Identification and Quantification of 29 Active Substances by HPLC–ESI-MS/MS in Lyophilized Swine Manure Samples
Abstract
1. Introduction
2. Results and Discussion
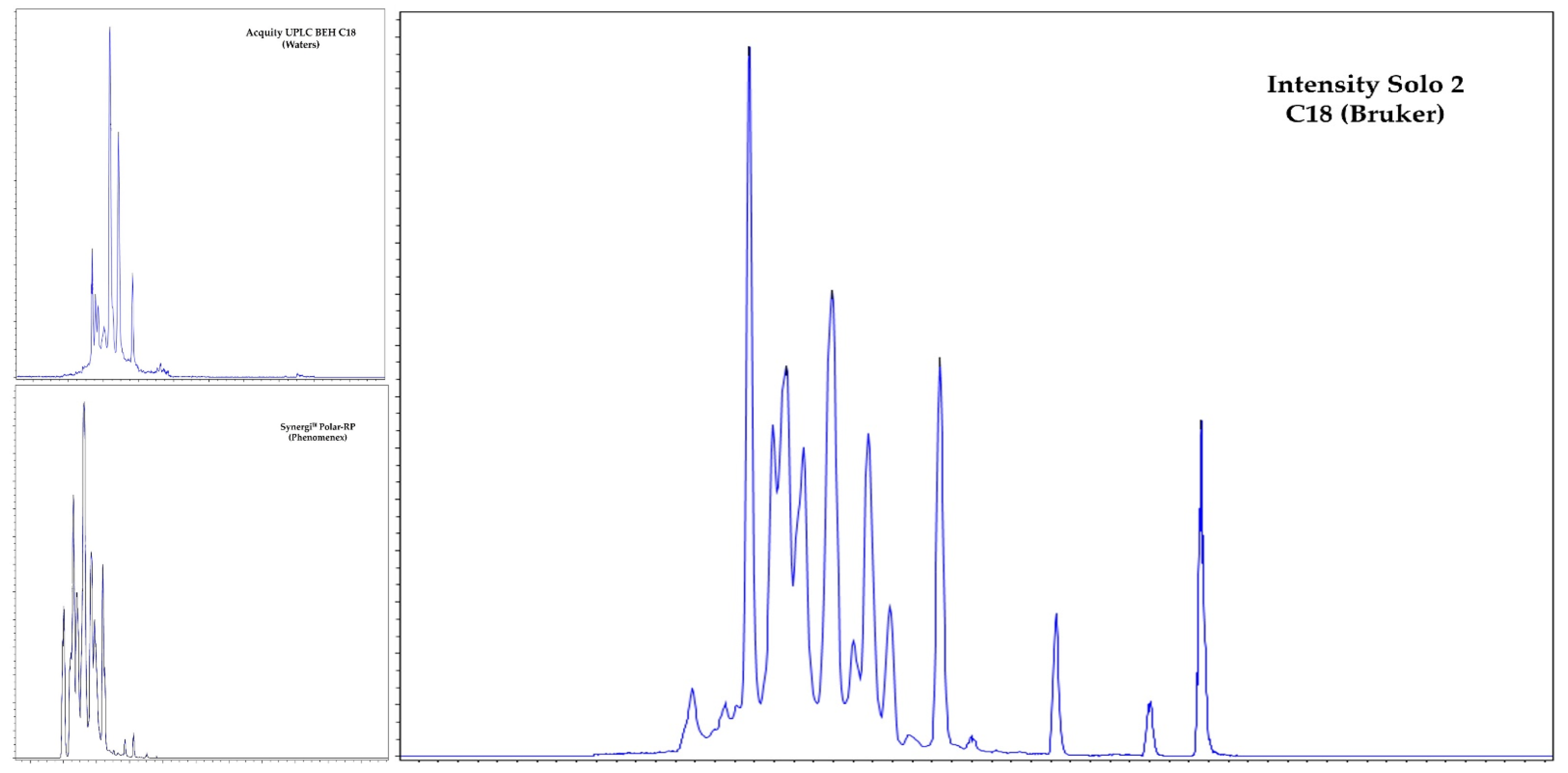
2.1. Optimization of the LC–MS/MS Method
2.2. Extraction Procedure
2.3. Method Validation
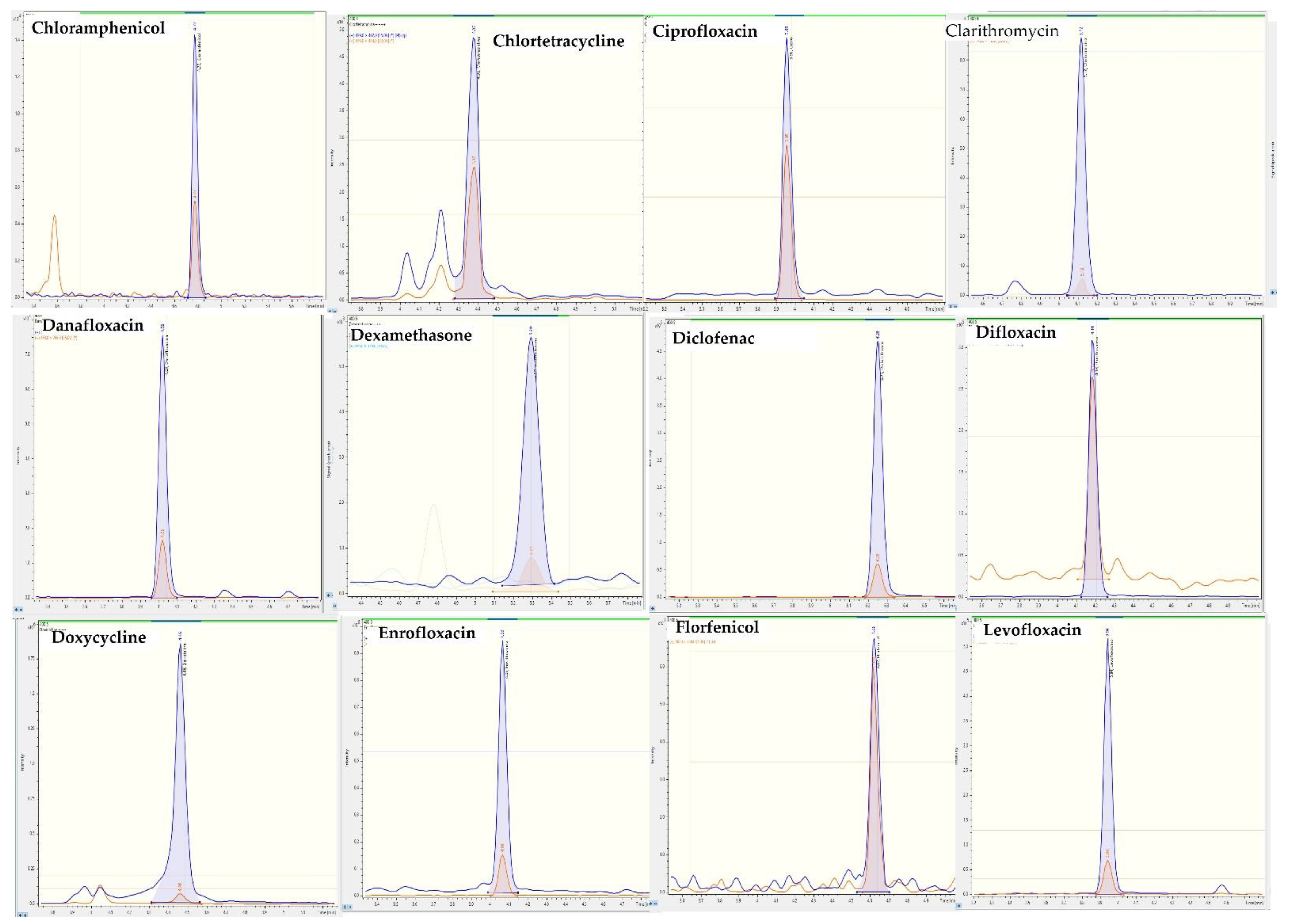
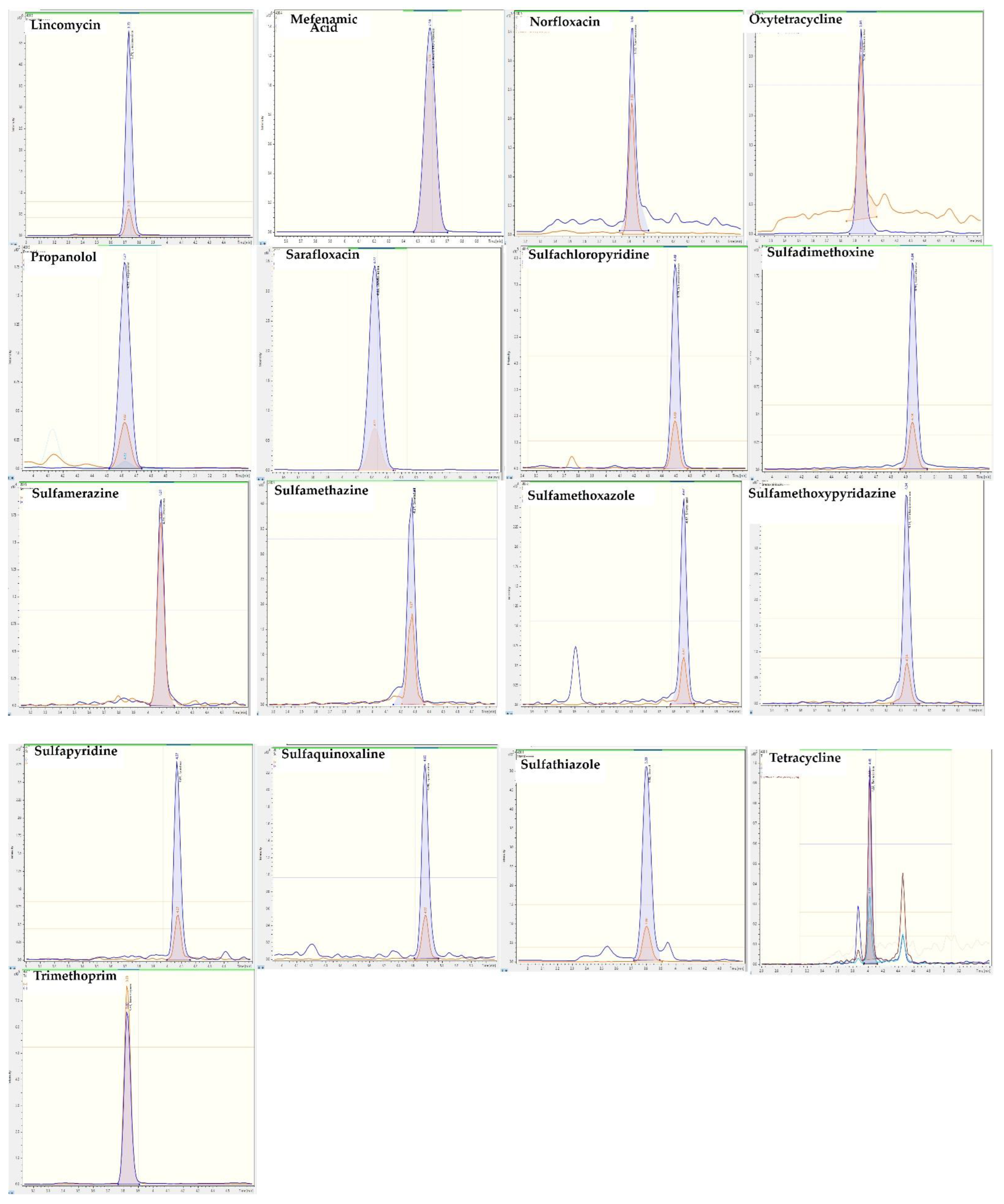
2.4. Application to Feces Samples
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Reagents and Standard Solutions
3.3. Equipment
3.4. Swine Manure Samples Extraction
3.5. LC–MS/MS Conditions
3.6. Validation
3.7. Swine Manure Collection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Radostits, O.M.; Gay, C.; Hinchcliff, K.W.; Constable, P.D. (Eds.) Veterinary Medicine E-Book: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats; Elsevier Health Sciences: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Coyne, L.A.; Latham, S.M.; Williams, N.J.; Dawson, S.; Donald, I.J.; Pearson, R.B.; Smith, R.F.; Pinchbeck, G.L. Understanding the culture of antimicrobial prescribing in agriculture: A qualitative study of UK pig veterinary surgeons. J. Antimicrob. Chemother. 2016, 71, 3300–3312. [Google Scholar] [CrossRef] [PubMed]
- Qui, J.; Zhao, T.; Liu, Q.; He, J.; Él, D.; Wu, G.; Li, Y.; Jiang, C.; Xu, Z. Residual veterinary antibiotics in pig excreta after oral admin-istration of sulfonamides. Environ. Geochem. Health 2016, 38, 549–556. [Google Scholar]
- Nouws, J.F.M.; Vree, T.B.; Degen, M.; Mevius, D. Pharmacokinetics of a sulphamethoxazole/trimethoprim formulation in pigs after intravenous administration. Vet. Q. 1991, 13, 148–154. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; Barceló, D. Trace organic chemicals contamination in ground water recharge. Chemosphere 2008, 72, 333–342. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total. Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Concha-Graña, E.; López-Mahía, P.; Muniategui, S.; Prada-Rodríguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef]
- Guo, X.Y.; Hao, L.J.; Qiu, P.Z.; Chen, R.; Xu, J.; Kong, X.J.; Shan, Z.J.; Wang, N. Pollution characteristics of 23 veterinary antibiotics in livestock manure and manure-amended soils in Jiangsu province, China. J. Environ. Sci. Health Part B 2016, 51, 383–392. [Google Scholar] [CrossRef]
- Berendsen, B.J.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef]
- Janusch, F.; Scherz, G.; Mohring, S.A.; Hamscher, G. Determination of fluoroquinolones in chicken faeces–A new liquid–liquid extraction method combined with LC–MS/MS. Environ. Toxicol. Pharmacol. 2014, 38, 792–799. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total. Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carballo, E.; González-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Karcı, A.; Balcıoğlu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial com-pounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, C.; Yue, L.; Sun, Y.; Ding, H.; Liu, Y. Excretion of enrofloxacin in pigs and its effect on ecological environment. Environ. Toxicol. Pharmacol. 2008, 26, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Martín-Esteban, A.; Tadeo, J.L. Multiresidue analysis of quinolones and fluoroquinolones in soil by ultrasonic-assisted extraction in small columns and HPLC-UV. Anal. Chim. Acta 2006, 562, 30–35. [Google Scholar] [CrossRef]
- Christian, T.; Schneider, R.J.; Färber, H.A.; Skutlarek, D.; Meyer, M.T.; Goldbach, H.E. Determination of Antibiotic Residues in Manure, Soil, and Surface Waters. Acta Hydrochim. Hydrobiol. 2003, 31, 36–44. [Google Scholar] [CrossRef]
