Molecular Transport within Polymer Brushes: A FRET View at Aqueous Interfaces
Abstract
:1. Introduction
2. Results
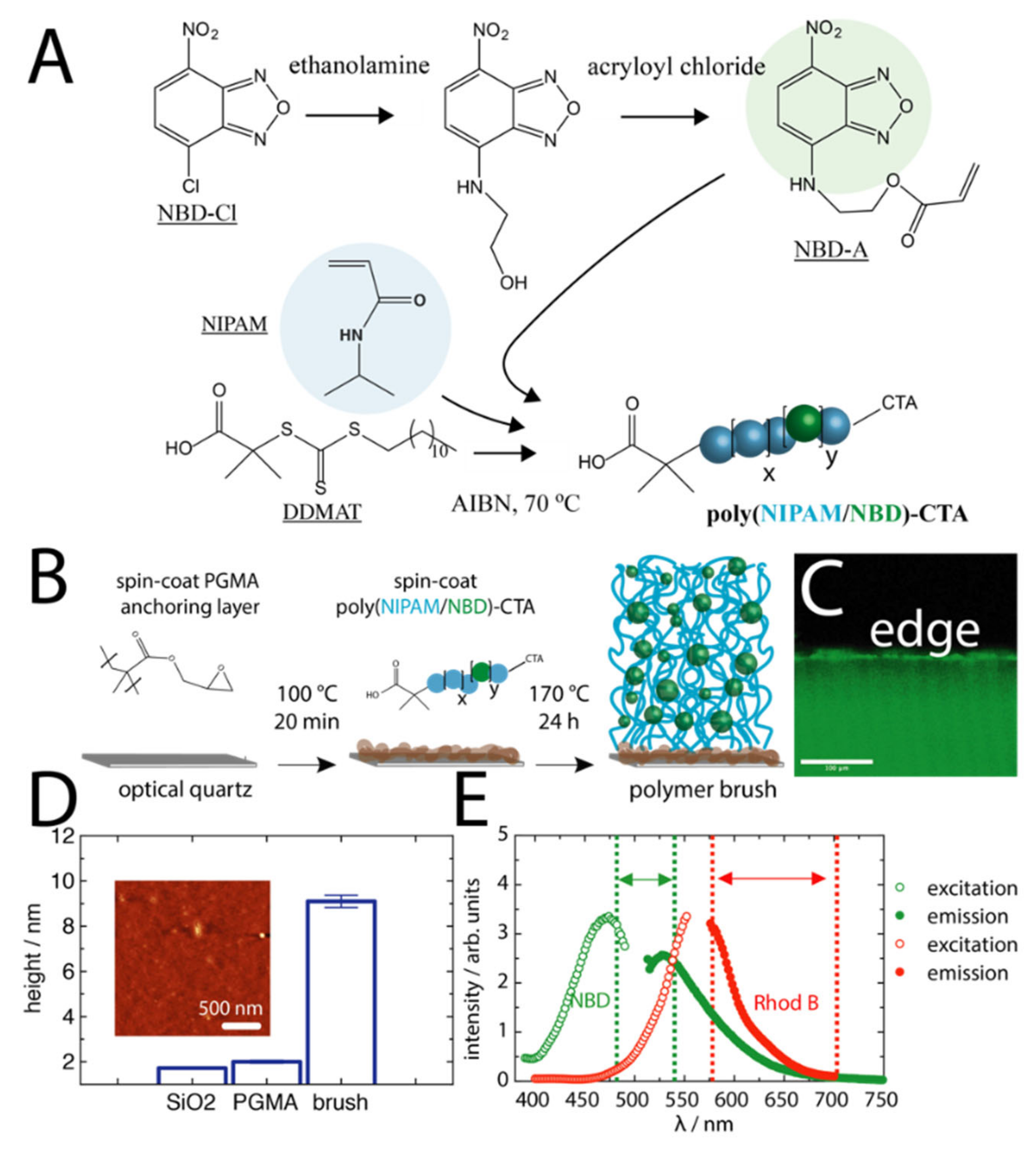
2.1. Polymer Brush Assembly
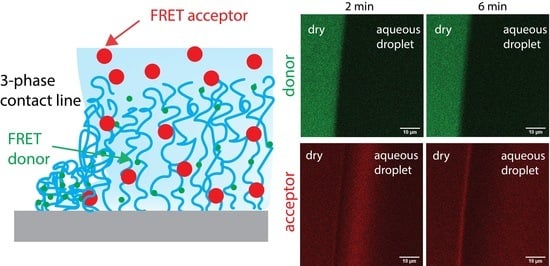
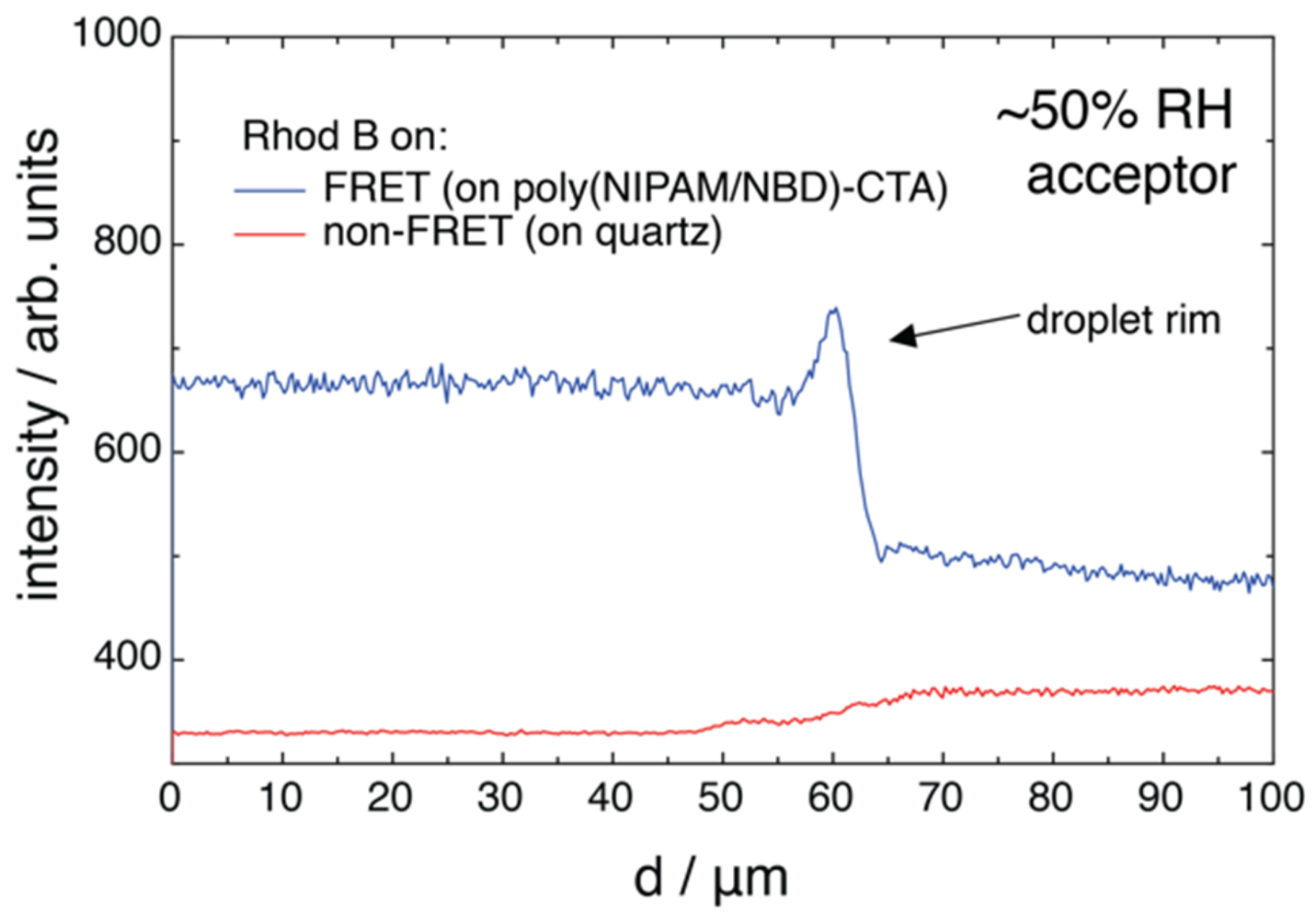
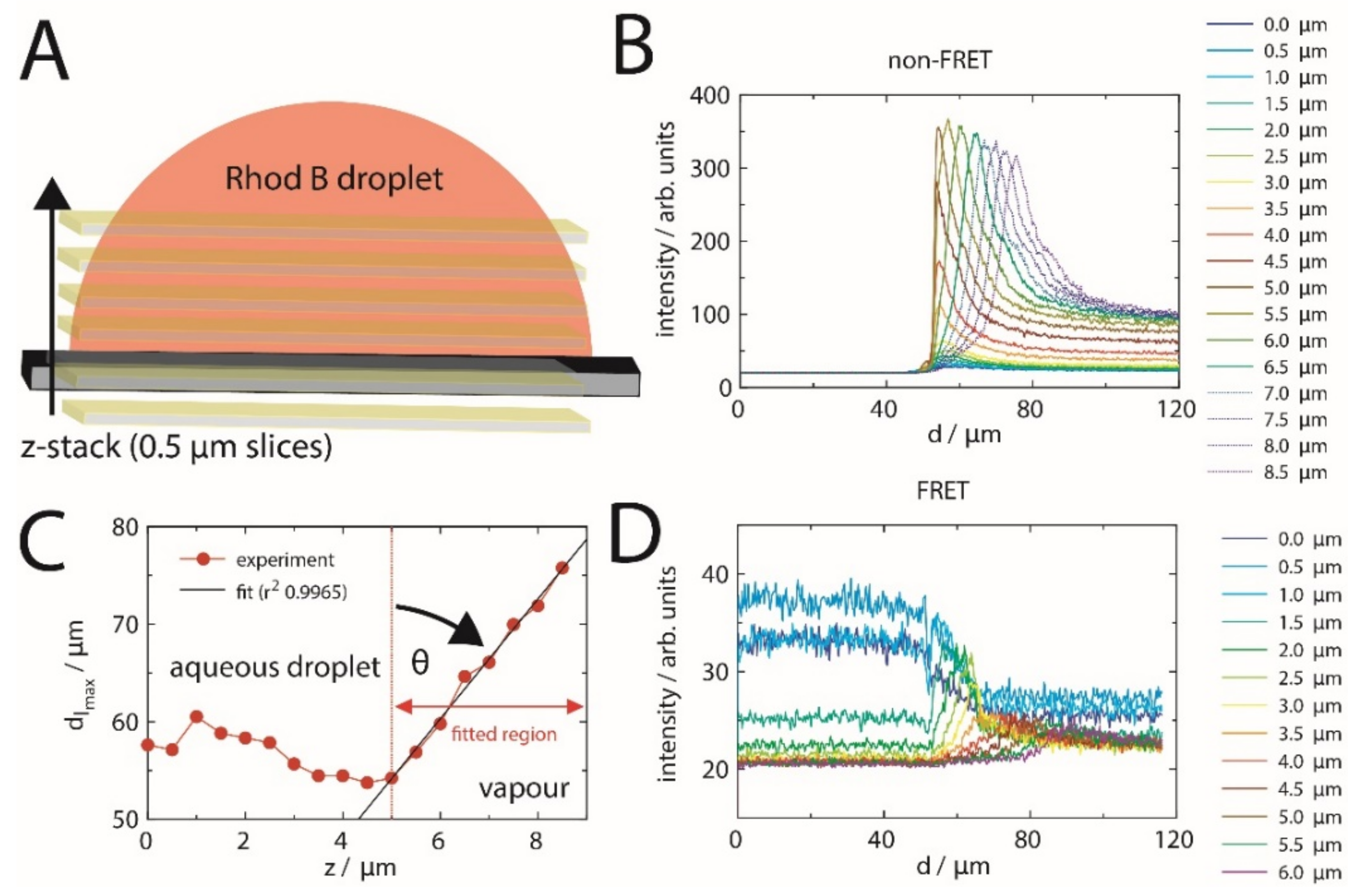
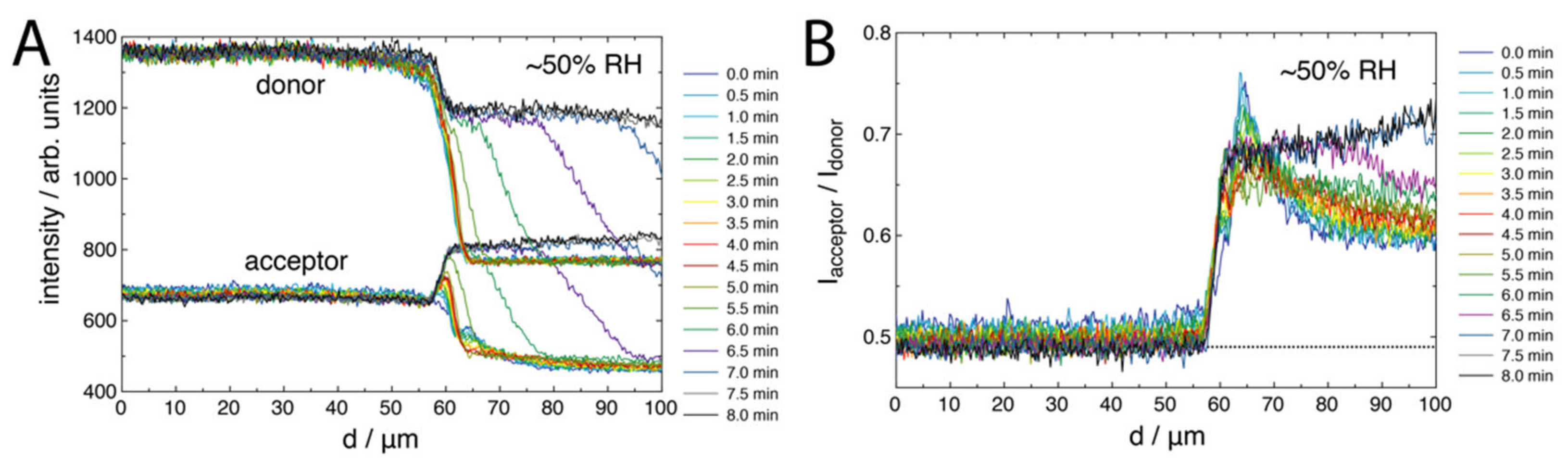
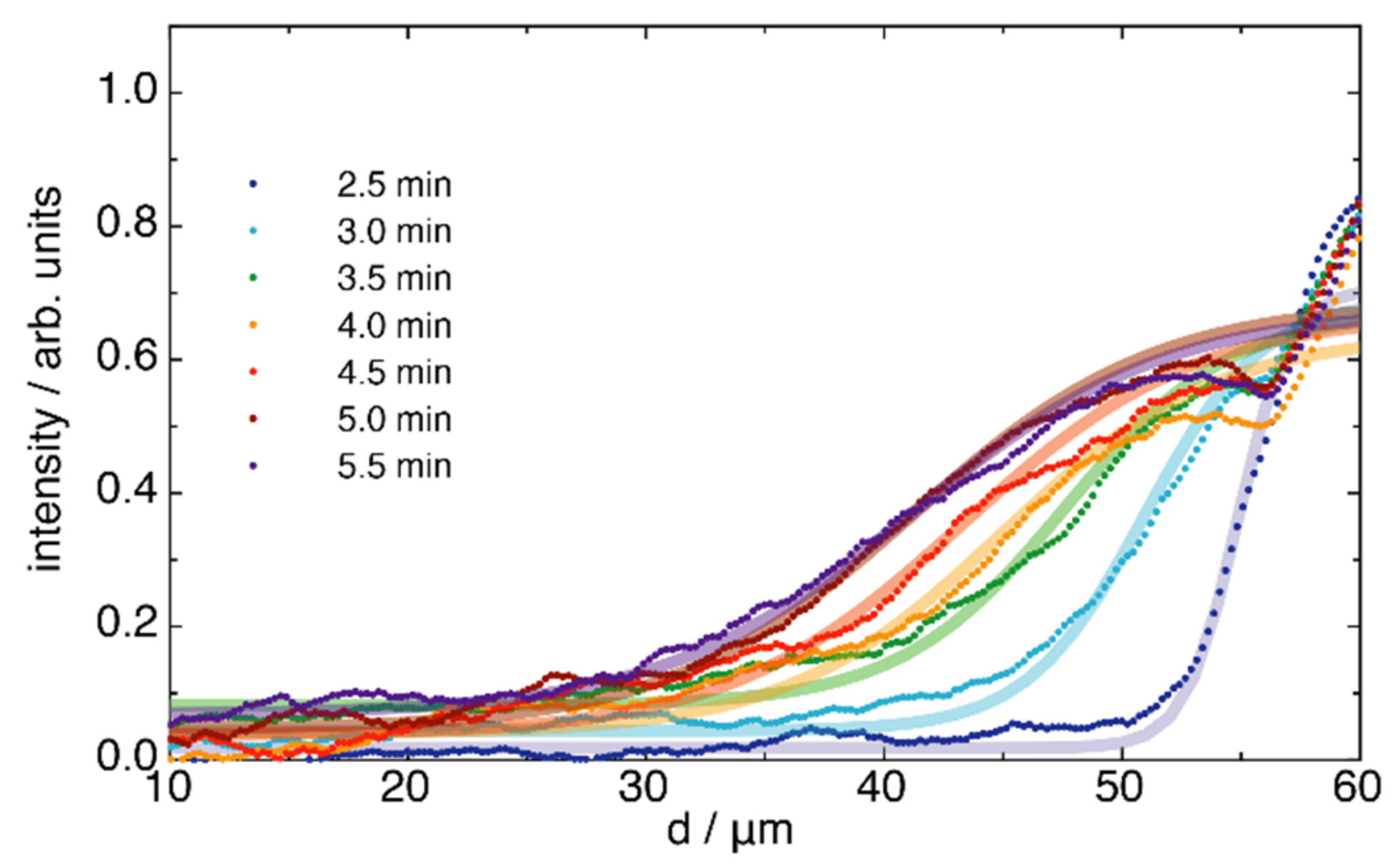
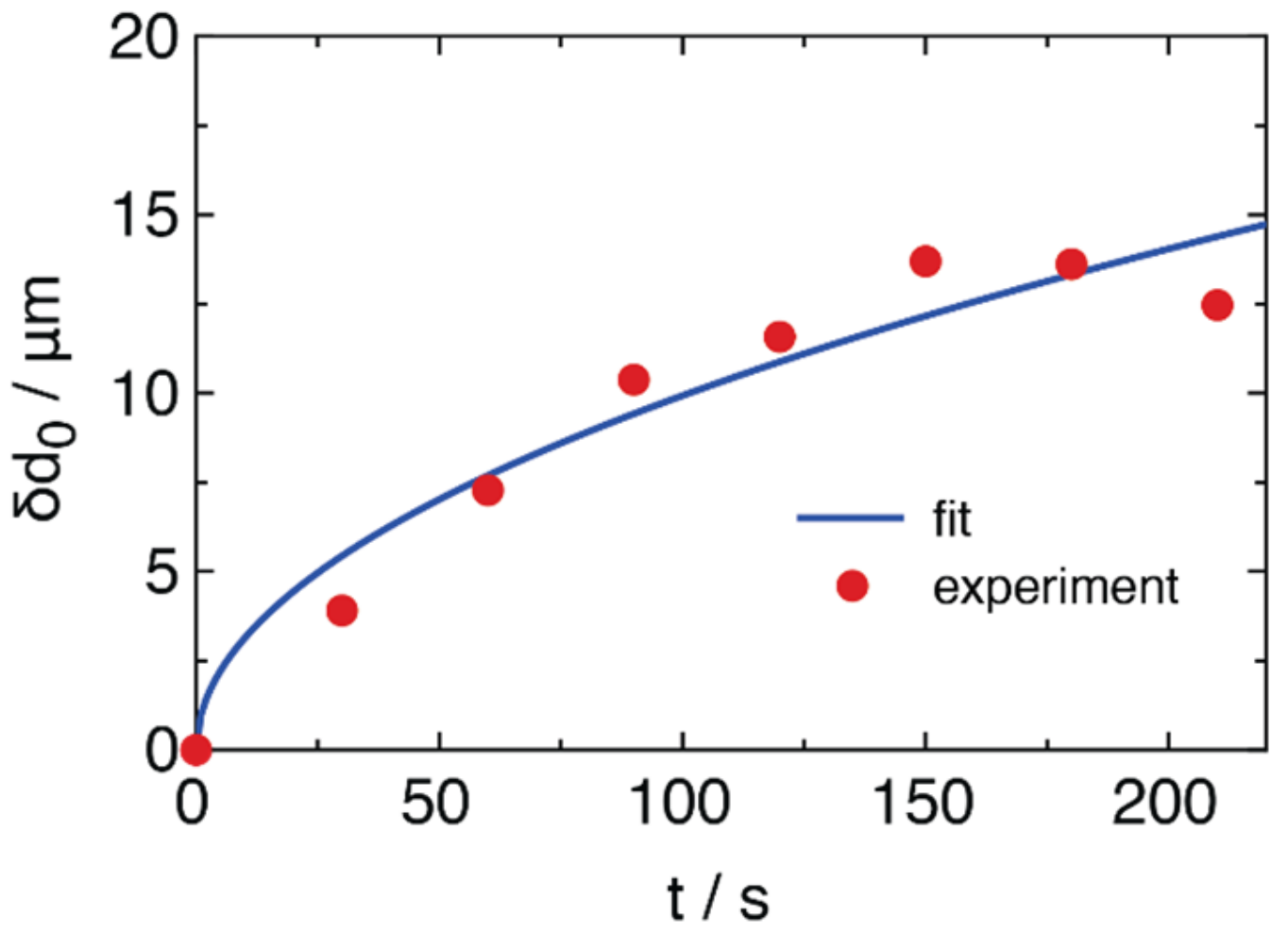
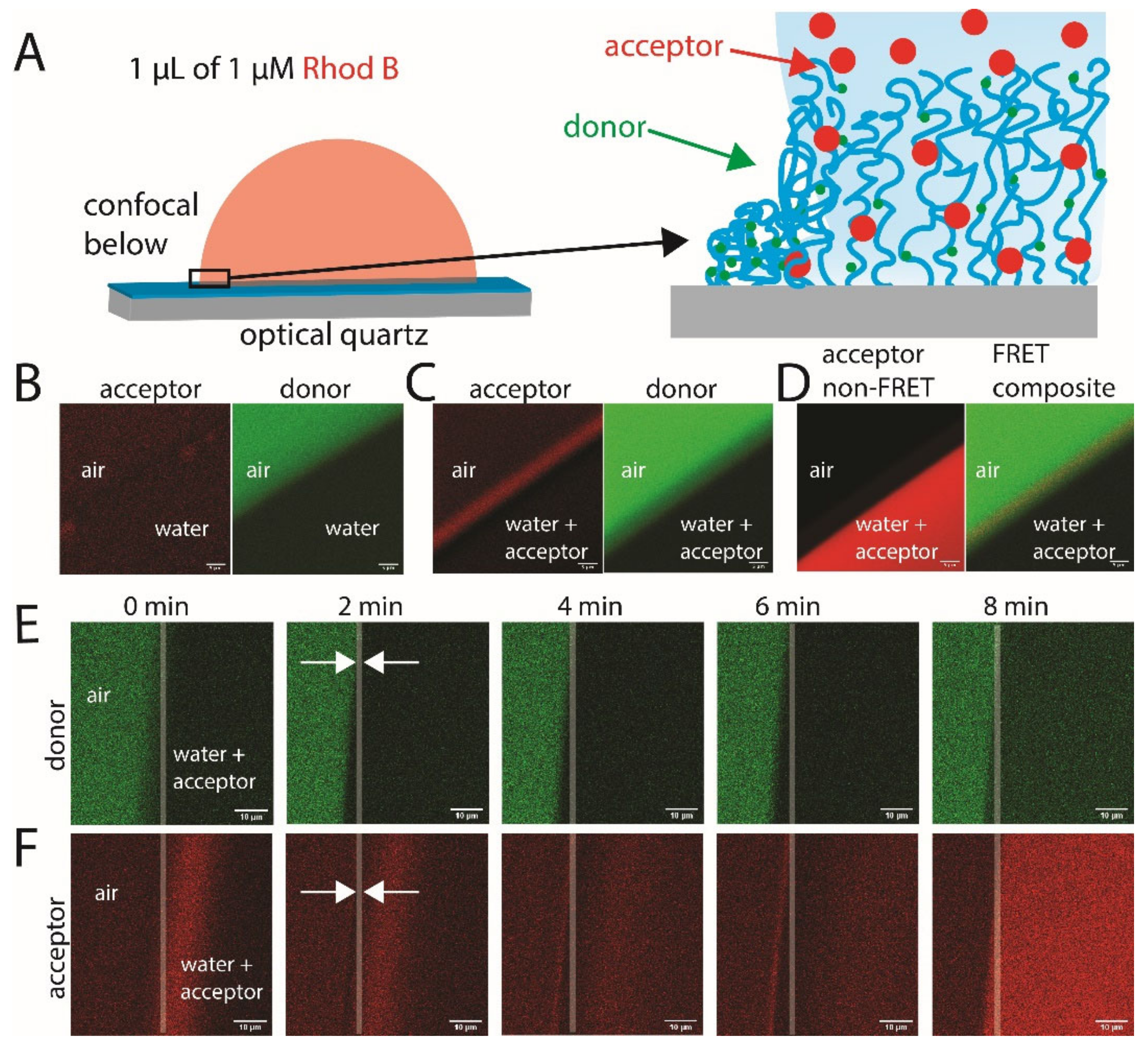
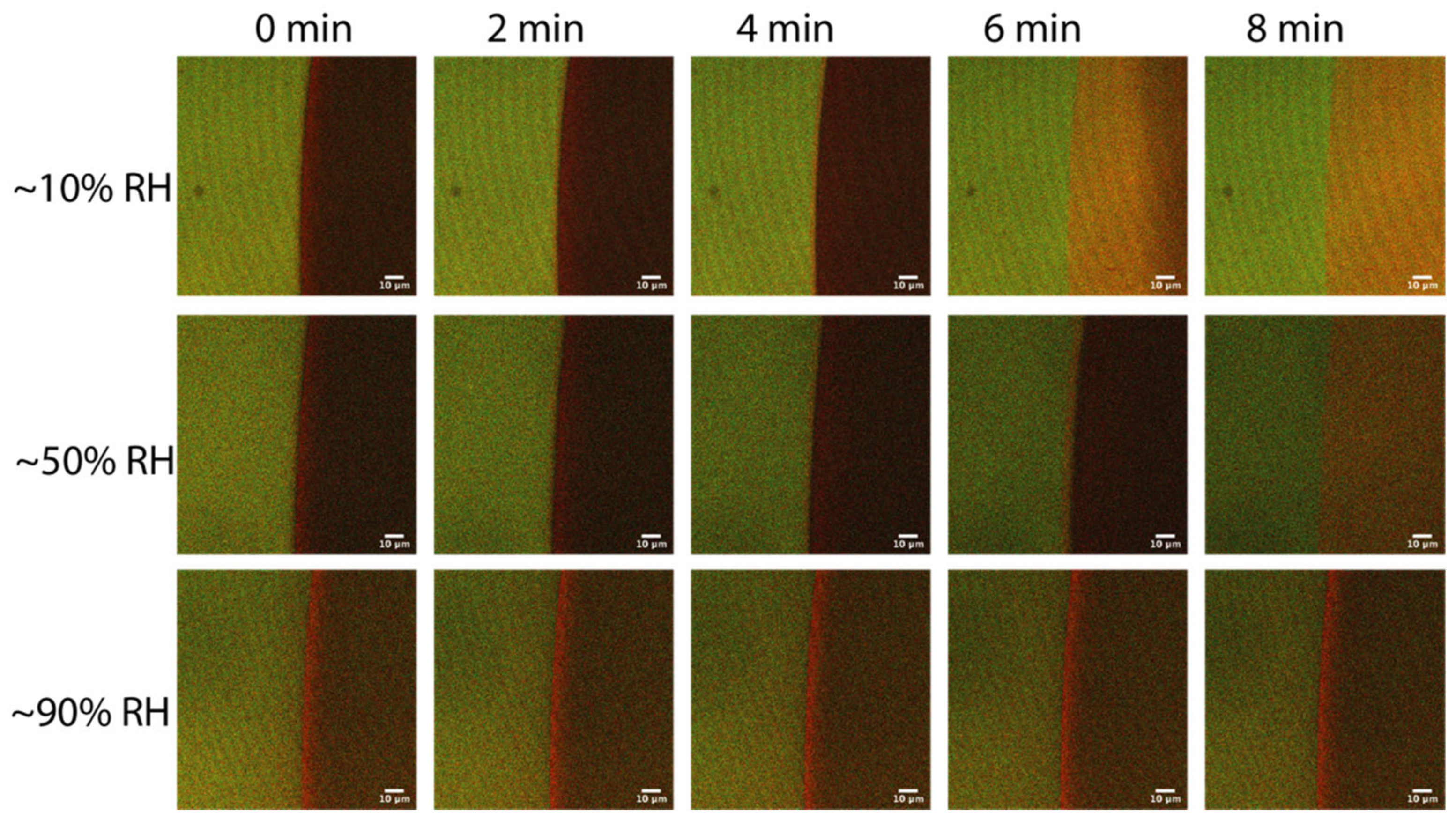
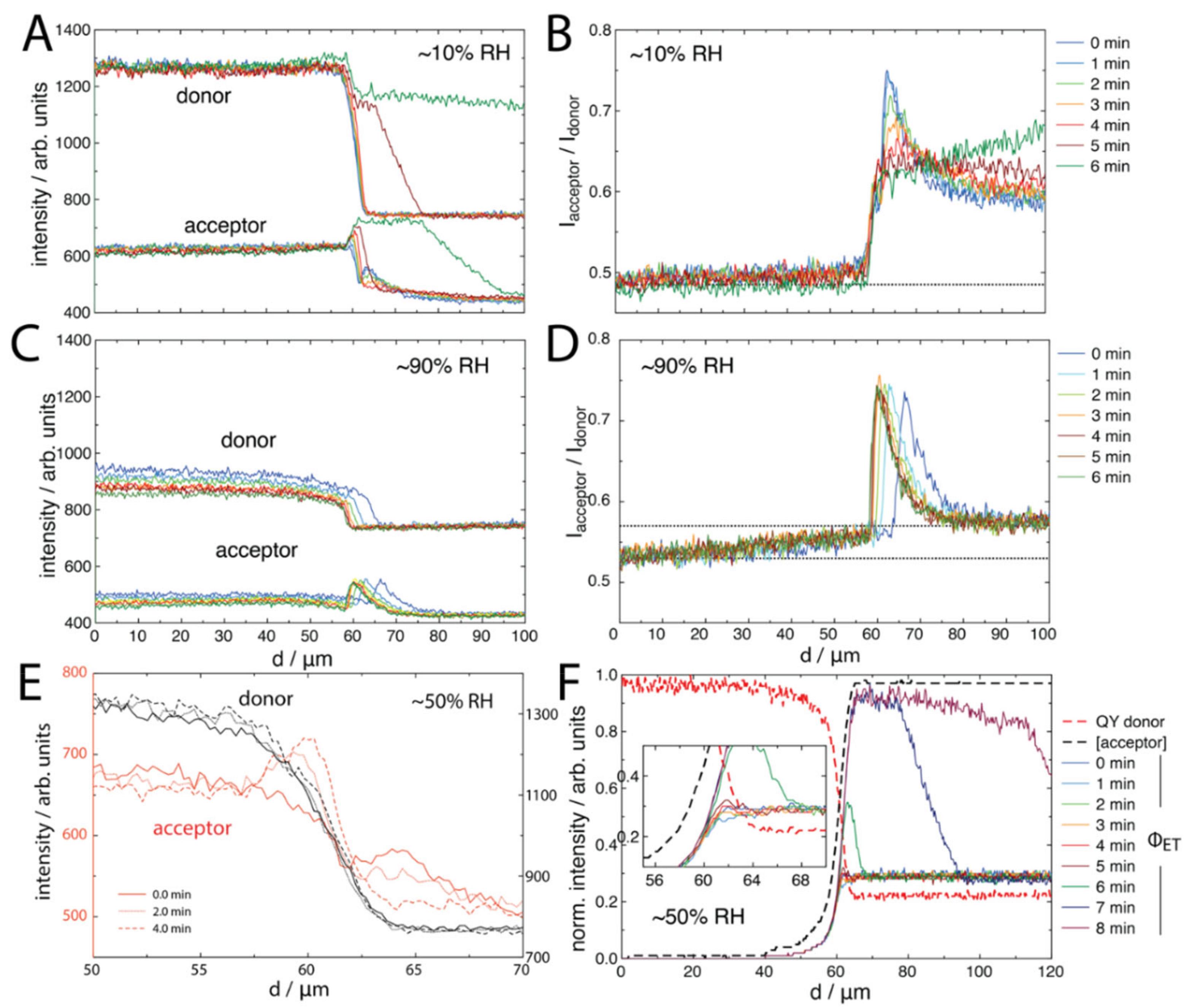
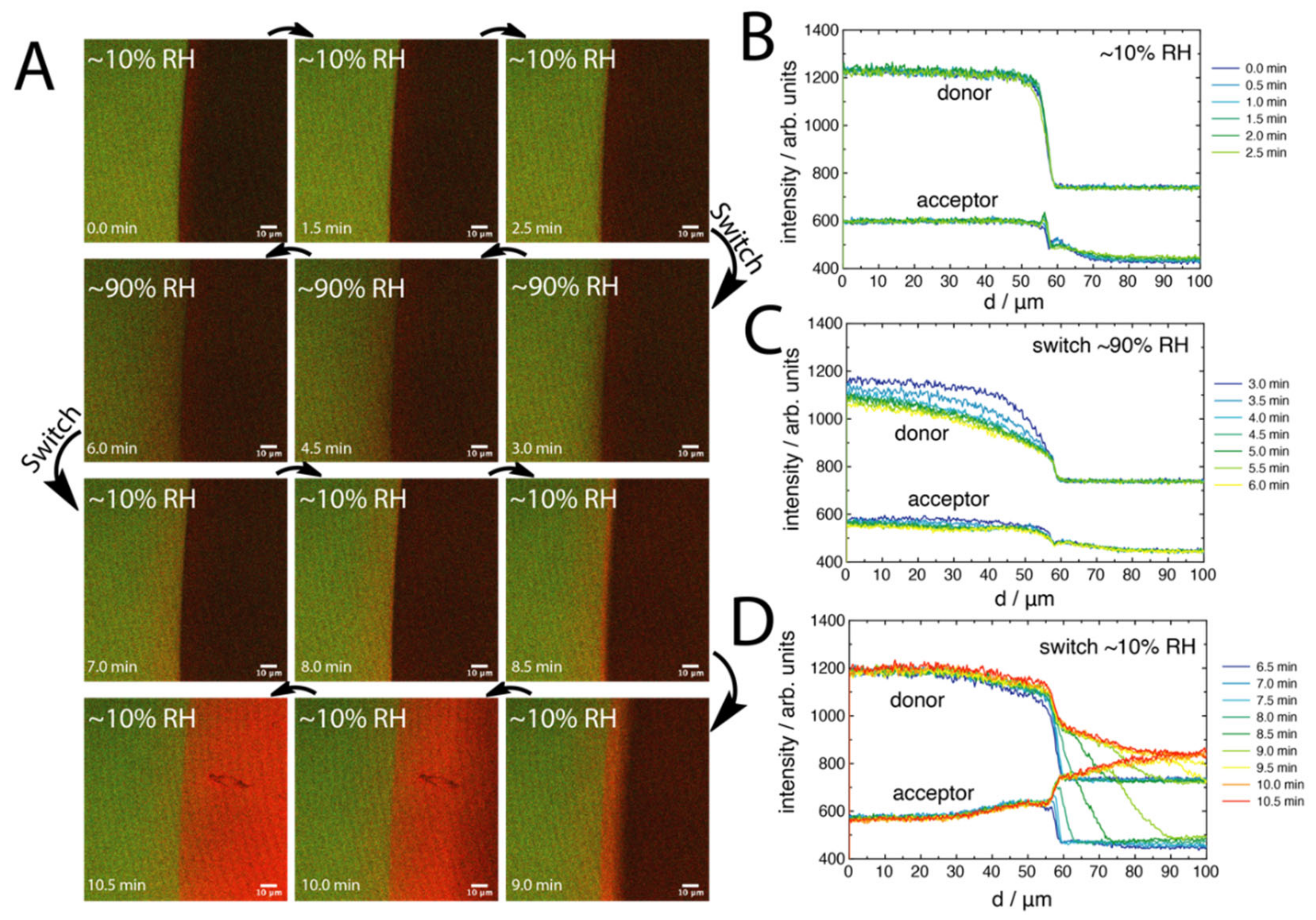
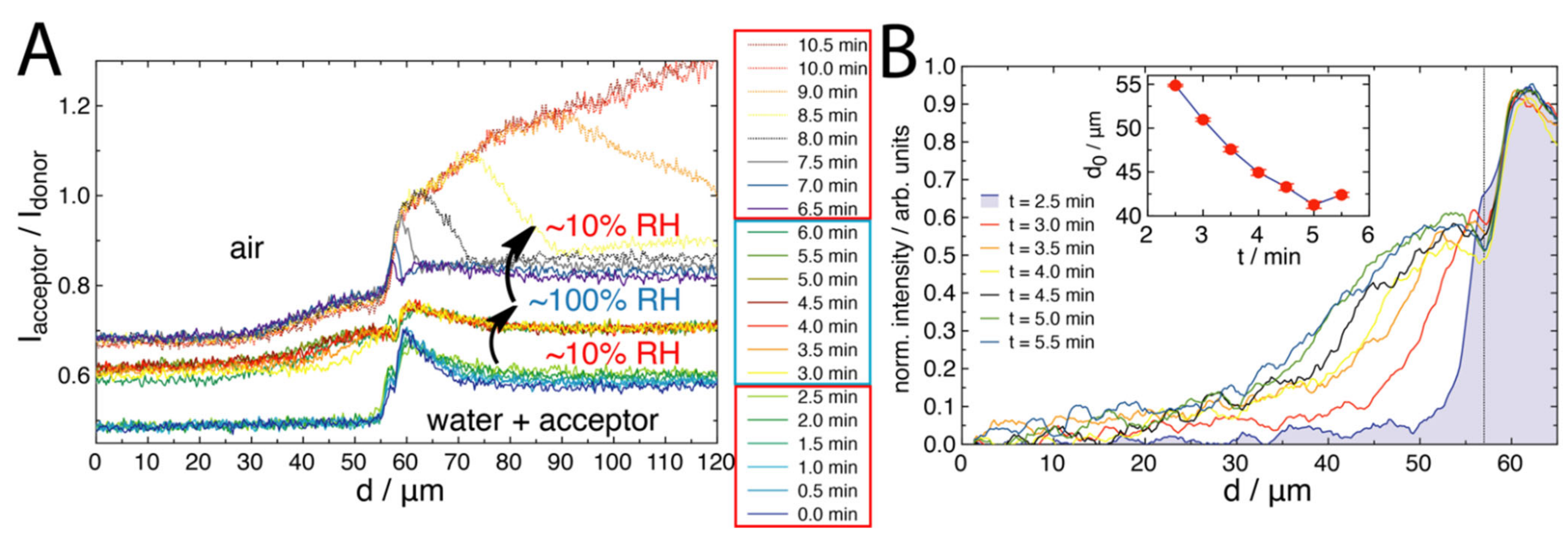
2.2. FRET of Aqueous Droplets on Polymer Brushes
3. Materials and Methods
3.1. Materials
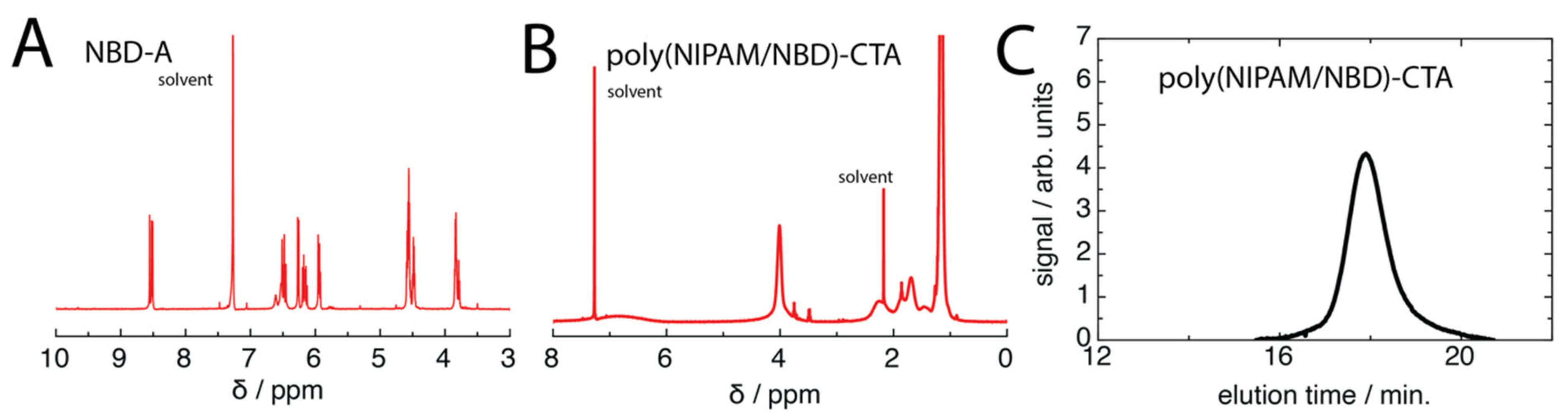
3.2. Synthesis of NBD-A
3.3. RAFT Polymer Synthesis
3.4. Grafting-To of Polymer Brushes
3.5. Fluorescence Measurements
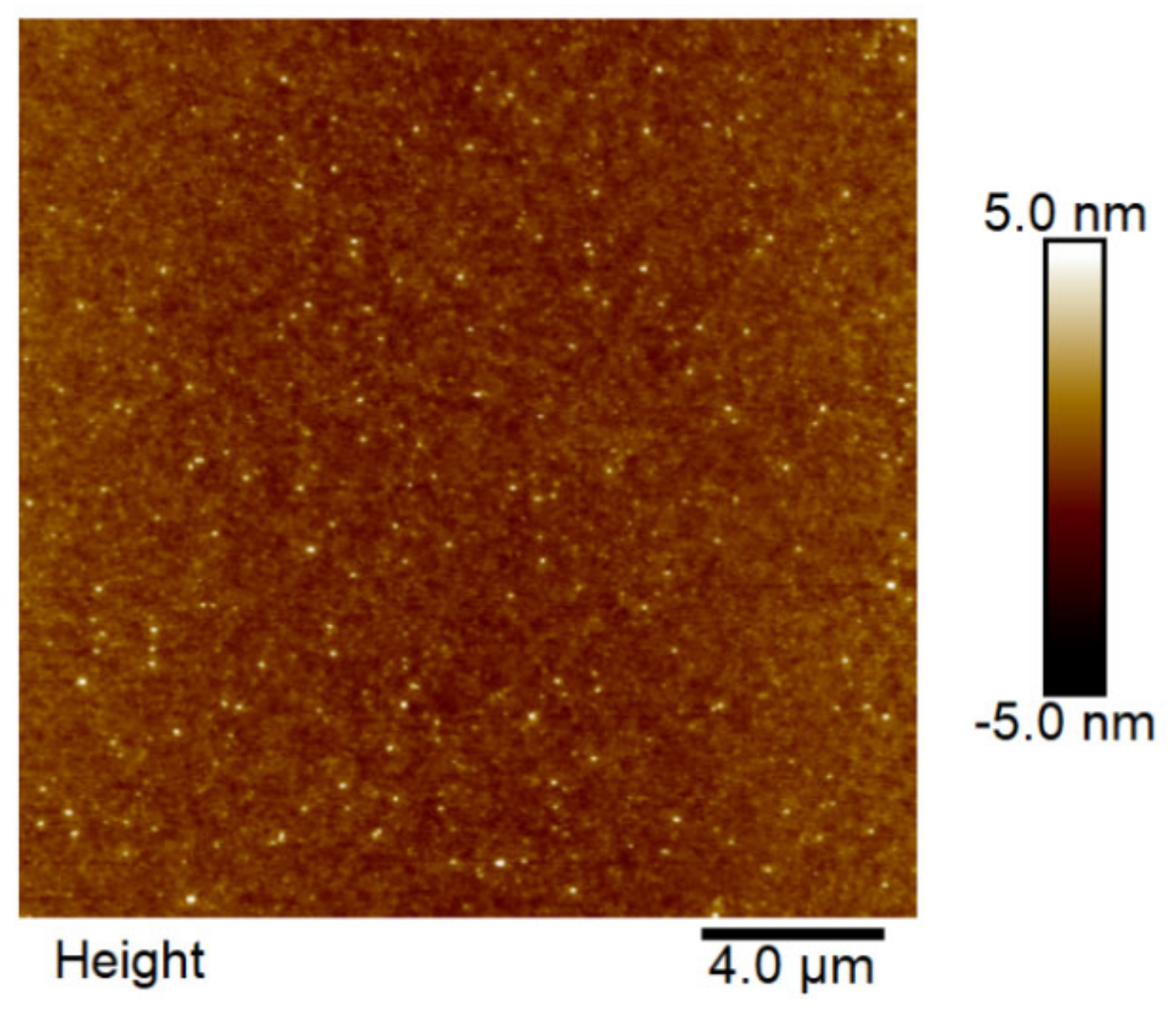
3.6. Atomic Force Microscopy (AFM)
3.7. Spectroscopic Ellipsometry
3.8. Confocal Laser Scanning Microscopy (CLSM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Kobayashi, M.; Terayama, Y.; Yamaguchi, H.; Terada, M.; Murakami, D.; Ishihara, K.; Takahara, A. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir 2012, 28, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Takahara, A. Tribological properties of hydrophilic polymer brushes under wet conditions. Chem. Rec. 2010, 10, 208–216. [Google Scholar] [CrossRef] [PubMed]
