Multi-Element Analysis and Origin Discrimination of Panax notoginseng Based on Inductively Coupled Plasma Tandem Mass Spectrometry (ICP-MS/MS)
Abstract
:1. Introduction
2. Results
2.1. Analytical Performance of the ICP-MS/MS for P. notoginseng
2.2. The Accumulation Dynamics of Elements during the Growth of P. notoginseng
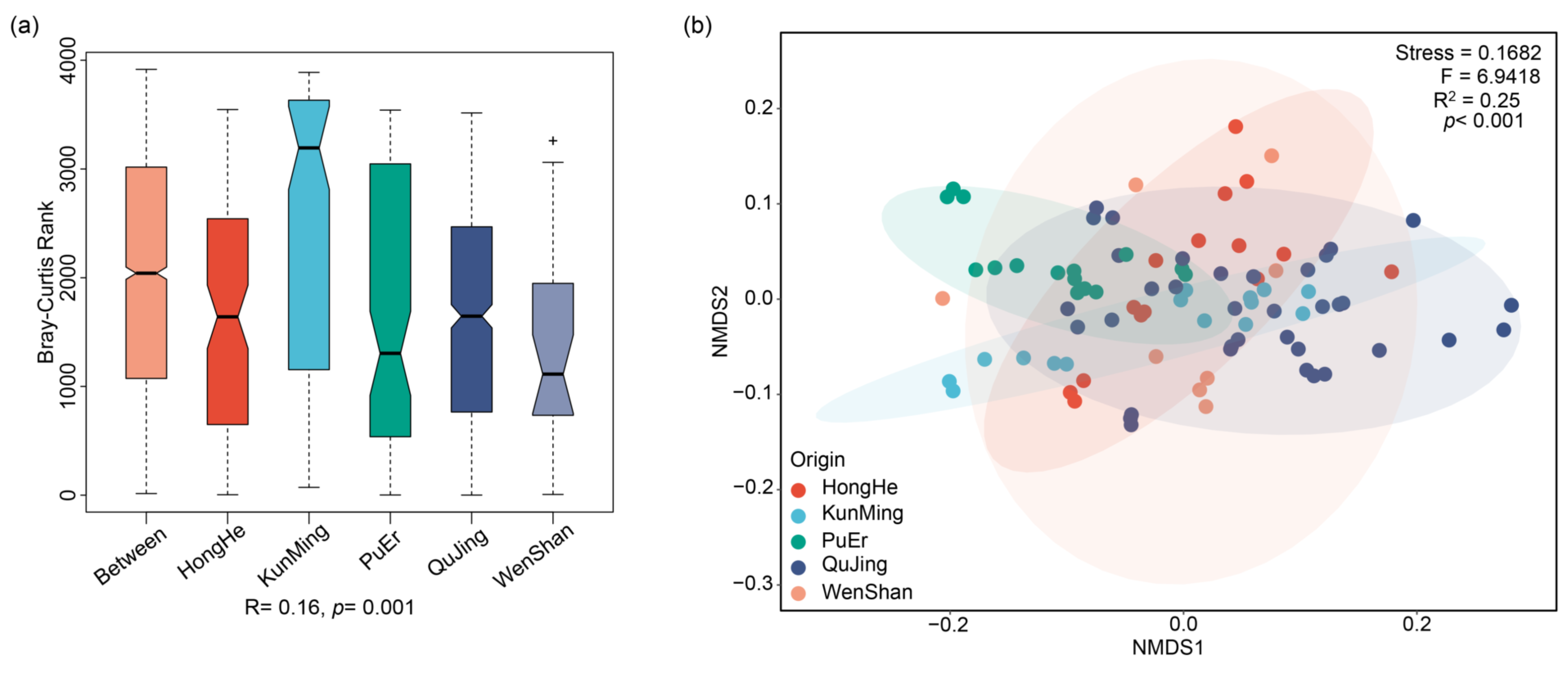
2.3. Modeling Analysis of P. notoginseng from Different Origins
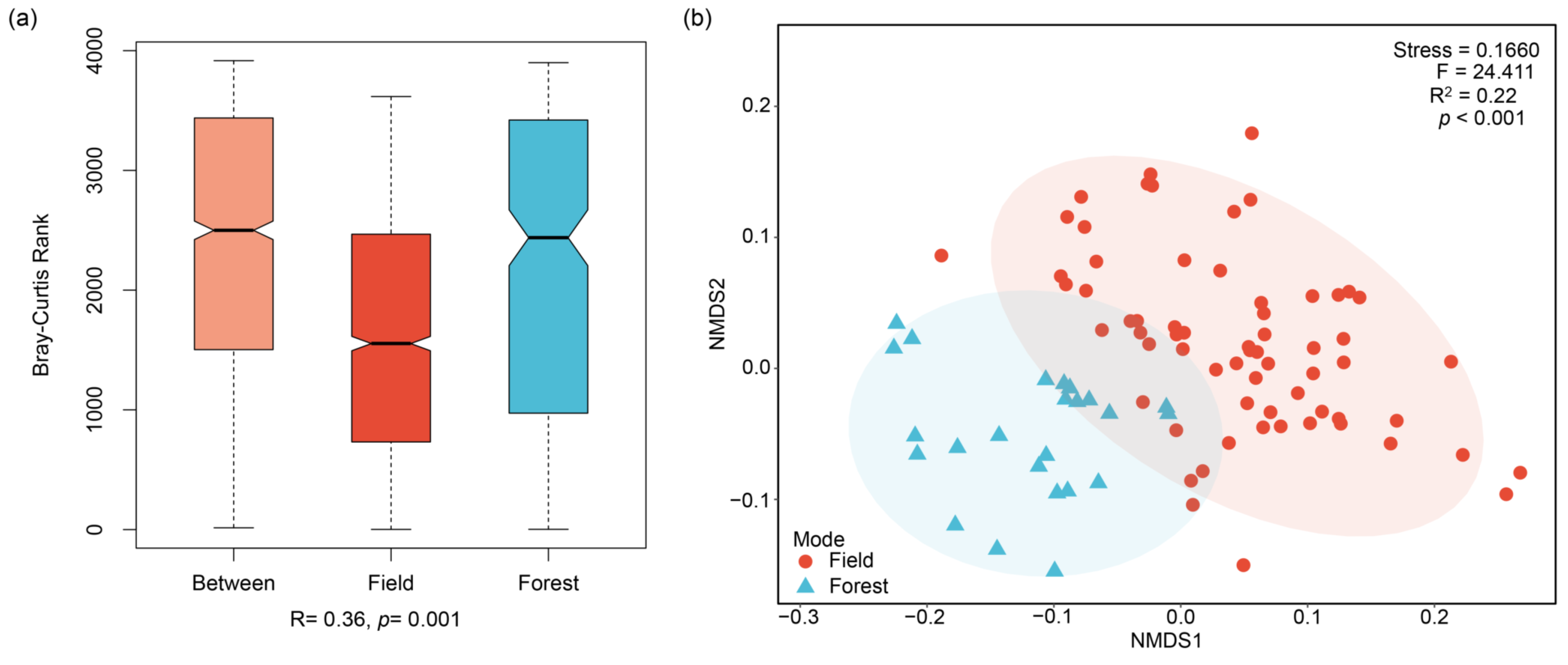
2.4. Modeling Analysis of P. notoginseng from Different Cultivation Models
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Collection and Pretreatment of P. notoginseng
4.3. Microwave-Assisted Acid Digestion Procedure
4.4. ICP-MS/MS Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yue, J.; Zuo, Z.; Huang, H.; Wang, Y. Application of Identification and Evaluation Techniques for Ethnobotanical Medicinal Plant of Genus Panax: A Review. Crit. Rev. Anal. Chem. 2021, 51, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Y.; Qin, X.; Wen, P.; Liu, J.; Yang, M. Global gene expression analysis using RNA-seq reveals the new roles of Panax notoginseng saponins in ischemic cardiomyocytes. J. Ethnopharmacol. 2021, 268, 113639. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Liao, Y.; Tian, G.; Li, H.; Jia, Y.; Zhang, H.; Tan, Z.; Li, X.; Deng, W.; Liu, K.; et al. Panax notoginseng saponins inhibit zymosan a induced atherosclerosis by suppressing integrin expression, FAK activation and NF-κB translocation. J. Ethnopharmacol. 2011, 138, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-M.; Chen, M.-H.; Han, F.; Wang, J.W.; Tu, Y.X. Role of Bioactive Constituents of Panax notoginseng in the Modulation of Tumorigenesis: A Potential Review for the Treatment of Cancer. Front. Pharmacol. 2021, 12, 738914. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Liu, P.; Wang, Y.; Huang, C.; Lan, L.; Yang, Y.; Cui, X. Stereoscopic cultivation of Panax notoginseng: A new approach to overcome the continuous cropping obstacle. Ind. Crop. Prod. 2018, 126, 38–47. [Google Scholar] [CrossRef]
- Wang, Y.L.; Cui, X.M.; Lan, L.; Chen, W.D.; Li, R.B.; Wang, C.X.; Yang, X.Y.; Liu, D.H.; Yang, Y. Light and temperature and their effects on photosynthesis characteristics of stereoscopic cultivation in Panax notoginseng. China J. Chin. Mater. Med. 2015, 15, 2921–2929. [Google Scholar] [CrossRef]
- Ye, C.; Fang, H.Y.; Liu, H.J.; Yang, M.; Zhu, S.S. Current status of soil sickness research on Panax notoginseng in Yunnan, China. Allelopath. J. 2019, 47, 1–14. [Google Scholar] [CrossRef]