- Morales-Muñoz, S.; Luque-García, J.L.; de Castro, M.L. Continuous microwave-assisted extraction coupled with derivatization and fluorimetric monitoring for the determination of fluoroquinolone antibacterial agents from soil samples. J. Chromatogr. A 2004, 1059, 25–31. [Google Scholar] [CrossRef]
- Sunderland, J.; Lovering, A.M.; Tobin, C.M.; MacGowan, A.P.; Roe, J.M.; Delsol, A.A. A reverse-phase HPLC assay for the simultaneous determination of enrofloxacin and ciprofloxacin in pig faeces. Int. J. Antimicrob. Agents 2004, 23, 390–393. [Google Scholar] [CrossRef]
- Bin Ho, Y.; Zakaria, M.P.; Latif, P.A.; Saari, N. Occurrence of veterinary antibiotics and progesterone in broiler manure and agricultural soil in Malaysia. Sci. Total Environ. 2014, 488–489, 261–267. [Google Scholar] [CrossRef]
- Rossi, R.; Saluti, G.; Moretti, S.; Diamanti, I.; Giusepponi, D.; Galarini, R. Multiclass methods for the analysis of antibiotic residues in milk by liquid chromatography coupled to mass spectrometry: A review. Food Addit. Contam. Part A 2018, 35, 241–257. [Google Scholar] [CrossRef]
- Moyo, B.; Tavengwa, N.T. Critical review of solid phase extraction for multiresidue clean-up and pre-concentration of antibiotics from livestock and poultry manure. Food Addit. Contam. Part A 2021, 39, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Giorgio, S.; Roberta, G. Residue determination in honey. Honey Anal. 2017, 1, 325–365. [Google Scholar]
- Jansen, L.J.; van de Schans, M.G.; de Boer, D.; Bongers, I.E.; Schmitt, H.; Hoeksma, P.; Berendsen, B.J. A new extraction procedure to abate the burden of non-extractable antibiotic residues in manure. Chemosphere 2019, 224, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Shelver, W.L.; Chakrabarty, S.; Young, J.M.; Byrd, C.J.; Smith, D.J. Evaluation of rapid and standard tandem mass spectrometric methods to analyse veterinary drugs and their metabolites in antemortem bodily fluids from food animals. Food Addit. Contam. Part A 2022, 39, 462–474. [Google Scholar] [CrossRef]
- Popova, I.E.; Bair, D.A.; Tate, K.W.; Parikh, S.J. Sorption, Leaching, and Surface Runoff of Beef Cattle Veterinary Pharmaceuticals under Simulated Irrigated Pasture Conditions. J. Environ. Qual. 2013, 42, 1167–1175. [Google Scholar] [CrossRef]
- Schlüsener, M.P.; Bester, K.; Spiteller, M. Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC–MS/MS. Anal. Bioanal. Chem. 2003, 375, 942–947. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU). 2021/808 of 22 March 2021 on the performance of analytical methods for residues of pharmacologically active substances used in food-producing animals and on the interpretation of results as well as on the methods to be used for sampling and repealing Decisions 2002/657/EC and 98/179/EC. Off. J. Eur. Union 2021, 180, 84–109. [Google Scholar]
- Nebot, C.; Iglesias, A.; Regal, P.; Miranda, J.M.; Fente, C.; Cepeda, A. A sensitive and validated HPLC–MS/MS method for simultaneous determination of seven coccidiostats in bovine whole milk. Food Control 2012, 27, 29–36. [Google Scholar] [CrossRef]
- Gavilán, R.E.; Nebot, C.; Patyra, E.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Simultaneous analysis of coccidiostats and sulphonamides in non-target feed by HPLC-MS/MS and validation following the Commission Decision 2002/657/EC. Food Addit. Contam. Part A 2018, 35, 1093–1106. [Google Scholar] [CrossRef]
