Dimerization/Elimination of β-Styrylmalonates under Action of TiCl4
Abstract
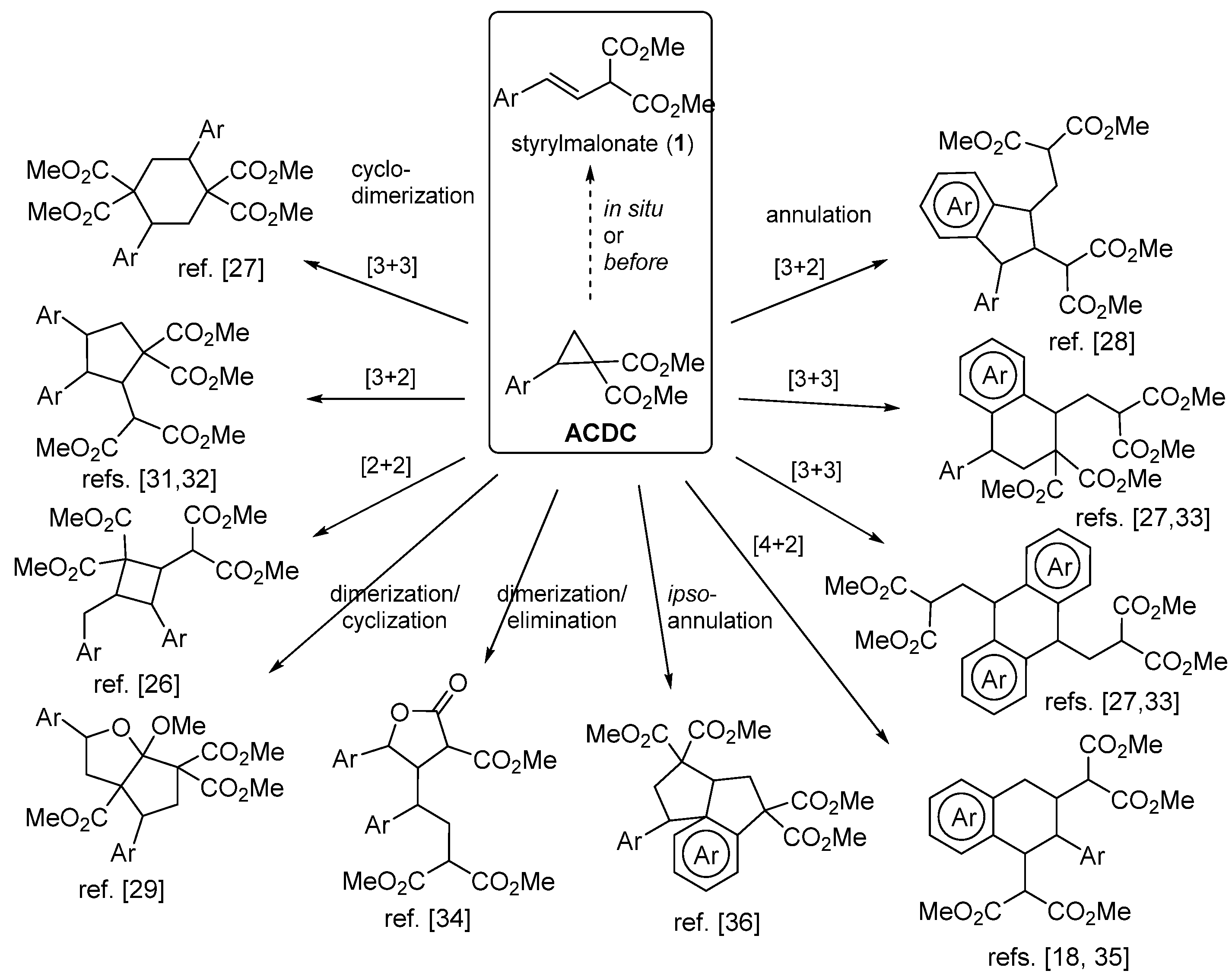
1. Introduction
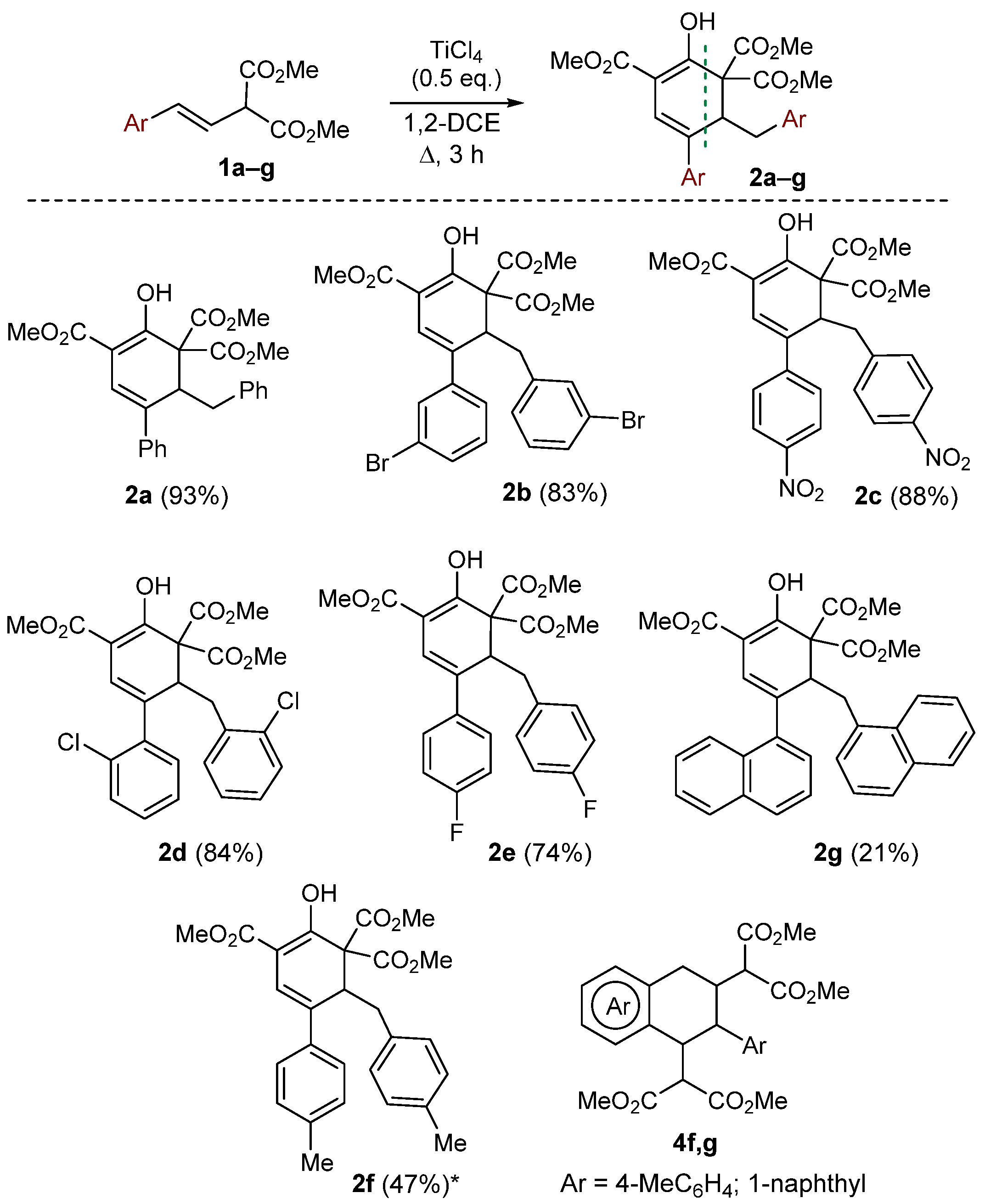
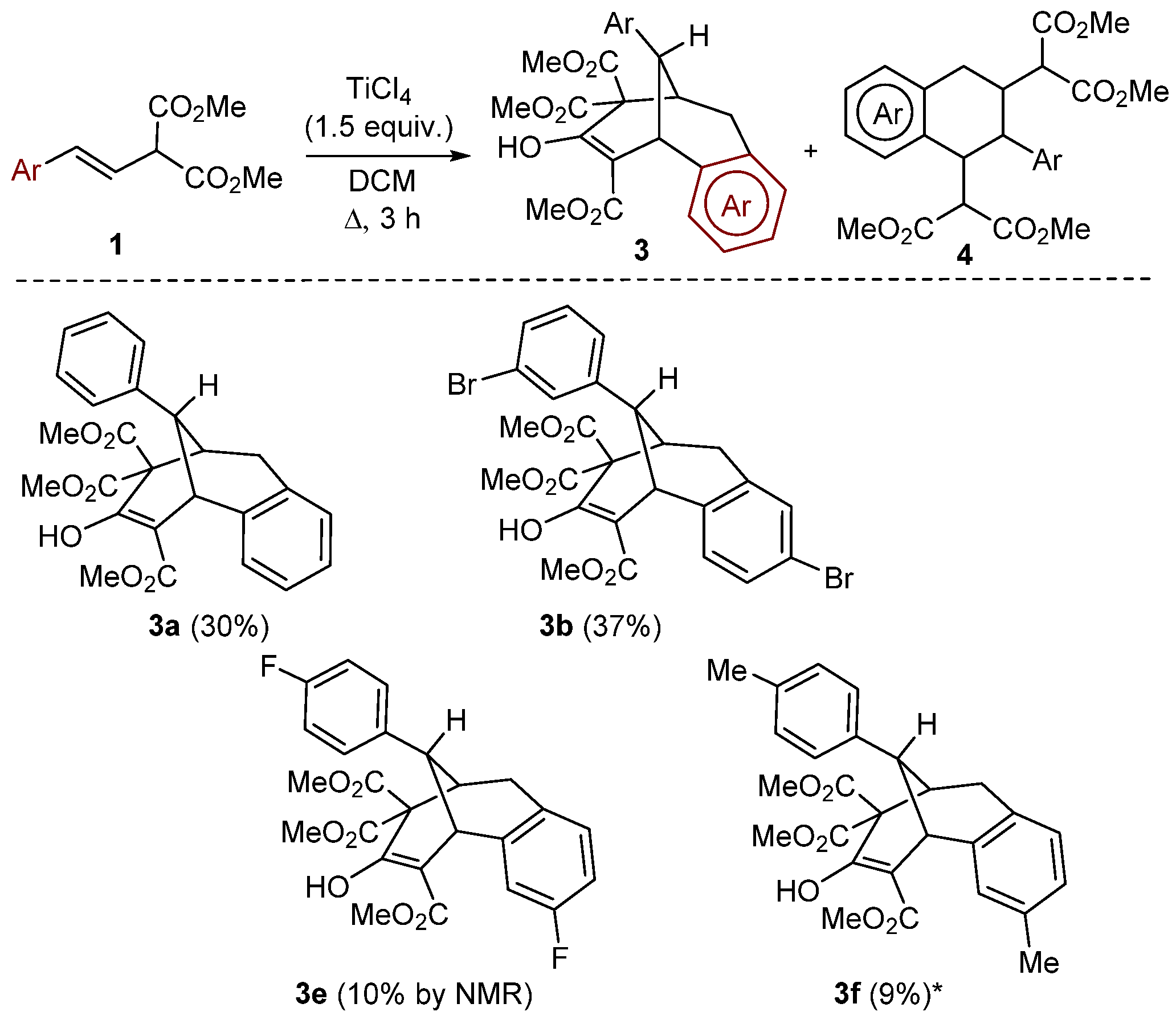
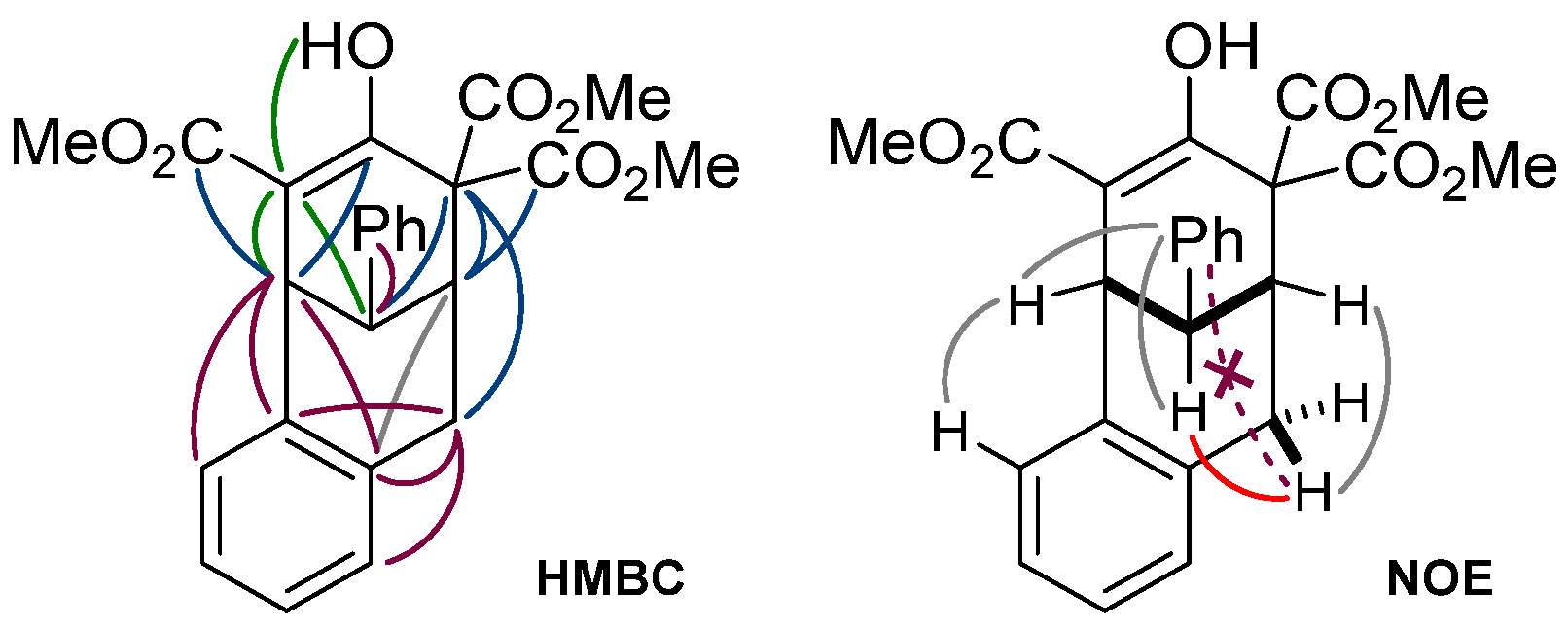
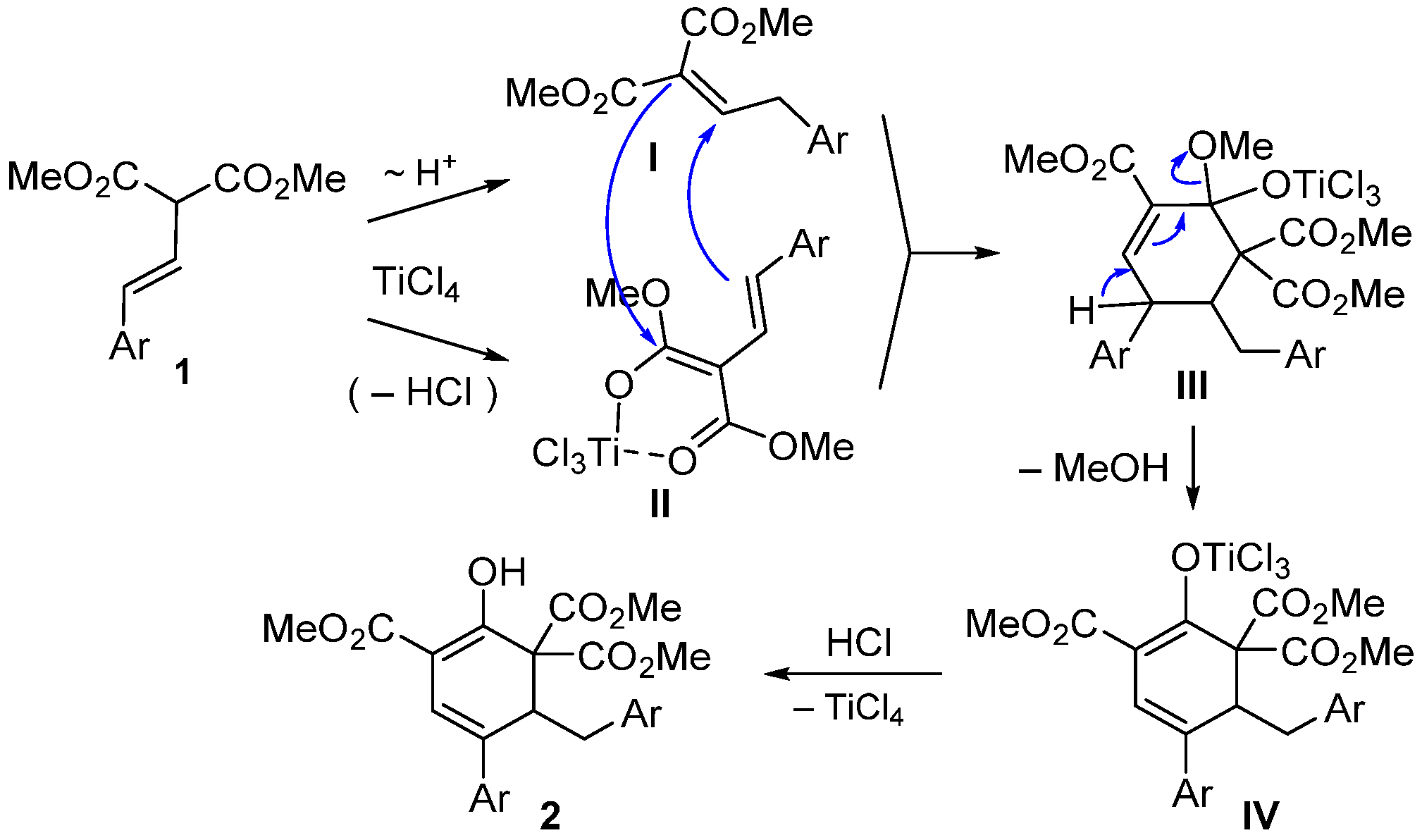
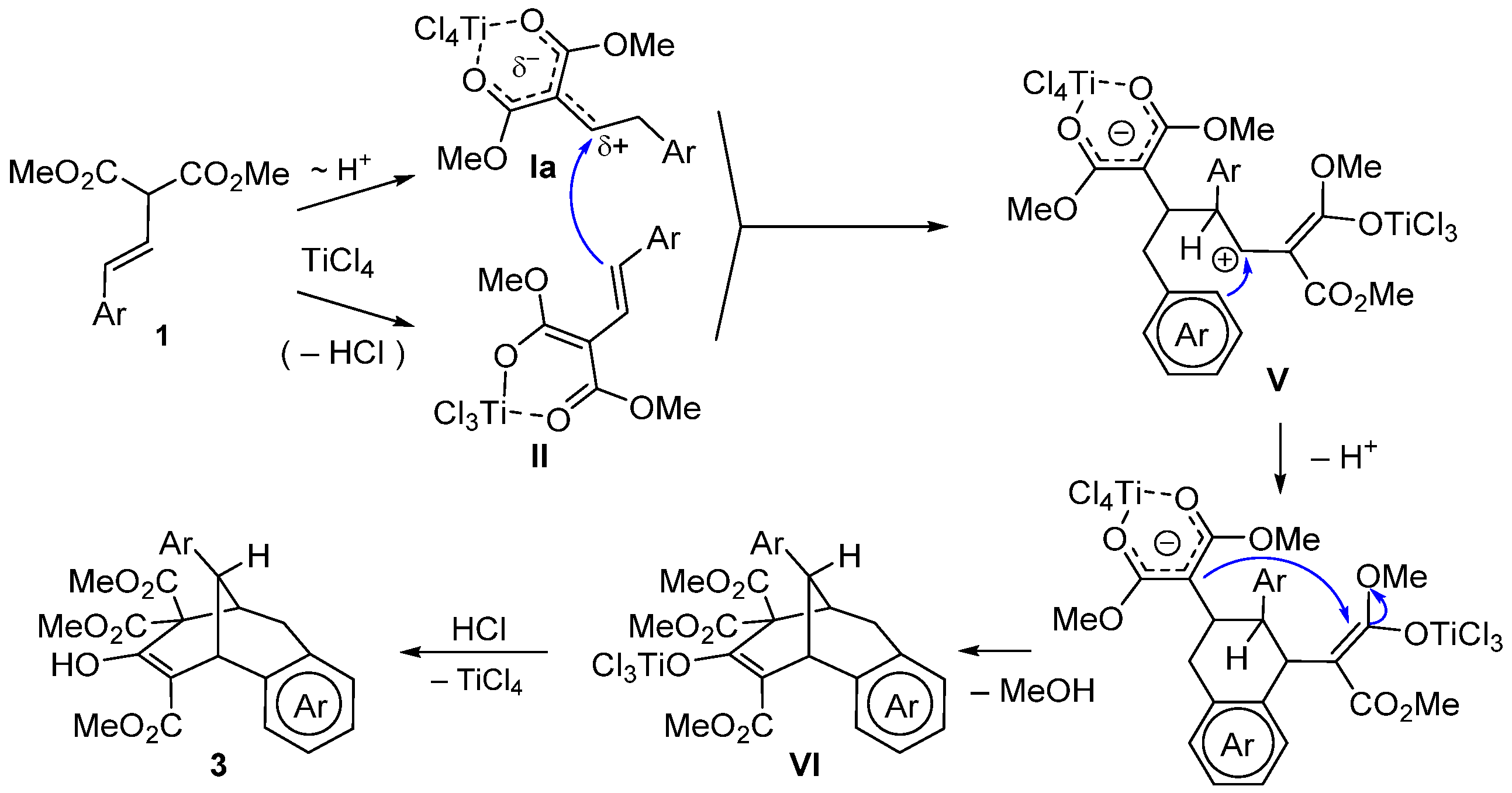
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Reissig, H.U.; Zimmer, R. Donor-acceptor-substituted cyclopropane derivatives and their application in organic synthesis. Chem. Rev. 2003, 103, 1151–1196. [Google Scholar] [CrossRef] [PubMed]
- De Simone, F.; Waser, J. Cyclization and cycloaddition reactions of cyclopropyl carbonyls and imines. Synthesis 2009, 20, 3353–3374. [Google Scholar] [CrossRef]
- Carson, C.A.; Kerr, M.A. Heterocycles from cyclopropanes: Applications in natural product synthesis. Chem. Soc. Rev. 2009, 38, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, M.Y.; Budynina, E.M.; Ivanova, O.A.; Trushkov, I.V. Recent advances in ring-forming reactions of donor–acceptor cyclopropanes. Mendeleev Commun. 2011, 21, 293–301. [Google Scholar] [CrossRef]
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A new golden age for donor-acceptor cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef] [PubMed]
- De Nanteuil, F.; De Simone, F.; Frei, R.; Benfatti, F.; Serrano, E.; Waser, J. Cyclization and annulation reactions of nitrogen-substituted cyclopropanes and cyclobutanes. Chem. Commun. 2014, 50, 10912–10928. [Google Scholar] [CrossRef]
