Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines
Abstract
1. Introduction
2. Results
2.1. Yields and Total Phenolic Contents (TPC) and Antioxidant Activities of Extracts from C. speciosa Roots
2.2. Fractionation by Column Chromatography
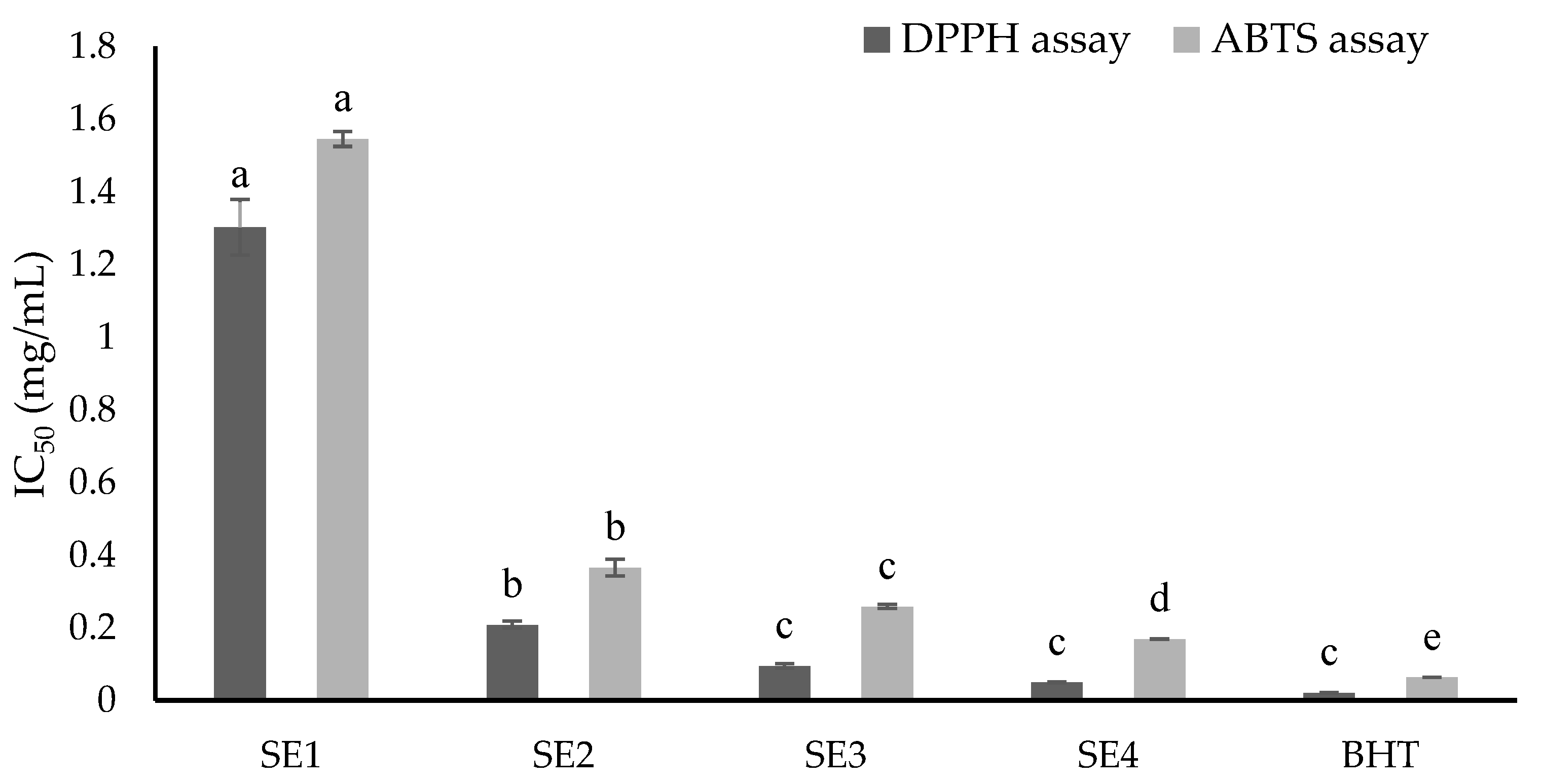
2.3. Antioxidant Capacities of Fractions from SE Extracts
2.4. Cytotoxic Activities of Fractions from SE Extracts
2.5. Apoptosis-Inducing Activities of Fractions from SE Extracts
2.6. Identification of Phytochemical Components of Active Fractions from SE Extract by LC-ESI-MS/MS
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Materials
4.1.2. Chemicals and Cell Lines
4.2. Sample Preparation and Extraction
4.3. Determination of Total Phenolic Content
4.4. In Vitro Antioxidant Capacity Assays
4.5. MTT Assays
4.6. Annexin V Assay
4.7. Identification of Phytochemical Components by Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (LC-ESI-MS/MS)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Hanamura, I. Gain/amplification of chromosome Arm 1q21 in multiple myeloma. Cancers 2021, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Seymour, J.F.; Wang, M.L. Mantle cell lymphoma. J. Clin. Oncol. 2016, 34, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Karnan, S.; Hanamura, I.; Ota, A.; Takasugi, S.; Nakamura, A.; Takahashi, M.; Uchino, K.; Murakami, S.; Wahiduzzaman, M.; Vu, Q.L.; et al. CD52 is a novel target for the treatment of FLT3-ITD-mutated myeloid leukemia. Cell Death Discov. 2021, 7, 121. [Google Scholar] [CrossRef] [PubMed]
- Ambade, A.; Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T.D. Active nature based ingredients for drug discovery with pivotal role of clinical efficacy: Review and prospective. J. Mod. Med. Chem. 2020, 8, 4–18. [Google Scholar] [CrossRef]
- Ding, P.; Qiu, J.; Ying, G.; Dai, L. Chemical constituents of Millettia speciosa. Chin. Herb. Med. 2014, 6, 332–334. [Google Scholar] [CrossRef]
- Fu, M.; Xiao, G.; Xu, Y.; Wu, J.; Chen, Y.; Qiu, S.-X. Chemical constituents from roots of Millettia speciosa. Chin. Herb. Med. 2016, 8, 385–389. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, Y.J.; Chen, X.; Luo, S.Y.; Pu, L.; Li, F.Z.; Zong, M.H.; Lou, W.Y. A novel polysaccharide from the roots of Millettia speciosa Champ.: Preparation, structural characterization and immunomodulatory activity. Int. J. Biol. Macromol. 2020, 145, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yin, T.; Ling, X.; Liang, H.; Zhao, Y. Interactions between thrombin and natural products of Millettia speciosa Champ. using capillary zone electrophoresis. Electrophoresis 2008, 29, 3391–3397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, X.; Liao, J.; Guo, H.; Yu, X.; Liang, J.; Zhang, X.; Su, Z.; Zhang, X.; Zeng, H. Antifatigue effect of Millettiae speciosae Champ. (leguminosae) extract in mice. Trop. J. Pharm. Res. 2015, 14, 479–485. [Google Scholar] [CrossRef]