- Patyra, E.; Nebot, C.; Gavilán, R.E.; Cepeda, A.; Kwiatek, K. Development and validation of multi-residue and multi-class method for antibacterial substances analysis in non-target feed by liquid chromatography—Tandem mass spectrometry. Food Addit. Contam. Part A 2018, 35, 467–478. [Google Scholar] [CrossRef]
- Zhang, X.; Li, R.; Zhang, P.; Wu, X.; Hua, H.; Yang, L.; Lu, J.; Rong, Y. Rapid determination of 25 drug residues in aquatic products by ultra performance liquid chroma-tography-quadrupole/electrostatic field orbitrap high resolution mass spectrometry. Se Pu Chin. J. Chromatogr. 2018, 36, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Pueyo, V.; Barata, C.; Lacorte, S.; Marcé-Recasens, R.M. Development of predicted environmental concentrations to prioritize the occurrence of pharmaceuticals in rivers from Catalonia. Sci. Total Environ. 2019, 666, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.Y.; Müller, S.R.; McArdell, C.S.; Alder, A.C.; Suter, M.J.-F. Quantification of veterinary antibiotics (sulfonamides and trimethoprim) in animal manure by liquid chromatography–mass spectrometry. J. Chromatogr. A 2002, 952, 111–120. [Google Scholar] [CrossRef]
- Van den Meersche, T.; Van Pamel, E.; Van Poucke, C.; Herman, L.; Heyndrickx, M.; Rasschaert, G.; Daeseleire, E. Development, validation and application of an ultra high performance liquid chromato-graphic-tandem mass spectrometric method for the simultaneous detection and quantification of five different classes of vet-erinary antibiotics in swine manure. J. Chromatogr. A 2016, 1429, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Björklund, E.; Krogh, K.A.; Hansen, M. Development of an analytical methodology for the determination of the antiparasitic drug toltrazuril and its two metabolites in surface water, soil and animal manure. Anal. Chim. Acta 2012, 755, 69–76. [Google Scholar] [CrossRef]
- Hansen, M.; Krogh, K.A.; Halling-Sørensen, B.; Björklund, E. Determination of ten steroid hormones in animal waste manure and agricultural soil using inverse and integrated clean-up pressurized liquid extraction and gas chromatography-tandem mass spectrometry. Anal. Methods 2011, 3, 1087–1095. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Ji, X.; Wu, H.; Yang, H.; Zhang, H.; Zhang, X.; Li, Z.; Ni, X.; Qian, M. Determination of veterinary drug/pesticide residues in livestock and poultry excrement using selective accelerated solvent extraction and magnetic material purification combined with ultra-high-performance liquid chromatog-raphy–tandem mass spectrometry. J. Chromatogr. A 2020, 1617, 460808. [Google Scholar] [CrossRef]
- Argüeso-Mata, M.; Bolado, S.; Jiménez, J.J.; López-Serna, R. Determination of antibiotics and other veterinary drugs in the solid phase of pig manure. Chemosphere 2021, 275, 130039. [Google Scholar] [CrossRef]
- Guo, C.; Wang, M.; Xiao, H.; Huai, B.; Wang, F.; Pan, G.; Liao, X.; Liu, Y. Development of a modified QuEChERS method for the determination of veterinary antibiotics in swine manure by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2016, 1027, 110–118. [Google Scholar] [CrossRef]
- Zhi, S.; Zhou, J.; Liu, H.; Wu, H.; Zhang, Z.; Ding, Y.; Zhang, K. Simultaneous extraction and determination of 45 veterinary antibiotics in swine manure by liquid chroma-tography-tandem mass spectrometry. J. Chromatogr. B 2020, 1154, 122286. [Google Scholar] [CrossRef] [PubMed]