- Grover, H.K.; Emmett, M.R.; Kerr, M.A. Carbocycles from donor–acceptor cyclopropanes. Org. Biomol. Chem. 2015, 13, 655–671. [Google Scholar] [CrossRef]
- Reissig, H.-U.; Werz, D.B. (Guest Editorial), Special Issue: Chemistry of donor-acceptor cyclopropanes and cyclobutanes. Isr. J. Chem. 2016, 56, 365–577. [Google Scholar] [CrossRef]
- Wallbaum, J.; Garve, L.K.B.; Jones, P.G.; Werz, D.B. Ring-opening 1,3-halochalcogenation of cyclopropane dicarboxylates. Org. Lett. 2017, 19, 98–101. [Google Scholar] [CrossRef]
- Dey, R.; Banerjee, P. Lewis acid catalyzed diastereoselective cycloaddition reactions of donor–acceptor cyclopropanes and vinyl azides: Synthesis of functionalized azidocyclopentane and tetrahydropyridine derivatives. Org. Lett. 2017, 19, 304–307. [Google Scholar] [CrossRef]
- Chidley, T.; Vemula, N.; Carson, C.A.; Kerr, M.A.; Pagenkopf, B.L. Cascade reaction of donor–acceptor cyclopropanes: Mechanistic studies on cycloadditions with nitrosoarenes and cis-diazenes. Org. Lett. 2016, 18, 2922–2925. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chakrabarty, S.; Daniliuc, C.G.; Studer, A. Tetrahydroquinolines via stereospecific [3 + 3]-annulation of donor–acceptor cyclopropanes with nitrosoarenes. Org. Lett. 2016, 18, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Garve, L.K.B.; Pawliczek, M.; Wallbaum, J.; Jones, P.G.; Werz, D.B. [4+3] Cycloaddition of donor–acceptor cyclopropanes with amphiphilic benzodithioloimine as surrogate for ortho-bisthioquinone. Chem. Eur. J. 2016, 22, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Banerjee, P. Lewis acid-catalyzed [3+2] cycloaddition of donor-acceptor cyclopropanes and enamines: Enantioselective synthesis of nitrogen-functionalized cyclopentane derivatives. Adv. Synth. Catal. 2016, 358, 2053–2058. [Google Scholar] [CrossRef]