- De Groot, G.W.; Santonicola, M.G.; Sugihara, K.; Zambelli, T.; Reimhult, E.; Voros, J.; Vancso, G.J. Switching transport through nanopores with pH-responsive polymer brushes for controlled ion permeability. ACS Appl. Mater. Interfaces 2013, 5, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Speyer, K.; Pastorino, C. Pressure responsive gating in nanochannels coated by semiflexible polymer brushes. Soft Matter 2019, 15, 937–946. [Google Scholar] [CrossRef]
- Ng, G.; Li, M.; Yeow, J.; Jung, K.; Pester, C.W.; Boyer, C. Benchtop preparation of polymer brushes by SI-PET-RAFT: The effect of the polymer composition and structure on inhibition of a Pseudomonas biofilm. ACS Appl. Mater. Interfaces 2020, 12, 55243–55254. [Google Scholar] [CrossRef]
- Motornov, M.; Sheparovych, R.; Katz, E.; Minko, S. Chemical gating with nanostructured responsive polymer brushes: Mixed brush versus homopolymer brush. ACS Nano 2008, 2, 41–52. [Google Scholar] [CrossRef]
- Youssef, M.; Morin, A.; Aubret, A.; Sacanna, S.; Palacci, J. Rapid characterization of neutral polymer brush with a conventional zetameter and a variable pinch of salt. Soft Matter 2020, 16, 4274–4282. [Google Scholar] [CrossRef]
- Whiting, G.L.; Snaith, H.J.; Khodabakhsh, S.; Andreasen, J.W.; Breiby, D.W.; Nielsen, M.M.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Enhancement of charge-transport characteristics in polymer films using polymer brushes. Nano Lett. 2006, 6, 573–578. [Google Scholar] [CrossRef]