- Wu, H.; Xia, J.; Qin, X.; Wu, H.; Zhang, S.; Zhao, Y.; Rensing, C.; Lin, W. Underlying Mechanism of Wild Radix pseudostellariae in Tolerance to Disease Under the Natural Forest Cover. Front. Microbiol. 2020, 11, 1142. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Ginsenosides analysis of New Zealand-grown forest Panax ginseng by LC-QTOF-MS/MS. J. Ginseng Res. 2020, 44, 552–562. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.; Dou, D.; Huang, L. The distinct of chemical profiles of mountainous forest cultivated ginseng and garden ginseng based on ginsenosides and oligosaccharides. J. Food Compos. Anal. 2021, 104, 104165. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Y.; Zhang, J.; Wang, G.; Chen, L.; Zhang, M.; Liu, T.; Liu, X.; Lu, C. Determining the geographical origin of Chinese green tea by linear discriminant analysis of trace metals and rare earth elements: Taking Dongting Biluochun as an example. Food Control 2016, 59, 714–720. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, G.; Wang, X.; Zhang, Y.; Zhao, J.; Yu, Y.-J.; Wang, H. Chemometric discrimination of the geographical origin of licorice in China by untargeted metabolomics. Food Chem. 2022, 380, 132235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Dong, Y.; Shi, Y.C.; Yang, H.; Zhang, J.Q.; Khan, M.R.; Deng, S.; He, G.P.; He, Q.; Lv, Y.P.; et al. CRISPR-Cas12-Based Rapid Authentication of Halal Food. J. Agric. Food Chem. 2021, 35, 10321–10328. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Liu, J.; Li, H.T.; Zhang, T.; Dong, Y.; Deng, S.; Lv, Y.P.; He, Q.; Deng, R.J. CRISPR-Cas sys-tem meets DNA barcoding: Development of a universal nucleic acid test for food authentication. Sens. Actuators B Chem. 2022, 353, 131138. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Yin, G.; Musyimi, Z. Spatial distribution of geographical indications for agricultural products and their drivers in China. Environ. Earth. Sci. 2016, 75, 612. [Google Scholar] [CrossRef]
- Ndraha, N.; Hsiao, H.-I.; Chih Wang, W.C. Comparative study of imported food control systems of Tai-wan, Japan, the United States, and the European Union. Food Control 2017, 78, 331–341. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Ge, Y. C/N/H/O stable isotope analysis for determining the geographical origin of American ginseng (Panax quinquefolius). J. Food Compos. Anal. 2021, 96, 103756. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Gao, J.; Zhao, Y.-F.; Wen, H.-S.; Zhang, J.-J.; Zhang, A.; Yuan, C.-L. Geographical Origin Classification of Chinese Wines Based on Carbon and Oxygen Stable Isotopes and Elemental Profiles. J. Food Prot. 2020, 83, 1323–1334. [Google Scholar] [CrossRef]