- Sabbatani, J.; Maulide, N. Temporary generation of a cyclopropyl oxocarbenium ion enables highly diastereoselective donor–acceptor cyclopropane cycloaddition. Angew. Chem. Int. Ed. 2016, 55, 6780–6783. [Google Scholar] [CrossRef]
- Pitts, C.R.; Ling, B.; Snyder, J.A.; Bragg, A.E.; Lectka, T. Aminofluorination of cyclopropanes: A multifold approach through a common, catalytically generated intermediate. J. Am. Chem. Soc. 2016, 138, 6598–6609. [Google Scholar] [CrossRef]
- Novikov, R.A.; Borisov, D.D.; Tarasova, A.V.; Tkachev, Y.V.; Tomilov, Y.V. Three-component gallium(III)-promoted addition of halide anions and acetylenes to donor–acceptor cyclopropanes. Angew. Chem. Int. Ed. 2018, 57, 10293–10298. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, V.A.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. A new type of donor–acceptor cyclopropane reactivity: The generation of formal 1,2- and 1,4-dipoles. Angew. Chem. Int. Ed. 2014, 53, 3187–3191. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Mane, S.P.; Nanda, L.N.; Shukla, M.K. Stereoselective synthesis of donor-acceptor cyclopropapyranone by Intramolecular cyclopropanation of vinylogous carbonates: Application to the total synthesis of (±)-diospongin B. Isr. J. Chem. 2016, 56, 553–557. [Google Scholar] [CrossRef]