- Nebot, C.; Guarddon, M.; Seco, F.; Iglesias, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Monitoring the presence of residues of tetracyclines in baby food samples by HPLC-MS/MS. Food Control 2014, 46, 495–501. [Google Scholar] [CrossRef]
- Boscher, A.; Guignard, C.; Pellet, T.; Hoffmann, L.; Bohn, T. Development of a multi-class method for the quantifi-cation of veterinary drug residues in feedingstuffs by liquid chromatography-tandem mass spectrometry. J. Chroma-Tography A 2010, 1217, 6394–6404. [Google Scholar] [CrossRef]
- Łukaszewicz, P.; Białk-Bielińska, A.; Dołżonek, J.; Kumirska, J.; Caban, M.; Stepnowski, P. A new approach for the extraction of tetracyclines from soil matrices: Application of the microwave-extraction technique. Anal. Bioanal. Chem. 2018, 410, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Melekhin, A.O.; Tolmacheva, V.V.; Shubina, E.G.; Dmitrienko, S.G.; Apyari, V.V.; Grudev, A.I. Using Hyper-crosslinked Polystyrene for the Multicomponent Solid-Phase Extraction of Residues of 63 Veterinary Preparations in Their Determination in Chicken Meat by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Anal. Chem. 2021, 76, 946–959. [Google Scholar] [CrossRef]
- Sengeløv, G.; Agersø, Y.; Halling-Sørensen, B.; Baloda, S.B.; Andersen, J.S.; Jensen, L.B. Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ. Int. 2003, 28, 587–595. [Google Scholar] [CrossRef]
- Hölzel, C.S.; Müller, C.; Harms, K.S.; Mikolajewski, S.; Schäfer, S.; Schwaiger, K.; Bauer, J. Heavy metals in liquid pig manure in light of bacterial antimicrobial resistance. Environ. Res. 2012, 113, 21–27. [Google Scholar] [CrossRef]
- Joy, S.R.; Bartelt-Hunt, S.L.; Snow, D.D.; Gilley, J.E.; Woodbury, B.L.; Parker, D.B.; Marx, D.B.; Li, X. Fate and Transport of Antimicrobials and Antimicrobial Resistance Genes in Soil and Runoff Following Land Application of Swine Manure Slurry. Environ. Sci. Technol. 2013, 47, 12081–12088. [Google Scholar] [CrossRef] [PubMed]
- Pu, C.; Liu, H.; Ding, G.; Sun, Y.; Yu, X.; Chen, J.; Ren, J.; Gong, X. Impact of direct application of biogas slurry and residue in fields: In situ analysis of antibiotic resistance genes from pig manure to fields. J. Hazard. Mater. 2018, 344, 441–449. [Google Scholar] [CrossRef] [PubMed]
| Compound | Therapeutic Groups | CAS | MW | Stock Solution Concentration (µg/mL) | Solvent |
|---|---|---|---|---|---|
| Amoxicillin | Antibiotic | 26787-78-0 | 365.4 | 800 | Methanol |
| Azithromycin | Antibiotic | 83905-01-5 | 749.03 | 800 | Methanol |
| Cefuroxime | Antibiotic | 55268-75-2 | 424.38 | 800 | Methanol |
| Chloramphenicol | Antibiotic | 56-75-7 | 323.13 | 1000 | Methanol |
| Chlortetracycline | Antibiotic | 57-62-5 | 478.88 | 1000 | Methanol |
| Ciprofloxacin | Antibiotic | 85721-33-1 | 331.34 | 400 | Water:Methanol (3:1) |
| Clarithromycin | Antibiotic | 81103-11-9 | 747.96 | 400 | Methanol |
| Colistin | Antibiotic | 1066-17-7 | 1155.4 | 800 | Methanol |
| Danofloxacin | Antibiotic | 112398-08-0 | 357.38 | 800 | Methanol |
| Decoquinate | Antiparasitic Agent | 18507-89-6 | 417.5 | 100 | Methanol |
| Dexamethasone | Corticosteroids | 50-02-2 | 392.5 | 800 | Methanol |
| Diclofenac | Anti-Inflammatory | 15307-86-5 | 296.15 | 800 | Methanol |
| Difloxacin | Antibiotic | 98106-17-3 | 399.4 | 400 | Methanol |
| Doxycycline | Antibiotic | 564-25-0 | 444.44 | 1000 | Methanol |
| Enrofloxacin | Antibiotic | 93106-60-6 | 359.4 | 800 | Methanol |
| Erythromycin | Antibiotic | 114-07-8 | 733.9 | 800 | Methanol |
