Research Update of Emergent Sulfur Quantum Dots in Synthesis and Sensing/Bioimaging Applications
Abstract
:1. Introduction
2. Synthetic Methods of SQDs
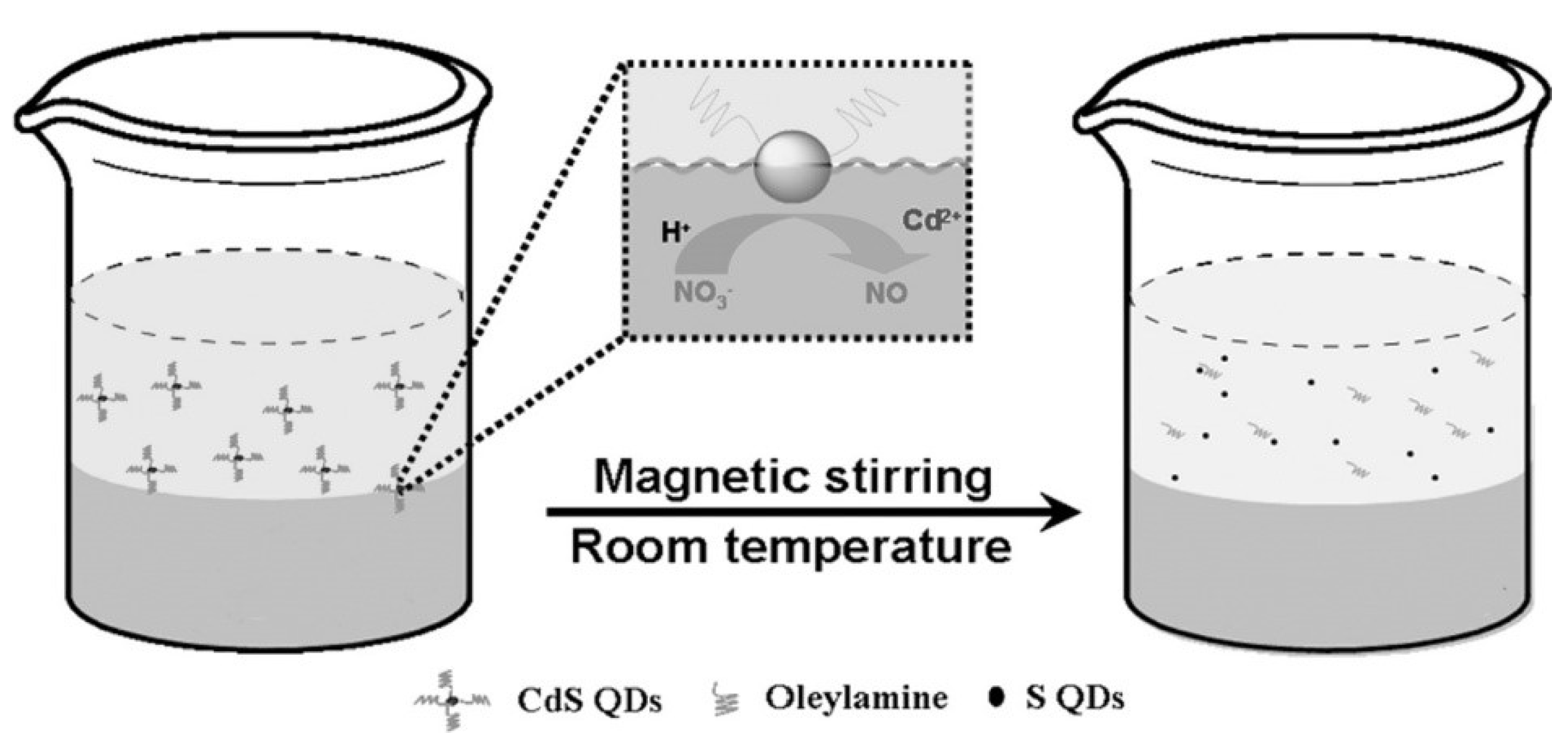
2.1. Acid Etching Oxidation Method
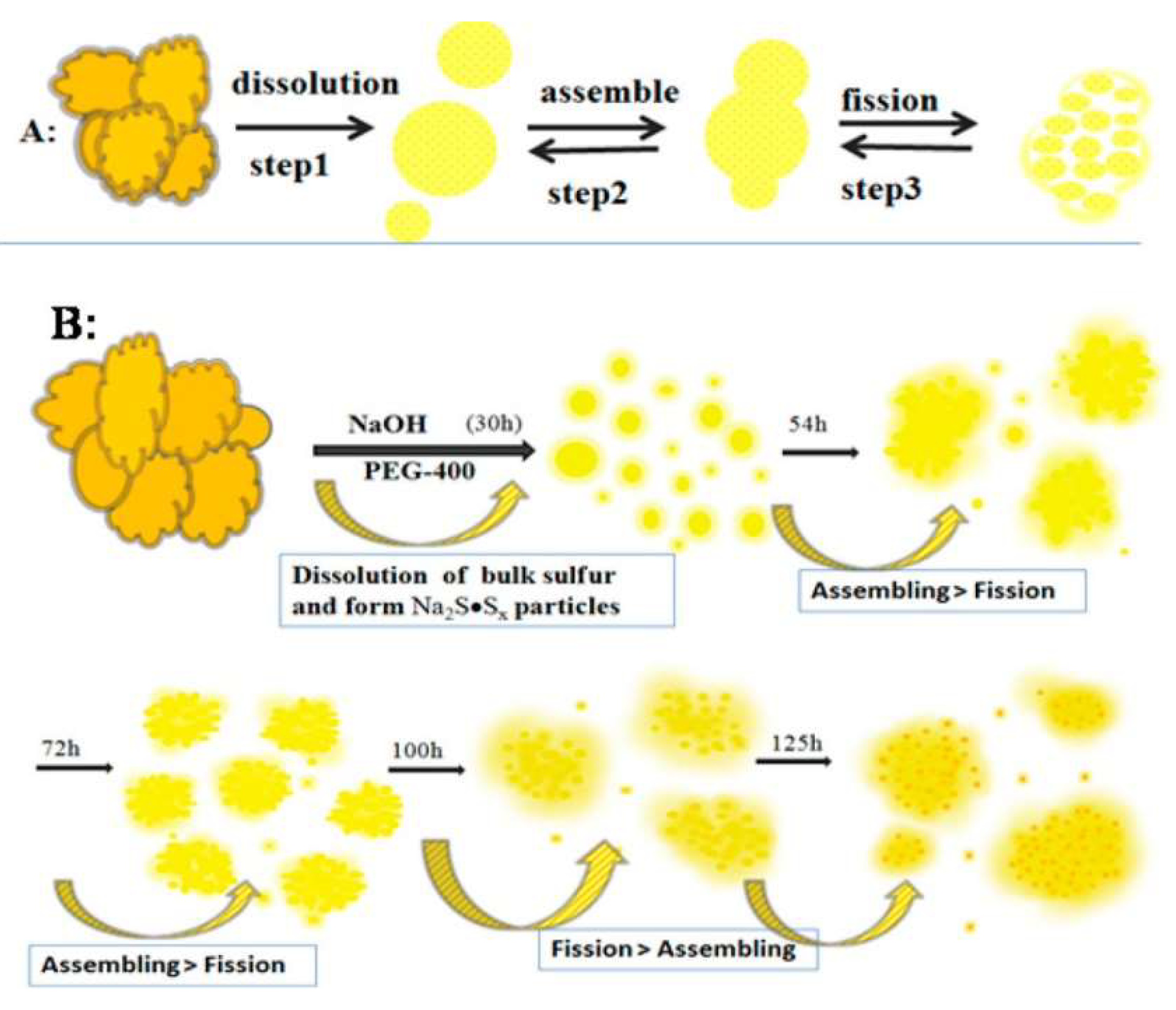
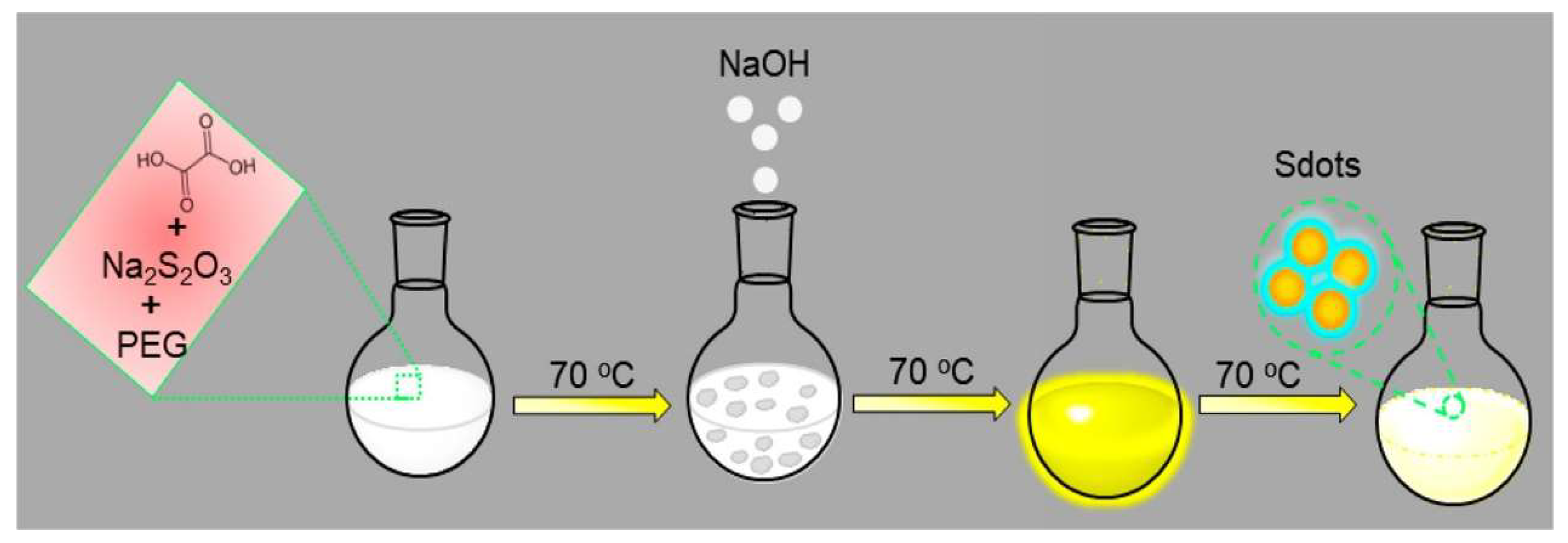
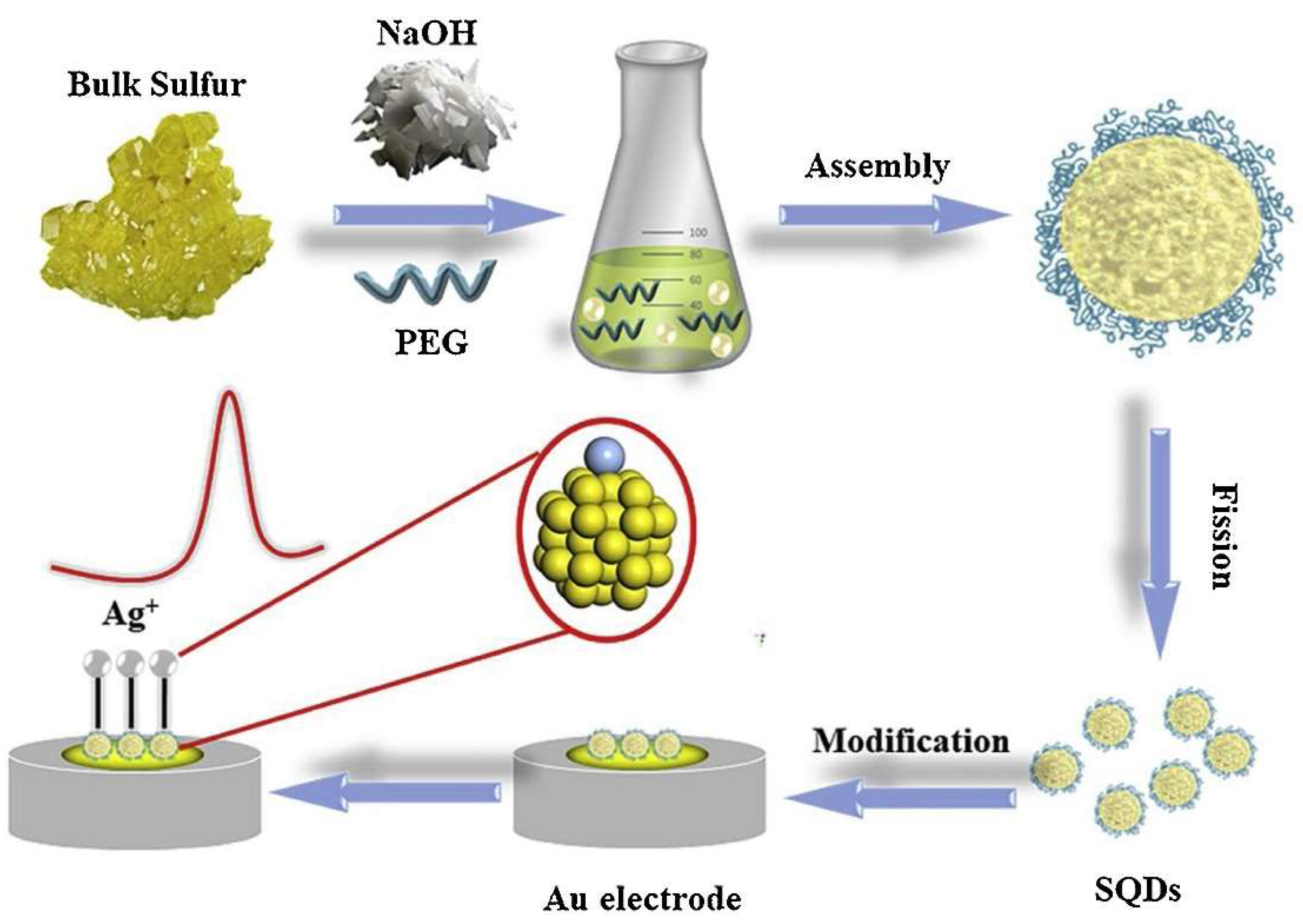
2.2. Assembly-Fission Method
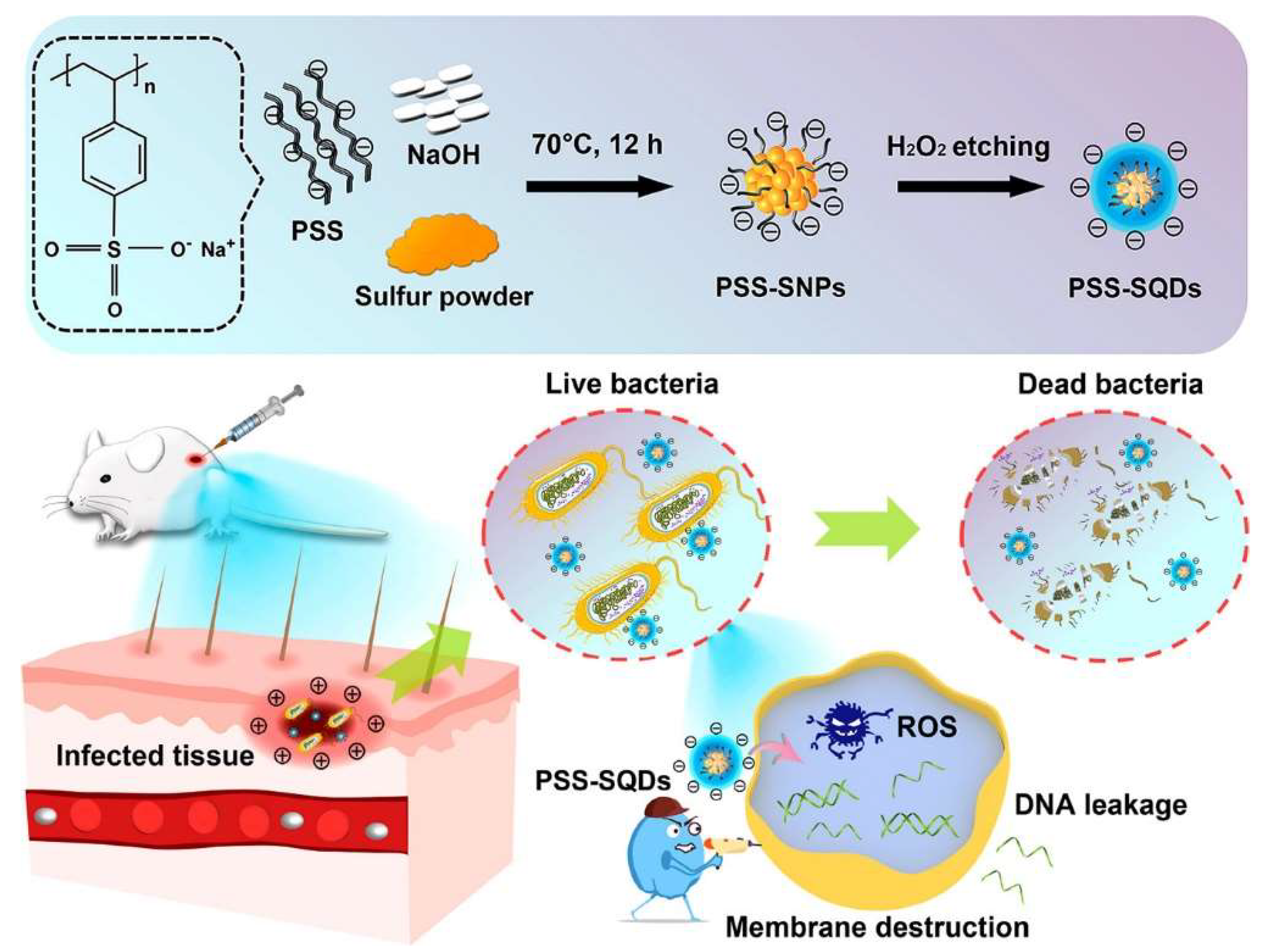
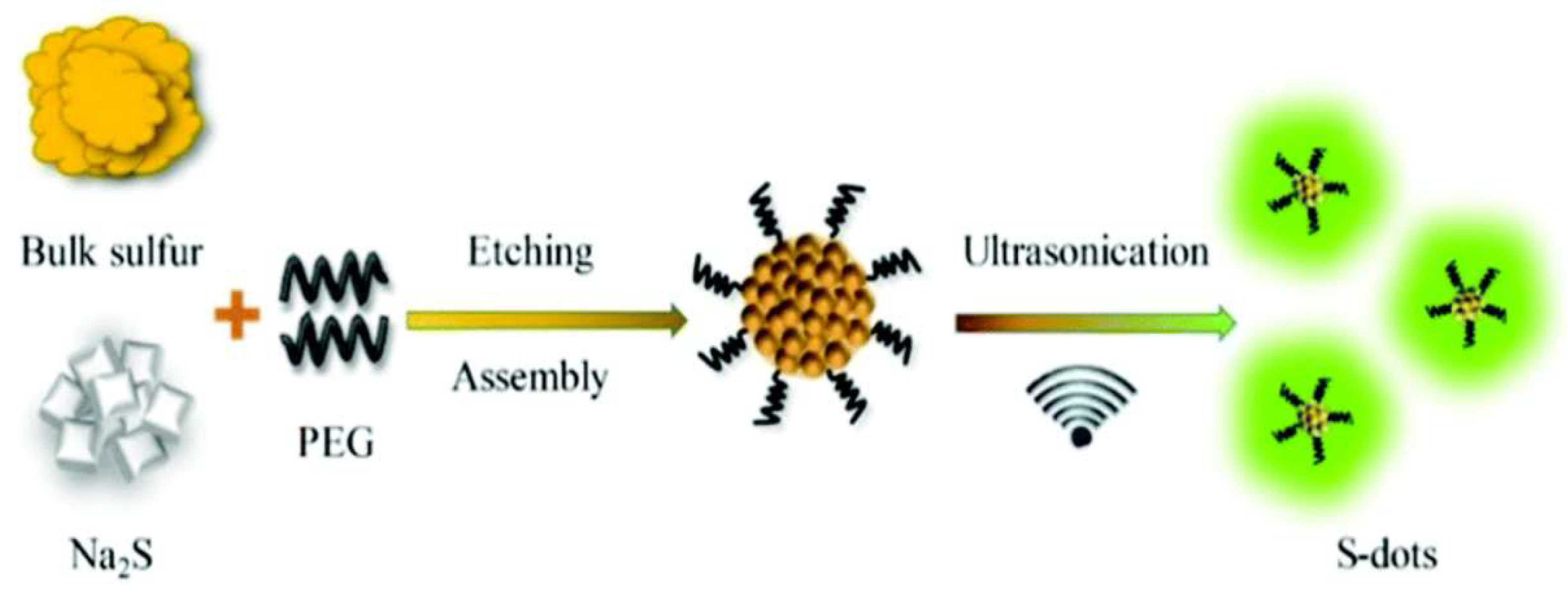
2.3. Surface-Etching Method
