Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy
Abstract
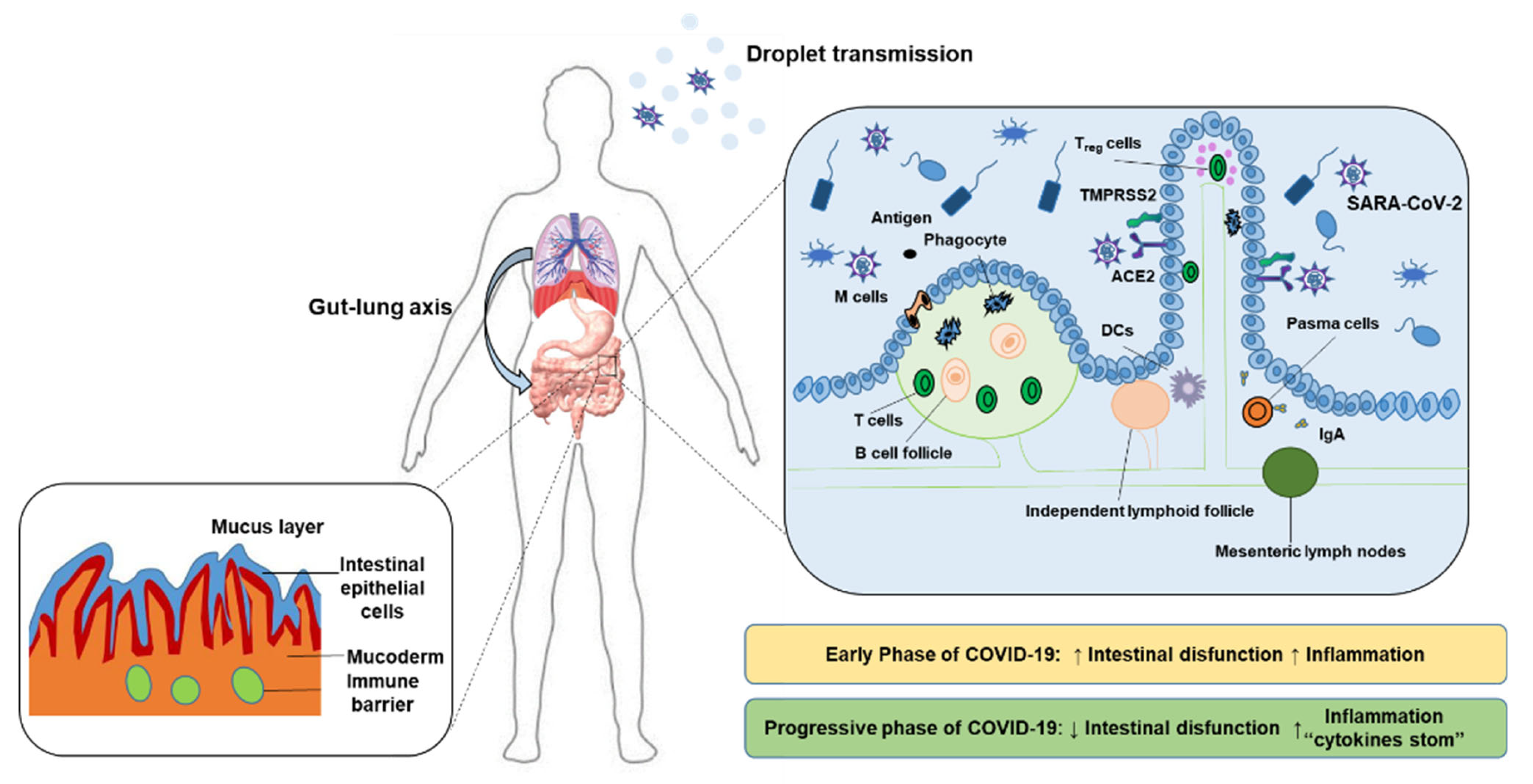
1. Introduction
2. Polysaccharides Ameliorate COVID-19
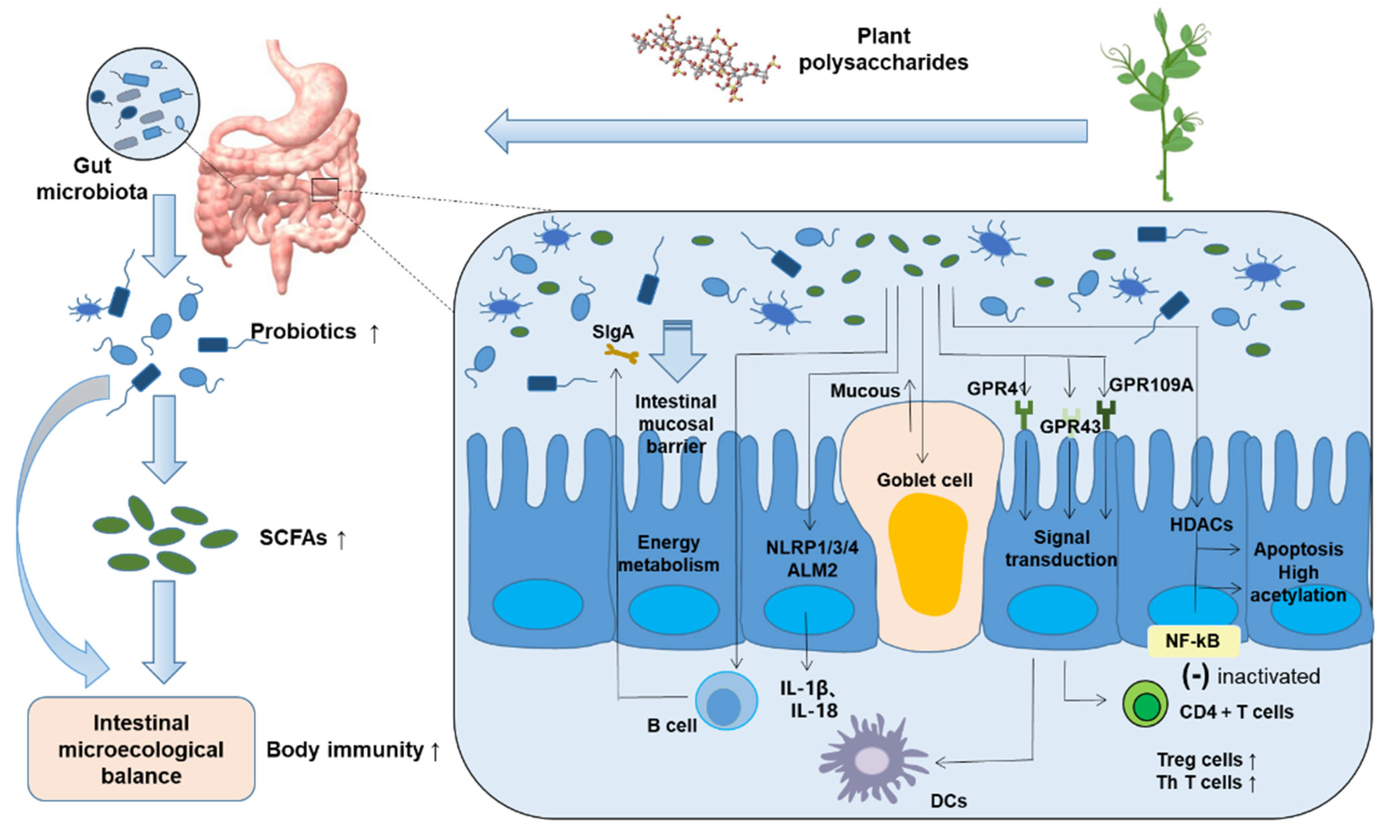
3. Mechanism of Polysaccharides Regulating Gut Flora
3.1. Increase the Number and Types of Probiotics in Intestinal Microecology
3.2. An Important Metabolite of Gut Flora—Short-Chain Fatty Acids
4. Gut Flora Regulates Host Immune System
5. Dietary Intervention to Maintain Intestinal Microecosystem Stability and Prevent COVID-19
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Hamid, S.; Mir, M.Y.; Rohela, G.K. Novel coronavirus disease (COVID-19): A pandemic (epidemiology, pathogenesis and potential therapeutics). New Microbes New Infect. 2020, 35, 100679. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical evidence does not support corticosteroid treatment for 2019—nCoV lung injury. Lancet 2020, 395, 473–485. [Google Scholar] [CrossRef]
- Dhar, D.; Mohanty, A. Gut microbiota and COVID-19—possible link and implications. Virus Res. 2020, 285, 198018. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Lankelma, J.M.; Birnie, E.; Weehuizen, T.A.F.; Scicluna, B.P.; Belzer, C.; Houtkooper, R.H.; Roelofs, J.J.T.H.; de Vos, A.F.; van der Poll, T.; Budding, A.E. The gut microbiota as a modulator of innate immunity during melioidosis. PLoS Neglect. Trop Dis. 2020, 11, e0005548. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Riksen, N.P.; Netea, M.G. Immunometabolic control of trained immunity. Mol. Aspects Med. 2021, 77, 100897. [Google Scholar] [CrossRef]
- Mörkl, S.; Butler, M.I.; Holl, A.; Cryan, J.F.; Dinan, T.G. Probiotics and the microbiota-gut-brain axis: Focus on Psychiatry. Curr. Nutr. Rep. 2020, 9, 171–182. [Google Scholar] [CrossRef]
- Mujagic, Z.; de Vos, P.; Boekschoten, M.V.; Govers, C.; Pieters, H.H.; de Wit, N.J.; Bron, P.A.; Masclee, A.A.; Troost, F.J. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; A randomized double-blind placebo controlled trial. Sci. Rep. 2017, 7, 40128. [Google Scholar] [CrossRef] [PubMed]
- Del Piano, M.; Carmagnola, S.; Anderloni, A.; Andorno, S.; Ballarè, M.; Balzarini, M.; Montino, F.; Orsello, M.; Pagliarulo, M.; Sartori, M. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: A double-blind, randomized, placebo-controlled study. J. Clin. Gastroenterol. 2010, 44, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Akour, A. Probiotics and COVID-19: Is there any link? Lett. Appl. Microbiol. 2020, 71, 229–234. [Google Scholar] [CrossRef]