| Florfenicol | Antibiotic | 73231-34-2 | 358.2 | 1000 | Methanol |
| Flumethasone | Glucocorticoid | 2135-17-3 | 410.5 | 800 | Methanol |
| Griseofulvin | Fungistatic Agent | 126-07-8 | 352.8 | 400 | Methanol |
| Ibuprofen | Nonsteroidal Anti-inflammatory | 15687-27-1 | 206.28 | 800 | Methanol |
| Levofloxacin | Antibiotic | 100986-85-4 | 361.37 | 800 | Methanol |
| Lincomycin | Antibiotic | 154-21-2 | 406.54 | 800 | Methanol |
| Maduramicin | Antiparasitic Agent | 84878-61-5 | 934.2 | 800 | Methanol |
| Mefenamic Acid | Anti-Inflammatory | 61-68-7 | 241.28 | 400 | Methanol |
| Monesin | Antiparasitic Agent | 17090-79-8 | 670.9 | 800 | Methanol |
| Narasin | Antiparasitic Agent | 555134-13-9 | 765.0 | 400 | Methanol |
| Nicarbazin | Antiparasitic Agent | 330-95-0 | 426.4 | 800 | Dimethyl Sulfoxide |
| Norfloxacin | Antibiotic | 70458-96-7 | 319.33 | 800 | Methanol |
| Oxytetracycline | Antibiotic | 79-57-2 | 460.44 | 1000 | Methanol |
| Paracetamol | Nonsteroidal Anti-Inflammatory | 103-90-2 | 151.16 | 800 | Methanol |
| Propranolol | Beta Blocker | 525-66-6 | 259.34 | 800 | Methanol |
| Robenidine | Antiparasitic Agent | 25875-51-8 | 334.2 | Methanol | |
| Sarafloxacin | Antibiotic | 98105-99-8 | 385.36 | 400 | Methanol |
| Salinomycin | Antiparasitic Agent | 53003-10-4 | 751.0 | Methanol | |
| Spectinomycin | Antibiotic | 1695-77-8 | 332.35 | 400 | Water:H+ |
| Sulfachloropyridazine | Antibiotic | 80-32-0 | 284.73 | 50 | Methanol |
| Sulfadiazine | Antibiotic | 68-35-9 | 250.28 | 50 | Methanol |
| Sulfadimethoxine | Antibiotic | 122-11-2 | 310.33 | 50 | Methanol |
| Sulfamerazine | Antibiotic | 127-79-7 | 264.31 | 50 | Methanol |
| Sulfamethazine | Antibiotic | 57-68-1 | 278.33 | 50 | Methanol |
| Sulfamethoxazole | Antibiotic | 723-46-6 | 253.28 | 50 | Methanol |
| Sulfamethoxypyridazine | Antibiotic | 80-35-3 | 280.3 | 50 | Methanol |
| Sulfapyridine | Antibiotic | 144.83-2 | 249.29 | 50 | Methanol |
| Sulfaquinoxaline | Antibiotic | 59-40-5 | 300.34 | 50 | Methanol |
| Sulfathiazole | Antibiotic | 72-14-0 | 255.32 | 50 | Methanol |
| Tetracycline | Antibiotic | 60-54-8 | 444.43 | 1000 | Methanol |
| Trimethoprim | Antibiotic | 738-70-5 | 290.32 | 800 | Methanol |
| Tylosin | Antibiotic | 1401-69-0 | 916.1 | 800 | Methanol |
| Amoxicillin | Antibiotic | 26787-78-0 | 365.4 | 800 | Methanol |
| Azithromycin | Antibiotic | 83905-01-5 | 749.03 | 800 | Methanol |
| Cefuroxime | Antibiotic | 55268-75-2 | 424.38 | 800 | Methanol |
| Chloramphenicol | Antibiotic | 56-75-7 | 323.13 | 1000 | Methanol |
| Chlortetracycline | Antibiotic | 57-62-5 | 478.88 | 1000 | Methanol |
| Ciprofloxacin | Antibiotic | 85721-33-1 | 331.34 | 400 | Water:Methanol (3:1) |
| Clarithromycin | Antibiotic | 81103-11-9 | 747.96 | 400 | Methanol |
| Colistin | Antibiotic | 1066-17-7 | 1155.4 | 800 | Methanol |
| Danofloxacin | Antibiotic | 112398-08-0 | 357.38 | 800 | Methanol |
| Decoquinate | Antiparasitic Agent | 18507-89-6 | 417.5 | 100 | Methanol |
| Dexamethasone | Corticosteroids | 50-02-2 | 392.5 | 800 | Methanol |
| Diclofenac | Anti-Inflammatory | 15307-86-5 | 296.15 | 800 | Methanol |
| Difloxacin | Antibiotic | 98106-17-3 | 399.4 | 400 | Methanol |
| Doxycycline | Antibiotic | 564-25-0 | 444.44 | 1000 | Methanol |
| Enrofloxacin | Antibiotic | 93106-60-6 | 359.4 | 800 | Methanol |
| Erythromycin | Antibiotic | 114-07-8 | 733.9 | 800 | Methanol |
| Florfenicol | Antibiotic | 73231-34-2 | 358.2 | 1000 | Methanol |
| Flumethasone | Glucocorticoid | 2135-17-3 | 410.5 | 800 | Methanol |
