Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells
Abstract
:1. Introduction
2. Results
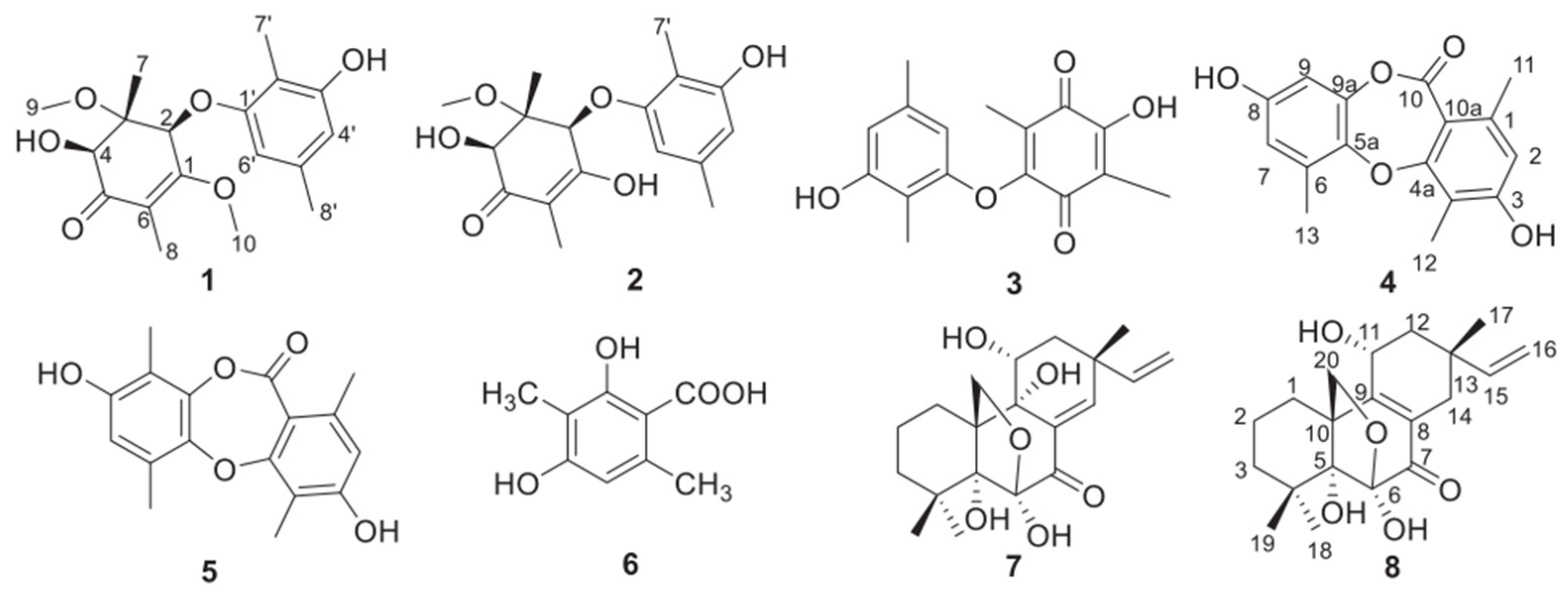
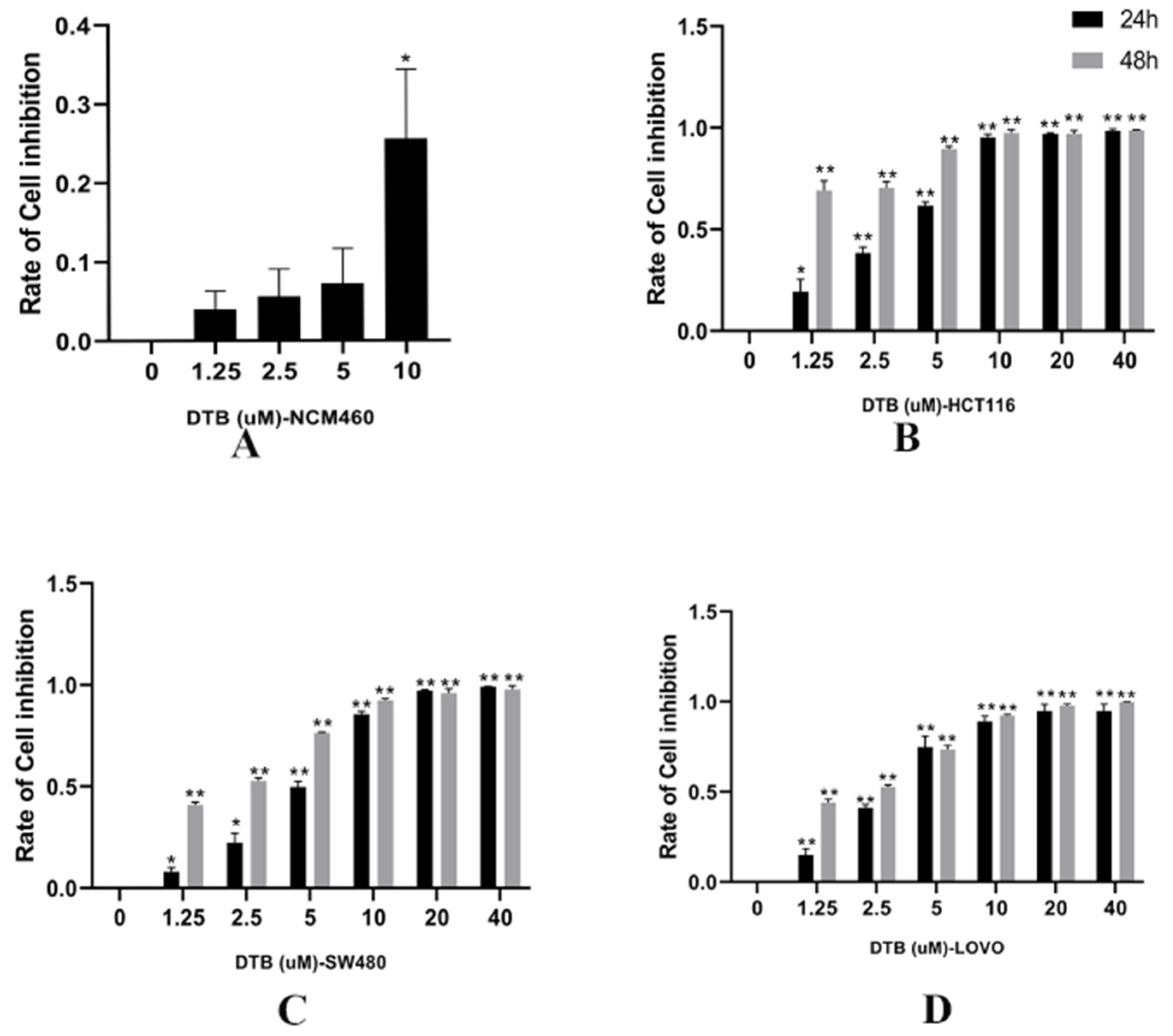
2.1. Inhibition of Colon Cancer Cell Proliferation by DTB
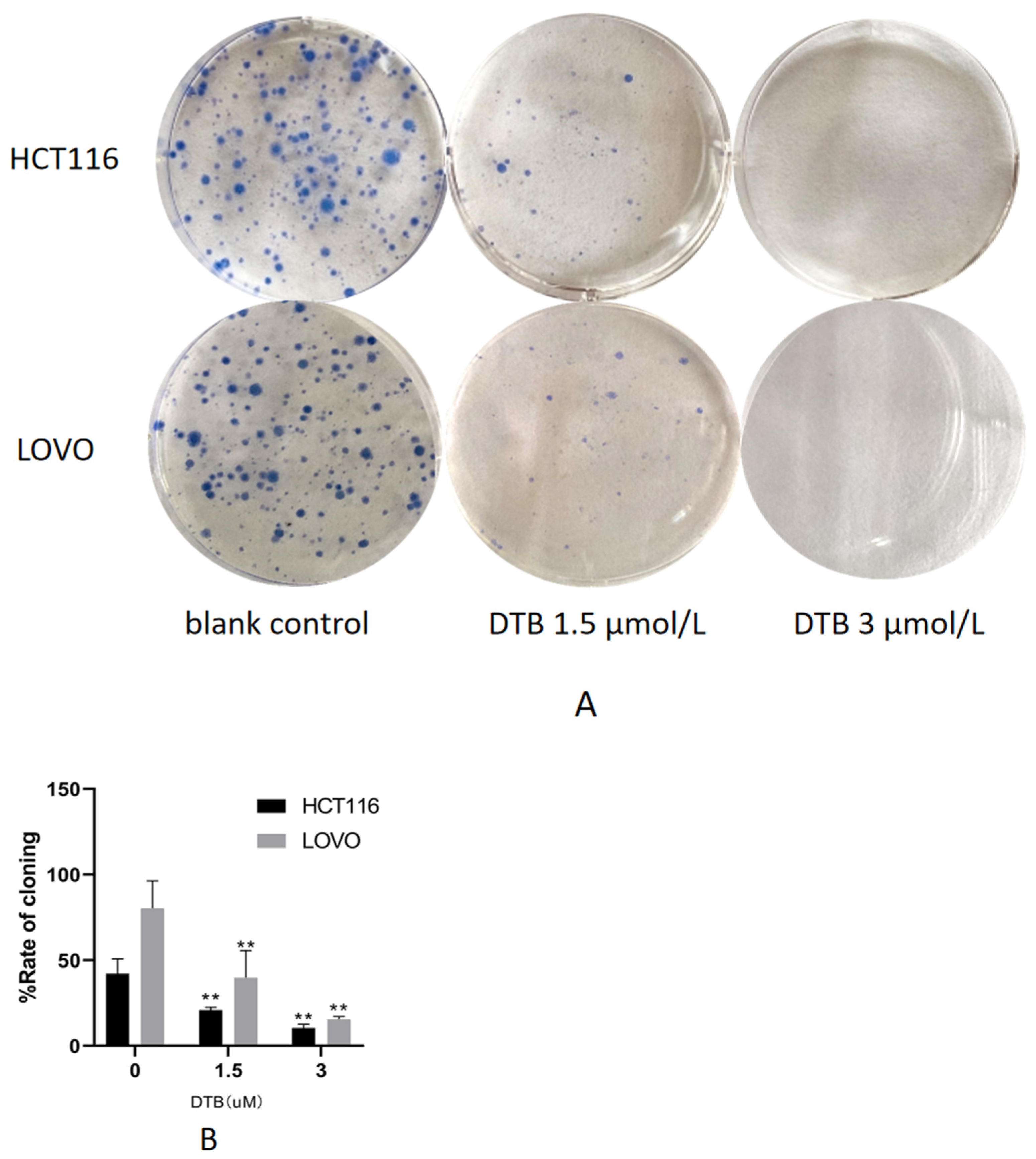
2.2. DTB Inhibits Cell Clonogenic Ability
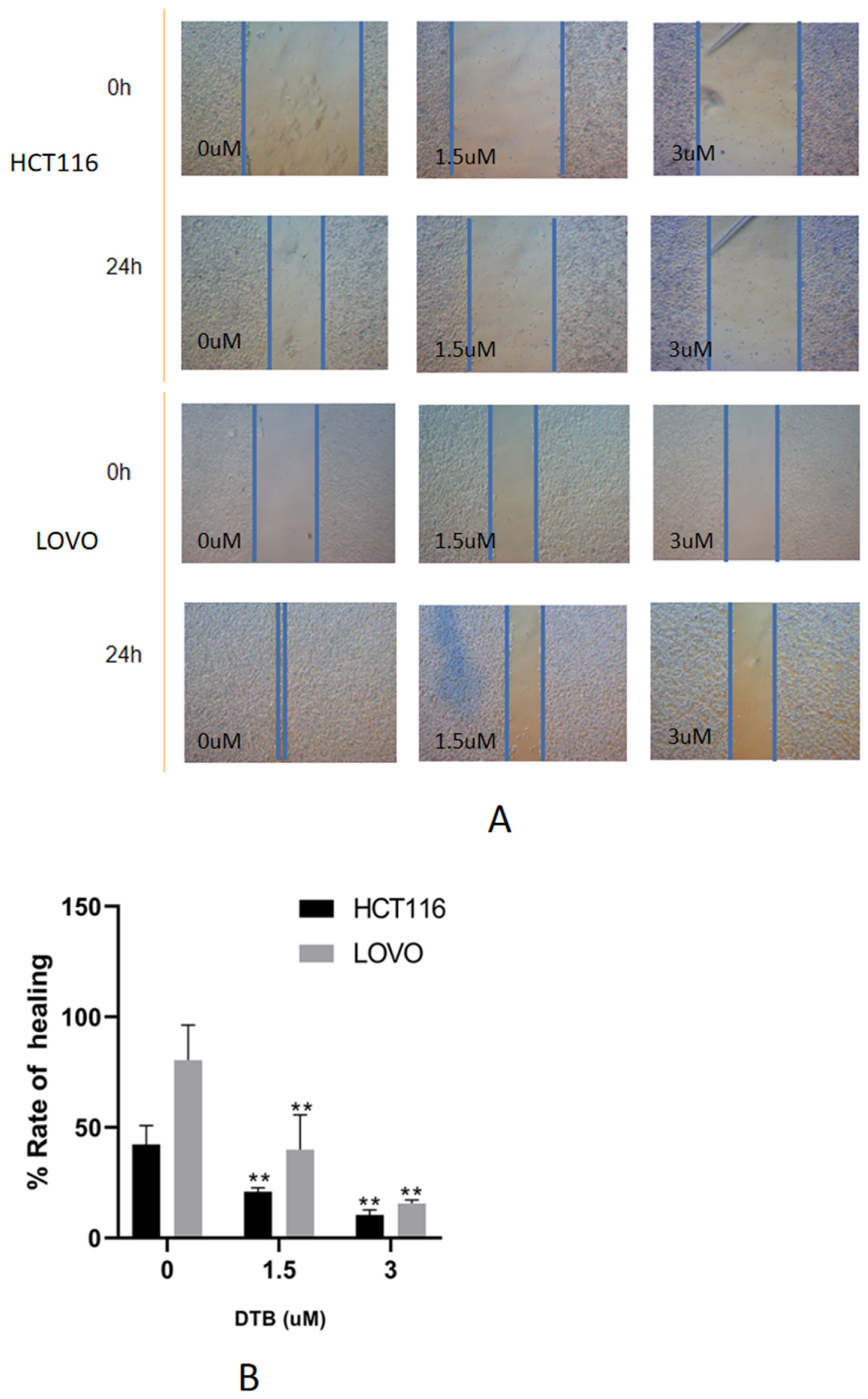
2.3. DTB Can Inhibit Migration of Colon Cancer Cells
2.4. Apoptotic Morphology Was Assessed by Fluorescence Microscopy
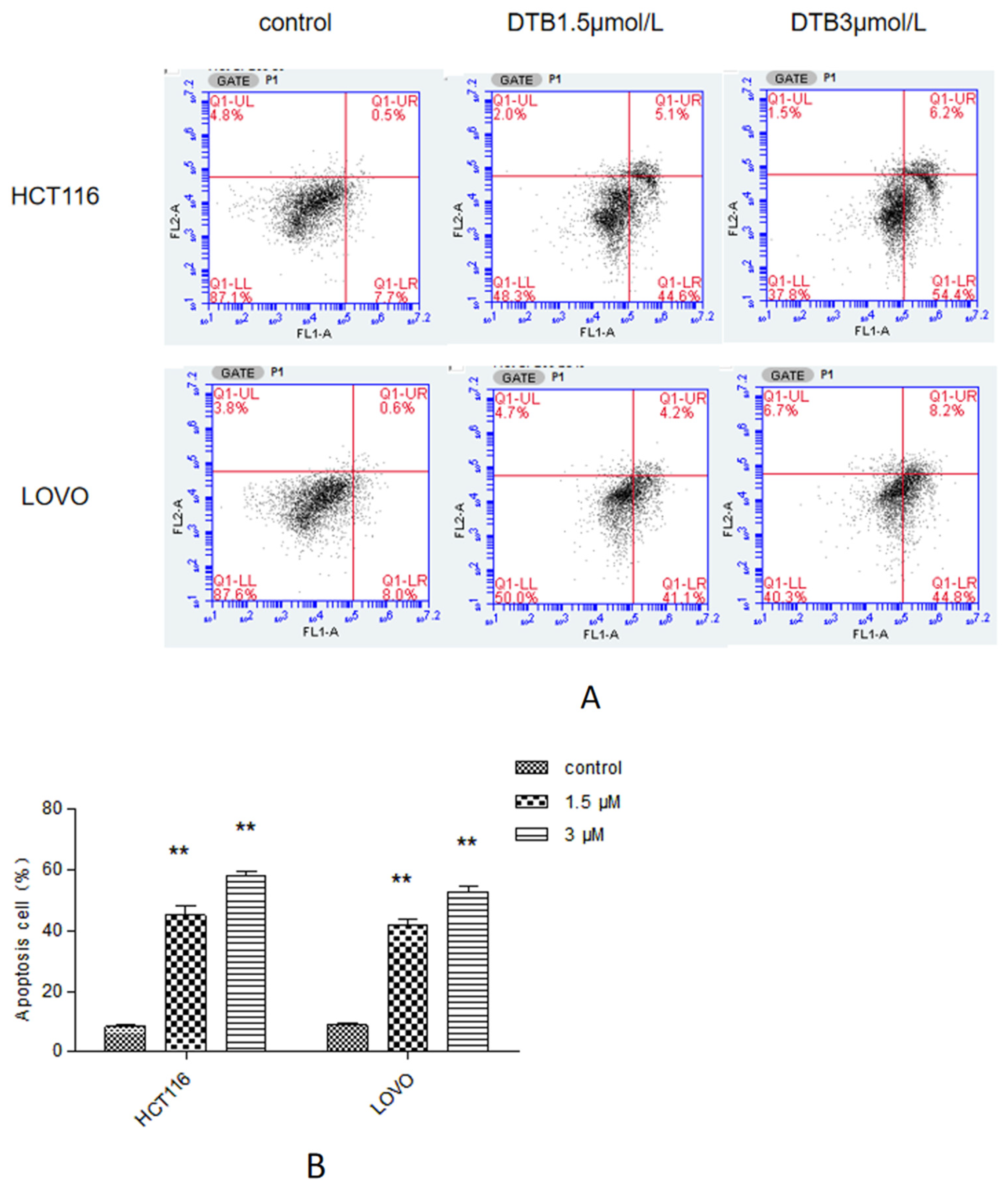
2.5. Apoptosis Was Assessed by Flow Cytometry
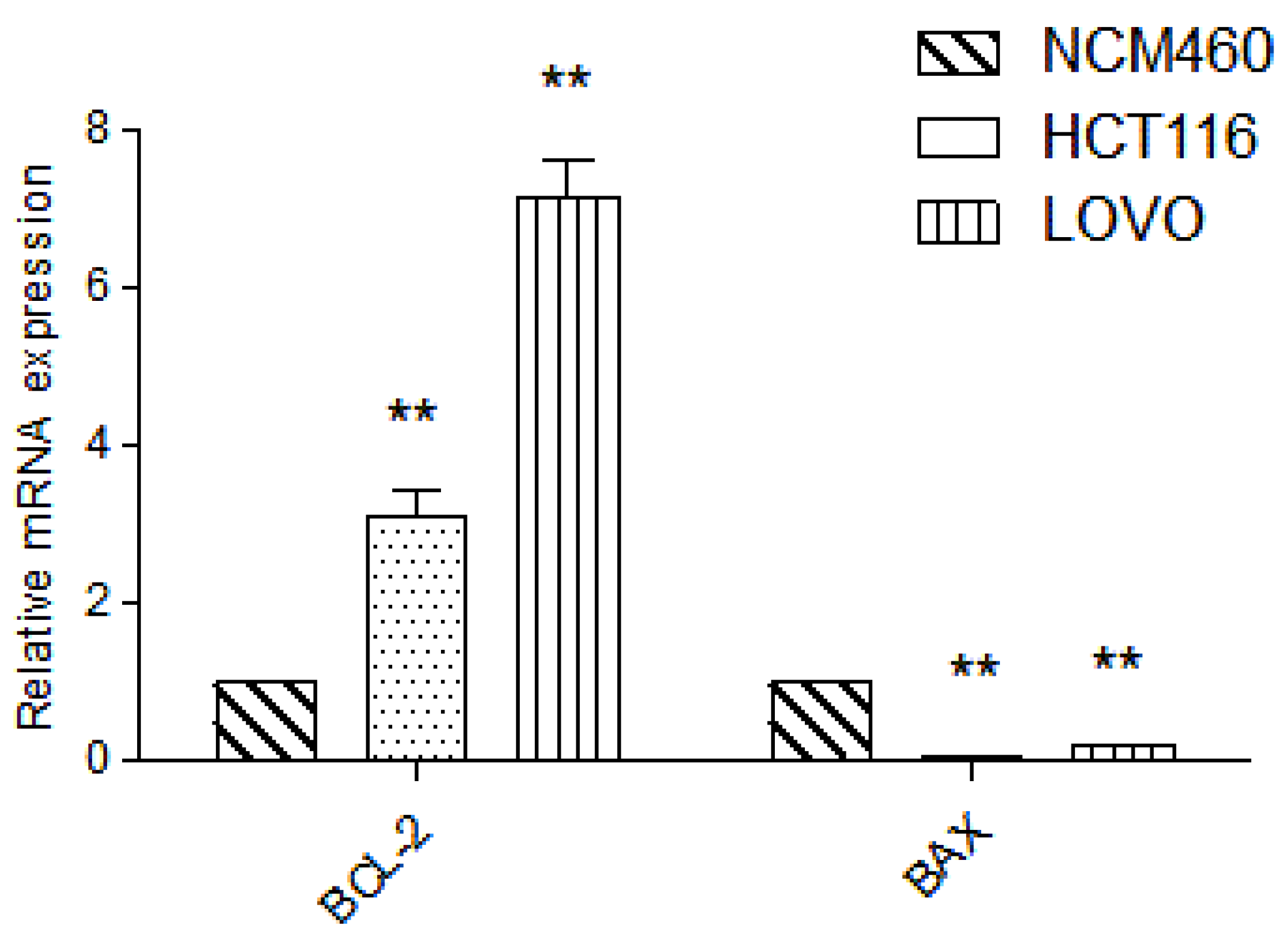
2.6. Detection of Target Gene by RT-PCR
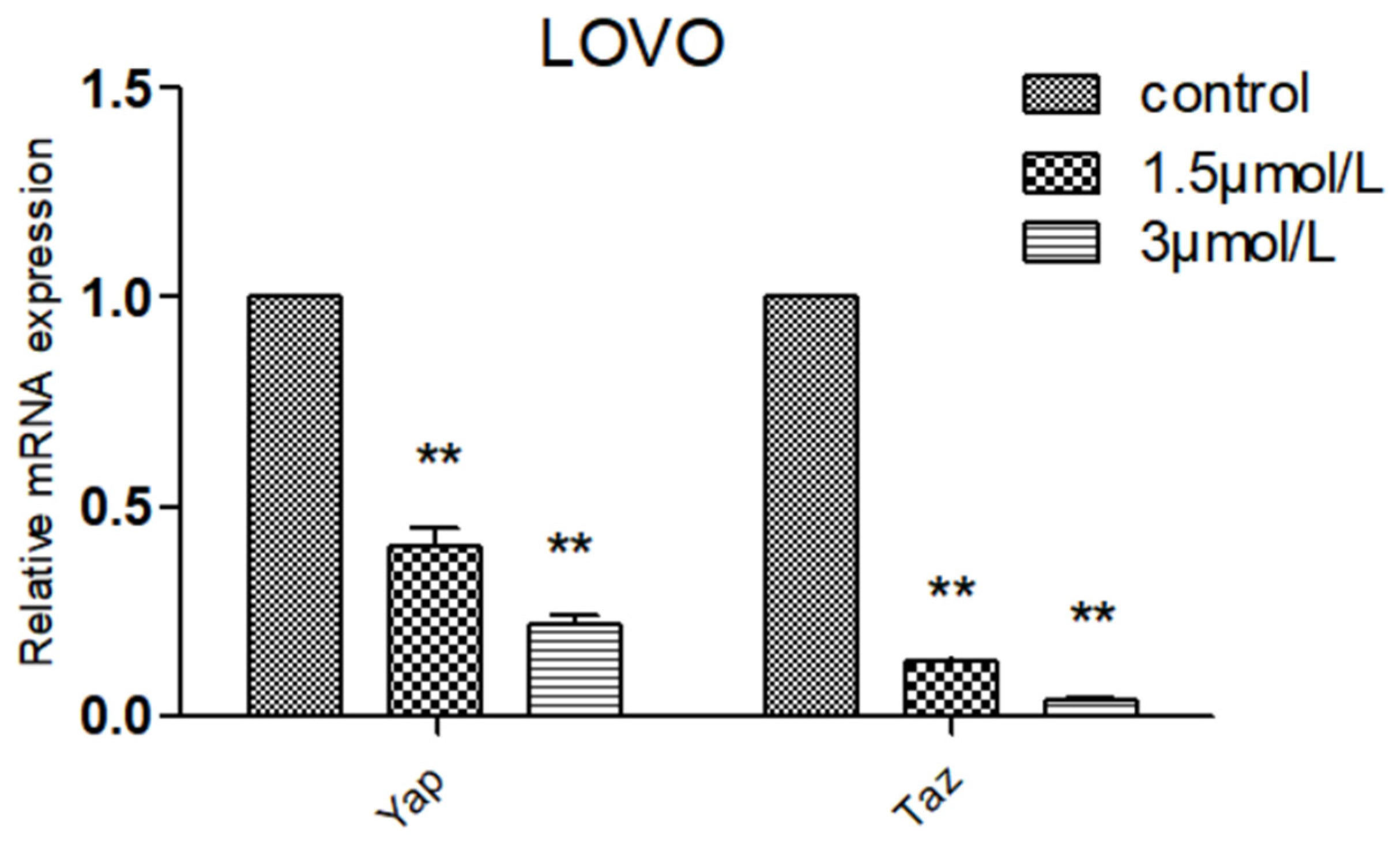
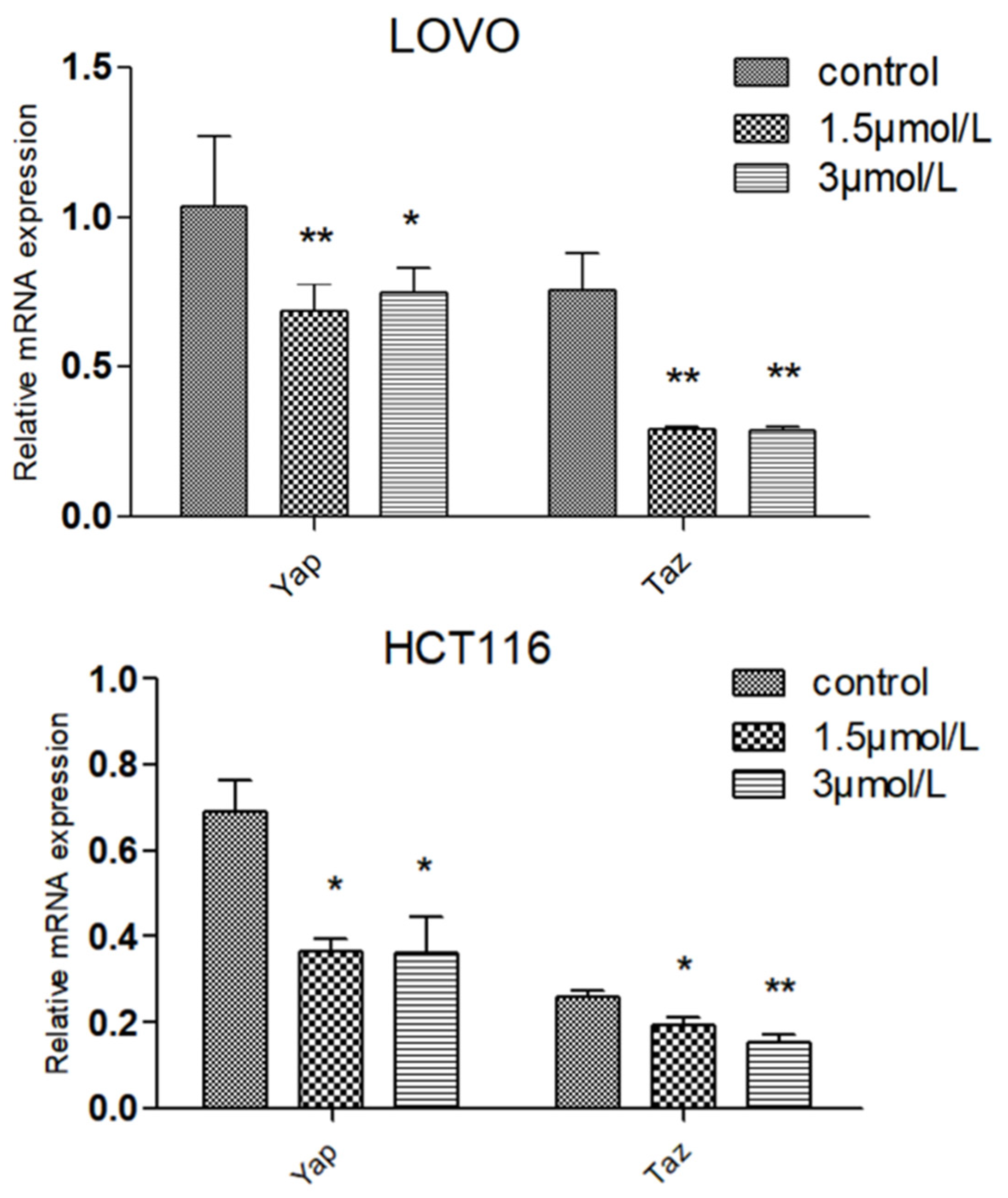
2.7. Effect of DTB on Hippo Signaling Pathway Effectors YAP and TAZ
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.1.1. Cells
4.1.2. Medicine
4.1.3. Experiment Reagents
4.2. Experiment Method
4.2.1. Drug Dissolution
4.2.2. CCK-8 Experiment
4.2.3. Plate Cloning Experiment
4.2.4. Wound Healing Assay
4.2.5. Hoechst 33342/PI Staining Experiments
4.2.6. Flow Cytometry
4.2.7. Real-Time PCR
4.2.8. Western Blot