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- Yuen, K.S.; Ye, Z.W.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020, 10, 40. [Google Scholar] [CrossRef]
- Villapol, S. Gastrointestinal symptoms associated with COVID-19: Impact on the gut microbiome. Transl. Res. 2020, 226, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, S.K.; Vitale, E.; Makarewicz, W. COVID-gastrointestinal and gut microbiota-related aspects. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10853–10859. [Google Scholar]
- Bozkurt, H.S.; Quigley, E.M. The probiotic Bifidobacterium in the management of Coronavirus: A theoretical basis. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420961304. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; de Carvalho, R.N., Jr. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef]
- Yim, S.K.; Kim, K.; Kim, I.H.; Chun, S.H.; Oh, T.H.; Kim, J.U.; Kim, J.W.; Jung, W.H.; Moon, H.S.; Ku, B.S. Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera In Vitro. Mar. Drugs 2021, 19, 219. [Google Scholar] [CrossRef]
- Mohan, K.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N.; Ganesan, A.; Velmurugan, K.; Sathishkumar, P.; Jayakumar, R.; Seedevi, P. Trends in the extraction, purification, characterisation and biological activities of polysaccharides from tropical and sub-tropical fruits—A comprehensive review. Carbohydr. Polym. 2020, 238, 116185. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Bezerra, I.L.; Caillot, A.R.C.; Palhares, L.C.G.F.; Santana-Filho, A.P.; Chavante, S.F.; Sassaki, G.L. Structural characterization of polysaccharides from Cabernet Franc, Cabernet Sauvignon and Sauvignon Blanc wines: Anti-inflammatory activity in LPS stimulated RAW 264.7 cells. Carbohydr. Polym. 2018, 186, 91–99. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides-dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2021, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietay fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Tan, X.; Zhou, X.; Chen, H.G. Structure-activity relationship of plant polysaccharides. Zhongguo Zhongyao Zazhi 2017, 42, 4104–4109. [Google Scholar]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 132, 143–160. [Google Scholar] [CrossRef]
- Xie, X.; He, Y.; Li, H.; Yu, D.; Na, L.; Sun, T.; Zhang, D.; Shi, X.; Xia, Y.; Jiang, T.; et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition 2019, 61, 132–142. [Google Scholar] [CrossRef]
