individuals [118]. Therefore, the relationship between body iron content and obesity is still a topic of debate and warrants further investigation.
4.5. Kidney Disease
Iron and iron-triggered oxidative stress and mitochondrial dysfunction are thought to be involved in the progression of multiple models of acute kidney injury [119,120,121]. Patients with chronic kidney disease (CKD) experience significant changes in iron balance and tissue distribution due to elevated iron losses, decreased iron absorption, and impaired mobilization of iron from stores [122]. If the iron metabolism is unbalanced, the accumulation of iron in the kidney and the increase of urinary iron concentration or iron deficiency will cause kidney damage and related complications [6,123,124]. Tubular cell lysosomal iron accumulation has been shown in patients with CKD [46], which is most likely due to excessive iron content, which catalyzes the formation of oxygen free radicals, disrupts mitochondrial oxidative metabolism, and leads to renal cell damage. According to the Fenton reaction, abnormal accumulation of iron creates oxidative stress. On the other hand, renal tubular epithelial cells have high energy demands and, therefore, have a large number of mitochondria, making them susceptible to oxidative stress [119]. In rat kidneys, iron in the form of myoglobin has been reported to generate oxidative stress, leading to mitochondrial dysfunction through lipid peroxidation of mitochondrial membranes, which leads to pro-inflammatory cells in a rat model of acute cerebral ischemia Factor production [123].
Ferroptosis, a new form of regulated cell death identified in recent years, is involved in the initiation and progression of diverse kidney diseases, such as renal ischemia-reperfusion injury, renal cell carcinoma, and acute kidney injury [125,126]. Unlike other types of known regulated cell death (e.g., pyroptosis, necrosis, autophagy, and apoptosis), ferroptosis is characterized by the iron-dependent overwhelming accumulation of lipid hydroperoxides and augmented mitochondrial membrane density [127]. The latest research demonstrated that mitochondrial iron overload can accelerate the process of ferroptosis [128]. Concerning the mechanism for iron overload-induced ferroptosis, recent studies using a model of aristolactam I-induced ferroptosis reported that Fe2+ overload-mediated mitochondrial ROS over-release would activate lipid peroxidation and inhibit the antioxidant system by inhibiting nuclear factor erythroid 2-related factor 2-heme oxygenase 1/glutathione peroxidase 4 pathway, which enhanced ferroptosis [129].
4.6. Neurodegenerative Disease (NDDs)
The brain is a metabolically active place compared to other organs [130]. Neuronal mitochondrial respiration accounts for about 20% of total oxygen consumption [131]. Cortical neurons in the human brain utilize approximately 4.7 billion ATP molecules produced by mitochondria per second to perform biological functions such as synaptic assembly, generation of action potentials, and synaptic transmission [132]. It has been reported that mitochondrial iron accumulation plays an important role in the initiation and progression of NDDs, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [133]. In detail, iron overload promotes mitochondrial dysfunction and catalyzes the production of ROS that triggers oxidative stress in the brain, resulting in neurological damage [134]. It has been reported that mitochondrial dysfunction is a common pathogenic feature of NDDs such as AD and PD [135,136]. Ferritin is a precursor of iron accumulation [137]. The two subunits of ferritin, L- ferritin (FTL) and H- ferritin (FTH), are essential for iron storage in vertebrate cells [138]. Compared with the liver, which mainly contains FTL, the brain and heart have more high iron oxidation activity, so it mainly contains FTH ferritin with significant antioxidant activity [139,140,141]. Differed from physiological ferritin, studies have shown that ferritin structures in NDDs are in the form of magnetite crystals [142]. This rare magnetic structure could help visualize brain tissue for the diagnosis of NDDs [143].
It is estimated that 10% of the world’s population may currently be affected by AD [144]. Patients with AD have diffuse accumulation of iron in the cerebral cortex and hippocampus, and the content of iron in senile plaques increases slightly [47]. Specifically, in AD (Figure 3), iron accumulation induces oxidative stress, lipid peroxidation, and inflammatory responses by disrupting mitochondrial function, depleting ATP, and inducing ROS production [135]. The combined effects of oxidative stress, lipid peroxidation and neuroinflammation lead to the production of amyloid-beta (Aβ) [132]. Through these mechanisms, iron accumulation induces apoptosis and/or necrosis, thus leading to cell death [135]. In addition, Aβ can induce lipid peroxidation in the presence of iron ions [145], as manifested by the increased expression of lipoxygenase in the brain of AD patients [146]. Knockout of lipoxygenase reduces iron-induced lipid peroxidation, which in turn reduces Aβ deposition in AD mouse brain and improves behavioral performance [147].
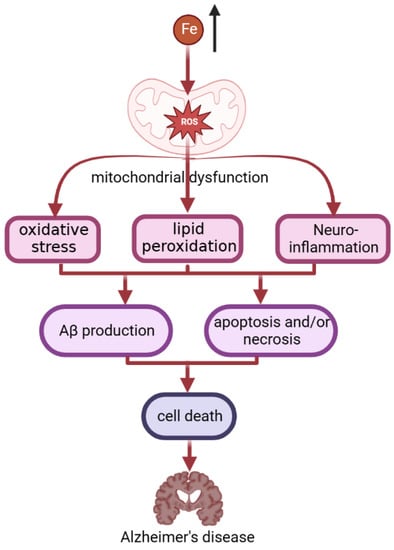
Figure 3.
The process of iron accumulation in AD patients. In AD, iron accumulation induces oxidative stress, lipid peroxidation, and inflammatory responses by disrupting mitochondrial function, and inducing ROS production. The combined effects of oxidative stress, lipid peroxidation, and neuroinflammation lead to the production of amyloid-beta (Aβ). Aβ can induce lipid peroxidation in the presence of iron ions. Through these mechanisms, iron accumulation induces apoptosis and/or necrosis, thus leading to cell death. This finally leads to becoming an AD patient.
Among other NDDs, PD is the second most common in people over 60 [148]. Focal accumulation of iron in the substantia nigra has been reported in patients with PD [48]. Iron is involved in the formation of α-synuclein aggregates in intracellular inclusions, called Lewy bodies, leading to synaptic dysfunction and disruption of axonal transport [149], which is a hallmark of PD. In murine models of PD, α-synuclein expression can be regulated to ameliorate PD injury by increasing mitochondrial ferritin [150,151]. Moreover, decreased mitochondrial complex I activity is observed in mitochondria isolated from human brain tissues and peripheral cells of sporadic PD patients, indicating an impairment of mitochondrial function [152]. Subsequently, such mitochondrial dysfunction may result in IRP1 activation, upregulated expression of DMT1 and TFR1, elevated uptake of iron, and elevated production of ROS [153]. The mitochondrial iron-specific changes in human and rodent models of PD have been demonstrated by a number of studies. For instance, mitochondrial iron uptake and the production of ROS were increased in SH-SY5Y dopaminergic neuroblastoma cells treated with rotenone (a mitochondrial complex I inhibitor) [154,155]. Further evidence comes from observations of the accumulation of transferrin in dopamine neurons (with much of it accumulating in the mitochondria) in a rodent rotenone model of PD [156].