- Jena, R.; Rath, D.; Rout, S.S.; Kar, D.M. A review on genus Millettia: Traditional uses, phytochemicals and pharmacological activities. Saudi Pharm. J. 2020, 28, 1686–1703. [Google Scholar] [CrossRef]
- Yu, D.; Liang, X. Characterization and identification of isoflavonoids in the roots of Millettia speciosa Champ. by UPLC-Q-TOF-MS/MS. Curr. Pharm. Anal. 2019, 15, 580–591. [Google Scholar] [CrossRef]
- Ayesh, B.M.; Abed, A.A.; Faris, D.M. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. extracts. BMC Res. Notes 2014, 7, 612. [Google Scholar] [CrossRef]
- Weerapreeyakul, N.; Nonpunya, A.; Barusrux, S.; Thitimetharoch, T.; Sripanidkulchai, B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin. Med. 2012, 7, 15. [Google Scholar] [CrossRef]
- Pfeffer, C.; Singh, A. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Koepke, J.I.; Wood, C.S.; Terlecky, L.J.; Walton, P.A.; Terlecky, S.R. Progeric effects of catalase inactivation in human cells. Toxicol. Appl. Pharmacol. 2008, 232, 99–108. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.-D.; Thuy, N.T.D.; Trang, L.T.; Huong, C.T.; Andriana, Y.; Tuyen, P.T. Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum Bark. Molecules 2019, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Mishra, A.K.; Mishra, A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell. Mol. Biol. 2012, 58, 142–147. [Google Scholar]
- Pietta, P.-G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Chaouhan, H.S.; Li, X.; Sun, K.-T.; Wang, I.-K.; Yu, T.-M.; Yu, S.-H.; Chen, K.-B.; Lin, W.-Y.; Li, C.-Y. Calycosin alleviates paraquat-induced neurodegeneration by improving mitochondrial functions and regulating autophagy in a drosophila model of Parkinson’s disease. Antioxidants 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Wang, D.; Zhou, D. Structural characterization and evaluation of the antioxidant activity of phenolic compounds from Astragalus taipaishanensis and their structure-activity relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef]
- Alrushaid, S.; Davies, N.; Martinez, S.; Sayre, C. Pharmacological characterization of liquiritigenin, a chiral flavonoid in licorice. Res. Pharm. Sci. 2016, 11, 355–365. [Google Scholar]
- Anh, L.H.; Quan, N.V.; Lam, V.Q.; Iuchi, Y.; Takami, A.; Teschke, R.; Xuan, T.D. Antioxidant, anti-tyrosinase, anti-α-amylase, and cytotoxic potentials of the invasive weed Andropogon virginicus. Plants 2020, 10, 69. [Google Scholar] [CrossRef]
- Anh, L.H.; Xuan, T.D.; Thuy, N.T.D.; Quan, N.V.; Trang, L.T. Antioxidant and α-amylase inhibitory activities and phytocompounds of Clausena indica Fruits. Medicines 2020, 7, 10. [Google Scholar] [CrossRef]
- Wcislo, G.; Szarlej-Wcislo, K. Colorectal cancer prevention by wheat consumption. In Wheat and Rice in Disease Prevention and Health; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 91–111. [Google Scholar]