2.4. Oxygen-Accelerated Method
2.5. Ultrasonication and Microwave Method
2.6. One-Step Hydrothermal Method
2.7. Other Methods
3. Applications
3.1. Sensing
3.1.1. Fluorescence Sensing
3.1.2. Colorimetric and Fluorescence Dual-Channel Sensing
3.1.3. Ratiometric Fluorescent Sensing
3.1.4. Electrochemical Sensing
3.1.5. Electrochemiluminescence Sensing
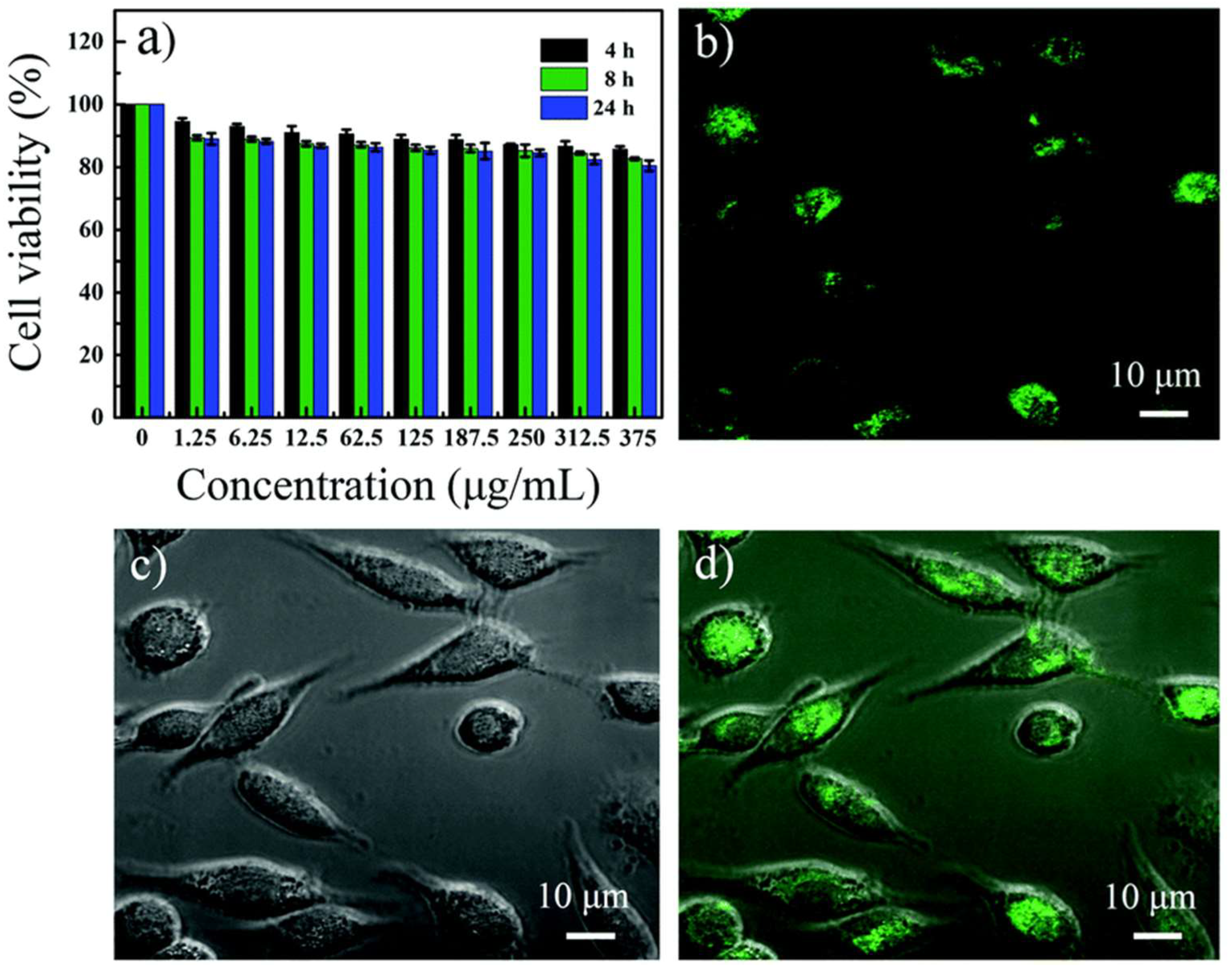
3.2. Bioimaging
4. Challenges and Prospects
- Currently, as shown in Table 1, for most reported synthetic methods, the synthesis time was shortened but with a relatively low QY, or QY was effectively improved but with a prolonged synthesis process. This suggested that more research efforts should be made to develop faster and simpler synthesis methods with higher QY and well-defined luminescence mechanisms. Meanwhile, the optical characteristics can be affected by side products, so the complex purification process should be simplified to achieve efficient product separation and purification.