- Mlostoń, G.; Kowalczyk, M.; Augustin, A.U.; Jones, P.G.; Werz, D.B. Ferrocenyl-substituted tetrahydrothiophenes via formal [3 + 2]-cycloaddition reactions of ferrocenyl thioketones with donor–acceptor cyclopropanes. Belstein J. Org. Chem. 2020, 16, 1288–1295. [Google Scholar] [CrossRef]
- Augustin, A.U.; Merz, J.L.; Jones, P.G.; Mlostoń, G.; Werz, D.B. (4 + 3)-Cycloaddition of donor–acceptor cyclopropanes with thiochalcones: A diastereoselective access to tetrahydrothiepines. Org. Lett. 2019, 21, 9405–9409. [Google Scholar] [CrossRef] [PubMed]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. GaCl3-Mediated reactions of donor–acceptor cyclopropanes with aromatic aldehydes. Angew. Chem. Int. Ed. 2016, 55, 12233–12237. [Google Scholar] [CrossRef] [PubMed]
- Borisov, D.D.; Novikov, R.A.; Eltysheva, A.S.; Tkachev, Y.V.; Tomilov, Y.V. Styrylmalonates as an alternative to donor–acceptor cyclopropanes in the reactions with aldehydes: A route to 5,6-dihydropyran-2-ones. Org. Lett. 2017, 19, 3731–3734. [Google Scholar] [CrossRef] [PubMed]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. Reactions of styrylmalonates with aromatic aldehydes: Detailed synthetic and mechanistic studies. J. Org. Chem. 2021, 86, 4457–4471. [Google Scholar] [CrossRef]
- Borisov, D.D.; Chermashentsev, G.R.; Novikov, R.A.; Tomilov, Y.V. Coupling of styrylmalonates with furan and benzofuran carbaldehydes: Synthesis and chemistry of substituted (4-oxocyclopent-2-enyl)malonates. J. Org. Chem. 2021, 86, 8489–8499. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tomilov, Y.V. Dimerization of donor–acceptor cyclopropanes. Mendeleev Commun. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Chagarovskiy, A.O.; Trushkov, I.V.; Melnikov, M.Y. (3 + 3)-Cyclodimerization of donor–acceptor cyclopropanes. Three routes to six-membered rings. J. Org. Chem. 2011, 76, 8852–8868. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Skvortsov, D.A.; Limoge, M.; Bakin, A.V.; Chagarovskiy, A.O.; Trushkov, I.V.; Melnikov, M.Y. A bioinspired route to indanes and cyclopentannulated hetarenes via (3+2)-cyclodimerization of donor–acceptor cyclopropanes. Chem. Commun. 2013, 49, 11482–11484. [Google Scholar] [CrossRef]
- Novikov, R.A.; Timofeev, V.P.; Tomilov, Y.V. Stereoselective double Lewis acid/organo-catalyzed dimerization of donor–acceptor cyclopropanes into substituted 2-oxabicyclo[3.3.0]octanes. J. Org. Chem. 2012, 77, 5993–6006. [Google Scholar] [CrossRef]
- Ma, H.; Hu, X.-Q.; Luo, Y.-C.; Xu, P.-F. 3,4,5-Trimethylphenol and Lewis acid dual-catalyzed cascade ring-opening/cyclization: Direct synthesis of naphthalenes. Org. Lett. 2017, 19, 6666–6669. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Budynina, E.M.; Trushkov, I.V.; Melnikov, M.Y. [3+2] Cyclodimerization of 2-arylcyclopropane-1,1-diesters. Lewis acid induced reversion of cyclopropane umpolung. Tetrahedron Lett. 2011, 52, 4421–4425. [Google Scholar] [CrossRef]
- Novikov, R.A.; Korolev, V.A.; Timofeev, V.P.; Tomilov, Y.V. New dimerization and cascade oligomerization reactions of dimethyl 2-phenylcyclopropan-1,1-dicarboxylate catalyzed by Lewis acids. Tetrahedron Lett. 2011, 52, 4996–4999. [Google Scholar] [CrossRef]