| Griseofulvin | Fungistatic Agent | 126-07-8 | 352.8 | 400 | Methanol |
| Ibuprofen | Nonsteroidal Anti-Inflammatory | 15687-27-1 | 206.28 | 800 | Methanol |
| Levofloxacin | Antibiotic | 100986-85-4 | 361.37 | 800 | Methanol |
| Lincomycin | Antibiotic | 154-21-2 | 406.54 | 800 | Methanol |
| Maduramicin | Antiparasitic Agent | 84878-61-5 | 934.2 | 800 | Methanol |
| Mefenamic Acid | Anti-Inflammatory | 61-68-7 | 241.28 | 400 | Methanol |
| Monesin | Antiparasitic Agent | 17090-79-8 | 670.9 | 800 | Methanol |
| Narasin | Antiparasitic Agent | 555134-13-9 | 765.0 | 400 | Methanol |
| Nicarbazin | Antiparasitic Agent | 330-95-0 | 426.4 | 800 | Dimethyl Sulfoxide |
| Norfloxacin | Antibiotic | 70458-96-7 | 319.33 | 800 | Methanol |
| Oxytetracycline | Antibiotic | 79-57-2 | 460.44 | 1000 | Methanol |
| Paracetamol | Nonsteroidal Anti-Inflammatory | 103-90-2 | 151.16 | 800 | Methanol |
| Propranolol | Beta Blocker | 525-66-6 | 259.34 | 800 | Methanol |
| Robenidine | Antiparasitic Agent | 25875-51-8 | 334.2 | Methanol | |
| Sarafloxacin | Antibiotic | 98105-99-8 | 385.36 | 400 | Methanol |
| Salinomycin | Antiparasitic Agent | 53003-10-4 | 751.0 | Methanol | |
| Spectinomycin | Antibiotic | 1695-77-8 | 332.35 | 400 | Water:H+ |
| Sulfachloropyridazine | Antibiotic | 80-32-0 | 284.73 | 50 | Methanol |
| Sulfadiazine | Antibiotic | 68-35-9 | 250.28 | 50 | Methanol |
| Sulfadimethoxine | Antibiotic | 122-11-2 | 310.33 | 50 | Methanol |
| Sulfamerazine | Antibiotic | 127-79-7 | 264.31 | 50 | Methanol |
| Sulfamethazine | Antibiotic | 57-68-1 | 278.33 | 50 | Methanol |
| Sulfamethoxazole | Antibiotic | 723-46-6 | 253.28 | 50 | Methanol |
| Sulfamethoxypyridazine | Antibiotic | 80-35-3 | 280.3 | 50 | Methanol |
| Sulfapyridine | Antibiotic | 144.83-2 | 249.29 | 50 | Methanol |
| Sulfaquinoxaline | Antibiotic | 59-40-5 | 300.34 | 50 | Methanol |
| Sulfathiazole | Antibiotic | 72-14-0 | 255.32 | 50 | Methanol |
| Tetracycline | Antibiotic | 60-54-8 | 444.43 | 1000 | Methanol |
| Trimethoprim | Antibiotic | 738-70-5 | 290.32 | 800 | Methanol |
| Tylosin | Antibiotic | 1401-69-0 | 916.1 | 800 | Methanol |
| Compound | Concentration | Matrix Effects | RSDME (%) | RSDr | RSDR | Trueness | a | b | R2 |
|---|---|---|---|---|---|---|---|---|---|
| (µg/kg) | (%) (n = 6) | (%) (n = 18) | (%) (n = 18) | ||||||
| Chloramphenicol | 200 | 0.9 | 7.5 | 13 | 11 | 118 | 3300.7 | 70.4 | 0.971 |
| 400 | 29 | 5 | 110 | ||||||
| 600 | 9 | 7 | 117 | ||||||
| Chlortetracycline | 200 | 1.3 | 15.8 | 20 | 13 | 141 | 22,883.9 | 3167.6 | 0.981 |
| 400 | 27 | 13 | 110 | ||||||
| 600 | 11 | 12 | 117 | ||||||
| Ciprofloxacin | 200 | 1.0 | 10.5 | 12 | 14 | 98 | 53,985.2 | 5468.9 | 0.986 |
| 400 | 21 | 14 | 113 | ||||||
| 600 | 3 | 14 | 107 | ||||||
| Clarithromycin | 200 | 0.5 | 13.0 | 21 | 5 | 107 | 68,053.0 | 906.3 | 0.966 |
| 400 | 41 | 7 | 111 | ||||||
| 600 | 8 | 16 | 136 | ||||||
| Danafloxacin | 200 | 0.6 | 8.1 | 7 | 18 | 99 | 16,787.8 | 4841.5 | 0.978 |
| 400 | 19 | 12 | 104 | ||||||
| 600 | 5 | 11 | 106 | ||||||
| Dexamethasone | 200 | 0.0 | 11.3 | 21 | 11 | 100 | 82.1 | 100.9 | 0.972 |
| 400 | 13 | 6 | 119 | ||||||
| 600 | 16 | 11 | 97 | ||||||
| Diclofenac | 200 | 0.4 | 2.9 | 10 | 12 | 110 | 49,795.8 | 3404.6 | 0.998 |
| 400 | 26 | 11 | 102 | ||||||
| 600 | 10 | 9 | 102 | ||||||
| Difloxacin | 200 | 0.3 | 3.0 | 9 | 18 | 113 | 30,438.3 | 2226.7 | 0.977 |
| 400 | 20 | 11 | 109 | ||||||
| 600 | 5 | 15 | 102 | ||||||