- In these reported methods, most of the precursors were bulk sulfur powder, PEG, and NaOH; thus requiring a large amount of bulk sulfur powder. Although Arshad et al. [45] used sodium thiosulfate as precursors to produce elemental sulfur, that is not enough, and it is necessary to find new precursors to design effective reaction systems.
- From the perspective of sensing and bioimaging applications, one limitation for the reported SQDs is that most of their emission colors were focused on blue and green, which could be easily interfered with by the self-fluorescence of biological samples. This problem can be solved by synthesizing SQDs with red or near infrared or even infrared-II fluorescence, and such emission modulation can be achieved by doping heteroatoms, changing passivators and reaction conditions, etc.
- For sensing applications, the target molecules are limited, including metal ions (Fe3+, Ag+, Hg2+, Co2+, Zn2+, Cr6+, Ce4+), Dox, AA, CQ, norfloxacin, BChE, miRNA-21, and GSH. One of the possible reasons for this is the limited functional groups on the surface of SQDs, resulting in their limited ability to recognize the target molecules.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, R.D.; Homan, B.S.; Kodaimati, M.; He, C.; Nepomnyashchii, A.B.; Swenson, N.K.; Lian, S.C.; Calzada, R.; Weiss, E.A. Electronic processes within quantum dot-molecule complexes. Chem. Rev. 2016, 116, 12865–12919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.D.; Coleman, J.J. Semiconductor quantum dots. Curr. Opin. Solid State Mater. Sci. 2016, 20, 352–360. [Google Scholar] [CrossRef]
- Yang, W.K.; Yang, Y.H.; Li, H.H.; Lin, D.Y.; Yang, W.T.; Guo, D.Y.; Pan, Q.H. Integration of Cd: ZnS QDs into ZIF-8 for enhanced selectivity toward Cu2+ detection. Inorg. Chem. Front. 2020, 7, 3718–3726. [Google Scholar] [CrossRef]
- Hu, J.C.; He, X.M.; Pu, H.B.; Yang, Y.X.; Chen, C.L. The influence of exposed surface on trap state of PbS quantum dots. Superlattices Microstruct. 2020, 145, 106616. [Google Scholar] [CrossRef]
- Melnychuk, C.; Guyot-Sionnest, P. Thermodynamic Limits to HgTe Quantum Dot Infrared Detector Performance. J. Electron. Mater. 2022, 51, 1428–1435. [Google Scholar] [CrossRef]
- Winnik, F.M.; Maysinger, D. Quantum dot cytotoxicity and ways to reduce it. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Y.; Lu, S.Y. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Ju, Y.Y.; Shi, X.X.; Xu, S.Y.; Ma, X.H.; Wei, R.J.; Hou, H.; Chu, C.C.; Sun, D.; Liu, G.; Tan, Y.Z. Atomically Precise Water-Soluble Graphene Quantum Dot for Cancer Sonodynamic Therapy. Adv. Sci. 2022, 2105034, Online ahead of print. [Google Scholar] [CrossRef]
- Ma, Y.G.; Mei, H.; Li, Y.Y.; Zhou, P.P.; Mao, G.Y.; Wang, H.L.; Wang, X.D. A novel raiometric fluorescence probe based on silicon quantum dots and copper nanoclusters for visual assay of L-cysteine in milks. Food Chem. 2022, 379, 132155. [Google Scholar] [CrossRef]
- Pansare, A.V.; Shedge, A.A.; Chhatre, S.Y.; Das, D.; Murkute, P.; Pansare, S.V.; Nagarkar, A.A.; Patil, V.R.; Chakrabarti, S. AgQDs employing black box synthetic strategy: Photocatalytic and biological behavior. J. Lumin. 2019, 212, 133–140. [Google Scholar] [CrossRef]
- Valappil, M.O.; Alwarappan, S.; Pillai, V.K. Phosphorene quantum dots: Synthesis, properties and catalytic applications. Nanoscale 2022, 14, 1037–1053. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B. Elemental Sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, D.; Qiu, Y.; Song, X.Q.; Zhang, Y.; Sun, X.; Huang, H.H.; Zhao, C.H.; Guo, Z.K.; Fan, L.S. High-Index Faceted Nanocrystals as Highly Efficient Bifunctional Electrocatalysts for High-Performance Lithium–Sulfur Batteries. Nanomicro Lett. 2022, 14, 40. [Google Scholar] [CrossRef]
- Wu, Y.J.; Meanwell, N.A. Geminal Diheteroatomic Motifs: Some Applications of Acetals, Ketals, and Their Sulfur and Nitrogen Homologues in Medicinal Chemistry and Drug Design. J. Med. Chem. 2021, 64, 9786–9874. [Google Scholar] [CrossRef]
