Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS
Abstract
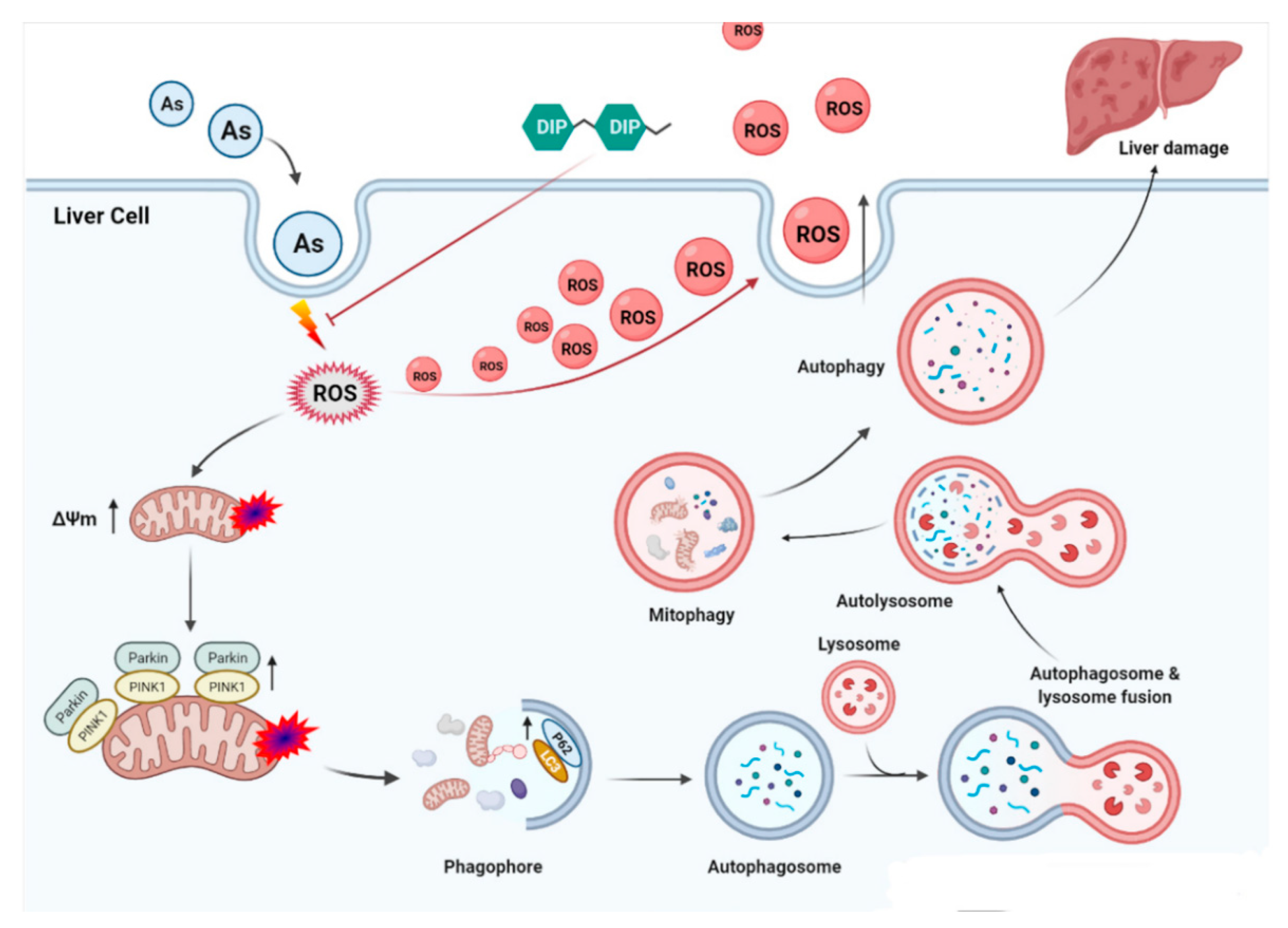
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Detection of ROS
2.5. Observe by Transmission Electron Microscopy
2.6. RT-qPCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
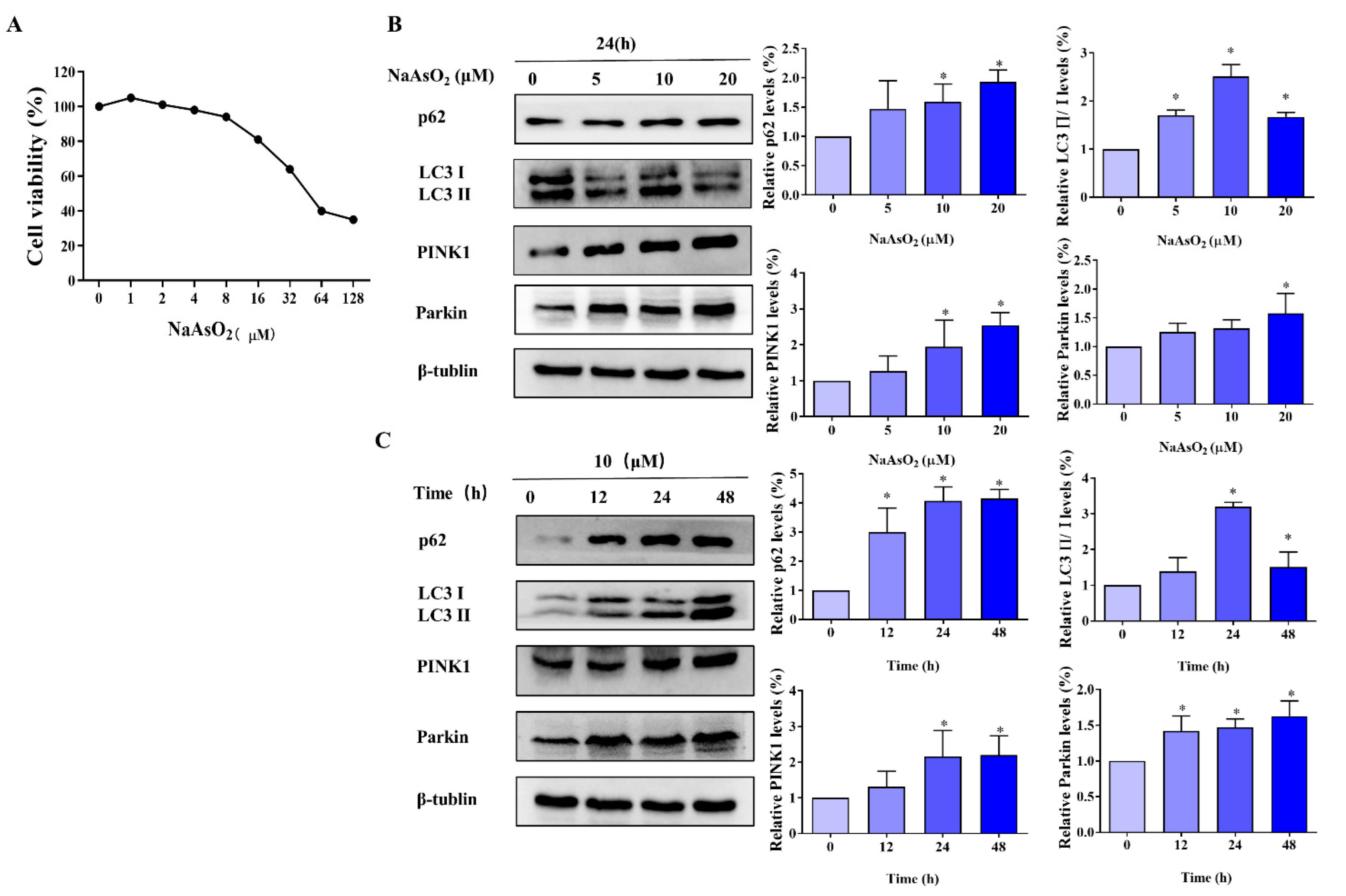
3.1. Effect of As on the Viability of L-02 Cells
3.2. As-Induces Mitophagy in L-02 Cells
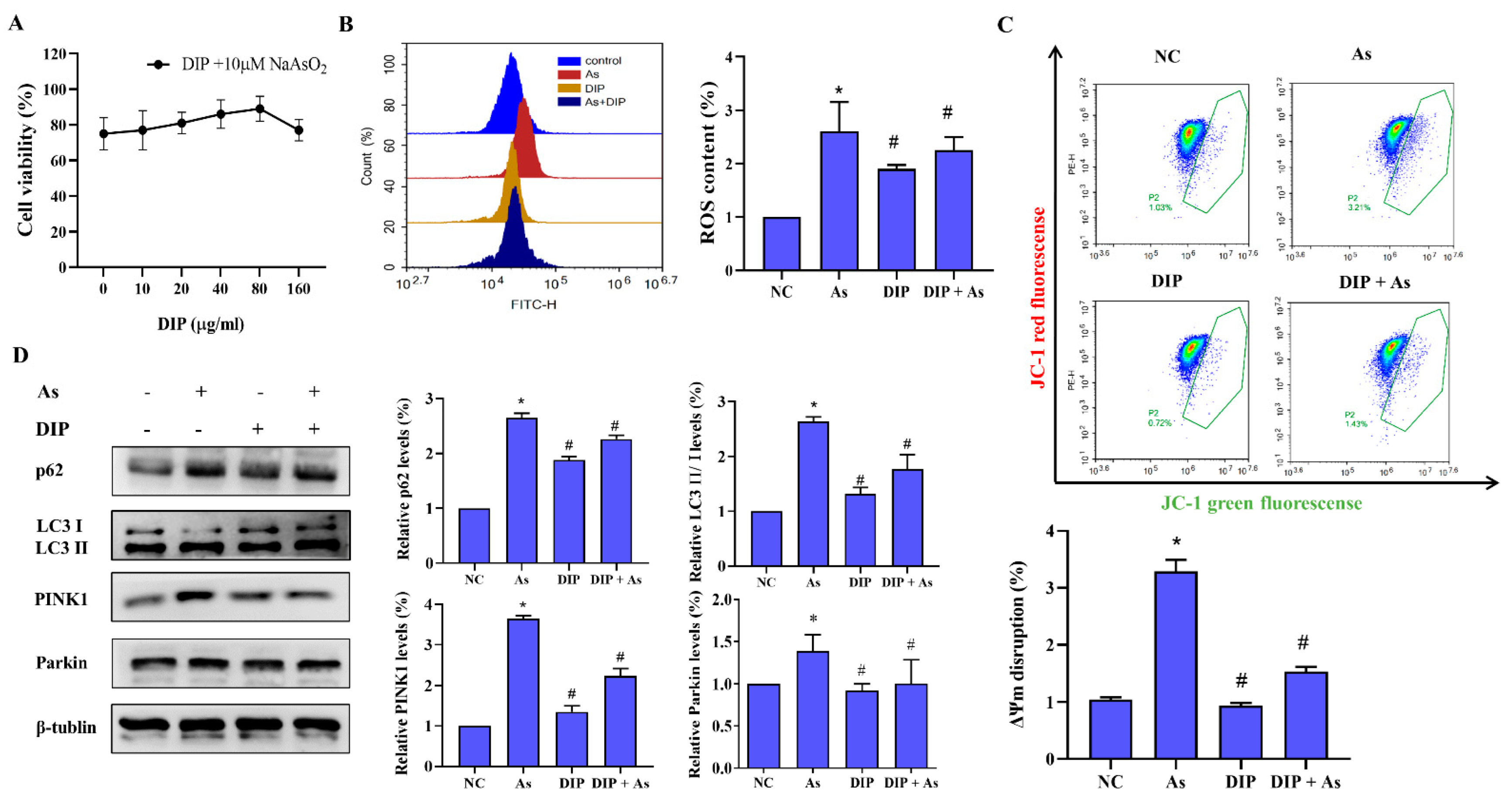
3.3. DIP Inhibited NaAsO2-Induced ROS and Mitophagy in L-02 Cells
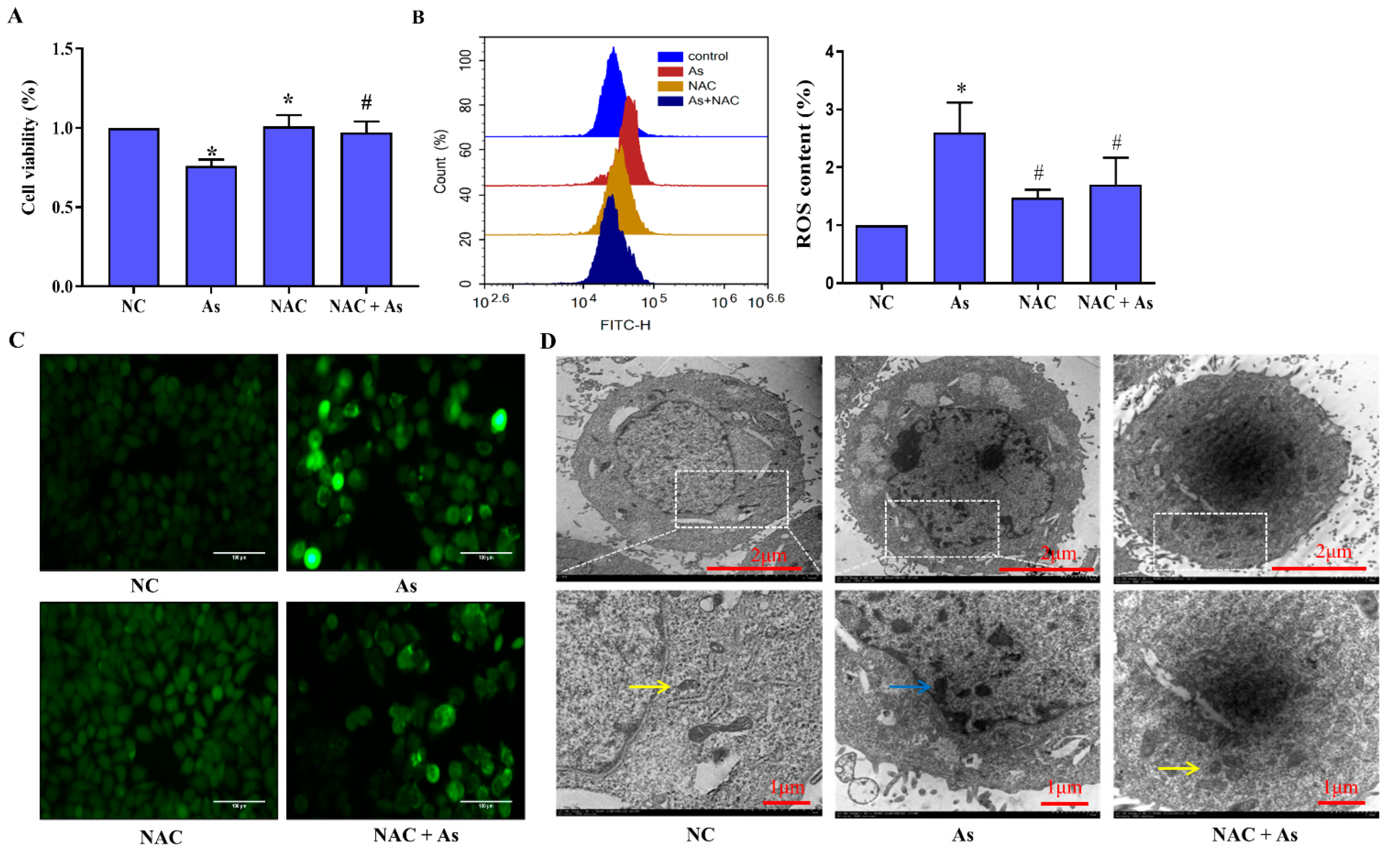
3.4. Effects of Scavenging ROS on NaAsO2-Induced Mitochondrial Structural Damage
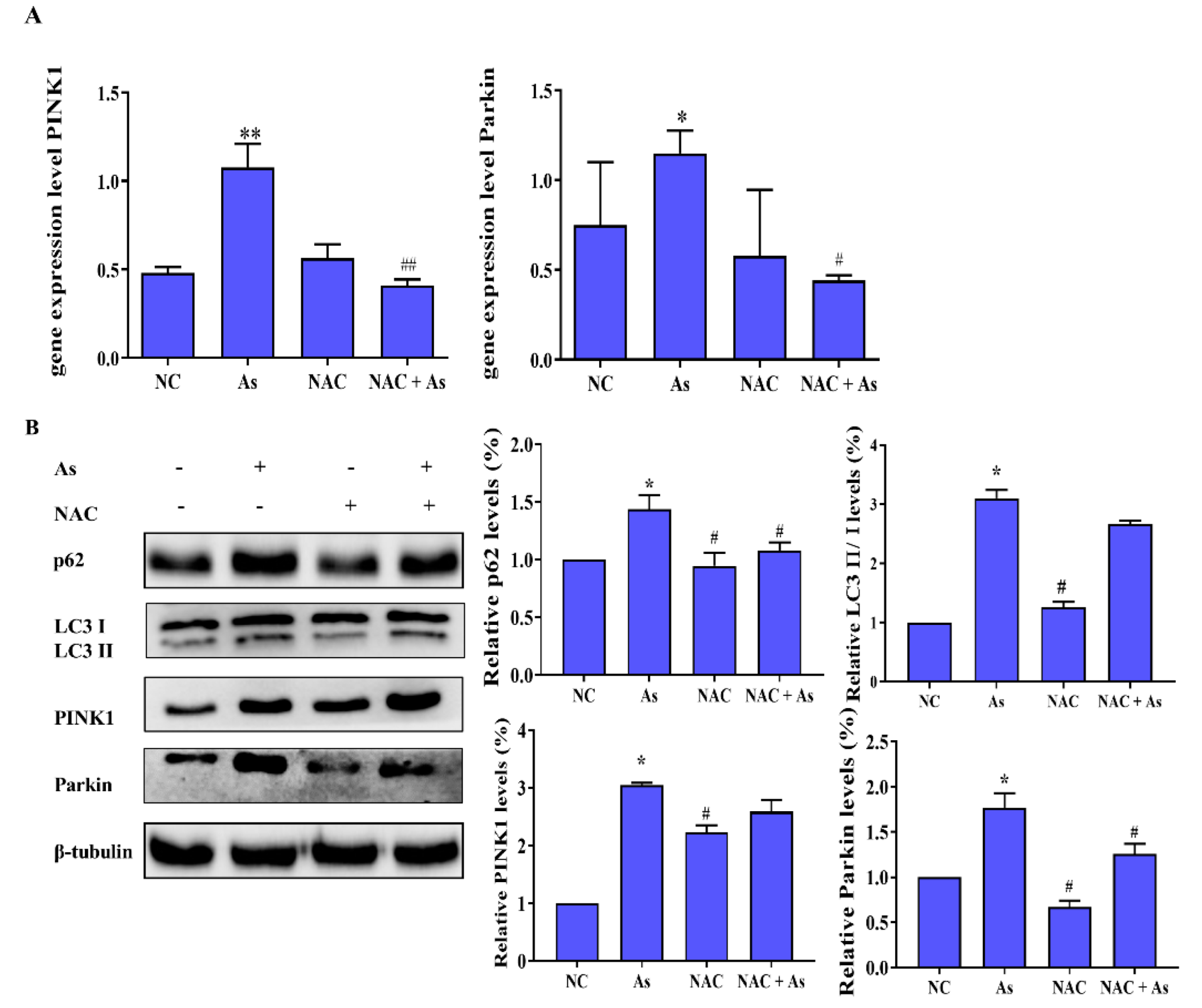
3.5. Effects of ROS Clearance on Mitophagy-Related Genes and Proteins in the PINK1/Prkin Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shrestha, A.; Sharma, S.; Gerold, J.; Erismann, S.; Sagar, S.; Koju, R.; Schindler, C.; Odermatt, P.; Utzinger, J.; Cissé, G. Water Quality, Sanitation, and Hygiene Conditions in Schools and Households in Dolakha and Ramechhap Districts, Nepal: Results from A Cross-Sectional Survey. Int. J. Environ. Res. 2017, 14, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akanda, M.; Kim, I.; Ahn, D.; Tae, H.; Tian, W.; Nam, H.; Choo, B.; Park, B. Geranium