- Yameen, B.; Ali, M.; Neumann, R.; Ensinger, W.; Knoll, W.; Azzaroni, O. Single Conical Nanopores Displaying pH-Tunable Rectifying Characteristics. Manipulating Ionic Transport with Zwitterionic Polymer Brushes. J. Am. Chem. Soc. 2009, 131, 2070–2071. [Google Scholar] [CrossRef]
- Anthi, J.; Kolivoska, V.; Holubova, B.; Vaisocherova-Lisalova, H. Probing polymer brushes with electrochemical impedance spectroscopy: A mini review. Biomater. Sci. 2021, 9, 7379–7391. [Google Scholar] [CrossRef]
- Wetzler, S.P.; Miller, K.A.; Kisley, L.; Stanton, A.L.D.; Braun, P.V.; Bailey, R.C. Real-time measurement of polymer brush dynamics using silicon photonic microring resonators: Analyte partitioning and interior brush kinetics. Langmuir 2020, 36, 10351–10360. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Hidaka, M.; Murase, Y.; Akimoto, A.M.; Nagase, K.; Okano, T.; Yoshida, R. Self-oscillating polymer brushes. Angew. Chem. Int. Ed. Engl. 2013, 52, 7468–7471. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.C.; Robertson, M.L. Towards mimicking biological function with responsive surface-grafted polymer brushes. Curr. Opin. Solid State Mater. Sci. 2019, 23, 1–12. [Google Scholar] [CrossRef]