- Samarasinghe, I.H.K.; Walpalage, S.; Edirisinghe, D.G.; Egodage, S.M. The use of diisopropyl xanthogen polysulfide as a potential accelerator in efficient sulfur vulcanization of natural rubber compounds. J. Appl. Polym. Sci. 2022, 139, 52063. [Google Scholar] [CrossRef]
- Najafi, S.; Razavi, S.M.; Khoshkam, M.; Asadi, A. Green Synthesized of Sulfur Nanoparticles and Its Application on Lettuce Plants Metabolic Profiling. BioNanoScience 2022, 12, 116–127. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, D.J.; Zheng, F.Y.; Zhou, H.F.; Jiang, S.X.; Wu, Y.J. Water-Soluble and Lowly Toxic Sulphur Quantum Dots. Adv. Funct. Mater. 2014, 24, 7133–7138. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, J.X.; Wu, X.H.; Tao, W.Q.; Li, Z. Ultrasensitive detection of Cr(VI) (Cr2O7(2−)/CrO4(2−)) ions in water environment with a fluorescent sensor based on metal-organic frameworks combined with sulfur quantum dots. Anal. Chim. Acta 2020, 1131, 68–79. [Google Scholar] [CrossRef] [PubMed]
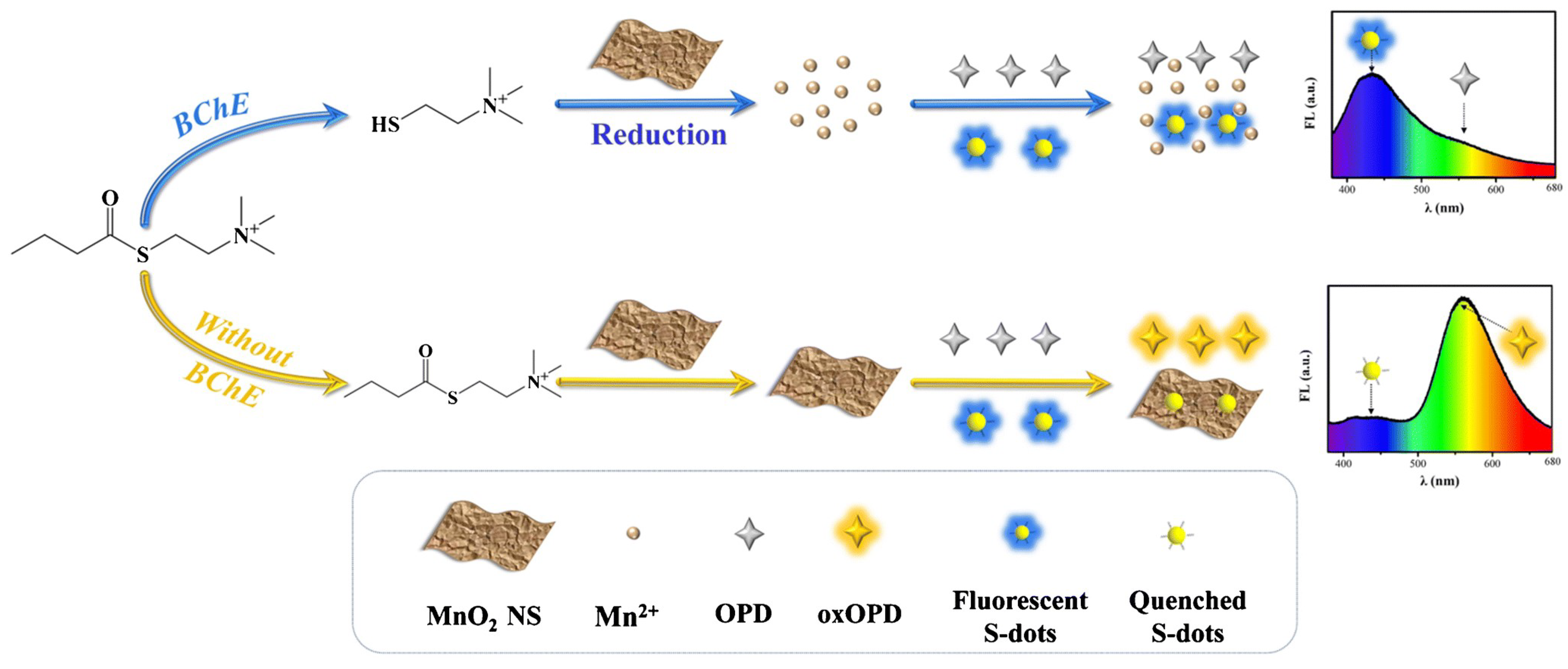
- Ma, Z.R.; Li, P.; Jiao, M.; Shi, Y.E.; Zhai, Y.Q.; Wang, Z.G. Ratiometric sensing of butyrylcholinesterase activity based on the MnO2 nanosheet-modulated fluorescence of sulfur quantum dots and o-phenylenediamine. Mikrochim. Acta 2021, 188, 294. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.W.; Shi, Y.E.; Zhai, Y.Q.; Lv, Y.K.; Zhang, P.; Wang, Z.G. Glutathione modulated fluorescence quenching of sulfur quantum dots by Cu2O nanoparticles for sensitive assay. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120365. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.W.; Shen, L.H.; Wei, J.H.; Li, Y.G.; Abbas, A.; Li, Y.Y.; Qu, M.N.; Zhang, D.D.; Zhang, C.X. Layered Sulfur Nanosheets Prepared by Assembly of Sulfur Quantum Dots: Implications for Wide Optical Absorption and Multiwavelength Photoluminescence. ACS Appl. Nano Mater. 2020, 3, 10749–10756. [Google Scholar] [CrossRef]
- Chen, H.Y.; Zhu, X.J.; Huang, H.; Wang, H.B.; Wang, T.; Zhao, R.B.; Zheng, H.G.; Asiri, A.M.; Luo, Y.L.; Sun, X.P. Sulfur dots–graphene nanohybrid: A metal-free electrocatalyst for efficient N2-to-NH3 fixation under ambient conditions. Chem. Commun. 2019, 55, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.R.; Lin, B.X.; Yu, Y.; Wang, Y.M.; Zhang, L.; Cao, Y.J.; Guo, M.L. A ratiometric fluorescent probe based on sulfur quantum dots and calcium ion for sensitive and visual detection of doxycycline in food. Food Chem. 2021, 356, 129720. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Sk, M.P.; Maurya, S.K.; Siddique, H.R. Mechanochemical Synthesis of Sulfur Quantum Dots for Cellular Imaging. ACS Appl. Nano Mater. 2021, 4, 3339–3344. [Google Scholar] [CrossRef]
- Wang, H.G.; Wang, Z.G.; Xiong, Y.; Kershaw, S.V.; Li, T.Z.; Wang, Y.; Zhai, Y.Q.; Rogach, A.L. Hydrogen Peroxide Assisted Synthesis of Highly Luminescent Sulfur Quantum Dots. Angew. Chem. Int. Ed. 2019, 58, 7040–7044. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.N.; Wu, J.L.; Li, M.; Tan, J.; Fu, W.S.; Tang, H.; Zhang, P. Negatively Charged Sulfur Quantum Dots for Treatment of Drug-Resistant Pathogenic Bacterial Infections. Nano Lett. 2021, 21, 9433–9441. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Wen, Q. Single element material sulfur quantum dots nonlinear optics and ultrafast photonic applications. Opt. Laser Technol. 2021, 138, 106858. [Google Scholar] [CrossRef]