- Qian, L.; Zuo, F.; Liu, H.; Zhang, C.; Chi, X.; Zhang, D. Determination of Geographical Origin of Wuchang Rice with the Geographical Indicator by Multielement Analysis. J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Ng, W.L.; Bay, L.J.; Goh, G.; Ang, T.H.; Kong, K.; Chew, P.; Koh, S.P.; Ch’ng, A.L.; Phang, H.; Chiew, P. Multivariate statistical analysis of stable isotope signatures and element concentrations to differentiate the geographical origin of retail milk sold in Singapore. Food Control 2021, 123, 107736. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.; Ma, Y.; Shao, F.; Huang, J.; Wu, H.; Tian, L. Origin Identification of the Sauce-Flavor Chinese Baijiu by Organic Acids, Trace Elements, and the Stable Carbon Isotope Ratio. J. Food Qual. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Fang, Y.; Shen, F.; Mao, B.; Zou, Y.; Li, P.; Pei, F.; Hu, Q. Multiangle discrimination of geographical origin of rice based on analysis of mineral elements and characteristic volatile components. Int. J. Food Sci. Technol. 2018, 53, 2088–2096. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Li, H. Discrimination between wild-grown and cultivated Gastrodia elata by near-infrared spectroscopy and chemometrics. Vib. Spectrosc. 2021, 113, 103203. [Google Scholar] [CrossRef]
- Arndt, M.; Drees, A.; Ahlers, C.; Fischer, M. Determination of the Geographical Origin of Walnuts (Juglans regia L.) Using Near-Infrared Spectroscopy and Chemometrics. Foods 2020, 9, 1860. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zeng, G.; Wang, B.; Xiao, J.; Zhang, L.; Cheng, W.; Wang, H.; Li, H.; Shi, X. Discrimination of the geographic origins and varieties of wine grapes using high-throughput sequencing assisted by a random forest model. LWT 2021, 145, 111333. [Google Scholar] [CrossRef]
- Orellana, S.; Johansen, A.M.; Gazis, C. Geographic classification of U.S. Washington State wines using elemental and water isotope composition. Food Chem. X 2019, 1, 100007. [Google Scholar] [CrossRef]
- Leder, R.; Petric, I.V.; Jusup, J.; Banović, M. Geographical Discrimination of Croatian Wines by Stable Isotope Ratios and Multielemental Composition Analysis. Front. Nutr. 2021, 8, 625613. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, L.; Zhao, Y.; Xiong, F.; Yuan, Y.; Nie, J.; Huang, L.; Yang, J. Stable isotope and multi-element profiling of Cassiae Semen tea combined with chemometrics for geographical discrimination. J. Food Compos. Anal. 2022, 107, 104359. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, C.; Zhang, J.; Qin, H.; Ma, G.; Liu, X.; Yin, J. Accurate discrimination of tea from multiple geographical regions by combining multi-elements with multivariate statistical analysis. J. Food Meas. Charaterization 2020, 14, 3361–3370. [Google Scholar] [CrossRef]