- Gao, J.; Liu, Z.J.; Chen, T.; Zhao, D. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm. Biol. 2014, 52, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wen, B.; Zhang, Z.; Cai, J. Ergosterol peroxide: An effect-directed detecting method from Anoectochilus elwesii and evaluation of anticancer activity. Nat. Prod. Res. 2021, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Y.; Yang, F.L.; Li, L.H.; Rao, Y.K.; Ju, T.C.; Wong, W.T.; Hsieh, C.Y.; Pivkin, M.V.; Hua, K.F.; Wu, S.H. Ergosterol peroxide from marine fungus Phoma sp. induces ROS-dependent apoptosis and autophagy in human lung adenocarcinoma cells. Sci. Rep. 2018, 8, 17956. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts cytotoxic activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM-p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Aratanechemuge, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Komiya, T. Induction of apoptosis by maackiain and trifolirhizin (maackiain glycoside) isolated from sanzukon (Sophora Subprostrate Chen et T. Chen) in human promyelotic leukemia HL-60 cells. Oncol. Rep. 2004, 12, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Maurya, R.; Mishra, D.P. Medicarpin, a legume phytoalexin sensitizes myeloid leukemia cells to TRAIL-induced apoptosis through the induction of DR5 and activation of the ROS-JNK-CHOP pathway. Cell Death Dis. 2014, 5, e1465. [Google Scholar] [CrossRef]
- Veeramuthu, D.; Raja, W.R.T.; Al-Dhabi, N.A.; Savarimuthu, I. Flavonoids: Anticancer properties. In Flavonoids: From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin elicits anti-cancer effects in tumour primary cultures and cell lines in vitro. Basic Clin. Pharmacol. Toxicol. 2009, 105, 425–432. [Google Scholar] [CrossRef]
- Cilla, A.; González-Sarrías, A.; Tomás-Barberán, F.A.; Espín, J.C.; Barberá, R. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chem. 2009, 114, 813–820. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Teschke, R. Potential hepatotoxins found in herbal medicinal products: A systematic review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Anh, L.H.; Tran, H.-D. Bio-guided isolation of prospective bioactive constituents from roots of Clausena indica (Dalzell) Oliv. Molecules 2019, 24, 4442. [Google Scholar] [CrossRef] [PubMed]


| Sample | TPC (mg GAE/g DW) | DPPH Assay IC50 (mg/mL) | ABTS Assay IC50 (mg/mL) |
|---|---|---|---|
| SM | 1.77 ± 0.29 a | 0.79 ± 0.06 c | 1.75 ± 0.14 c |
| SH | 0.06 ± 0.01 c | 0.82 ± 0.04 c | 1.84 ± 0.05 c |
| SE | 0.09 ± 0.02 c | 0.20 ± 0.01 c | 0.39 ± 0.01 d |
| SB | 0.18 ± 0.01 b | 2.43 ± 0.08 b | 3.98 ± 0.06 b |
| SW | 0.84 ± 0.05 b | 15.20 ± 0.76 a | 5.41 ± 0.25 a |
| BHT | - | 0.02 ± 0.00 d | 0.08 ± 0.00 e |
| No. | Fractions | Mobile Solvent | Code | Amount (g) | Yield (%) |
|---|---|---|---|---|---|
| 1 | F1-F20 | C 100%; CM 0.1% | SE1 | 0.01 | 0.30 |
| 2 | F21-F37 | C 100%; CM 0.1% | SE2 | 0.07 | 2.12 |