- Shen, L.H.; Wang, H.N.; Liu, S.N.; Bai, Z.W.; Zhang, S.C.; Zhang, X.R.; Zhang, C.X. Assembling of Sulfur Quantum Dots in Fission of Sublimed Sulfur. J. Am. Chem. Soc. 2018, 140, 7878–7884. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.L.; Shi, L.X.; Du, K.; Qin, Y.; Qu, S.J.; Xia, D.Q.; Zhou, Z.; Huang, Z.G.; Ding, S.N. Copper-Ion-Assisted Precipitation Etching Method for the Luminescent Enhanced Assembling of Sulfur Quantum Dots. ACS Omega 2020, 5, 5407–5411. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Tan, J.S.; Wang, G.; Gao, P.X.; Lei, J.H.; Zhou, L. Oxygen accelerated scalable synthesis of highly fluorescent sulfur quantum dots. Chem. Sci. 2019, 11, 772–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Wang, H.G.; Feng, A.R.; Chang, J.Y.; Zhang, C.C.; Shi, Y.E.; Zhai, Y.Q.; Biju, V.; Wang, Z.G. Photoluminescence investigations of sulfur quantum dots synthesized by a bubbling-assisted strategy. Nanoscale Adv. 2021, 3, 4271–4275. [Google Scholar] [CrossRef]
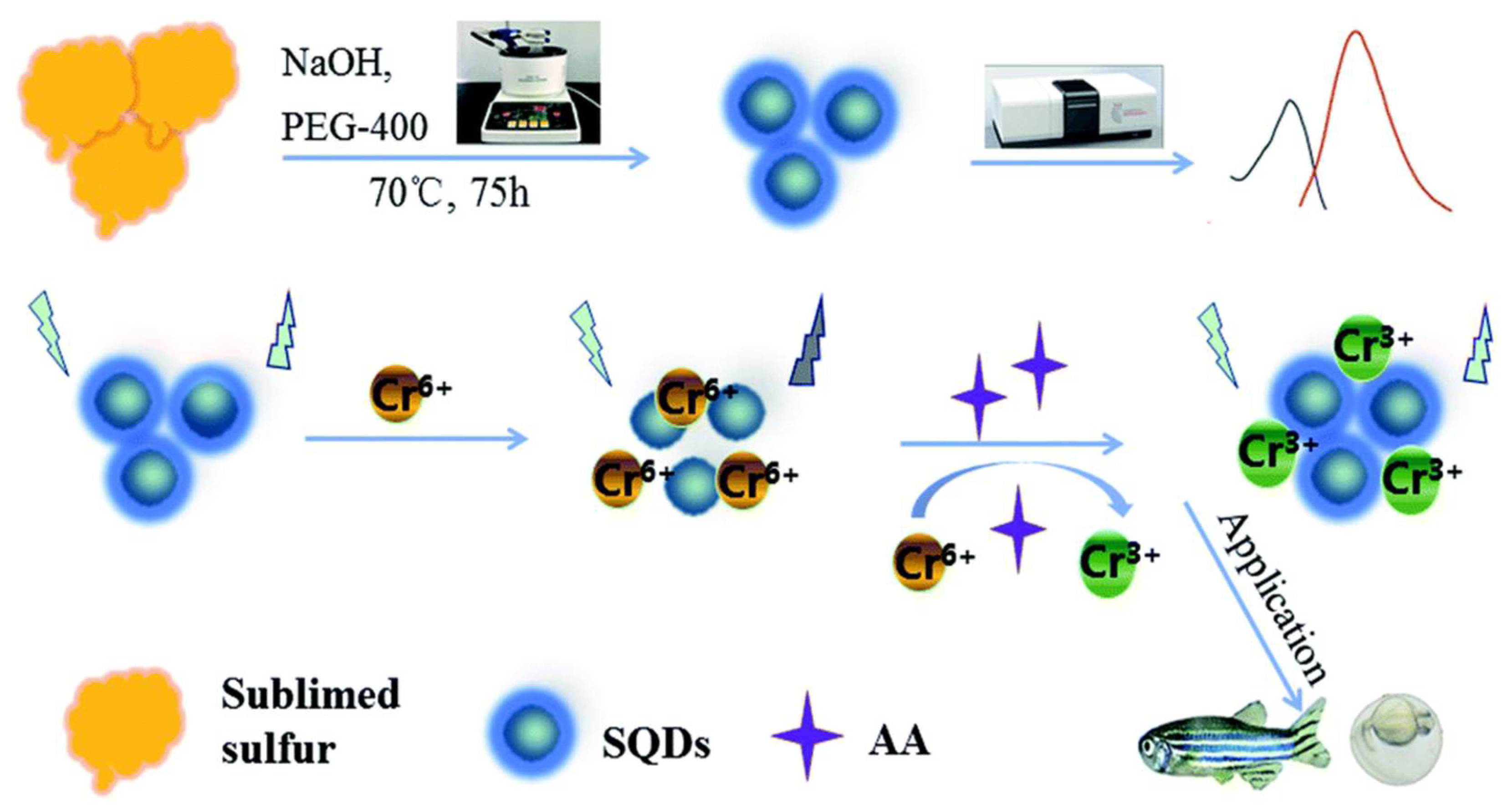
- Duan, Y.X.; Tan, J.S.; Huang, Z.M.; Deng, Q.M.; Liu, S.J.; Wang, G.; Li, L.G.; Zhou, L. Facile synthesis of carboxymethyl cellulose sulfur quantum dots for live cell imaging and sensitive detection of Cr(VI) and ascorbic acid. Carbohydr. Polym. 2020, 249, 116882. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Lei, J.H.; Ruan, H.; Gong, Y.Y.; Wang, G.; Zhou, L. One-pot synthesis of hydroxypropyl-β-cyclodextrin capped fluorescent sulfur quantum dots for highly sensitive and selective recognition of tartrazine. Microchem. J. 2021, 164, 106031. [Google Scholar] [CrossRef]
- Lei, J.H.; Huang, Z.M.; Gao, P.X.; Sun, J.H.; Zhou, L. Polyvinyl Alcohol Enhanced Fluorescent Sulfur Quantum Dots for Highly Sensitive Detection of Fe3+ and Temperature in Cells. Part Part Syst Charact. 2021, 38, 2000332. [Google Scholar] [CrossRef]
- Zhang, C.C.; Zhang, P.; Ji, X.J.; Wang, H.G.; Kuang, H.Z.; Cao, W.L.; Pan, M.Y.; Shi, Y.E.; Wang, Z.G. Ultrasonication-promoted synthesis of luminescent sulfur nano-dots for cellular imaging applications. Chem. Commun. 2019, 55, 13004–13007. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Dai, H.Q.; Wei, X.; Su, D.L.; Wei, C.; Chen, Y.Y.; Xie, F.X.; Zhang, W.L.; Guo, R.Q.; Qu, S.N. 49.25% efficient cyan emissive sulfur dots via a microwave-assisted route. RSC Adv. 2020, 10, 17266–17269. [Google Scholar] [CrossRef]
- Sheng, Y.L.; Huang, Z.N.; Zhong, Q.; Deng, H.H.; Lai, M.C.; Yang, Y.; Chen, W.; Xia, X.H.; Peng, H.P. Size-focusing results in highly photoluminescent sulfur quantum dots with a stable emission wavelength. Nanoscale 2021, 13, 2519–2526. [Google Scholar] [CrossRef]
- Xiao, L.; Du, Q.C.; Huang, Y.; Wang, L.; Cheng, S.J.; Wang, Z.; Wong, T.N.; Yeow, E.K.L.; Sun, H.D. Rapid Synthesis of Sulfur Nanodots by One-Step Hydrothermal Reaction for Luminescence-Based Applications. ACS Appl. Nano Mater. 2019, 2, 6622–6628. [Google Scholar] [CrossRef]
- Wang, C.X.; Wei, Z.T.; Pan, C.W.; Pan, Z.W.; Wang, X.M.; Liu, J.; Wang, H.Z.; Huang, G.Y.; Wang, M.; Mao, L.Q. Dual Functional Hydrogen Peroxide Boosted One Step Solvothermal Synthesis of Highly Uniform Sulfur Quantum Dots at Elevated Temperature and Their Fluorescent Sensing. Sens. Actuators B Chem. 2021, 344, 130326. [Google Scholar] [CrossRef]
- Arshad, F.; Sk, M.P. Luminescent Sulfur Quantum Dots for Colorimetric Discrimination of Multiple Metal Ions. ACS Appl. Nano Mater. 2020, 3, 3044–3049. [Google Scholar] [CrossRef]
- Caires, A.J.; Mansur, H.S.; Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; Dos Reis, J.K.P. Gold nanoparticle-carboxymethyl cellulose nanocolloids for detection of human immunodeficiency virus type-1 (HIV-1) using laser light scattering immunoassay. Colloids Surf. B. 2019, 177, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.L.; Ma, X.Z.; Zhou, S.F.; Huang, J.; Liu, Y.Q.; Liu, Y.Z. Highly efficient removal of heavy metal ions by carboxymethyl cellulose-immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr. Polym. 2019, 213, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, S. Nanophase materials by hydrothermal, microwave-hydrothermal and microwave solvothermal methods. Curr. Sci. 2003, 85, 1730–1734. Available online: https://www.jstor.org/stable/24109979 (accessed on 13 March 2022).