- Endaye, M.; Atlabachew, M.; Mehari, B.; Alemayehu, M.; Mengistu, D.A.; Kerisew, B. Combining Multi-Element Analysis with Statistical Modeling for Tracing the Origin of Green Coffee Beans from Amhara Region, Ethiopia. Biol. Trace Elem. Res. 2020, 195, 669–678. [Google Scholar] [CrossRef]
- Beltrán, M.; Sánchez-Astudillo, M.; Aparicio, R.; García-González, D.L. Geographical traceability of virgin olive oils from south-western Spain by their multi-elemental composition. Food Chem. 2015, 169, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.C.; Mazumder, D.; Gadd, P.; Doubleday, Z.A. Tracking the provenance of octopus using isotopic and multi-elemental analysis. Food Chem. 2022, 371, 131133. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, Y.; Sun, Y.; Duan, Y. Elemental characteristics of Sanqi (Panax notoginseng) in Yunnan province of China: Multielement determination by ICP-AES and ICP-MS and statistical analysis. Microchem. J. 2019, 146, 931–939. [Google Scholar] [CrossRef]
- Yin, Q.; Zhu, Y.; Ju, S.; Liao, W.; Yang, Y. Rapid determination of copper and lead in Panax notoginseng by magnetic solid-phase extraction and flame atomic absorption spectrometry. Res. Chem. Intermed. 2016, 42, 4985–4998. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, W.; Yang, J.; Min, Y.; Huang, Z. Determination of Arsenic in Panax notoginseng by Hydride Generation Atomic Fluorescence Spectrometry. Asian J. Chem. 2013, 25, 2959–2961. [Google Scholar] [CrossRef]
- Zheng, Y.; Xia, P.; Chai, W.; Liang, Z.; Yan, K. Accumulation dynamics of elements in Panax notoginseng during its whole growing seasons. Ind. Crop. Prod. 2020, 158, 113023. [Google Scholar] [CrossRef]
- Leyden, E.; Farkas, J.; Gilbert, S.; Hutson, J.; Mosley, L.M. A simple and rapid ICP-MS/MS determination of sulfur isotope ratios (34S/32S) in complex natural waters: A new tool for tracing seawater intrusion in coastal systems. Talanta 2021, 235, 122708. [Google Scholar] [CrossRef]
- Hirata, J.; Itabashi, D.; Aimoto, M. Determination of Ultra-trace Tellurium in Steel by ID-ICP-MS/MS with Liquid-Liquid Extraction. Anal. Sci. 2021, 37, 1757–1763. [Google Scholar] [CrossRef]
- Simpson, A.; Gilbert, S.; Tamblyn, R.; Hand, M.; Spandler, C.; Gillespie, J.; Nixon, A.; Glorie, S. In Situ Lu Hf geochronology of garnet, apatite and xenotime by LA ICP MS/MS. Chem. Geol. 2021, 577, 120299. [Google Scholar] [CrossRef]