- Wang, S.; Jing, B.; Zhu, Y. Molecule motion at polymer brush interfaces from single-molecule experimental perspectives. J. Polym. Sci. B Polym. Phys. 2014, 52, 85–103. [Google Scholar] [CrossRef]
- Frost, R.; Debarre, D.; Jana, S.; Bano, F.; Schunemann, J.; Gorlich, D.; Richter, R.P. A method to quantify molecular diffusion within thin solvated polymer films: A case study on films of natively unfolded Nucleoporins. ACS Nano 2020, 14, 9938–9952. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, L.; Saez, A.E.; Pemberton, J.E. Flow Field penetration in thin nanoporous polymer films under laminar flow by Förster resonance energy transfer coupled with total internal reflectance fluorescence microscopy. Anal. Chem. 2015, 87, 11746–11754. [Google Scholar] [CrossRef]
- Wang, H.; Pemberton, J.E. Effect of solvent quality on laminar slip flow penetration of poly(N-isopropylacrylamide) films with an exploration of the mass transport mechanism. Langmuir 2017, 33, 7468–7478. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pemberton, J.E. Direct nanoscopic measurement of laminar slip flow penetration of deformable polymer brush surfaces: Synergistic effect of grafting density and solvent quality. Langmuir 2019, 35, 13646–13655. [Google Scholar] [CrossRef]
- Schubotz, S.; Honnigfort, C.; Nazari, S.; Fery, A.; Sommer, J.U.; Uhlmann, P.; Braunschweig, B.; Auernhammer, G.K. Memory effects in polymer brushes showing co-nonsolvency effects. Adv. Colloid Interface Sci. 2021, 294, 102442. [Google Scholar] [CrossRef]