| Doxycycline | 200 | 2.8 | 8.4 | 11 | 16 | 95 | 1,294,454.7 | 14660.6 | 0.998 |
| 400 | 18 | 6 | 103 | ||||||
| 600 | 6 | 17 | 108 | ||||||
| Enrofloxacin | 200 | 1.2 | 9.0 | 18 | 10 | 90 | 236,205.7 | 7664.0 | 0.982 |
| 400 | 12 | 8 | 117 | ||||||
| 600 | 14 | 9 | 92 | ||||||
| Florfenicol | 200 | 0.9 | 9.0 | 18 | 10 | 111 | 2720.1 | 37.8 | 0.975 |
| 400 | 24 | 9 | 118 | ||||||
| 600 | 13 | 6 | 139 | ||||||
| Levofloxacin | 200 | 0.5 | 14.4 | 13 | 16 | 102 | 93,029.5 | 3682.9 | 0.971 |
| 400 | 23 | 5 | 102 | ||||||
| 600 | 3 | 14 | 98 | ||||||
| Lincomycin | 200 | 5.5 | 2.1 | 34 | 16 | 70 | 178,170.3 | 43379.3 | 0.977 |
| 400 | 28 | 15 | 66 | ||||||
| 600 | 20 | 10 | 74 | ||||||
| Mefenamic Acid | 200 | 2.3 | 19.1 | 24 | 16 | 118 | 273,977.7 | 13018.5 | 0.994 |
| 400 | 38 | 12 | 82 | ||||||
| 600 | 8 | 14 | 95 | ||||||
| Norfloxacin | 200 | 0.5 | 1.1 | 12 | 8 | 101 | 210,771.6 | 2414.2 | 0.969 |
| 400 | 22 | 9 | 110 | ||||||
| 600 | 4 | 9 | 114 | ||||||
| Oxytetracycline | 200 | 0.5 | 1.1 | 8 | 20 | 124 | 183,941.1 | 2705.8 | 0.977 |
| 400 | 24 | 13 | 84 | ||||||
| 600 | 13 | 10 | 109 | ||||||
| Propranolol | 200 | 0.4 | 3.8 | 13 | 20 | 118 | 77,055.7 | 1916.3 | 0.991 |
| 400 | 21 | 17 | 118 | ||||||
| 600 | 10 | 7 | 126 | ||||||
| Sarafloxacin | 200 | 0.7 | 3.6 | 7 | 18 | 109 | 96,223.4 | 4575.7 | 0.98 |
| 400 | 19 | 10 | 111 | ||||||
| 600 | 6 | 15 | 96 | ||||||
| Sulfachloropyridine | 200 | 1.0 | 3.7 | 10 | 22 | 113 | 239,127.4 | 5107.6 | 0.984 |
| 400 | 25 | 13 | 123 | ||||||
| 600 | 6 | 11 | 144 | ||||||
| Sulfadimethoxine | 200 | 0.8 | 3.6 | 6 | 15 | 117 | 480,417.2 | 13543.4 | 0.975 |
| 400 | 23 | 11 | 113 | ||||||
| 600 | 5 | 11 | 115 | ||||||
| Sulfamerazine | 200 | 1.8 | 3.6 | 5 | 22 | 117 | 67,678.2 | 4582.8 | 0.979 |
| 400 | 24 | 12 | 108 | ||||||
| 600 | 3 | 11 | 116 | ||||||
| Sulfamethazine | 200 | 1.5 | 2.5 | 6 | 25 | 132 | 645,449.5 | 13561.1 | 0.974 |
| 400 | 23 | 13 | 122 | ||||||
| 600 | 4 | 12 | 125 | ||||||
| Sulfamethoxazole | 200 | 0.9 | 5.5 | 12 | 22 | 111 | 185,586.8 | 5934.0 | 0.974 |
| 400 | 28 | 18 | 106 | ||||||
| 600 | 8 | 9 | 128 | ||||||
| Sulfamethoxypyridazine | 200 | 1.6 | 3.6 | 6 | 23 | 117 | 153,709.0 | 11111.6 | 0.976 |
| 400 | 22 | 12 | 110 | ||||||
| 600 | 3 | 12 | 115 | ||||||
| Sulfapyridine | 200 | 1.2 | 4.9 | 5 | 22 | 111 | 90,339.4 | 8788.6 | 0.983 |
| 400 | 27 | 13 | 112 | ||||||
| 600 | 5 | 11 | 123 | ||||||
| Sulfaquinoxaline | 200 | 0.8 | 4.6 | 6 | 21 | 115 | 216,109.5 | 4361.8 | 0.985 |
| 400 | 34 | 14 | 118 | ||||||
| 600 | 5 | 13 | 137 | ||||||
| Sulfathiazole | 200 | 0.5 | 5.4 | 6 | 21 | 116 | 70,093.4 | 6651.6 | 0.97 |
| 400 | 25 | 18 | 117 | ||||||
| 600 | 18 | 4 | 125 | ||||||
| Tetracycline | 200 | 0.1 | 5.2 | 7 | 18 | 107 | 16,481.5 | 1369.9 | 0.997 |
| 400 | 28 | 6 | 109 | ||||||
| 600 | 5 | 17 | 115 | ||||||
| Trimethoprim | 200 | 0.3 | 5.2 | 8 | 19 | 111 | 58,128.0 | 6639.5 | 0.975 |
| 400 | 27 | 12 | 119 | ||||||
| 600 | 5 | 11 | 121 |
| Compound | Rt (min) | RSD of Rt (%) | MRM 1 | MRM 2 |
|---|---|---|---|---|
| Chloramphenicol | 4.82 | 0.5 | (−) 323.0 > 152.0 [14.0 V] | (−) 323.0 > 194.1 [9.0 V] |
| Chlortetracycline | 4.43 | 0.2 | (+) 479.0 > 462.0 [15.0 V] | (+) 479.0 > 444.0 [22.0 V] |
| Ciprofloxacin | 4.00 | 0.4 | (+) 332.2 > 314.1 [16.0 V] | (+) 332.2 > 231.0 [32.0 V] |
| Clarithromycin | 5.17 | 0.3 | (+) 749.0 > 158.0 [25.0 V] | (+) 749.0 > 116.0 [50.0 V] |
| Danafloxacin | 4.07 | 0.3 | (+) 358.0 > 340.0 [25.0 V] | (+) 358.0 > 255.0 [35.0 V] |