4.7. Cancers
Iron overload is related to the occurrence of various cancers such as liver, colon, rectum, lung, esophagus and bladder cancers (Figure 4) [157,158] because iron is needed in all stages of tumor development, survival, proliferation and metastasis [159]. There are two well-defined mechanisms of cancer development induced by iron excess [160,161]. One is associated with the pre-oxidant effects of iron, which can lead to DNA damage and subsequently promote oncogenesis [162]. The dependence of cancer cells on iron to maintain their rapid growth rate constitutes the other mechanism [161,163]. During rapid cell proliferation, more iron may be imported to mitochondria of cancer cells, in order to produce heme and ISC and to satisfy increasing demands for these cofactors [164]. For instance, the rates of heme-synthesis are elevated in non-small cell lung cancer cells compared to normal nonmalignant lung cells [165]. Intriguingly, the expression of iron homeostasis proteins associated with iron accumulation is altered in multiple cancer cell types, such as an elevated expression of the iron uptake-related protein TFR1, a reduced expression of the iron export-related protein FPN, and an elevated production of hepcidin [160,161,166,167,168]. Tumor growth and survival can be greatly influenced by altered expression of these proteins. Evidence for this is provided by observations found in breast cancer that a high expression of FPN and low expression of hepcidin predicts a favorable prognosis, while a low expression of FPN is related to metastatic progression and reduced survival [169,170,171]. In addition, the expression of mitoferrin-2 (related to mitochondrial iron uptake) is altered in head and neck cancers [172]. The demand for iron in cancer cells is an important strategy for the anti-cancer targeting of chelating agents. Iron chelators affect the initiation, growth, proliferation, and metastasis of cancer cells by targeting different stages of disease progression, including associated iron metabolic pathways and iron-containing proteins [160,173]. The first iron chelator for clinical trials is desferrioxamine (DFO) [174,175], which was originally used as a treatment for iron overload [176]. It can also target ferritin through autophagy degradation [177]. Quercetin can not only effectively form complexes with iron, but also induce iron deficiency behaviors in cancer cells, such as induction of transferrin receptor-1 and iron regulatory protein-2 expression and decreased ferritin expression. In addition, quercetin can regulate the expression of iron metabolism genes in rats and reduce the expression of DMT1, Dcytb, FPN, and hepcidin. This reduces the level of iron absorption [178]. In addition, the new iron chelator CN128 has great potential in the treatment of clinical skin cancer, with good oral bioavailability and tissue distribution [179]. It is worth mentioning that, according to a new study, the increase in iron can promote estrogen-induced carcinogenesis by producing additional ROS [49]. This may be a new breakthrough in the treatment of cancer.
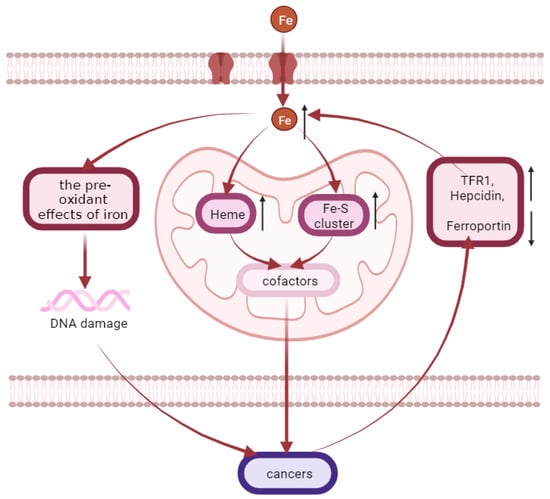
Figure 4.
Iron accumulation is related to the occurrence of various cancers. Iron overload is related to the occurrence of various cancers such as liver, colon, rectum, lung, esophagus, and bladder cancers. There are two well-defined mechanisms of cancer development induced by iron excess. One is associated with the pre-oxidant effects of iron, which can lead to DNA damage and subsequently promote oncogenesis. The other one is that more iron may be imported to mitochondria of cancer cells during rapid cell proliferation, in order to produce heme and Fe-S cluster and to satisfy increasing demands for these cofactors. Meanwhile, the expression of iron homeostasis proteins associated with iron accumulation is altered in multiple cancer cell types, such as an elevated expression of the iron uptake-related protein TFR1, a reduced expression of the iron export-related protein ferroportin, and an elevated production of hepcidin.
5. Summary
The literature reviewed here indicates that iron has important physiological and pathological significance in the body. Disorders of mitochondrial iron metabolism underlie the pathogenesis of many diseases (Figure 5). In detail, mitochondrial iron deficiency or overload can result in dysfunctional mitochondrial synthesis of heme and/or ISC, causing mitochondrial dysfunction and consequent oxidative damage. This may lead to further downstream signals to induce various diseases. However, information is limited to the optimal iron treatment strategy for the diseases. In the near future, more efforts should be made to find better diagnostic parameters for accurately gauging iron status and to take measures to maintain the mitochondrial iron balance, ultimately promoting the healthy growth of the body.
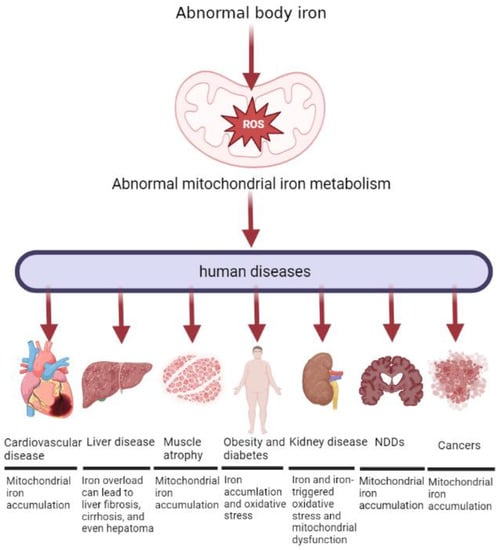
Figure 5.