koreanumIn Vivo and In Vitro Hepatoprotective Effects of Methanolic Extract via Downregulation of MAPK/Caspase-3 Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 8137627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Luo, P.; Zou, Z.; Wang, Q.; Yao, M.; Yu, C.; Wei, S.; Sun, B.; Zhu, K.; Zeng, Q.; et al. Alterations of arsenic levels in arsenicosis residents and awareness of its risk factors: A population-based 20-year follow-up study in a unique coal-borne arsenicosis County in Guizhou, China. Environ. Int. 2019, 129, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xi, S. A review on arsenic carcinogenesis: Epidemiology, metabolism, genotoxicity and epigenetic changes. Regul. Toxicol. Pharmacol. 2018, 99, 78–88. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Q.; Wang, D.; Luo, F.; Liu, X.; Xue, J.; Yang, P.; Xu, H.; Lu, J.; Zhang, A.; et al. MicroRNA-191, regulated by HIF-2alpha, is involved in EMT and acquisition of a stem cell-like phenotype in arsenite-transformed human liver epithelial cells. Toxicol. Vitr. 2017, 48, 128–136. [Google Scholar] [CrossRef]
- Ma, X.; McKeen, T.; Zhang, J.; Ding, W. Role and Mechanisms of Mitophagy in Liver Diseases. Cells 2020, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Chen, M.; Wei, J. Involvement of phosphatase and tensin homolog-induced putative kinase 1/Parkin-mediated autophagy in angiotensin II-induced cardiac hypertrophy in C57BL/6 mice. J. Int. Med Res. 2020, 48, 300060519896143. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wang, T.; Liu, Y.; Li, X.; Xu, S.; Wu, C.; Zou, H.; Cao, M.; Jin, G.; Lang, J.; et al. Mitophagy promotes sorafenib resistance through hypoxia-inducible ATAD3A dependent Axis. J. Exp. Clin. Cancer Res. 2020, 39, 274. [Google Scholar] [CrossRef]
- Duan, T.; Hu, T.; Wu, C.; Yeh, Y.; Lu, J.; Zhang, Q.; Li, X.; Jian, W.; Luo, P. PINK1/Parkin-mediated mitophagy is involved in NaAsO-induced apoptosis of human hepatic cells through activation of ERK signaling. Toxicol. Vitr. 2020, 66, 104857. [Google Scholar] [CrossRef]
- Springer, M.; Macleod, K. In Brief: Mitophagy: Mechanisms and role in human disease. J. Pathol. 2016, 240, 253–255. [Google Scholar] [CrossRef]
- Fu, Y.; Lin, S.; Lu, M.; Wei, S.; Zhou, J.; Zhao, L.; Zhang, Q.; Lin, D.; Liu, Y.; Chen, H.; et al. Dictyophora indusiataQuantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Using an Improved Fluorometric Assay. Polymers 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gui, X.; Wu, L.; Tian, S.; Wang, H.; Xie, L.; Wu, W. Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: A metabonomics study. BMC Neurol. 2020, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Luo, P.; Shen, X.C.; Jiang, F.F.; Zeng, D.; Fang, Y.; Long, Y.G.; Zhang, A.H. Effects of intervention by Dictyophora polysaccharide on liver function in early and late stage of arsenic poisoning among rats. J. Environ. Health 2016, 33, 1039–1043. [Google Scholar]
- Hu, T.; Shen, L.; Huang, Q.; Wu, C.; Zhang, H.; Zeng, Q.; Wang, G.; Wei, S.; Zhang, S.; Zhang, J.; et al. Protective Effect of Dictyophora Polysaccharides on Sodium Arsenite-Induced Hepatotoxicity: A Proteomics Study. Front. Pharmacol. 2021, 12, 749035. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, K.; Pan, D.; Pan, X.; Yang, H.; Xiao, J.; Shen, X.; Luo, P. Inhibition Effect of Dictyophora Polysaccharides on Human Hepatocellular Carcinoma Cell Line HCC-LM3. Med. Sci. Monit. 2020, 26, e918870. [Google Scholar] [CrossRef]
- Wang, W.; Song, X.; Zhang, J.; Li, H.; Liu, M.; Gao, Z.; Wang, X.; Jia, L. Antioxidation, hepatic- and renal-protection of water-extractable polysaccharides by Dictyophora indusiata on obese mice. Int. J. Biol. Macromol. 2019, 134, 290–301. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Yu, B.; Lei, Z.; Wu, X.; Yang, J.; Ren, J. Cell-based evaluation of a novel Dictyophora indusiata polysaccharide against oxidative-induced erythrocyte hemolysis. Cell. Mol. Biol. 2016, 62, 38–44. [Google Scholar]