- Ariyama, K.; Nishida, T.; Noda, T.; Kadokura, M.; Yasui, A. Effects of fertilization, crop year, variety, and provenance factors on mineral concentrations in onions. J. Agric. Food Chem. 2006, 9, 3341–3350. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, S.; Zhang, Z. Relationship between multi-element composition in tea leaves and in provenance soils for geographical traceability. Food Control 2017, 76, 82–87. [Google Scholar] [CrossRef]
- Carini, F.; Bengtsson, G. Post-deposition transport of radionuclides in fruit. J. Environ. Radioact. 2001, 52, 215–236. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, S.C. Plant Physiology, 8th ed.; Higher Education Press: Beijing, China, 2019; ISBN 978-7-04-050044-8. [Google Scholar]
- Kotsiantis, S.B.; Zaharakis, I.D.; Pintelas, P.E. Machine learning: A review of classification and combining techniques. Artif. Intell. Rev. 2006, 3, 159–190. [Google Scholar] [CrossRef]
- Yuan, Y.; Hu, G.; Chen, T.; Zhao, M.; Zhang, Y.; Li, Y.; Xu, X.; Shao, S.; Zhu, J.; Wang, Q.; et al. Improved Discrimination for Brassica Vegetables Treated with Agricultural Fertilizers Using a Combined Chemometric Approach. J. Agric. Food Chem. 2016, 28, 5633–5643. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zeng, Y.-T.; Zhao, X.; Tong, H.-R. Improved geographical origin discrimination for tea using ICP-MS and ICP-OES techniques in combination with chemometric approach. J. Sci. Food Agric. 2020, 100, 3507–3516. [Google Scholar] [CrossRef]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J., III. The Collinearity Problem in Linear Regression. The Partial Least Squares (PLS) Approach to Generalized Inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yao, L.; Xia, Z.; Gao, Y.; Gong, Z. Geographical discrimination and adulteration analysis for edible oils using two-dimensional correlation spectroscopy and convolutional neural networks (CNNs). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 118973. [Google Scholar] [CrossRef]
- Drivelos, S.A.; Danezis, G.P.; Halagarda, M.; Popek, S.; Georgiou, C.A. Geographical origin and botanical type honey authentication through elemental metabolomics via chemometrics. Food Chem. 2021, 338, 127936. [Google Scholar] [CrossRef]
- Wu, H.; Lin, G.; Tian, L.; Yan, Z.; Yi, B.; Bian, X.; Jin, B.; Xie, L.; Zhou, H.; Rogers, K.M. Origin verification of French red wines using isotope and elemental analyses coupled with chemometrics. Food Chem. 2021, 339, 127760. [Google Scholar] [CrossRef]