4.2.9. Statistical Approach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, B.M.; Cosby, R.; Quereshy, F.; Jonker, D. Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review. Clin. Oncol. 2017, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Ping, X.; Kuang, H.X.; Li, X.L.; Wang, Y.; Zhang, B.M.; Bu, H.; Wang, Z.B.; Meng, Y.H.; Wang, Y.H.; Wang, Q.H. Proteomics and Its Application to Determine Mechanism of Action of Traditional Chinese Medicine. China J. Chin. Mater. Med. 2018, 43, 904. [Google Scholar]
- Ke, Z.; Chungeng, L. Advances in the Basic Research of Traditional Chinese Medicine for the Treatment of Colon Cancer. Int. J. Trad. Chin. Med. 2020, 42, 936–938. [Google Scholar]
- Cui, H.; Lin, Y.; Luo, M.; Lu, Y.; Huang, X.; She, Z. Diaporisoindoles A-C: Three isoprenylisoindole alkaloid derivatives from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. Org. Lett. 2017, 19, 5621–5624. [Google Scholar] [CrossRef]
- Khalil, Z.G.; Huang, X.-C.; Raju, R.; Piggott, A.M.; Capon, R.J. Shornephine a: Structure, chemical stability, and P-glycoprotein inhibitory properties of a rare diketomorpholine from an Australian marine-derived Aspergillus sp. J. Organomet. Chem. 2014, 79, 8700–8705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schueffler, A.; Anke, T. Fungal natural products in research and development. Nat. Prod. Rep. 2014, 31, 1425–1448. [Google Scholar] [CrossRef]