- Habtemariam, S. Dictyophora indusiataThe Chemistry, Pharmacology and Therapeutic Potential of the Edible Mushroom (ex.) Fischer (Synn.). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Ma, C.; Hu, M.; Wang, Y.; Ma, F.; Tao, N.; Qin, Z. A polysaccharide from Dictyophora indusiata inhibits the immunosuppressive function of cancer-associated fibroblasts. Cell Biochem. Funct. 2017, 35, 414–419. [Google Scholar] [CrossRef]
- Kanwal, S.; Joseph, T.; Owusu, L.; Xiaomeng, R.; Meiqi, L.; Yi, X. A Polysaccharide Isolated from Dictyophora indusiata Promotes Recovery from Antibiotic-Driven Intestinal Dysbiosis and Improves Gut Epithelial Barrier Function in a Mouse Model. Nutrients 2018, 10, 1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ji, X.; Yan, M.; Chen, X.; Kang, M.; Teng, L.; Wu, X.; Chen, J.; Deng, C. Protective effect and mechanism of polysaccharide from Dictyophora indusiata on dextran sodium sulfate-induced colitis in C57BL/6 mice. Int. J. Biol. Macromol. 2019, 140, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zeng, Q.; Luo, P.; Sun, B.; Liang, B.; Wei, S.; Xu, Y.; Wang, Q.; Liu, Q.; Zhang, A. Assessing the risk of coal-burning arsenic-induced liver damage: A population-based study on hair arsenic and cumulative arsenic. Environ. Sci. Pollut. Res. Int. 2021, 28, 50489–50499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liu, T.; Deng, X.; Ding, W.; Yue, Z.; Yang, W.; Lv, X.; Li, W. Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy in type 2 diabetic rats. Respir. Res. 2021, 22, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Y.; Chu, B.; Liu, N.; Chen, S.; Wang, J. Lactobacillus rhamnosus GR-1 Prevents -Induced Apoptosis Through PINK1/Parkin-Mediated Mitophagy in Bovine Mastitis. Front. Immunol. 2021, 12, 715098. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Gong, L.; Chen, L.; Xu, M.; Abou-Hamdan, H.; Tang, M.; Désaubry, L.; Song, Z. PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis. Front. Immunol. 2020, 16, 419–434. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C. Astrocytes autophagy in aging and neurodegenerative disorders. Biomed. Pharmacother. 2020, 122, 109691. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, Q.; Ma, W.; Zhang, Q.; Qiu, J.; Gu, X.; Yang, H.; Sun, H. Skeletal Muscle Atrophy Was Alleviated by Salidroside Through Suppressing Oxidative Stress and Inflammation During Denervation. Front. Pharmacol. 2019, 10, 997. [Google Scholar] [CrossRef]