- Qi, J.; Li, Y.; Zhang, C.; Wang, C.; Wang, J.; Guo, W.; Wang, S. Geographic origin discrimination of pork from different Chinese regions using mineral elements analysis assisted by machine learning techniques. Food Chem. 2021, 337, 127779. [Google Scholar] [CrossRef] [PubMed]
- Boser, B.E.; Guyon, I.M.; Vapnik, V.N. A Training Algorithm for Optimal Margin Classifiers. In Proceedings of the COLT 92: The 5th Annual Workshop on Computational Learning Theory, Pittsburgh, PA, USA, 27–29 July 1992; Haussler, D., Ed.; ACM Press: New York, NY, USA, 1992; pp. 144–152. [Google Scholar]
- Kabir, M.H.; Guindo, M.L.; Chen, R.; Liu, F. Geographic Origin Discrimination of Millet Using Vis-NIR Spectroscopy Combined with Machine Learning Techniques. Foods 2021, 10, 2767. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Liu, X.; Wang, Y.; Ren, X.; Dong, Y.; Song, R.; Ma, J.; Fan, Q.; Wei, J.; et al. Fast discrimination and quantification analysis of Curcumae Radix from four botanical origins using NIR spectroscopy coupled with chemometrics tools. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Wang, Y.-J.; Yan, H.; Chang, X.-W.; Zhou, G.-S.; Zhu, L.; Liu, P.; Guo, S.; Dong, T.T.X.; Duan, J.-A. Rapid Geographical Origin Identification and Quality Assessment of Angelicae Sinensis Radix by FT-NIR Spectroscopy. J. Anal. Methods Chem. 2021, 2021, 127779. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Z.; Qian, Q.; Song, W.; Rogers, K.M.; Rao, Q.; Wang, S.; Zhang, Q.; Shao, S.; Tian, M.L.; et al. Isotope chemometrics determines farming methods and geographical origin of vegetables from Yangtze River Delta Region, China. Food Chem. 2021, 342, 128379. [Google Scholar] [CrossRef]
- Nasr, E.G.; Epova, E.N.; de Diego, A.; Souissi, R.; Hammami, M.; Abderrazak, H.; Donard, O.F.X. Trace Elements Analysis of Tunisian and European Extra Virgin Olive Oils by ICP-MS and Chemometrics for Geographical Discrimination. Foods 2021, 1, 82. [Google Scholar] [CrossRef]
- Nguyen-Quang, T.; Bui-Quang, M.; Truong-Ngoc, M. Rapid Identification of Geographical Origin of Commercial Soybean Marketed in Vietnam by ICP-MS. J. Anal. Methods Chem. 2021, 2021, 9. [Google Scholar] [CrossRef]
- de Souza, M.J.; Barciela-Alonso, M.C.; Aboal-Somoza, M.; Bermejo-Barrera, P. Determination of the Trace Element Contents of Fruit Juice Samples by ICP OES and ICP-MS. Braz. J. Anal. Chem. 2021, 9, 49–61. [Google Scholar] [CrossRef]
- Gómez-Rubio, V. ggplot2-Elegant Graphics for Data Analysis, 2nd ed. ; J. Stat. Soft. 2017, 77, 1–3. [Google Scholar] [CrossRef] [Green Version]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 1 January 2020).