- Vanegas, S.M.; Meydani, M.; Barnett, J.B.; Goldin, B.; Kane, A.; Rasmussen, H.; Brown, C.; Vangay, P.; Knights, D.; Jonnalagadda, S.; et al. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017, 105, 635–650. [Google Scholar] [CrossRef]
- Dongarrà, M.L.; Rizzello, V.; Muccio, L.; Fries, W.; Cascio, A.; Bonaccorsi, I.; Ferlazzo, G. Mucosal immunology and probiotics. Curr. Allergy Asthma Rep. 2013, 13, 19–26. [Google Scholar] [CrossRef]
- Maldonado, G.C.; Cazorla, S.I.; Lemme, D.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed]
- Karakula-Juchnowicz, H.; Rog, J.; Juchnowicz, D.; Łoniewski, I.; Skonieczna-Żydecka, K.; Krukow, P.; Futyma-Jedrzejewska, M.; Kaczmarczyk, M. The study evaluating the effect of probiotic supplementation on the mental status, inflammation, and intestinal barrier in major depressive disorder patients using gluten-free or gluten-containing diet (SANGUT study): A 12-week, randomized, double-blind, and placebo-controlled clinical study protocol. Nutr. J. 2019, 18, 50. [Google Scholar] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysachharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Holota, Y.; Dovbynchuk, T.; Kaji, I.; Vareniuk, I.; Dzyubenko, N.; Chervinska, T.; Zakordonets, L.; Stetska, V.; Ostapchenko, L.; Serhiychuk, T.; et al. The long-term consequences of antibiotic therapy: Role of colonic short-chain fatty acids (SCFA) system and intestinal barrier integrity. PLoS ONE 2019, 14, e0220642. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Perera, A.P.; Vemuri, R.; Gondalia, S.V.; Beale, D.J.; Karpe, A.V.; Shastri, S.; Basheer, W.; Southam, B.; Eri, R.; et al. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur. J. Nutr. 2020, 59, 3669–3689. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty (SCFAs)-mediated gut epithelial and immune regulatiom and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mices. Gastroenterology 2013, 145, 396–406. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 111–119. [Google Scholar]
- Smelt, M.J.; de Haan, B.J.; Bron, P.A.; van Swam, I.; Meijerink, M.; Wells, J.M.; Faas, M.M.; de Vos, P. Probiotics can generate foxp3 T-cell responses in the small intestine and simultaneously inducing CD4 and CD8 T cell activation in the large intestine. PLoS ONE 2013, 8, e68952. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fibre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Otake, N.; Nakashima, S.; Matsuoka, T.; Aoe, S. Ingestion of High β-Glucan Barley Flour Enhances the Intestinal Immune System of Diet-Induced Obese Mice by Prebiotic Effects. Nutrients 2021, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 736739. [Google Scholar] [CrossRef]