- Georgakopoulos, N.; Wells, G.; Campanella, M. The pharmacological regulation of cellular mitophagy. Nat. Chem. Biol. 2017, 13, 136–146. [Google Scholar] [CrossRef]
- Salazar, C.; Ruiz-Hincapie, P.; Ruiz, L. The Interplay among PINK1/PARKIN/Dj-1 Network during Mitochondrial Quality Control in Cancer Biology: Protein Interaction Analysis. Cells 2018, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, J.; Han, S.; Fan, Y.; Guo, L.; Aziz, K.; Nowsheen, S.; Kim, S.; Park, S.; Luo, Q.; et al. The AMPK-Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome. Nat. Cell Biol. 2019, 21, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.; Moreira, P.; Ambrósio, A.; Alves, C. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Rüb, C.; Wilkening, A.; Voos, W. Mitochondrial quality control by the Pink1/Parkin system. Cell Tissue Res. 2017, 367, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Soya, N.; Truong, L.; Croteau, N.; Lukacs, G.; Trempe, J. PINK1 autophosphorylation is required for ubiquitin recognition. Eur. Mol. Biol. Organ. 2018, 19, e44981. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Wu, L.; Wu, X.; Huang, Y.; Liu, B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int. J. Biol. Macromol. 2017, 103, 175–181. [Google Scholar] [CrossRef]