- Gao, P.X.; Wang, G.; Zhou, L. Luminescent Sulfur Quantum Dots: Synthesis, Properties and Potential Applications. ChemPhotoChem 2020, 4, 5235–5244. [Google Scholar] [CrossRef]
- Pal, A.; Arshad, F.; Sk, M.P. Emergence of sulfur quantum dots: Unfolding their synthesis, properties, and applications. Adv. Colloid Interface Sci. 2020, 285, 102274. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.E.; Zhang, P.; Yang, D.; Wang, Z. Synthesis, photoluminescence properties and sensing applications of luminescent sulfur nanodots. Chem. Commun. 2020, 56, 10982–10988. [Google Scholar] [CrossRef] [PubMed]
- Sansalone, L.; Tang, S.; Zhang, Y.; Thapaliya, E.R.; Raymo, F.M.; Garcia-Amorós, J. Semiconductor quantum dots with photoresponsive ligands. Top. Curr. Chem. 2016, 374, 73. [Google Scholar] [CrossRef]
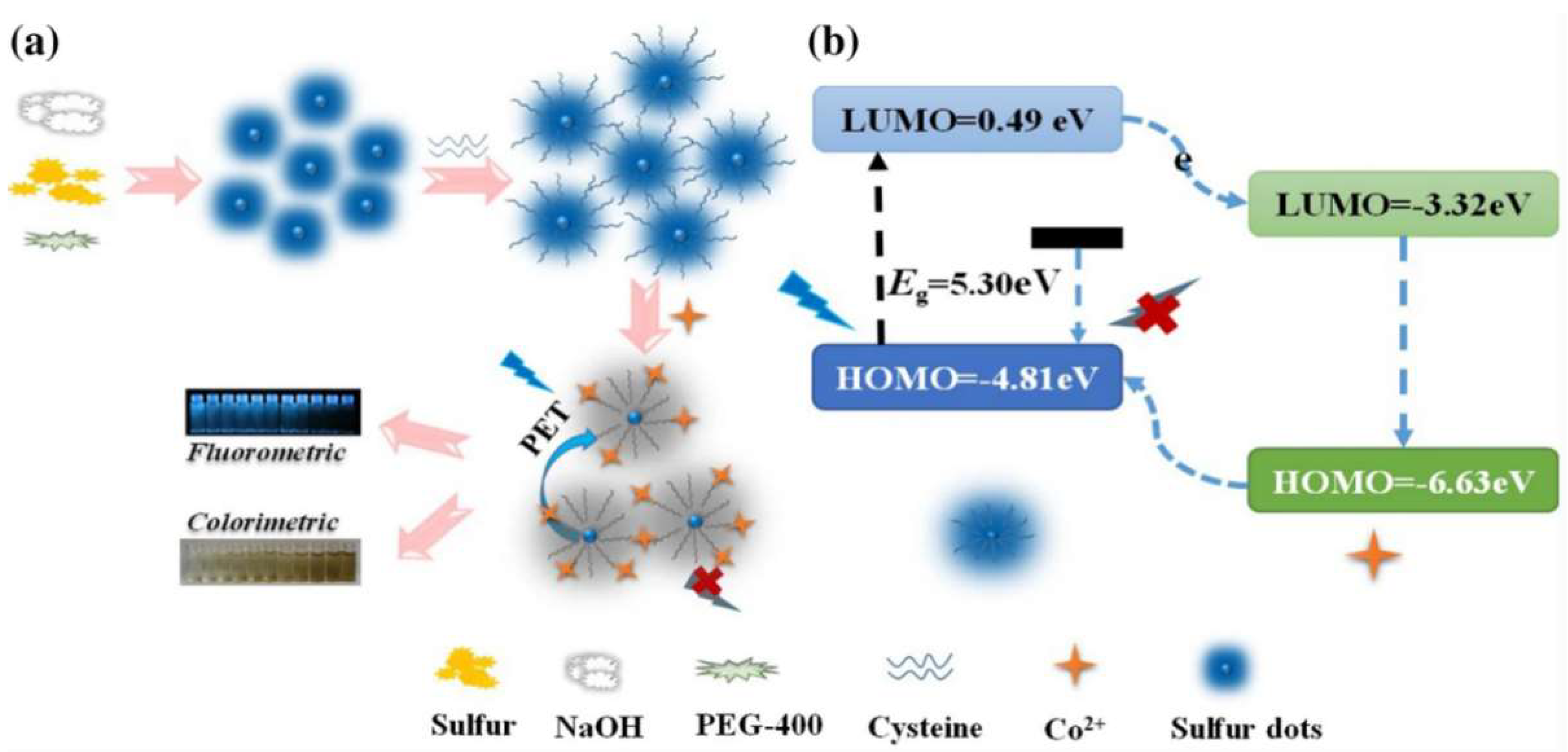
- Wang, S.; Bao, X.; Gao, B.; Li, M. A novel sulfur quantum dot for the detection of cobalt ions and norfloxacin as a fluorescent “switch”. Dalton Trans. 2019, 48, 8288–8296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fan, Z.F. Using zinc ion-enhanced fluorescence of sulfur quantum dots to improve the detection of the zinc(II)-binding antifungal drug clioquinol. Mikrochim. Acta 2020, 187, 3. [Google Scholar] [CrossRef] [PubMed]
- Zu, F.L.; Yan, F.Y.; Bai, Z.J.; Xu, J.X.; Wang, Y.Y.; Huang, Y.C.; Zhou, X.G. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Li, T.Z.; Gao, Y.T.; Li, H.Y.; Zhang, C.Y.; Xing, Y.F.; Jiao, M.; Shi, Y.E.; Li, W.; Zhai, Y.Q.; Wang, Z.G. Ultrasensitive detection of butyrylcholinesterase activity based on the inner filter effect of MnO2 nanosheets on sulfur nanodots. Analyst 2020, 145, 5206–5212. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; An, X.X.; Pan, S.; Liu, H.; Hu, X.L. Hydrogen peroxide assisted synthesis of sulfur quantum dots for the detection of chromium (VI) and ascorbic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 247, 119122. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.K.; Mei, H.; Qian, Q.H.; Dahlgren, R.A.; Gao, M.; Wang, X.D. Sulfur quantum dot-based “ON–OFF–ON” fluorescence platform for detection and bioimaging of Cr(VI) and ascorbic acid in complex environmental matrices and biological tissues. RSC Adv. 2021, 11, 10572–10581. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.H.; Wang, Y.; Luo, Z.; Zhang, B.W.; Mei, X.L.; Yang, X.P. Facile synthesis of fluorescent sulfur quantum dots for selective detection of p-nitrophenol in water samples. Microchem. J. 2021, 170, 106735. [Google Scholar] [CrossRef]
- Lu, H.X.; Zhang, H.Q.; Li, Y.F.; Gan, F. Sensitive and selective determination of tetracycline in milk based on sulfur quantum dot probes. RSC Adv. 2021, 11, 22960–22968. [Google Scholar] [CrossRef]
- Huang, Z.M.; Gao, Y.; Huang, Z.Y.; Chen, D.L.; Sun, J.H.; Zhou, L. Sulfur quantum dots: A novel fluorescent probe for sensitive and selective detection of Fe3+ and phytic acid. Microchem. J. 2021, 170, 106656. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.X.; Liu, W.; Zhao, S.; Lu, Z.W.; Wang, Y.Y.; Wang, G.T.; Zou, P.; Wang, X.X.; Zhao, Q.B.; et al. Smartphone based platform for ratiometric fluorometric and colorimetric determination H2O2 and glucose. Sens. Actuators B. 2020, 305, 127524. [Google Scholar] [CrossRef]
- Liu, W.; Ding, F.; Wang, Y.Y.; Mao, L.; Liang, R.X.; Zou, P.; Wang, X.X.; Zhao, Q.B.; Rao, H.B. Fluorometric and colorimetric sensor array for discrimination of glucose using enzymatic-triggered dual-signal system consisting of Au@Ag nanoparticles and carbon nanodots. Sens. Actuators B. 2018, 265, 310–317. [Google Scholar] [CrossRef]
- Qiao, G.X.; Liu, L.; Hao, X.X.; Zheng, J.K.; Liu, W.Q.; Gao, J.W.; Zhang, C.C.; Wang, Q.M. Signal transduction from small particles: Sulfur nanodots featuring mercury sensing, cell entry mechanism and in vitro tracking performance. Chem. Eng. J. 2020, 382, 122907. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Li, Y.; Nie, Y.L.; Tian, X.K. Sulfur quantum dot-based portable paper sensors for fluorometric and colorimetric dual-channel detection of cobalt. J. Mater. Sci. 2020, 56, 4782–4796. [Google Scholar] [CrossRef]
- Lu, C.F.; Wang, Y.; Xu, B.Y.; Zhang, W.; Xie, Y.; Chen, Y.Y.; Wang, L.Z.; Wang, X.X. A colorimetric and fluorescence dual-signal determination for iron (II) and H2O2 in food based on sulfur quantum dots. Food Chem. 2022, 366, 130613. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.Q.; Li, X.Y.; Yang, P.; Yue, J.Y.; Jiang, Y.; Tang, B. Linker-Eliminated Nano Metal–Organic Framework Fluorescent Probe for Highly Selective and Sensitive Phosphate Ratiometric Detection in Water and Body Fluids. Anal. Chem. 2020, 92, 3722–3727. [Google Scholar] [CrossRef]
- Tan, H.L.; Wu, X.Y.; Weng, Y.H.; Lu, Y.J.; Huang, Z.Z. Self-Assembled FRET Nanoprobe with Metal-Organic Framework As a Scaffold for Ratiometric Detection of Hypochlorous Acid. Anal. Chem. 2020, 92, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Xu, B.J.; Dong, C. Recent advances in colorimetric strategies for acetylcholinesterase assay and their applications. TrAC Trends Anal. Chem. 2021, 142, 116320. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Shoba, A.; Kavitha, B.; Aswathaman, H.; Ganesan, H.; Senthil Kumar, N. Synthesis, characterization and electrochemical sensors application of MnO2 nanoparticles. Mater. Today 2022, 48, 521–526. [Google Scholar] [CrossRef]