- Huang, W.; Man, Y.; Gao, C.; Zhou, L.; Gu, J.; Xu, H.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.; et al. Short-Chain Fatty Acids Ameliorate Diabetic Nephropathy via GPR43-Mediated Inhibition of Oxidative Stress and NF-κB Signaling. Oxid Med. Cell Longevity 2020, 2020, 4074832. [Google Scholar] [CrossRef] [PubMed]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The gut-lung axis in respiratory disease. Ann. Am. Thorac Soc. 2015, 12, 150–156. [Google Scholar]
- Kusumo, P.D.; Bela, B.; Wibowo, H.; Munasir, Z.; Surono, I.S. Lactobacillus plantarum IS-10506 supplementation increase faecal SIgA and immune response in children younger than two years. Benefic Microbes 2019, 10, 245–252. [Google Scholar] [CrossRef]
- Li, K.L.; Wang, B.Z.; Li, Z.P.; Li, Y.L.; Liang, J.J. Alterations of intestinal flora and the effects of probiotics in children with recurrent respiratory tract infection. World J. Pediatr. 2019, 15, 255–261. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Chang, C.S.; Kao, C.Y. Current understanding of the gut microbiota shaping mechanisms. J. Biomed. Sci. 2019, 21, 59. [Google Scholar] [CrossRef]
- Ganal, S.C.; Sanos, S.L.; Kallfass, C.; Oberle, K.; Johner, C.; Kirschning, C.; Lienenklaus, S.; Weiss, S.; Staeheli, P.; Aichele, P.; et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity 2012, 37, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Marchesi, J.R.; McDonald, J.A.K.; Pass, D.A.; Masetti, G.; Michael, D.R.; Plummer, S.; Jack, A.A.; Davies, T.S.; Hughes, T.R.; et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes 2021, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Kidane, D. Gut microbiota imbalance and base excision repair dynamics in colon cancer. J. Cancer 2016, 7, 1421–1430. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fong, W.; Li, Q.; Yu, J. Gut microbiota modulation: A novel strategy for prevention and treatment of colorectal cancer. Oncogene 2020, 39, 4925–4943. [Google Scholar] [CrossRef]
- Guldris, S.C.; Parra, E.G.; Amenós, A.C. Gut microbiota in chronic kidney disease. J. Bras. Nefrol. 2017, 37, 9–19. [Google Scholar] [CrossRef]
- Sang, S.; Idehen, E.; Zhao, Y.; Chu, Y. Emerging science on whole grain intake and inflammation. Nutr. Rev. 2020, 78, 21–28. [Google Scholar] [CrossRef]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef]