- Liau, P.; Wu, M.; Lee, C. Scutellaria baicalensisInhibitory Effects of Root Extract on Linoleic Acid Hydroperoxide-induced Lung Mitochondrial Lipid Peroxidation and Antioxidant Activities. Molecules 2019, 24, 2143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Kume, S.; Koya, D. Autophagy: A Novel Therapeutic Target for Diabetic Nephropathy. Diabetes Metab. J. 2015, 39, 451–460. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Lu, J.; Wu, C.; Duan, T.; Luo, P. Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS. Molecules 2022, 27, 2806. https://doi.org/10.3390/molecules27092806
Hu T, Lu J, Wu C, Duan T, Luo P. Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS. Molecules. 2022; 27(9):2806. https://doi.org/10.3390/molecules27092806
Chicago/Turabian StyleHu, Ting, Ju Lu, Changyan Wu, Tianxiao Duan, and Peng Luo. 2022. "Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS" Molecules 27, no. 9: 2806. https://doi.org/10.3390/molecules27092806
APA StyleHu, T., Lu, J., Wu, C., Duan, T., & Luo, P. (2022). Dictyophora Polysaccharide Attenuates As-Mediated PINK1/Parkin Pathway-Induced Mitophagy in L-02 Cell through Scavenging ROS. Molecules, 27(9), 2806. https://doi.org/10.3390/molecules27092806