- Besford, Q.A.; Merlitz, H.; Schubotz, S.; Yong, H.; Chae, S.; Schnepf, M.J.; Weiss, A.C.G.; Auernhammer, G.K.; Sommer, J.U.; Uhlmann, P.; et al. Mechanofluorescent polymer brush surfaces that spatially resolve surface solvation. ACS Nano 2022, 16, 3383–3393. [Google Scholar] [CrossRef]
- Besford, Q.A.; Yong, H.; Merlitz, H.; Christofferson, A.J.; Sommer, J.U.; Uhlmann, P.; Fery, A. FRET-integrated polymer brushes for spatially resolved sensing of changes in polymer conformation. Angew. Chem. Int. Ed. Engl. 2021, 60, 16600–16606. [Google Scholar] [CrossRef] [PubMed]
- Shiomoto, S.; Higuchi, H.; Yamaguchi, K.; Takaba, H.; Kobayashi, M. Spreading dynamics of a precursor film of ionic liquid or water on a micropatterned polyelectrolyte brush surface. Langmuir 2021, 37, 3049–3056. [Google Scholar] [CrossRef] [PubMed]
- Leong, F.Y.; Le, D.-V. Dynamics of a droplet on a polymer brush in channel flow. Phys. Fluids 2021, 33, 043303. [Google Scholar] [CrossRef]
- Ritsema van Eck, G.C.; Chiappisi, L.; de Beer, S. Fundamentals and applications of polymer brushes in air. ACS Appl. Polym. Mater. 2022. [Google Scholar] [CrossRef]
- Eggenberger, O.M.; Ying, C.; Mayer, M. Surface coatings for solid-state nanopores. Nanoscale 2019, 11, 19636–19657. [Google Scholar] [CrossRef] [Green Version]