Abnormal mitochondrial iron metabolism can cause different diseases in the human body. Both insufficient and excessive levels of iron can be detrimental to mitochondrial function. Mitochondria are found in human cells, and normal cellular physiology depends on mitochondrial function. Consequently, these cells are vulnerable to diseases associated with failure of mitochondrial iron homeostasis and consequent mitochondrial dysfunction. The diseases described in the paper are: Sideroblastic anemia, Cardiovascular disease, liver disease, muscle atrophy, obesity and diabetes, kidney disease, Neurodegenerative diseases, and cancers.
Author Contributions
Conceptualization, Y.D. and C.L.; writing—original draft preparation, G.D. and J.L.; writing—review and editing, C.Z., Q.G., F.L., J.Z., J.Y., P.Z. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hunan Province (2021JJ20044), the Changsha Natural Science Funds for Distinguished Young Scholar (kq2009020), The Science and Technology Innovation Program of Hunan Province (2022RC1159), the National Key Research and Development Programs of China (2022YFD1300503), and China Agriculture Research System of MOF and MARA (CARS-35).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21 (Suppl. S1), S6–S20. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Pantopoulos, K. Oxidative stress and iron homeostasis: Mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Ferring-Appel, D.; Sauer, S.W.; Kaden, S.; Lyoumi, S.; Puy, H.; Kolker, S.; Grone, H.J.; Hentze, M.W. Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab. 2010, 12, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S. Iron Homeostasis Pathways as Therapeutic Targets in Acute Kidney Injury. Nephron 2018, 140, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2021, 513, 6–12. [Google Scholar] [CrossRef]
- Levi, S.; Rovida, E. The role of iron in mitochondrial function. Biochim. Biophys. Acta 2009, 1790, 629–636. [Google Scholar] [CrossRef]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Andrews, N.C. Disorders of iron metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pantopoulos, K. Regulation of cellular iron metabolism. Biochem. J. 2011, 434, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Masaratana, P.; Latunde-Dada, G.O.; Arno, M.; Simpson, R.J.; McKie, A.T. Duodenal reductase activity and spleen iron stores are reduced and erythropoiesis is abnormal in Dcytb knockout mice exposed to hypoxic conditions. J. Nutr. 2012, 142, 1929–1934. [Google Scholar] [CrossRef]
- Muir, A.; Hopfer, U. Regional specificity of iron uptake by small intestinal brush-border membranes from normal and iron-deficient mice. Am. J. Physiol. 1985, 248 Pt 1, G376–G379. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ramakrishnan, S.K.; Weisz, K.; Triner, D.; Xie, L.; Attili, D.; Pant, A.; Gyorffy, B.; Zhan, M.; Carter-Su, C.; et al. Iron Uptake via DMT1 Integrates Cell Cycle with JAK-STAT3 Signaling to Promote Colorectal Tumorigenesis. Cell Metab. 2016, 24, 447–461. [Google Scholar] [CrossRef]
- Theil, E.C.; Chen, H.; Miranda, C.; Janser, H.; Elsenhans, B.; Nunez, M.T.; Pizarro, F.; Schumann, K. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J. Nutr. 2012, 142, 478–483. [Google Scholar] [CrossRef]
- Troadec, M.B.; Ward, D.M.; Lo, E.; Kaplan, J.; De Domenico, I. Induction of FPN1 transcription by MTF-1 reveals a role for ferroportin in transition metal efflux. Blood 2010, 116, 4657–4664. [Google Scholar] [CrossRef]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef]
- Strzyz, P. Iron expulsion by exosomes drives ferroptosis resistance. Nat. Rev. Mol. Cell Biol. 2020, 21, 4–5. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [PubMed]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.S.; Ghoti, H.; Breuer, W.; Rachmilewitz, E.; Attar, S.; Weiss, G.; Cabantchik, Z.I. The role of endocytic pathways in cellular uptake of plasma non-transferrin iron. Haematologica 2012, 97, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.C. Guidelines for quantifying iron overload. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Galy, B.; Hentze, M.W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008, 28, 197–213. [Google Scholar] [CrossRef]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Pan, G.; Song, Z.; He, Y.; Zhang, S.; Ye, X.; Yang, X.; Xie, E.; Wang, X.; et al. Transferrin Receptor 1 Regulates Thermogenic Capacity and Cell Fate in Brown/Beige Adipocytes. Adv. Sci. 2020, 7, 1903366. [Google Scholar] [CrossRef]
- Rensvold, J.W.; Krautkramer, K.A.; Dowell, J.A.; Denu, J.M.; Pagliarini, D.J. Iron Deprivation Induces Transcriptional Regulation of Mitochondrial Biogenesis. J. Biol. Chem. 2016, 291, 20827–20837. [Google Scholar] [CrossRef]
- Swenson, S.A.; Moore, C.M.; Marcero, J.R.; Medlock, A.E.; Reddi, A.R.; Khalimonchuk, O. From Synthesis to Utilization: The Ins and Outs of Mitochondrial Heme. Cells 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Braymer, J.J.; Lill, R. Iron-sulfur cluster biogenesis and trafficking in mitochondria. J. Biol. Chem. 2017, 292, 12754–12763. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pain, J.; Dancis, A.; Pain, D. Mitochondria export iron-sulfur and sulfur intermediates to the cytoplasm for iron-sulfur cluster assembly and tRNA thiolation in yeast. J. Biol. Chem. 2019, 294, 9489–9502. [Google Scholar] [CrossRef]
- Lill, R.; Muhlenhoff, U. Iron-sulfur protein biogenesis in eukaryotes: Components and mechanisms. Annu. Rev. Cell Dev. Biol. 2006, 22, 457–486. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Krasnikov, B.F.; Kuzminova, A.E.; Vysokikh, M.; Zorova, L.D. Mitochondria revisited. Alternative functions of mitochondria. Biosci. Rep. 1997, 17, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and Iron: Current questions. Expert Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef]