- Deehan, E.C.; Walter, J. The fiber gap and the disappearing gut microbiome: Implications for human nutrition. Trends Endocrinol. Metab. 2016, 27, 239–242. [Google Scholar] [CrossRef]
- Yuan, H.; Jiang, S.; Liu, Y.; Daniyal, M.; Jian, Y.; Peng, C.; Shen, J.; Liu, S.; Wang, W. The flower head of Chrysanthemum morifolium Ramat. (Juhua): A paradigm of flowers serving as Chinese dietary herbal medicine. J. Ethnopharmacol. 2020, 261, 113043. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Wang, S.; Jiao, Z.; Sun, T.; Liu, T.; Song, K. Characterization and anti-tumor bioactivity of astragalus polysaccharides by immunomodulation. Int. J. Biol. Macromol. 2019, 145, 985–997. [Google Scholar] [CrossRef]
- Wang, H.; Lian, P.; Niu, X.; Zhao, L.; Mu, X.; Feng, B.; Li, J.; Liang, Z.; Qiao, J. TLR4 deficiency reduces pulmonary resistance to streptococcus pneumoniae in gut microbiota-disrupted mice. PLoS ONE 2018, 13, e0209183. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.F.; Zhang, Y.Z.; Wu, X.; Yin, Y.L.; Tan, Z.L.; Feng, Y.; Yan, F.Y.; Bo, M.J.; Huang, R.L.; Li, T.J. Fermentation characterization of chinese yam polysaccharide and its effects on the gut microbiota of rats. Int. J. Microbiol. 2009, 2009, 598152. [Google Scholar] [CrossRef] [PubMed]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C.; Gnanapavan, S. Evidence of clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. 2018, 25, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ren, R.; Zhang, Q.; Wu, J.; Zhang, Y.; Xue, M.; Yin, D.; Yang, Y. Coptis chinensis Franch polysaccharides provide a dynamically regulation on intestinal microenvironment, based on the intestinal flora and mucosal immunity. J. Ethnopharmacol. 2021, 267, 113542. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, X.; Luo, S. Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy. Molecules 2022, 27, 2773. https://doi.org/10.3390/molecules27092773
Tang M, Cheng L, Liu Y, Wu Z, Zhang X, Luo S. Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy. Molecules. 2022; 27(9):2773. https://doi.org/10.3390/molecules27092773
Chicago/Turabian StyleTang, Mengsheng, Lu Cheng, Yanan Liu, Zufang Wu, Xin Zhang, and Songmei Luo. 2022. "Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy" Molecules 27, no. 9: 2773. https://doi.org/10.3390/molecules27092773
APA StyleTang, M., Cheng, L., Liu, Y., Wu, Z., Zhang, X., & Luo, S. (2022). Plant Polysaccharides Modulate Immune Function via the Gut Microbiome and May Have Potential in COVID-19 Therapy. Molecules, 27(9), 2773. https://doi.org/10.3390/molecules27092773