- Kassambara, A. ggpubr R Package: ggplot2-Based Publication Ready Plots. Available online: https://rpkgs.datanovia.com/ggpubr (accessed on 1 January 2020).
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. Available online: https://rdrr.io/cran/pheatmap/ (accessed on 1 January 2020).
- Kumar, A. Pre-processing and Modelling using Caret Package in R. Int. J. Comput. Appl. 2018, 181, 39–42. [Google Scholar] [CrossRef]

| Modes | Region | Sensitivity | Specificity | Balanced Accuracy | Accuracy | p-Value [Acc > NIR] | Kappa Value | Area under ROC Curve (AUC) |
|---|---|---|---|---|---|---|---|---|
| PLS-DA | HongHe | 0.7500 | 0.8696 | 0.8098 | 0.4815 | 2.76 × 10−1 | 0.3844 | 0.8750 |
| KunMing | 1.0000 | 0.5652 | 0.7862 | |||||
| PuEr | 0.5000 | 0.9565 | 0.7283 | |||||
| QuJing | 0.0910 | 1.0000 | 0.5455 | |||||
| WenShan | 0.7500 | 1.0000 | 0.8750 | |||||
| LDA | HongHe | 0.2500 | 0.9565 | 0.6033 | 0.7037 | 1.78 × 10−3 | 0.6129 | 0.7881 |
| KunMing | 0.7500 | 0.9565 | 0.8533 | |||||
| PuEr | 1.0000 | 0.9565 | 0.9783 | |||||
| QuJing | 0.6364 | 0.8750 | 0.7557 | |||||
| WenShan | 1.0000 | 0.8696 | 0.9348 | |||||
| RF | HongHe | 0.7500 | 0.9565 | 0.8533 | 0.8889 | 2.89 × 10−7 | 0.8472 | 0.8750 |
| KunMing | 0.7500 | 1.0000 | 0.8750 | |||||
| PuEr | 0.7500 | 1.0000 | 0.8750 | |||||
| QuJing | 1.0000 | 0.8750 | 0.9375 | |||||
| WenShan | 1.0000 | 1.0000 | 1.0000 | |||||
| NNs | HongHe | 1.0000 | 0.9565 | 0.9783 | 0.9259 | 2.32 × 10−8 | 0.902 | 0.9750 |
| KunMing | 0.7500 | 0.9565 | 0.8533 | |||||
| PuEr | 1.0000 | 1.0000 | 1.0000 | |||||
| QuJing | 0.9091 | 1.0000 | 0.9545 | |||||
| WenShan | 1.0000 | 1.0000 | 1.0000 | |||||
| kNNs | HongHe | 1.0000 | 0.9565 | 0.9783 | 0.6667 | 5.82 × 10−3 | 0.595 | 0.8920 |
| KunMing | 0.7500 | 0.8261 | 0.7880 | |||||
| PuEr | 1.0000 | 0.9565 | 0.9783 | |||||
| QuJing | 0.2727 | 1.0000 | 0.6364 | |||||
| WenShan | 1.0000 | 0.8696 | 0.9348 | |||||
| NB | HongHe | 0.7500 | 0.9565 | 0.8533 | 0.7407 | 4.63 × 10−4 | 0.6655 | 0.9071 |
| KunMing | 1.0000 | 0.8261 | 0.9130 | |||||
| PuEr | 0.7500 | 1.0000 | 0.8750 | |||||
| QuJing | 0.6364 | 0.9375 | 0.7869 | |||||
| WenShan | 0.7500 | 0.9565 | 0.8533 | |||||
| SVMs | HongHe | 1.0000 | 0.9565 | 0.9783 | 0.8889 | 2.89 × 10−7 | 0.8548 | 0.9625 |