- Ward, D.M.; Cloonan, S.M. Mitochondrial Iron in Human Health and Disease. Annu. Rev. Physiol. 2019, 81, 453–482. [Google Scholar] [CrossRef]
- Santambrogio, P.; Dusi, S.; Guaraldo, M.; Rotundo, L.I.; Broccoli, V.; Garavaglia, B.; Tiranti, V.; Levi, S. Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Neurobiol. Dis. 2015, 81, 144–153. [Google Scholar] [CrossRef]
- Tavsan, Z.; Ayar Kayali, H. The Variations of Glycolysis and TCA Cycle Intermediate Levels Grown in Iron and Copper Mediums of Trichoderma harzianum. Appl. Biochem. Biotechnol. 2015, 176, 76–85. [Google Scholar] [CrossRef]
- Manceau, H.; Ausseil, J.; Masson, D.; Feugeas, J.P.; Sablonniere, B.; Guieu, R.; Puy, H.; Peoc’h, K. Neglected Comorbidity of Chronic Heart Failure: Iron Deficiency. Nutrients 2022, 14, 3214. [Google Scholar] [CrossRef]
- Corradi, F.; Fischetti, I.; De Caterina, R. Addenda online Ferro e cardiopatia ischemica stabile-lezioni dallo scompenso cardiaco. G. Ital. Cardiol. 2019, 20. [Google Scholar] [CrossRef]
- Payne, R.M. The Heart in Friedreich’s Ataxia: Basic Findings and Clinical Implications. Prog. Pediatr. Cardiol. 2011, 31, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Iron and the liver. Liver Int. 2016, 36 (Suppl. S1), 116–123. [Google Scholar] [CrossRef]
- DeRuisseau, K.C.; Park, Y.M.; DeRuisseau, L.R.; Cowley, P.M.; Fazen, C.H.; Doyle, R.P. Aging-related changes in the iron status of skeletal muscle. Exp. Gerontol. 2013, 48, 1294–1302. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Ghaben, A.L.; Morley, T.S.; Samms, R.J.; Adams, A.C.; An, Y.; Johnson, J.A.; Joffin, N.; Onodera, T.; Crewe, C.; et al. A Novel Model of Diabetic Complications: Adipocyte Mitochondrial Dysfunction Triggers Massive beta-Cell Hyperplasia. Diabetes 2020, 69, 313–330. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Boadle, R.A.; Harris, D.C. Iron accumulation in human chronic renal disease. Am. J. Kidney Dis. 1992, 20, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Mostile, G.; Cicero, C.E.; Giuliano, L.; Zappia, M.; Nicoletti, A. Iron and Parkinson’s disease: A systematic review and meta-analysis. Mol. Med. Rep. 2017, 15, 3383–3389. [Google Scholar] [CrossRef]
- Bajbouj, K.; Shafarin, J.; Abdalla, M.Y.; Ahmad, I.M.; Hamad, M. Estrogen-induced disruption of intracellular iron metabolism leads to oxidative stress, membrane damage, and cell cycle arrest in MCF-7 cells. Tumour Biol. 2017, 39, 1010428317726184. [Google Scholar] [CrossRef]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014, 114, 524–537. [Google Scholar] [CrossRef]
- Gammella, E.; Recalcati, S.; Rybinska, I.; Buratti, P.; Cairo, G. Iron-induced damage in cardiomyopathy: Oxidative-dependent and independent mechanisms. Oxid. Med. Cell. Longev. 2015, 2015, 230182. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Petrak, J.; Mracek, T.; Benes, J.; Borlaug, B.A.; Nuskova, H.; Pluhacek, T.; Spatenka, J.; Kovalcikova, J.; Drahota, Z.; et al. Myocardial iron content and mitochondrial function in human heart failure: A direct tissue analysis. Eur. J. Heart Fail. 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.K.; Anker, S.D. Anaemia, iron deficiency and heart failure in 2020: Facts and numbers. ESC Heart Fail. 2020, 7, 2007–2011. [Google Scholar] [CrossRef] [PubMed]
- Hoes, M.F.; Grote Beverborg, N.; Kijlstra, J.D.; Kuipers, J.; Swinkels, D.W.; Giepmans, B.N.G.; Rodenburg, R.J.; van Veldhuisen, D.J.; de Boer, R.A.; van der Meer, P. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur. J. Heart Fail. 2018, 20, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Rineau, E.; Gaillard, T.; Gueguen, N.; Procaccio, V.; Henrion, D.; Prunier, F.; Lasocki, S. Iron deficiency without anemia is responsible for decreased left ventricular function and reduced mitochondrial complex I activity in a mouse model. Int. J. Cardiol. 2018, 266, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Willett, W.C.; Rimm, E.B.; Giovannucci, E.L.; Stampfer, M.J. Dietary iron intake and risk of coronary disease among men. Circulation 1994, 89, 969–974. [Google Scholar] [CrossRef]
- Van der, A.D.; Peeters, P.H.; Grobbee, D.E.; Marx, J.J.; van der Schouw, Y.T. Dietary haem iron and coronary heart disease in women. Eur. Heart J. 2005, 26, 257–262. [Google Scholar] [CrossRef]
- Kiechl, S.; Willeit, J.; Egger, G.; Poewe, W.; Oberhollenzer, F. Body iron stores and the risk of carotid atherosclerosis: Prospective results from the Bruneck study. Circulation 1997, 96, 3300–3307. [Google Scholar] [CrossRef]
- Vinchi, F.; Porto, G.; Simmelbauer, A.; Altamura, S.; Passos, S.T.; Garbowski, M.; Silva, A.M.N.; Spaich, S.; Seide, S.E.; Sparla, R.; et al. Atherosclerosis is aggravated by iron overload and ameliorated by dietary and pharmacological iron restriction. Eur. Heart J. 2020, 41, 2681–2695. [Google Scholar] [CrossRef]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loreal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta 2012, 1820, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Petrocine, S.V.; Qian, J.; Lamarche, J.B.; Knutson, M.D.; Garrick, M.D.; Koeppen, A.H. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich’s ataxia. Cerebellum 2006, 5, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Whitnall, M.; Suryo Rahmanto, Y.; Sutak, R.; Xu, X.; Becker, E.M.; Mikhael, M.R.; Ponka, P.; Richardson, D.R. The MCK mouse heart model of Friedreich’s ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc. Natl. Acad. Sci. USA 2008, 105, 9757–9762. [Google Scholar] [CrossRef] [PubMed]
- Puccio, H.; Simon, D.; Cossee, M.; Criqui-Filipe, P.; Tiziano, F.; Melki, J.; Hindelang, C.; Matyas, R.; Rustin, P.; Koenig, M. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat. Genet. 2001, 27, 181–186. [Google Scholar] [CrossRef]