- Khalil, A.; Rostami, P.; Auernhammer, G.K.; Andrieu-Brunsen, A. Mesoporous coatings with simultaneous light-triggered transition of water imbibition and droplet coalescence. Adv. Mater. Interfaces 2021, 8, 2100252. [Google Scholar] [CrossRef]
- Fery-Forgues, S.; Fayet, J.P.; Lopez, A. Drastic changes in the fluorescence properties of NBD probes with the polarity of the medium: Involvement of a TICT state? J. Photochem. Photobiol. A Chem. 1993, 70, 229–243. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, C.; Qi, L.; Liu, M.; Dong, P.; Jiang, Q.; Yang, X.; Mu, X.; Mao, L. Intracellular temperature sensing by a ratiometric fluorescent polymer thermometer. J. Mater. Chem. B 2014, 2, 7544–7550. [Google Scholar] [CrossRef]
- Yu, Y.; Kieviet, B.D.; Liu, F.; Siretanu, I.; Kutnyanszky, E.; Vancso, G.J.; de Beer, S. Stretching of collapsed polymers causes an enhanced dissipative response of PNIPAM brushes near their LCST. Soft Matter 2015, 11, 8508–8516. [Google Scholar] [CrossRef]
- Yong, H.; Bittrich, E.; Uhlmann, P.; Fery, A.; Sommer, J.-U. Co-nonsolvency transition of poly(N-isopropylacrylamide) brushes in a series of binary mixtures. Macromolecules 2019, 52, 6285–6293. [Google Scholar] [CrossRef]
- Rauch, S.; Eichhorn, K.-J.; Oertel, U.; Stamm, M.; Kuckling, D.; Uhlmann, P. Temperature responsive polymer brushes with clicked rhodamine B: Synthesis, characterization and swelling dynamics studied by spectroscopic ellipsometry. Soft Matter 2012, 8, 10260. [Google Scholar] [CrossRef] [Green Version]