- Zhao, M.; Ruan, Q.; Pan, W.; Tang, Y.; Zhao, Z.; Cui, H. New polyketides and diterpenoid derivatives from the fungus Penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Fitoterapia 2020, 143, 104561. [Google Scholar] [CrossRef]
- Li, S.; Dongli, L.; Yuchan, C.; Meihua, T.; Feijun, D.; Weimin, Z. Secondary Metabolites From the Marine Fungus Broom Like Curcuma Curvifolia and Their Antitumor Activity in South China Sea. Chin. Trad. Herb. Drugs 2011, 42, 432–436. [Google Scholar]
- Alba, Z.; Wei, W.; Arnoud, S. Crosstalk between Cell Adhesion Complexes in Regulation of Mechanotransduction. BioEssays 2020, 42, 2000119. [Google Scholar]
- Richard, O.H. Integrins: Bidirectional, Allosteric Signaling Machines. Sci. Direct. Cell 2002, 110, 673–687. [Google Scholar]
- Murphy, J.M.; Park, H.; Lim, S.-T.S. FAK and Pyk2 in Diseases. Front. Biol. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- He, Y.; Li, N.; Cook, S.L.; Yoon, M.-S.; Kapoor, A.; Rao, C.V.; Kenis, P.J.A.; Chen, J.; Wang, F. Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton. Mol. Biol. Cell 2013, 24, 3369–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourdon, J.C.; Deguin-Chambon, V.; Lelong, J.C.; Dessen, P.; May, P.; Debuire, B.; May, E. Further Characterisation of the p53 Responsive Element—Identification of New Candidate Genes for Transactivation by p53. Oncogene 1997, 14, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Deiry, W.S.; Kern, S.E.; Pietenpol, J.A.; Kinzler, K.W.; Vogelstein, B. Definition of a Consensus Binding Site for p53. Nat. Genet. 1992, 1, 45–49. [Google Scholar] [CrossRef]
- Mack, D.H.; Vartikar, J.; Pipas, J.M.; Laimins, L.A. Specific Repression of TATA-Mediated but Not Initiator-Mediated Transcription by Wild-Type p53. Nature 1993, 363, 281–283. [Google Scholar] [CrossRef]
- Seto, E.; Usheva, A.; Zambetti, G.P.; Momand, J.; Horikoshi, N.; Weinmann, R.; Levine, A.J.; Shenk, T. Wild-Type p53 Binds to the TATA-Binding Protein and Represses Transcription. Proc. Natl. Acad. Sci. USA 1992, 89, 12028–12032. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.; Meimei, L.; Na, L.; Xiaoyu, C.; Hanjing, D.; Jun, Y. Effect of Stilbene Glycoside on the Expression of p53, BCL-2, and BAX in Gerbil Brain Subjected to Cerebral Ischemia-Reperfusion. Chin. J. Histochem. Cytochem. 