- Gao, X.; Qian, M.; Campian, J.L.; Marshall, J.; Zhou, Z.; Roberts, A.M.; Kang, Y.J.; Prabhu, S.D.; Sun, X.F.; Eaton, J.W. Mitochondrial dysfunction may explain the cardiomyopathy of chronic iron overload. Free Radic. Biol. Med. 2010, 49, 401–407. [Google Scholar] [CrossRef]
- Chang, H.C.; Wu, R.; Shang, M.; Sato, T.; Chen, C.; Shapiro, J.S.; Liu, T.; Thakur, A.; Sawicki, K.T.; Prasad, S.V.; et al. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol. Med. 2016, 8, 247–267. [Google Scholar] [CrossRef]
- Velasco-Sanchez, D.; Aracil, A.; Montero, R.; Mas, A.; Jimenez, L.; O’Callaghan, M.; Tondo, M.; Capdevila, A.; Blanch, J.; Artuch, R.; et al. Combined therapy with idebenone and deferiprone in patients with Friedreich’s ataxia. Cerebellum 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Sohn, Y.S.; Breuer, W.; Munnich, A.; Cabantchik, Z.I. Redistribution of accumulated cell iron: A modality of chelation with therapeutic implications. Blood 2008, 111, 1690–1699. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Bayeva, M.; Ghanefar, M.; Potini, V.; Sun, L.; Mutharasan, R.K.; Wu, R.; Khechaduri, A.; Jairaj Naik, T.; Ardehali, H. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc. Natl. Acad. Sci. USA 2012, 109, 4152–4157. [Google Scholar] [CrossRef]
- Elas, M.; Bielanska, J.; Pustelny, K.; Plonka, P.M.; Drelicharz, L.; Skorka, T.; Tyrankiewicz, U.; Wozniak, M.; Heinze-Paluchowska, S.; Walski, M.; et al. Detection of mitochondrial dysfunction by EPR technique in mouse model of dilated cardiomyopathy. Free Radic. Biol. Med. 2008, 45, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Galvano, F.; Orlandi, C.; Bianchi, A.; Di Giacomo, C.; La Fauci, L.; Acquaviva, R.; De Lorenzo, A. Oxidative stress in normal-weight obese syndrome. Obesity 2010, 18, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef]
- Itabe, H.; Obama, T.; Kato, R. The Dynamics of Oxidized LDL during Atherogenesis. J. Lipids 2011, 2011, 418313. [Google Scholar] [CrossRef] [PubMed]
- Fantuzzi, G.; Mazzone, T. Adipose tissue and atherosclerosis: Exploring the connection. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Burgos Alves, M.I.; Aviles Plaza, F.; Martinez-Tomas, R.; Sanchez-Campillo, M.; Larque, E.; Perez-Llamas, F.; Martinez Hernandez, P.; Parra Pallares, S. Oxidized LDL and its correlation with lipid profile and oxidative stress biomarkers in young healthy Spanish subjects. J. Physiol. Biochem. 2010, 66, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Di Renzo, L.; Carraro, A.; Valente, R.; Iacopino, L.; Colica, C.; De Lorenzo, A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: A randomized crossover trial. Oxid. Med. Cell. Longev. 2014, 2014, 681318. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron deficiency in chronic heart failure: An international pooled analysis. Am. Heart J. 2013, 165, 575–582.e3. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Luscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br. J. Haematol. 2016, 172, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Fracanzani, A.L.; Bugianesi, E.; Dongiovanni, P.; Galmozzi, E.; Vanni, E.; Canavesi, E.; Lattuada, E.; Roviaro, G.; Marchesini, G.; et al. HFE genotype, parenchymal iron accumulation, and liver fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2010, 138, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Metals, oxidative stress, and hepatic fibrogenesis. Semin. Liver Dis. 1996, 16, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Pietrangelo, A. Pathogens, Metabolic Adaptation, and Human Diseases—An Iron-Thrifty Genetic Model. Gastroenterology 2015, 149, 834–838. [Google Scholar] [CrossRef]
- Ikeda, Y.; Imao, M.; Satoh, A.; Watanabe, H.; Hamano, H.; Horinouchi, Y.; Izawa-Ishizawa, Y.; Kihira, Y.; Miyamoto, L.; Ishizawa, K.; et al. Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway. J. Trace Elem. Med. Biol. 2016, 35, 66–76. [Google Scholar] [CrossRef]
- Evans, W.J. What is sarcopenia? J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, 5–8. [Google Scholar] [CrossRef]
- Fulster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschope, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Marzetti, E.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Dupont-Versteegden, E.E.; Carter, C.S.; Bernabei, R.; Leeuwenburgh, C. Mitochondrial death effectors: Relevance to sarcopenia and disuse muscle atrophy. Biochim. Biophys. Acta 2010, 1800, 235–244. [Google Scholar] [CrossRef]
- Kramer, I.F.; Snijders, T.; Smeets, J.S.J.; Leenders, M.; van Kranenburg, J.; den Hoed, M.; Verdijk, L.B.; Poeze, M.; van Loon, L.J.C. Extensive Type II Muscle Fiber Atrophy in Elderly Female Hip Fracture Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1369–1375. [Google Scholar] [CrossRef]
- Sayed, R.K.; de Leonardis, E.C.; Guerrero-Martinez, J.A.; Rahim, I.; Mokhtar, D.M.; Saleh, A.M.; Abdalla, K.E.; Pozo, M.J.; Escames, G.; Lopez, L.C.; et al. Identification of morphological markers of sarcopenia at early stage of aging in skeletal muscle of mice. Exp. Gerontol. 2016, 83, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Altun, M.; Edstrom, E.; Spooner, E.; Flores-Moralez, A.; Bergman, E.; Tollet-Egnell, P.; Norstedt, G.; Kessler, B.M.; Ulfhake, B. Iron load and redox stress in skeletal muscle of aged rats. Muscle Nerve 2007, 36, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Knutson, M.D.; Carter, C.S.; Leeuwenburgh, C. Iron accumulation with age, oxidative stress and functional decline. PLoS ONE 2008, 3, e2865. [Google Scholar] [CrossRef] [PubMed]