- Kumar, N.; Shetti, N.P.; Jagannath, S.; Aminabhavi, T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022, 430, 132966. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, R.; Ling, H.; Zhao, Z.L.; Han, W.J.; Shi, X.W.; Payne, G.F.; Wang, X.H. Toward scalable fabrication of electrochemical paper sensor without surface functionalization. NPJ Flex. Electron. 2022, 6, 12. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.W.; Xie, K.F.; Zhu, J.W.; Chen, F.; Wang, H.G.; Zhang, H.W.; Su, W.T.; Wang, Z.G.; Zhou, C.T.; et al. Electrochemical detection of silver ions by using sulfur quantum dots modified gold electrode. Sens. Actuators B Chem. 2020, 304, 127390. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, W.L.; Wei, H.F.; Ma, C.; Lin, Y.H.; Zhu, J.J. Stable and Monochromatic All-Inorganic Halide Perovskite Assisted by Hollow Carbon Nitride Nanosphere for Ratiometric Electrochemiluminescence Bioanalysis. Anal. Chem. 2020, 92, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.C.; Zhu, X.R.; Wang, N.N.; Li, Y.F.; Ju, H.X.; Lei, J.P. Electroactive Metal-Organic Frameworks as Emitters for Self-Enhanced Electrochemiluminescence in Aqueous Medium. Angew. Chem. Int. Ed. 2020, 59, 10446–10450. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.L.; Zhang, C.X. Electrogenerated Chemiluminescence Biosensing. Anal. Chem. 2020, 92, 524–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.L.; Zhang, Y.; Yuan, R.; Wang, H.J. Ultrasensitive Electrochemiluminescence Biosensor Using Sulfur Quantum Dots as an Emitter and an Efficient DNA Walking Machine with Triple-Stranded DNA as a Signal Amplifier. Anal. Chem. 2020, 92, 15112–15119. [Google Scholar] [CrossRef] [PubMed]
- Han, T.T.; Yang, J.L.; Wang, Y.; Cao, Y.; Wang, Y.Y.; Chen, H.Y.; Zhu, J.J. Boosted anodic electrochemiluminescence from blue-emissive sulfur quantum dots and its bioanalysis of glutathione. Electrochim. Acta 2021, 381, 138281. [Google Scholar] [CrossRef]



| Method | Precursor | Solvent | Ligand | Temperature | Reaction Time | QY | Emission Wavelength | Application | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Acid etching oxidation | CdS or ZnS QDs, HNO3aqueous solution | n-hexane, H2O | Not mention | RT | 36 h | 0.549% | 428 nm | Quenching by Fe3+ | [19] |
| Assembly-fission | Sublimed sulfur, NaOH | H2O | PEG-400 | 70 °C | 125 h | 3.8% | 529−488 nm | ECL inannihilation reaction, CL from oxidation | [30] |
| Surface etching | Sulfur powder, NaOH, H2O2 | H2O | PEG | 70 °C | 5 h | 23% | 440–500 nm | LEDs | [27] |
| Surface etching | Sulfur powder, NaOH, Cu2+ | H2O | PEG-400 | 70 °C | 72 h | 32.8% | 425–525 nm | No | [31] |
| Surface etching | Sulfur powder, NaOH, H2O2 | H2O | PSS | 70 °C | 12 h | 5.13% | 420 nm | Anti-bacteria | [28] |
| Oxygen accelerated | Sublimed sulfur, NaOH, pure O2 | H2O | PEG-400 | 90 °C | 10 h | 21.5% | 490 nm | Cellular imaging | [32] |
| Oxygen accelerated | Sublimed sulfur, NaOH, N2 or air | H2O | PEG-400 | 70 °C | 72 h | 8% | 425–500 nm | No | [33] |
| Oxygen accelerated | Sublimed sulfur, NaOH, pure O2 | H2O | CMC | 90 °C | 24 h | 7.1% | 434 nm | Detection of Cr6+ and AA, cell imaging | [34] |
| Oxygen accelerated | Sublimed sulfur, NaOH, pure O2 | H2O | HP-β-CD | 85 °C | 12 h | 4.66% | 443 nm | Detection of TTZ, cell imaging | [35] |
| Oxygen accelerated | Sublimed sulfur, NaOH, pure O2 | H2O | PVA | 75 °C | 12 h | 4.62% | 443 nm | Quenching by Fe3+, Nanothermometer to monitor cell temperature | [36] |
| Ultrasonication and microwave | Sublimated sulfur, Na2S | H2O | PEG-400 | RT, ultrasonication | 12 h | 2.1% | 515–562 nm | Cellular imaging | [37] |
| Ultrasonication and microwave | Sulfur powder, NaOH, H2O2 | H2O | PEG-400 | 70, 80, 90 and 95 °C via microwave, 70 °C | 5 min for microwave, 40 h | 49.25% | 445–506 nm | No | [38] |
| Ultrasonication and microwave | Sublimed sulfur, NaOH, H2O2 | H2O | PEG-400 | 70 °C, ultrasound-microwave | 2 h | 58.6% | 440 nm | Ce4+ and AA detection | [39] |
| One-step hydrothermal | Sulfur (monoclinic phase), NaOH | H2O | PEG | 170 °C | 4 h | 4.02% | 554 nm | No | [40] |
| One-step hydrothermal | Sublimed sulfur, H2O2 | H2O | PEG-400 | 220 °C | 42 h | 10.3% | 365 nm | Fe3+ detection, cellular imaging | [41] |
| Situ reaction | Sodium thiosulfate, oxalic acid, NaOH | H2O | PEG-400 | 70 °C | 6 h | 2.5% | 462 nm | Colorimetric discrimination of multiple metal ions | [42] |
| Mechanochemical | Sodium thiosulfate, oxalic acid, NaOH | H2O | PEG-400 | RT | 1 h | 4.8% | 461 nm | Cellular imaging | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, K.; Sun, Y.; Liu, J.; Fu, Y.; Ye, K.; Liang, J.; Wu, Y. Research Update of Emergent Sulfur Quantum Dots in Synthesis and Sensing/Bioimaging Applications. Molecules 2022, 27, 2822. https://doi.org/10.3390/molecules27092822
Ning K, Sun Y, Liu J, Fu Y, Ye K, Liang J, Wu Y. Research Update of Emergent Sulfur Quantum Dots in Synthesis and Sensing/Bioimaging Applications. Molecules. 2022; 27(9):2822. https://doi.org/10.3390/molecules27092822
Chicago/Turabian StyleNing, Keke, Yujie Sun, Jiaxin Liu, Yao Fu, Kang Ye, Jiangong Liang, and Yuan Wu. 2022. "Research Update of Emergent Sulfur Quantum Dots in Synthesis and Sensing/Bioimaging Applications" Molecules 27, no. 9: 2822. https://doi.org/10.3390/molecules27092822
APA StyleNing, K., Sun, Y., Liu, J., Fu, Y., Ye, K., Liang, J., & Wu, Y. (2022). Research Update of Emergent Sulfur Quantum Dots in Synthesis and Sensing/Bioimaging Applications. Molecules, 27(9), 2822. https://doi.org/10.3390/molecules27092822