2018, 27, 18–22. [Google Scholar]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. p53 Has a Direct Apoptogenic Role at the Mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef]
- Yee, K.S.; Vousden, K.H. Complicating the Complexity of p53. Carcinogenesis 2005, 26, 1317–1322. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct Activation of BAX by p53 Mediates Mitochondrial Membrane Permeabilization and Apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, J.I.; Dumont, P.; Hafey, M.; Murphy, M.E.; George, D.L. Mitochondrial p53 Activates Bak and Causes Disruption of a Bak-Mcl1 Complex. Nat. Cell Biol. 2004, 6, 443–450. [Google Scholar] [CrossRef]
- Yaeger, R.; Shah, M.A.; Miller, V.A.; Kelsen, J.R.; Wang, K.; Heins, Z.J.; Ross, J.; He, Y.; Sanford, E.; Yantiss, R.; et al. Genomic Alterations Observed in Colitis-Associated Cancers Are Distinct from Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology 2016, 151, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghdaei, H.A.; Kadijani, A.A.; Sorrentino, D.; Mirzaei, A.; Shahrokh, S.; Balaii, H.; Geraci, M.; Zali, M.R. An increased BAX/BCL-2 ratio in circulating inflammatory cells predicts primary response to infliximab in inflammatory bowel disease patients. United Eur. Gastroent 2018, 6, 1074–1081. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.P. Kakkar Lupeol prevents acetaminophen-induced in vivo hepatotoxicity by altering BAX/BCL-2 and oxidative stress-mediated mitochondrial signalingcascade. Life Sci. 2012, 90, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Lopes, G.S.; Jurkiewicz, A.; Garcez-do-Carmo, L.; Smaili, S.S. BCL-2modulates endoplasmic reticulum and mitochondria cal-cium stores in PC 12 cells. Neurochem. Res. 2012, 37, 238–243. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Li, T.Y.; Liu, D.N.; Zhang, H.T. PI3K/Akt pathway involving into apoptosis and invasion in human colon cancer cells Lo Vo. Mol. Biol. Rep. 2014, 41, 3359–3367. [Google Scholar] [CrossRef]
- Lu, X.; Li, C.; Wang, Y.K.; Jiang, K.; Gai, X.-D. Sorbitol induces apoptosis of human colorectal cancer cells via p38 MAPK signal transduction. Oncol. Lett. 2014, 7, 1992–1996. [Google Scholar] [CrossRef]