- Kasztura, M.; Dziegala, M.; Kobak, K.; Bania, J.; Mazur, G.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Both iron excess and iron depletion impair viability of rat H9C2 cardiomyocytes and L6G8C5 myocytes. Kardiol. Pol. 2017, 75, 267–275. [Google Scholar] [CrossRef]
- Ding, H.; Chen, S.; Pan, X.; Dai, X.; Pan, G.; Li, Z.; Mai, X.; Tian, Y.; Zhang, S.; Liu, B.; et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kang, H.T.; Lee, D.C.; Lee, H.R.; Lee, Y.J. Body composition and its association with cardiometabolic risk factors in the elderly: A focus on sarcopenic obesity. Arch. Gerontol. Geriatr. 2013, 56, 270–278. [Google Scholar] [CrossRef]
- Perna, S.; Peroni, G.; Faliva, M.A.; Bartolo, A.; Naso, M.; Miccono, A.; Rondanelli, M. Sarcopenia and sarcopenic obesity in comparison: Prevalence, metabolic profile, and key differences. A cross-sectional study in Italian hospitalized elderly. Aging Clin. Exp. Res. 2017, 29, 1249–1258. [Google Scholar] [CrossRef]
- Kim, T.H.; Hwang, H.J.; Kim, S.H. Relationship between Serum Ferritin Levels and Sarcopenia in Korean Females Aged 60 Years and Older Using the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-2, 3), 2008–2009. PLoS ONE 2014, 9, e90105. [Google Scholar] [CrossRef]
- Xu, J.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Knutson, M.D.; Judge, A.R.; Dupont-Versteegden, E.E.; Marzetti, E.; Leeuwenburgh, C. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol. 2012, 47, 100–108. [Google Scholar] [CrossRef]
- Argiles, J.M.; Fontes-Oliveira, C.C.; Toledo, M.; Lopez-Soriano, F.J.; Busquets, S. Cachexia: A problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 2014, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Halon-Golabek, M.; Borkowska, A.; Herman-Antosiewicz, A.; Antosiewicz, J. Iron Metabolism of the Skeletal Muscle and Neurodegeneration. Front. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Reardon, T.F.; Allen, D.G. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp. Physiol. 2009, 94, 720–730. [Google Scholar] [CrossRef]
- Kobak, K.; Kasztura, M.; Dziegala, M.; Bania, J.; Kapusniak, V.; Banasiak, W.; Ponikowski, P.; Jankowska, E.A. Iron limitation promotes the atrophy of skeletal myocytes, whereas iron supplementation prevents this process in the hypoxic conditions. Int. J. Mol. Med. 2018, 41, 2678–2686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, J.; Wang, F.; Gong, J.; Wang, L.; Wu, Q.; Li, W.; Liu, H.; Wang, J.; Zhang, K.; et al. Hemojuvelin is a novel suppressor for Duchenne muscular dystrophy and age-related muscle wasting. J. Cachexia Sarcopenia Muscle 2019, 10, 557–573. [Google Scholar] [CrossRef]
- Ikeda, Y.; Funamoto, M.; Tsuchiya, K. The role of iron in obesity and diabetes. J. Med. Investig. 2022, 69, 1–7. [Google Scholar] [CrossRef]
- Yan, H.F.; Liu, Z.Y.; Guan, Z.A.; Guo, C. Deferoxamine ameliorates adipocyte dysfunction by modulating iron metabolism in ob/ob mice. Endocr. Connect. 2018, 7, 604–616. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Lopez-Bermejo, A.; Ricart, W. Cross-talk between iron metabolism and diabetes. Diabetes 2002, 51, 2348–2354. [Google Scholar] [CrossRef]
- Orr, J.S.; Kennedy, A.; Anderson-Baucum, E.K.; Webb, C.D.; Fordahl, S.C.; Erikson, K.M.; Zhang, Y.; Etzerodt, A.; Moestrup, S.K.; Hasty, A.H. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 2014, 63, 421–432. [Google Scholar] [CrossRef]
- Tajima, S.; Ikeda, Y.; Sawada, K.; Yamano, N.; Horinouchi, Y.; Kihira, Y.; Ishizawa, K.; Izawa-Ishizawa, Y.; Kawazoe, K.; Tomita, S.; et al. Iron reduction by deferoxamine leads to amelioration of adiposity via the regulation of oxidative stress and inflammation in obese and type 2 diabetes KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E77–E86. [Google Scholar] [CrossRef]
- Xue, H.; Chen, D.; Zhong, Y.K.; Zhou, Z.D.; Fang, S.X.; Li, M.Y.; Guo, C. Deferoxamine ameliorates hepatosteatosis via several mechanisms in ob/ob mice. Ann. N. Y. Acad. Sci. 2016, 1375, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, Y.; Ikeda, Y.; Tamaki, T. Body iron accumulation in obesity, diabetes and its complications, and the possibility of therapeutic application by iron regulation. Nihon Yakurigaku Zasshi 2019, 154, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells 2021, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wu, L.; Yang, G.; Zhang, C.; Liu, X.; Sun, X.; Chen, X.; Wang, N. The role of iron metabolism in chronic diseases related to obesity. Mol. Med. 2022, 28, 130. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Moreno, M.; Ricart, W.; Fernandez-Real, J.M. Fine-tuned iron availability is essential to achieve optimal adipocyte differentiation and mitochondrial biogenesis. Diabetologia 2014, 57, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Medina-Gomez, G. Mitochondria and endocrine function of adipose tissue. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 791–804. [Google Scholar] [CrossRef]
- Rumberger, J.M.; Peters, T., Jr.; Burrington, C.; Green, A. Transferrin and iron contribute to the lipolytic effect of serum in isolated adipocytes. Diabetes 2004, 53, 2535–2541. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Wylie-Rosett, J.; Gunter, M.J.; Negassa, A.; Kabat, G.C.; Rohan, T.E.; Crandall, J.; Diabetes Prevention Program Research Group. Biomarkers of body iron stores and risk of developing type 2 diabetes. Diabetes Obes. Metab. 2009, 11, 472–479. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Baliga, R.; Ueda, N.; Shah, S.V. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem. J. 1993, 291 Pt 3, 901–905. [Google Scholar] [CrossRef]