- Ivanova, O.A.; Budynina, E.M.; Khrustalev, V.N.; Skvortsov, D.A.; Trushkov, I.V.; Melnikov, M.Y. A straightforward approach to tetrahydroindolo[3,2-b]carbazoles and 1-indolyltetrahydrocarbazoles through [3+3] cyclodimerization of indole-derived cyclopropanes. Chem. Eur. J. 2016, 22, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Dousset, M.; Parrain, J.-L.; Chouraqui, G. Intriguing electrophilic reactivity of donor–acceptor cyclopropanes: Experimental and theoretical studies. Eur. J. Org. Chem. 2017, 35, 5238–5245. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tarasova, A.V.; Tomilov, Y.V. Synthesis of substituted naphthalenes by GaCl3-mediated cross-dimerization—Fragmentation of 2-arylcyclopropane-1,1-dicarboxylates. Russ. Chem. Bull. Int. Ed. 2014, 63, 2737–2740. [Google Scholar] [CrossRef]
- Novikov, R.A.; Tomilov, Y.V. Dimerization of dimethyl 2-(naphthalen-1-yl)cyclopropane-1,1-dicarboxylate in the presence of GaCl3 to [3+2], [3+3], [3+4], and spiroannulation products. Helv. Chim. Acta 2013, 96, 2068–2080. [Google Scholar] [CrossRef]
- Borisov, D.D.; Chermashentsev, G.R.; Novikov, R.A.; Tomilov, Y.V. Synthesis of substituted β-styrylmalonates by sequential isomerization of 2-arylcyclopropane-1,1-dicarboxylates and (2-arylethylidene)malonates. Synthesis 2021, 53, 2253–2259. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, X.; Feng, X. Asymmetric catalytic reactions of donor–acceptor cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 9192–9204. [Google Scholar] [CrossRef]