| KunMing | 0.7500 | 0.9130 | 0.8315 | |||||
| PuEr | 1.0000 | 1.0000 | 1.0000 | |||||
| QuJing | 0.8182 | 1.0000 | 0.9091 | |||||
| WenShan | 1.0000 | 1.0000 | 1.0000 |
| Modes | Sensitivity | Specificity | Accuracy | p-Value [Acc > NIR] | Kappa Value | Area under ROC Curve (AUC) |
|---|---|---|---|---|---|---|
| PLS-DA | 1.0000 | 0.8571 | 0.9615 | 0.0030 | 0.8976 | 0.9286 |
| LR | 0.7368 | 1.0000 | 0.8077 | 0.2605 | 0.8261 | 0.8684 |
| LDA | 0.6842 | 1.0000 | 0.8571 | 0.4258 | 0.5385 | 0.8421 |
| RF | 1.0000 | 0.8571 | 0.9615 | 0.0030 | 0.8976 | 0.9286 |
| NNs | 0.8947 | 0.8571 | 0.8846 | 0.0531 | 0.7194 | 0.8759 |
| kNNs | 1.0000 | 0.5714 | 0.0531 | 0.0531 | 0.6609 | 0.7857 |
| NB | 0.7368 | 1.0000 | 0.8077 | 0.26045 | 0.6012 | 0.8684 |
| SVMs | 1.0000 | 1.0000 | 1.0000 | 0.0003 | 1.0000 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Liu, J.; Zhang, Q.; Li, J.; Wu, Z.; Wang, X.; Xie, Y.; Zhao, J.; Shi, R.; Ma, X.; et al. Multi-Element Analysis and Origin Discrimination of Panax notoginseng Based on Inductively Coupled Plasma Tandem Mass Spectrometry (ICP-MS/MS). Molecules 2022, 27, 2982. https://doi.org/10.3390/molecules27092982
Ji C, Liu J, Zhang Q, Li J, Wu Z, Wang X, Xie Y, Zhao J, Shi R, Ma X, et al. Multi-Element Analysis and Origin Discrimination of Panax notoginseng Based on Inductively Coupled Plasma Tandem Mass Spectrometry (ICP-MS/MS). Molecules. 2022; 27(9):2982. https://doi.org/10.3390/molecules27092982
Chicago/Turabian StyleJi, Chao, Jinyu Liu, Qin Zhang, Juan Li, Zhiqiang Wu, Xingyu Wang, Yuxin Xie, Jiangchao Zhao, Rui Shi, Xing Ma, and et al. 2022. "Multi-Element Analysis and Origin Discrimination of Panax notoginseng Based on Inductively Coupled Plasma Tandem Mass Spectrometry (ICP-MS/MS)" Molecules 27, no. 9: 2982. https://doi.org/10.3390/molecules27092982
APA StyleJi, C., Liu, J., Zhang, Q., Li, J., Wu, Z., Wang, X., Xie, Y., Zhao, J., Shi, R., Ma, X., Khan, M. R., Busquets, R., He, X., Zhu, Y., Zhu, S., & Zheng, W. (2022). Multi-Element Analysis and Origin Discrimination of Panax notoginseng Based on Inductively Coupled Plasma Tandem Mass Spectrometry (ICP-MS/MS). Molecules, 27(9), 2982. https://doi.org/10.3390/molecules27092982