- Baliga, R.; Zhang, Z.; Baliga, M.; Ueda, N.; Shah, S.V. In vitro and in vivo evidence suggesting a role for iron in cisplatin-induced nephrotoxicity. Kidney Int. 1998, 53, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Wish, J.B.; Aronoff, G.R.; Bacon, B.R.; Brugnara, C.; Eckardt, K.U.; Ganz, T.; Macdougall, I.C.; Nunez, J.; Perahia, A.J.; Wood, J.C. Positive Iron Balance in Chronic Kidney Disease: How Much is Too Much and How to Tell? Am. J. Nephrol. 2018, 47, 72–83. [Google Scholar] [CrossRef] [PubMed]
- van Swelm, R.P.L.; Wetzels, J.F.M.; Swinkels, D.W. The multifaceted role of iron in renal health and disease. Nat. Rev. Nephrol. 2020, 16, 77–98. [Google Scholar] [CrossRef] [PubMed]
- ElAlfy, M.S.; Khalil Elsherif, N.H.; Ebeid, F.S.E.; Ismail, E.A.R.; Ahmed, K.A.; Darwish, Y.W.; Ibrahim, A.S.; Elghamry, I.R.F.; Shokrey, N.A.; Alajeil, D.N. Renal iron deposition by magnetic resonance imaging in pediatric beta-thalassemia major patients: Relation to renal biomarkers, total body iron and chelation therapy. Eur. J. Radiol. 2018, 103, 65–70. [Google Scholar] [CrossRef]
- Linkermann, A.; Skouta, R.; Himmerkus, N.; Mulay, S.R.; Dewitz, C.; De Zen, F.; Prokai, A.; Zuchtriegel, G.; Krombach, F.; Welz, P.S.; et al. Synchronized renal tubular cell death involves ferroptosis. Proc. Natl. Acad. Sci. USA 2014, 111, 16836–16841. [Google Scholar] [CrossRef]
- Tang, S.; Xiao, X. Ferroptosis and kidney diseases. Int. Urol. Nephrol. 2020, 52, 497–503. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.F.; Yue, L.X.; Wang, N.N.; Zhou, Y.Q.; Zhou, W.; Liu, X.; Ni, Y.H.; Huang, C.S.; Qiu, L.Z.; Liu, H.; et al. Mitochondrial Iron Overload-Mediated Inhibition of Nrf2-HO-1/GPX4 Assisted ALI-Induced Nephrotoxicity. Front. Pharmacol. 2020, 11, 624529. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. The metabolism of neuronal iron and its pathogenic role in neurological disease: Review. Ann. N. Y. Acad. Sci. 2004, 1012, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Cardaioli, E.; Da Pozzo, P.; Formichi, P.; Gallus, G.N.; Radi, E. Mitochondria, oxidative stress and neurodegeneration. J. Neurol. Sci. 2012, 322, 254–262. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Ageing, neuroinflammation and neurodegeneration. Front. Biosci. Schol. Ed. 2015, 7, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Garza-Lombo, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, K.F.; Haass, C. Mitochondrial dysfunction in Parkinson’s disease. Biochim. Biophys. Acta 2010, 1802, 29–44. [Google Scholar] [CrossRef]
- Quintana, C.; Cowley, J.M.; Marhic, C. Electron nanodiffraction and high-resolution electron microscopy studies of the structure and composition of physiological and pathological ferritin. J. Struct. Biol. 2004, 147, 166–178. [Google Scholar] [CrossRef]
- Cheng, R.; Dhorajia, V.V.; Kim, J.; Kim, Y. Mitochondrial iron metabolism and neurodegenerative diseases. Neurotoxicology 2022, 88, 88–101. [Google Scholar] [CrossRef]
- Worwood, M. Ferritin in human tissues and serum. Clin. Haematol. 1982, 11, 275–307. [Google Scholar] [CrossRef]
- Powell, L.W.; Alpert, E.; Isselbacher, K.J.; Drysdale, J.W. Human isoferritins: Organ specific iron and apoferritin distribution. Br. J. Haematol. 1975, 30, 47–55. [Google Scholar] [CrossRef]
- Zhang, N.; Yu, X.; Xie, J.; Xu, H. New Insights into the Role of Ferritin in Iron Homeostasis and Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 2812–2823. [Google Scholar] [CrossRef] [PubMed]
- Bossoni, L.; Grand Moursel, L.; Bulk, M.; Simon, B.G.; Webb, A.; van der Weerd, L.; Huber, M.; Carretta, P.; Lascialfari, A.; Oosterkamp, T.H. Human-brain ferritin studied by muon spin rotation: A pilot study. J. Phys. Condens. Matter 2017, 29, 415801. [Google Scholar] [CrossRef] [PubMed]
- Strbak, O.; Balejcikova, L.; Mihalikova, M.; Kopcansky, P.; Dobrota, D. Quantitative Analysis of Magnetoferritin-Induced Relaxivity Enhancement in MRI. Acta Phys. Pol. A 2020, 137, 720–722. [Google Scholar] [CrossRef]
- Meraz-Rios, M.A.; Franco-Bocanegra, D.; Toral Rios, D.; Campos-Pena, V. Early onset Alzheimer’s disease and oxidative stress. Oxid. Med. Cell. Longev. 2014, 2014, 375968. [Google Scholar] [CrossRef]
- Huang, X.; Atwood, C.S.; Hartshorn, M.A.; Multhaup, G.; Goldstein, L.E.; Scarpa, R.C.; Cuajungco, M.P.; Gray, D.N.; Lim, J.; Moir, R.D.; et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 1999, 38, 7609–7616. [Google Scholar] [CrossRef]
- Pratico, D.; Zhukareva, V.; Yao, Y.; Uryu, K.; Funk, C.D.; Lawson, J.A.; Trojanowski, J.Q.; Lee, V.M. 12/15-lipoxygenase is increased in Alzheimer’s disease: Possible involvement in brain oxidative stress. Am. J. Pathol. 2004, 164, 1655–1662. [Google Scholar] [CrossRef]
- Yang, H.; Zhuo, J.M.; Chu, J.; Chinnici, C.; Pratico, D. Amelioration of the Alzheimer’s disease phenotype by absence of 12/15-lipoxygenase. Biol. Psychiatry 2010, 68, 922–929. [Google Scholar] [CrossRef]
- Feeney, C.J.; Frantseva, M.V.; Carlen, P.L.; Pennefather, P.S.; Shulyakova, N.; Shniffer, C.; Mills, L.R. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008, 1198, 1–15. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Carmichael, J. Huntington’s disease: Molecular basis of neurodegeneration. Expert Rev. Mol. Med. 2003, 5, 1–21. [Google Scholar] [CrossRef]
- Shi, Z.H.; Nie, G.; Duan, X.L.; Rouault, T.; Wu, W.S.; Ning, B.; Zhang, N.; Chang, Y.Z.; Zhao, B.L. Neuroprotective mechanism of mitochondrial ferritin on 6-hydroxydopamine-induced dopaminergic cell damage: Implication for neuroprotection in Parkinson’s disease. Antioxid. Redox Signal. 2010, 13, 783–796. [Google Scholar] [CrossRef]
- Guan, H.; Yang, H.; Yang, M.; Yanagisawa, D.; Bellier, J.P.; Mori, M.; Takahata, S.; Nonaka, T.; Zhao, S.; Tooyama, I. Mitochondrial ferritin protects SH-SY5Y cells against H2O2-induced oxidative stress and modulates alpha-synuclein expression. Exp. Neurol. 2017, 291, 51–61. [Google Scholar] [CrossRef]