| Dexamethasone | 5.34 | 0.2 | (+) 393.0 > 373.0 [7.0 V] | (+) 393.0 > 354.6 [10.0 V] |
| Diclofenac | 6.29 | 0.2 | (+) 296.0 > 215.0 [15.0 V] | (+) 296.0 > 151.0 [60.0 V] |
| Difloxacin | 4.23 | 0.2 | (+) 386.0 > 299.0 [25.0 V] | (+) 386.0 > 299.0 [25.0 V] |
| Doxycycline | 4.52 | 1.7 | (+) 445.0 > 428.0 [15.0 V] | (+) 445.0 > 154.0 [30.0 V] |
| Enrofloxacin | 4.11 | 3.9 | (+) 360.0 > 342.1 [17.0 V] | (+) 360.0 > 286.0 [31.0 V] |
| Florfenicol | 4.66 | 1.1 | (−) 358.0 > 185.0 [15.0 V] | (−) 358.0 > 338.0 [5.0 V] |
| Levofloxacin | 3.98 | 1.1 | (+) 362.0 > 261.0 [30.0 V] | (+) 362.0 > 179.0 [40.0 V] |
| Lincomycin | 3.73 | 0.6 | (+) 407.3 > 126.2 [22.0 V] | (+) 407.3 > 359.2 [12.0 V] |
| Mefenamic Acid | 6.61 | 0.2 | (+) 242.0 > 223.8 [15.0 V] | (+) 242.0 > 209.0 [27.0 V] |
| Norfloxacin | 3.96 | 0.3 | (+) 320.0 > 302.0 [15.0 V] | (+) 320.0 > 276.0 [15.0 V] |
| Oxytetracycline | 3.96 | 0.4 | (+) 461.0 > 426.0 [20.0 V] | (+) 461.0 > 443.0 [10.0 V] |
| Paracetamol | 3.58 | 2.9 | (+) 152.3 > 110.0 [23.0 V] | (+) 152.3 > 92.7 [23.0 V] |
| Propranolol | 4.67 | 1.4 | (+) 260.0 > 116.0 [20.0 V] | (+) 260.0 > 154.5 [20.0 V] |
| Sarafloxacin | 4.27 | 0.2 | (+) 400.0 > 299.0 [30.0 V] | (+) 400.0 > 382.0 [30.0 V] |
| Sulfachloropyridine | 4.52 | 0.2 | (+) 285.0 > 156.0 [11.0 V] | (+) 285.0 > 108.0 [18.0 V] |
| Sulfadiazine | 3.74 | 1.6 | (+) 251.1 > 156.0 [12.0 V] | (+) 251.1 > 108.0 [19.0 V] |
| Sulfadimethoxine | 4.97 | 0.2 | (+) 311.0 > 156.0 [20.0 V] | (+) 311.0 > 108.0 [18.0 V] |
| Sulfamerazine | 4.05 | 1.3 | (+) 265.0 > 156.0 [16.0 V] | (+) 265.0 > 172.0 [16.0 V] |
| Sulfamethazine | 4.25 | 0.6 | (+) 279.0 > 186.0 [15.0 V] | (+) 279.0 > 156.0 [15.0 V] |
| Sulfamethoxazole | 4.64 | 0.2 | (+) 254.0 > 156.0 [11.0 V] | (+) 254.0 > 92.0 [18.0 V] |
| Sulfamethoxypyridazine | 4.27 | 0.2 | (+) 281.0 > 156.0 [13.0 V] | (+) 281.0 > 92.0 [24.0 V] |
| Sulfapyridine | 3.93 | 4.1 | (+) 250.0 > 156.0 [13.0 V] | (+) 250.0 > 92.0 [23.0 V] |
| Sulfaquinoxaline | 4.98 | 0.2 | (+) 301.0 > 156.0 [15.0 V] | (+) 301.0 > 92.0 [25.0 V] |
| Sulfathiazole | 3.86 | 3.1 | (+) 256.0 > 156.0 [12.0 V] | (+) 256.0 > 92.0 [22.0 V] |
| Tetracycline | 4.08 | 5.9 | (+) 445.4 > 410.0 [20.0 V] | (+) 445.4 > 427.0 [15.0 V] |
| Trimethoprim | 3.88 | 0.4 | (+) 291.0 > 123.0 [20.0 V] | (+) 291.0 > 230.0 [24.0 V] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebot, C.; Cardelle-Cobas, A.; García-Presedo, I.; Patyra, E.; Cepeda, A.; Franco, C.M. Identification and Quantification of 29 Active Substances by HPLC–ESI-MS/MS in Lyophilized Swine Manure Samples. Molecules 2023, 28, 216. https://doi.org/10.3390/molecules28010216
Nebot C, Cardelle-Cobas A, García-Presedo I, Patyra E, Cepeda A, Franco CM. Identification and Quantification of 29 Active Substances by HPLC–ESI-MS/MS in Lyophilized Swine Manure Samples. Molecules. 2023; 28(1):216. https://doi.org/10.3390/molecules28010216
Chicago/Turabian StyleNebot, Carolina, Alejandra Cardelle-Cobas, Ignacio García-Presedo, Ewelina Patyra, Alberto Cepeda, and Carlos M. Franco. 2023. "Identification and Quantification of 29 Active Substances by HPLC–ESI-MS/MS in Lyophilized Swine Manure Samples" Molecules 28, no. 1: 216. https://doi.org/10.3390/molecules28010216
APA StyleNebot, C., Cardelle-Cobas, A., García-Presedo, I., Patyra, E., Cepeda, A., & Franco, C. M. (2023). Identification and Quantification of 29 Active Substances by HPLC–ESI-MS/MS in Lyophilized Swine Manure Samples. Molecules, 28(1), 216. https://doi.org/10.3390/molecules28010216