- Pirenne, V.; Muriel, B.; Waser, J. Catalytic enantioselective ring-opening reactions of cyclopropanes. Chem. Rev. 2021, 121, 227–263. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y. Research progress on asymmetric synthesis of donor-acceptor cyclopropanes and their enantioselective ring-opening/annulation reactions. Chin. J. Appl. Chem. 2018, 35, 1037–1056. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y. Asymmetric ring-opening reactions of donor-acceptor cyclopropanes and cyclobutanes. Isr. J. Chem. 2016, 56, 463–475. [Google Scholar] [CrossRef]
- Oertling, H.; Reckziegel, A.; Surburg, H.; Bertram, H.-J. Applications of menthol in synthetic chemistry. Chem. Rev. 2007, 107, 2136–2164. [Google Scholar] [CrossRef] [PubMed]
- Obydennov, D.L.; Chernyshova, E.V.; Sosnovskikh, V.Y. Self-condensation of enaminodiones as a method for benzene ring construction: Synthesis of diacyl-substituted phenols and catechols. J. Org. Chem. 2019, 84, 6491–6501. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Burgart, Y.V.; Saloutin, V.I. Catalyst-free transformations of diethyl 2-ethoxymethylenemalonate and diethyl polyfluorobenzoylmalonates in water. Tetrahedron Lett. 2012, 53, 1961–1963. [Google Scholar] [CrossRef]
- Chagarovskiy, A.O.; Ivanova, O.A.; Rakhmankulov, E.R.; Budynina, E.M.; Trushkov, I.V.; Melnikov, M.Y. Lewis Acid-Catalyzed Isomerization of 2-Arylcyclopropane-1,1-dicarboxylates: A New Efficient Route to 2-Styrylmalonates. Adv. Synth. Catal. 2010, 352, 3179–3184. [Google Scholar] [CrossRef]
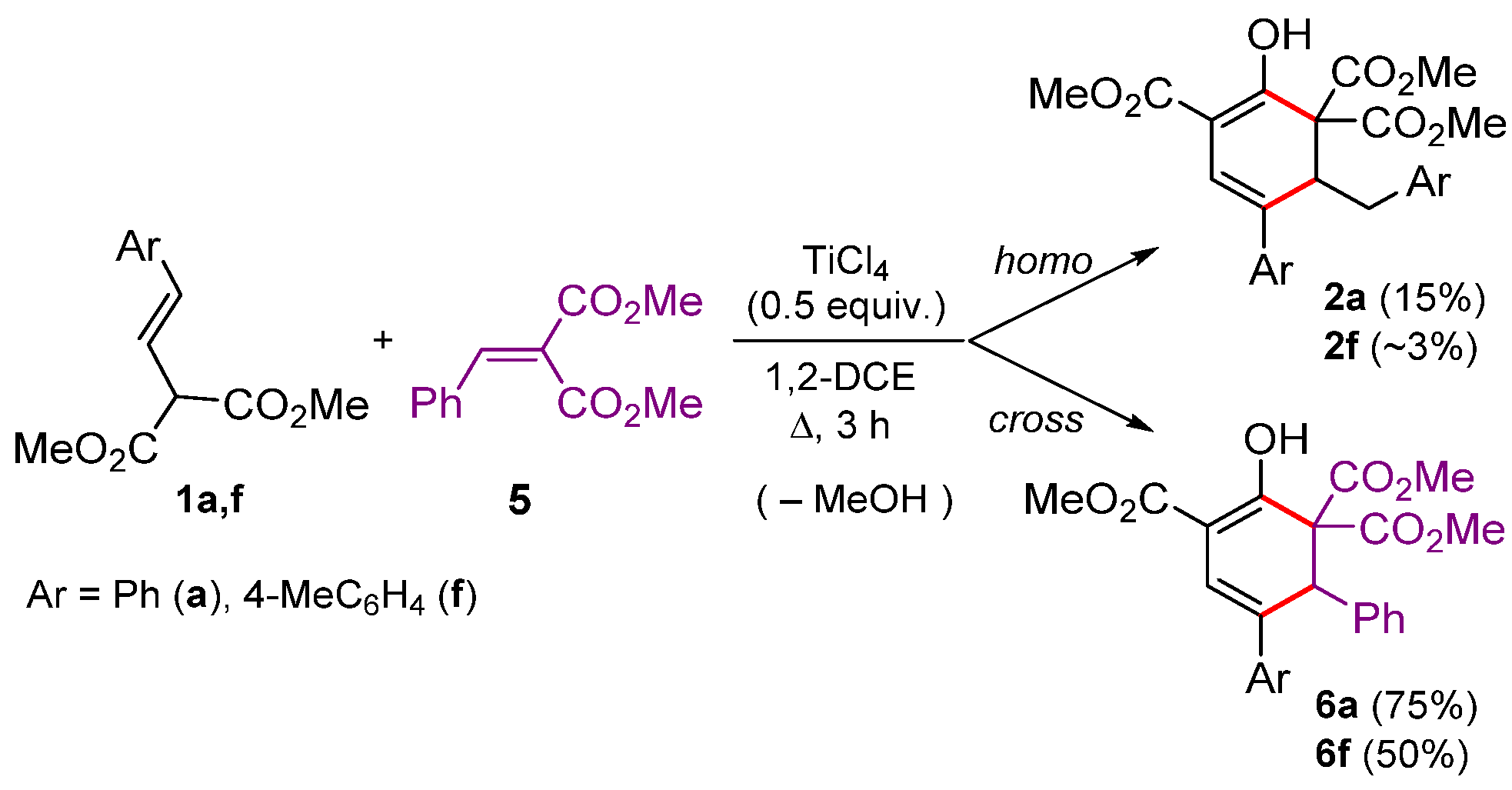
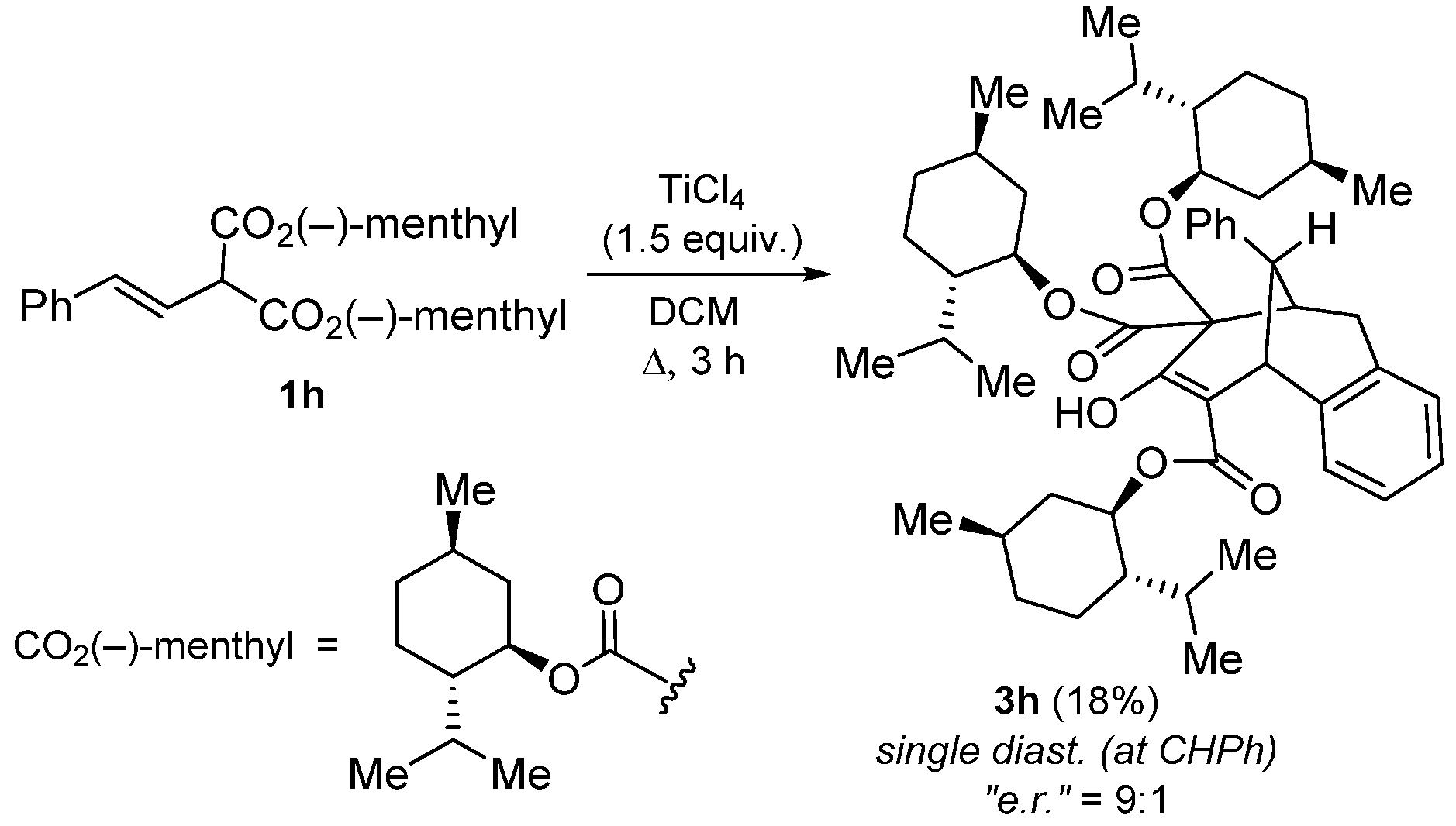
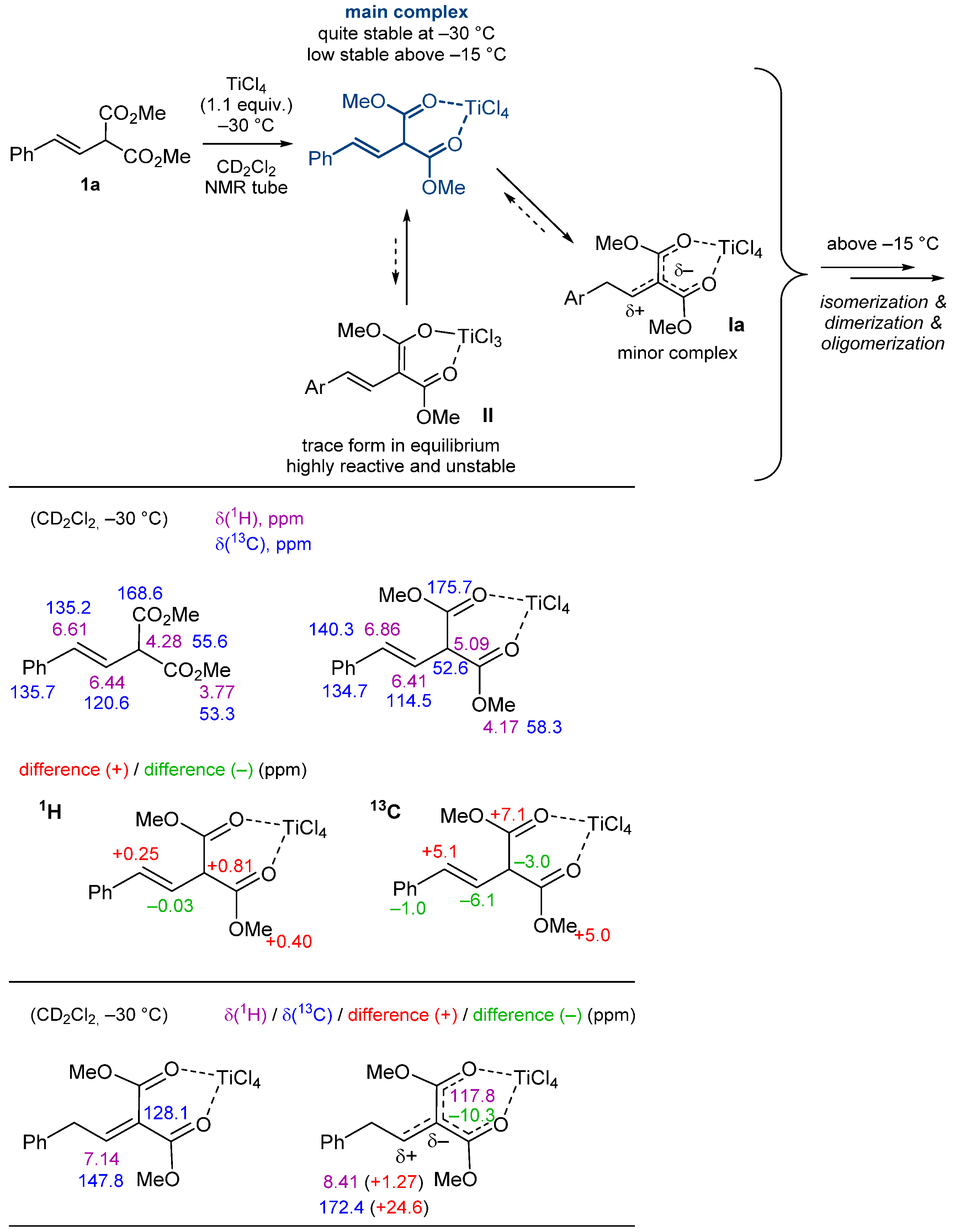
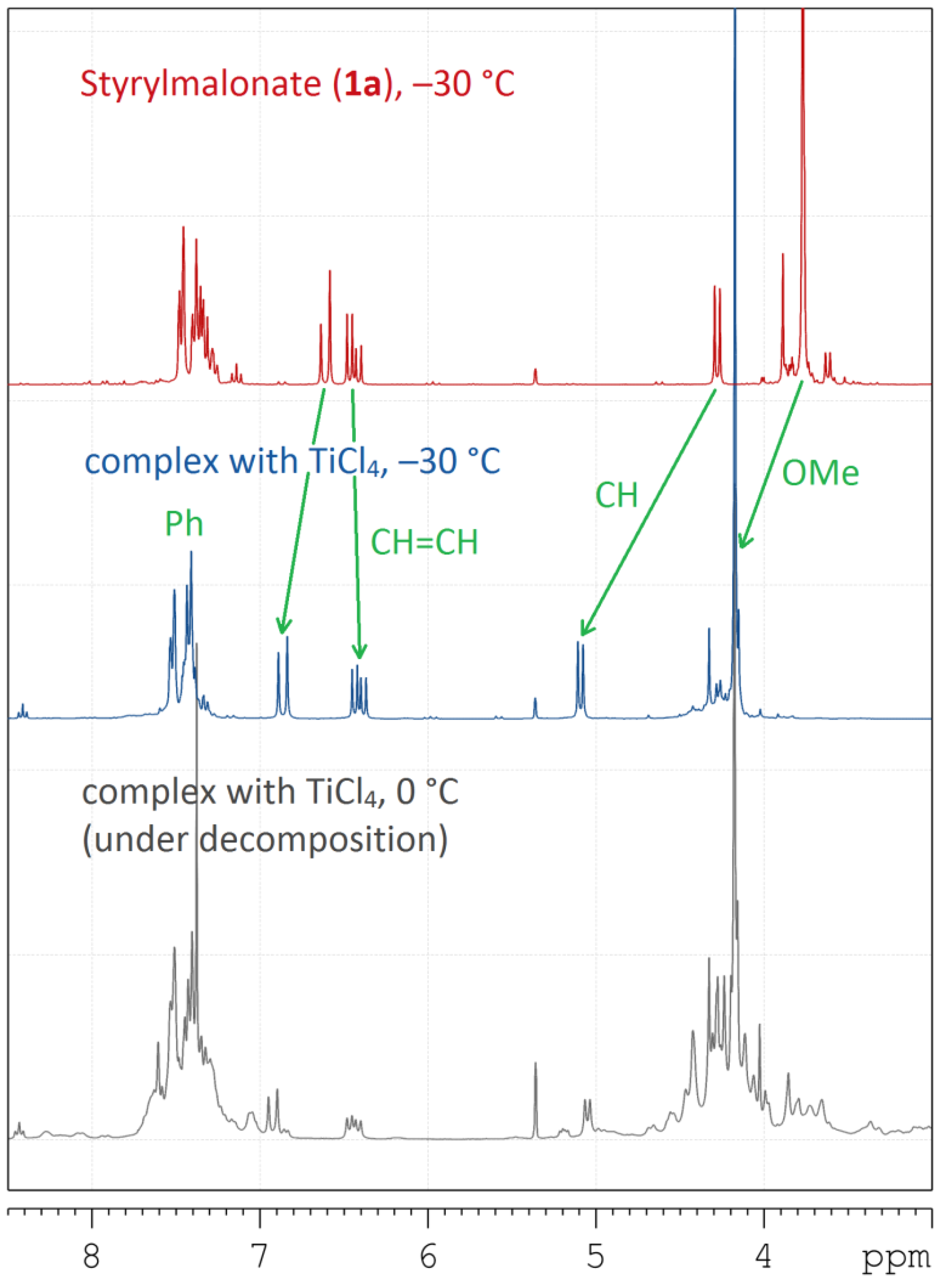