- Farinha, J.P.S.; Martinho, J.M.G. Resonance energy transfer in polymer nanodomains. J. Phys. Chem. C 2008, 112, 10591–10601. [Google Scholar] [CrossRef]
- Pathak, P.; London, E. Measurement of lipid nanodomain (raft) formation and size in sphingomyelin/POPC/cholesterol vesicles shows TX-100 and transmembrane helices increase domain size by coalescing preexisting nanodomains but do not induce domain formation. Biophys. J. 2011, 101, 2417–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farinha, J.P.S.; Winnik, M.A. Imaging an oil droplet under a latex film. Langmuir 1999, 15, 7088–7091. [Google Scholar] [CrossRef]
- Farinha, J.P.S.; Vorobyova, O.; Winnik, M.A. An energy tranfer study of the interface thickness in blends of poly(butyl methacrylate) and poly(2-ethylhexyl methacrylate). Macromolecules 2000, 33, 5863–5873. [Google Scholar] [CrossRef]
- Gendron, P.O.; Avaltroni, F.; Wilkinson, K.J. Diffusion coefficients of several rhodamine derivatives as determined by pulsed field gradient-nuclear magnetic resonance and fluorescence correlation spectroscopy. J. Fluoresc. 2008, 18, 1093–1101. [Google Scholar] [CrossRef]
- Besford, Q.A.; Liu, M.; Christofferson, A.J. Stabilizing dipolar interactions drive specific molecular structure at the water liquid-vapor interface. J. Phys. Chem. B 2018, 122, 8309–8314. [Google Scholar] [CrossRef]