- Mizuno, Y.; Ikebe, S.; Hattori, N.; Nakagawa-Hattori, Y.; Mochizuki, H.; Tanaka, M.; Ozawa, T. Role of mitochondria in the etiology and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta 1995, 1271, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Munoz, Y.; Carrasco, C.M.; Campos, J.D.; Aguirre, P.; Nunez, M.T. Parkinson’s Disease: The Mitochondria-Iron Link. Park. Dis. 2016, 2016, 7049108. [Google Scholar]
- Carroll, C.B.; Zeissler, M.L.; Chadborn, N.; Gibson, K.; Williams, G.; Zajicek, J.P.; Morrison, K.E.; Hanemann, C.O. Changes in iron-regulatory gene expression occur in human cell culture models of Parkinson’s disease. Neurochem. Int. 2011, 59, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Mena, N.P.; Garcia-Beltran, O.; Lourido, F.; Urrutia, P.J.; Mena, R.; Castro-Castillo, V.; Cassels, B.K.; Nunez, M.T. The novel mitochondrial iron chelator 5-((methylamino)methyl)-8-hydroxyquinoline protects against mitochondrial-induced oxidative damage and neuronal death. Biochem. Biophys. Res. Commun. 2015, 463, 787–792. [Google Scholar] [CrossRef]
- Mastroberardino, P.G.; Hoffman, E.K.; Horowitz, M.P.; Betarbet, R.; Taylor, G.; Cheng, D.; Na, H.M.; Gutekunst, C.A.; Gearing, M.; Trojanowski, J.Q.; et al. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol. Dis. 2009, 34, 417–431. [Google Scholar] [CrossRef]
- Toyokuni, S.; Kong, Y.; Cheng, Z.; Sato, K.; Hayashi, S.; Ito, F.; Jiang, L.; Yanatori, I.; Okazaki, Y.; Akatsuka, S. Carcinogenesis as Side Effects of Iron and Oxygen Utilization: From the Unveiled Truth toward Ultimate Bioengineering. Cancers 2020, 12, 3320. [Google Scholar] [CrossRef]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef]
- Ludwig, H.; Evstatiev, R.; Kornek, G.; Aapro, M.; Bauernhofer, T.; Buxhofer-Ausch, V.; Fridrik, M.; Geissler, D.; Geissler, K.; Gisslinger, H.; et al. Iron metabolism and iron supplementation in cancer patients. Wien. Klin. Wochenschr. 2015, 127, 907–919. [Google Scholar] [CrossRef]
- Manz, D.H.; Blanchette, N.L.; Paul, B.T.; Torti, F.M.; Torti, S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016, 1368, 149–161. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Rivella, S. Cancer cells with irons in the fire. Free Radic. Biol. Med. 2015, 79, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Quiros Roldan, E.; Biasiotto, G.; Magro, P.; Zanella, I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against SARS-CoV-2 infection (COVID-19): A role for iron homeostasis? Pharmacol. Res. 2020, 158, 104904. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.; Fernandes, P.A.; Ramos, M.J. Ribonucleotide reductase: A critical enzyme for cancer chemotherapy and antiviral agents. Recent Pat. Anticancer Drug Discov. 2007, 2, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Akatsuka, S.; Motooka, Y.; Zheng, H.; Cheng, Z.; Shiraki, Y.; Mashimo, T.; Imaoka, T.; Toyokuni, S. BRCA1 haploinsufficiency promotes chromosomal amplification under Fenton reaction-based carcinogenesis through ferroptosis-resistance. Redox Biol. 2022, 54, 102356. [Google Scholar] [CrossRef] [PubMed]
- Hooda, J.; Cadinu, D.; Alam, M.M.; Shah, A.; Cao, T.M.; Sullivan, L.A.; Brekken, R.; Zhang, L. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PLoS ONE 2013, 8, e63402. [Google Scholar] [CrossRef]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P.; et al. Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, W.; Wu, H.; Wang, Y.H.; Gong, Y.W.; Zhao, Y.L.; Liu, S.H.; Wang, H.Z.; Svatek, R.S.; Rodriguez, R.; et al. Pan-cancer analysis of iron metabolic landscape across the Cancer Genome Atlas. J. Cell Physiol. 2020, 235, 1013–1024. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, C.X.; Shi, Y.B.; Lu, H.; He, X.Z. Decreased expression of ferroportin in prostate cancer. Oncol. Lett. 2015, 10, 913–916. [Google Scholar] [CrossRef]
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci. Transl. Med. 2010, 2, 43ra56. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, S.; Chen, Y.; Zhang, D.; Yuan, L.; Cong, H.; Liu, S. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim. Biophys. Sin. 2015, 47, 703–715. [Google Scholar] [CrossRef]
- Shan, Z.; Wei, Z.; Shaikh, Z.A. Suppression of ferroportin expression by cadmium stimulates proliferation, EMT, and migration in triple-negative breast cancer cells. Toxicol. Appl. Pharmacol. 2018, 356, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.I.; Schwartz, J.M.; Maldonado, E.N.; Lemasters, J.J.; Nieminen, A.L. Mitoferrin-2-dependent mitochondrial iron uptake sensitizes human head and neck squamous carcinoma cells to photodynamic therapy. J. Biol. Chem. 2013, 288, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget 2015, 6, 18748–18779. [Google Scholar] [CrossRef] [PubMed]
- Estrov, Z.; Tawa, A.; Wang, X.H.; Dube, I.D.; Sulh, H.; Cohen, A.; Gelfand, E.W.; Freedman, M.H. In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood 1987, 69, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, G.; Veuger, S. Reversing oncogenic transformation with iron chelation. Oncotarget 2021, 12, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef]
- De Domenico, I.; Ward, D.M.; Kaplan, J. Specific iron chelators determine the route of ferritin degradation. Blood 2009, 114, 4546–4551. [Google Scholar] [CrossRef]
- Lesjak, M.; Balesaria, S.; Skinner, V.; Debnam, E.S.; Srai, S.K.S. Quercetin inhibits intestinal non-haem iron absorption by regulating iron metabolism genes in the tissues. Eur. J. Nutr. 2019, 58, 743–753. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, X.; Li, Z.; Lu, Z.; Kong, S.; Jiang, H.; Du, H.; Pan, X.; Nandi, M.; Kong, X.; et al. CN128: A New Orally Active Hydroxypyridinone Iron Chelator. J. Med. Chem. 2020, 63, 4215–4226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).