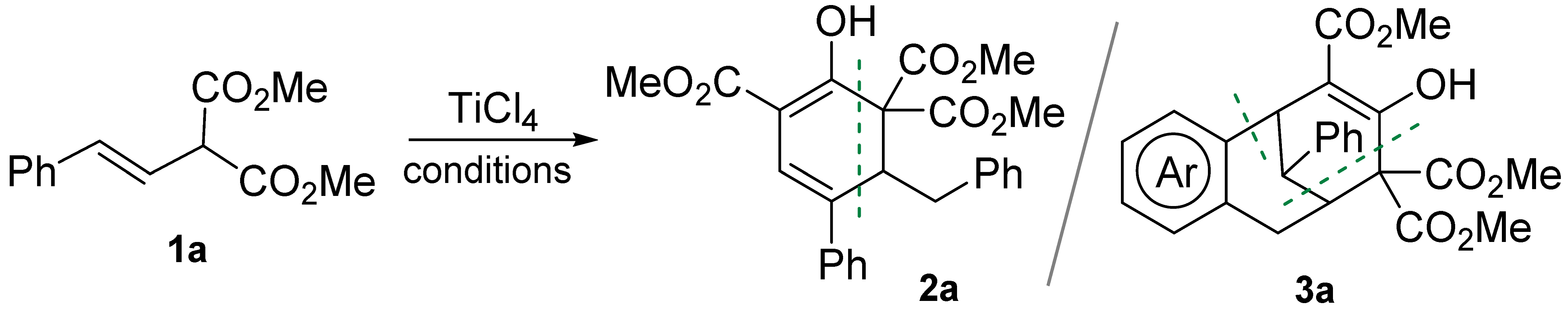 | ||||||
|---|---|---|---|---|---|---|
| Entry | Solvent [e] | T, °C | t, h | TiCl4 (Equiv.) | NMR Yields, % [a,b] | |
| 2a | 3a | |||||
| 1 | 1,2-DCE | 60 | 3 | 1 | 27 | 26 |
| 2 | CH2Cl2 | 40 | 3 | 1 | 10 | 30 |
| 3 [c] | CH2Cl2 | 25 | 3 | 1 | 0 | 0 |
| 4 | CH2Cl2 | 40 | 3 | 0.5 | 53 | 7 |
| 5 [c] | CH2Cl2 | 40 | 3 | 0.1 | traces | 0 |
| 6 | CH2Cl2 | 40 | 1.5 | 1 | 7 | 24 |
| 7 | CH2Cl2 | 40 | 3 | 1.5 | traces | 30 |
| 8 | CH2Cl2 | 40 | 3 | 2 | 2 | 5 |
| 9 | 1,2-DCE | 80 | 3 | 0.5 | 93 [d] | traces |
| 10 | 1,2-DCE | 80 | 1.5 | 0.5 | 86 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borisov, D.D.; Chermashentsev, G.R.; Potapov, K.V.; Novikov, R.A.; Tomilov, Y.V. Dimerization/Elimination of β-Styrylmalonates under Action of TiCl4. Molecules 2023, 28, 270. https://doi.org/10.3390/molecules28010270
Borisov DD, Chermashentsev GR, Potapov KV, Novikov RA, Tomilov YV. Dimerization/Elimination of β-Styrylmalonates under Action of TiCl4. Molecules. 2023; 28(1):270. https://doi.org/10.3390/molecules28010270
Chicago/Turabian StyleBorisov, D. D., G. R. Chermashentsev, K. V. Potapov, R. A. Novikov, and Yu. V. Tomilov. 2023. "Dimerization/Elimination of β-Styrylmalonates under Action of TiCl4" Molecules 28, no. 1: 270. https://doi.org/10.3390/molecules28010270
APA StyleBorisov, D. D., Chermashentsev, G. R., Potapov, K. V., Novikov, R. A., & Tomilov, Y. V. (2023). Dimerization/Elimination of β-Styrylmalonates under Action of TiCl4. Molecules, 28(1), 270. https://doi.org/10.3390/molecules28010270