- Harvey, K.F.; Zhang, X.; Thomas, D.M. The Hippo Pathway and Human Cancer. Nat. Rev. Cancer 2013, 13, 246–257. [Google Scholar] [CrossRef]
- Pan, D.J. The Hippo Signaling Pathway in Development and Cancer. Dev. Cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [Green Version]
- Zanconato, F.; Cordenonsi, M.; Yap, P.S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallahi, E.; O’Driscoll, N.A.; Matallanas, D. The MST/Hippo Pathway and Cell Death: A Non-Canonical Affair. Genes 2016, 7, 28. [Google Scholar] [CrossRef]
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef] [Green Version]
- Bai, N.; Zhang, C.Y.; Liang, N.; Zhang, Z.; Chang, A.; Yin, J.; Li, Z.; Luo, N.; Tan, X.; Luo, N.; et al. Yes-Associated Protein (YAP) Increases Chemosensitivity of Hepatocellular Carcinoma Cells by Modulation of p53. Cancer Biol. Ther. 2013, 14, 511–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lu, Y.; Akbani, R.; Ju, Z.; Roebuck, P.L.; Liu, W.; Yang, J.-Y.; Broom, B.M.; Verhaak, R.G.W.; Kane, D.W.; et al. TCPA: A resource for cancer functional proteomics data. Nat. Methods 2013, 10, 1046–1047. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Zhagn, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Wills, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Y es-associated protein (YAP) overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Shi, S.; Guo, Z.; Zhang, X.; Han, S.; Yang, A.; Wen, W.; Zhu, Q. Overexpression of Y AP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS ONE 2013, 8, 65539. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, P.; Liu, D.; Wu, Z.; Cui, H.; Zhang, R.; Kuang, Z. Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells. Molecules 2022, 27, 2944. https://doi.org/10.3390/molecules27092944
Tang P, Liu D, Wu Z, Cui H, Zhang R, Kuang Z. Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells. Molecules. 2022; 27(9):2944. https://doi.org/10.3390/molecules27092944
Chicago/Turabian StyleTang, Peihua, Dandan Liu, Zheli Wu, Hui Cui, Ren Zhang, and Zaoyuan Kuang. 2022. "Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells" Molecules 27, no. 9: 2944. https://doi.org/10.3390/molecules27092944
APA StyleTang, P., Liu, D., Wu, Z., Cui, H., Zhang, R., & Kuang, Z. (2022). Inhibitory Effects and Mechanism of the Natural Compound Diaporthein B Extracted from Marine-Derived Fungi on Colon Cancer Cells. Molecules, 27(9), 2944. https://doi.org/10.3390/molecules27092944