- Poisson, J.; Polgar, A.M.; Fromel, M.; Pester, C.W.; Hudson, Z.M. Preparation of patterned and multilayer thin films for organic electronics via oxygen-tolerant SI-PET-RAFT. Angew. Chem. Int. Ed. Engl. 2021, 60, 19988–19996. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besford, Q.A.; Schubotz, S.; Chae, S.; Özdabak Sert, A.B.; Weiss, A.C.G.; Auernhammer, G.K.; Uhlmann, P.; Farinha, J.P.S.; Fery, A. Molecular Transport within Polymer Brushes: A FRET View at Aqueous Interfaces. Molecules 2022, 27, 3043. https://doi.org/10.3390/molecules27093043
Besford QA, Schubotz S, Chae S, Özdabak Sert AB, Weiss ACG, Auernhammer GK, Uhlmann P, Farinha JPS, Fery A. Molecular Transport within Polymer Brushes: A FRET View at Aqueous Interfaces. Molecules. 2022; 27(9):3043. https://doi.org/10.3390/molecules27093043
Chicago/Turabian StyleBesford, Quinn A., Simon Schubotz, Soosang Chae, Ayşe B. Özdabak Sert, Alessia C. G. Weiss, Günter K. Auernhammer, Petra Uhlmann, José Paulo S. Farinha, and Andreas Fery. 2022. "Molecular Transport within Polymer Brushes: A FRET View at Aqueous Interfaces" Molecules 27, no. 9: 3043. https://doi.org/10.3390/molecules27093043
APA StyleBesford, Q. A., Schubotz, S., Chae, S., Özdabak Sert, A. B., Weiss, A. C. G., Auernhammer, G. K., Uhlmann, P., Farinha, J. P. S., & Fery, A. (2022). Molecular Transport within Polymer Brushes: A FRET View at Aqueous Interfaces. Molecules, 27(9), 3043. https://doi.org/10.3390/molecules27093043