| 3 | F38-F59 | CM 0.2% | SE3 | 0.04 | 1.21 |
| 4 | F60-F139 | CM 0.2%; CM 0.5%; CM 1% | SE4 | 0.14 | 4.25 |
| Sample | LCL | U266 | KMS11 | Mino | |||
|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | IC50 (µg/mL) | SI | |
| SE1 | ne | 353.55 ± 9.82 b | - | 179.04 ± 35.26 a | - | 335.54 ± 11.12 a | - |
| SE2 | 398.58 ± 9.06 a | 383.25 ± 6.65 a | 1.04 | 94.93 ± 3.78 b | 4.20 | 76.65 ± 0.84 c | 5.20 |
| SE3 | 324.40 ± 11.67 b | 87.86 ± 4.52 d | 3.69 | 169.06 ± 11.54 a | 1.92 | 159.82 ± 5.24 b | 2.03 |
| SE4 | 205.42 ± 6.52 c | 112.93 ± 5.06 c | 1.82 | 152.91 ± 6.28 a | 1.34 | 151.69 ± 0.83 b | 1.35 |
| Doxorubicin | 5.56 ± 0.75 d | 0.13 ± 0.00 e | 42.77 | 0.46 ± 0.01 c | 12.09 | 3.09 ± 0.12 d | 1.80 |
| No. | RT (min) | Detected Fraction | Tentative Identity | Precursor (m/z) | Exact Mass | Molecular Formula | Classification |
|---|---|---|---|---|---|---|---|
| 1 | 4.34 | SE1 | 4,6-Dioxoheptanoic acid | 159.1 | 158.1 | C7H10O4 | Keto acids |
| 2 | 4.41 | SE3 | 5-(1-Hydroxyethyl)oxolan-2-one | 113.1 | 130.1 | C6H10O3 | Lactones |
| 3 | 4.65 | SE4 | Tri(propylene glycol)methyl ether | 207.2 | 206.2 | C10H22O4 | Ethers |
| 4 | 4.69 | SE3 | 5-(4-Hydroxypentyl)benzene-1,3-diol | 197.1 | 196.1 | C11H16O3 | Resorcinols |
| 5 | 4.78 | SE2 | Indole-7-carboxaldehyde | 146.1 | 145.1 | C9H7NO | Indoles |
| 6 | 5.16 | SE4 | Calycosin | 285.1 | 284.1 | C16H12O5 | Isoflavones |
| 7 | 5.22 | SE2 | Syringaresinol | 401.2 | 418.2 | C22H26O8 | Lignans |
| 8 | 5.24 | SE3, SE4 | Odoratin | 315.1 | 314.1 | C17H14O6 | Isoflavones |
| 9 | 5.33 | SE2 | Pinoresinol | 341.1 | 358.1 | C20H22O6 | Lignans |
| 10 | 5.43 | SE3 | Barpisoflavone A | 301.1 | 300.1 | C16H12O6 | Isoflavones |
| 11 | 5.64 | SE4 | Sebacic acid | 185.1 | 202.1 | C10H18O4 | Esters |
| 12 | 5.78 | SE4 | Liquiritigenin | 257.1 | 256.1 | C15H12O4 | Flavanones |
| 13 | 5.80 | SE2, SE3 | Pseudo-baptigenin | 283.1 | 282.1 | C16H10O5 | Isoflavones |
| 14 | 5.87 | SE3 | Formononetin | 269.1 | 268.1 | C16H12O4 | Isoflavones |
| 15 | 5.97 | SE1, SE2 | 7-hydroxy-6,4′-dimethoxyisoflavone | 299.1 | 298.1 | C17H14O5 | Isoflavones |
| 16 | 6.14 | SE1, SE2 | Maackiain | 285.1 | 284.1 | C16H12O5 | Flavones |
| 17 | 6.25 | SE1, SE2 | Medicarpin | 271.1 | 270.1 | C16H14O4 | Pterocarpans |
| 18 | 6.51 | SE2, SE4 | 2S-Amino-4E-pentadecene-1,3R-diol | 258.3 | 257.3 | C15H31NO2 | Amines |
| 19 | 6.60 | SE1 | Dimethyl azelate | 217.1 | 216.1 | C11H20O4 | Fatty acid esters |
| 20 | 7.48 | SE4 | 9Z,11E,13E-Octadecatrienoic acid | 279.2 | 278.2 | C18H30O2 | Fatty acyls |
| 21 | 7.64 | SE1, SE2, SE3 | 13-Keto-9Z,11E-octadecadienoic acid | 295.2 | 294.2 | C18H30O3 | Fatty acyls |
| 22 | 7.96 | SE4 | Friedelin | 427.4 | 426.4 | C30H50O | Triterpenoids |
| 23 | 8.18 | SE4 | Linoleoyl ethanolamide | 324.3 | 323.3 | C20H37NO2 | Carboximidic acids |
| 24 | 8.64 | SE3, SE4 | Bacosine | 439.4 | 456.4 | C30H48O3 | Triterpenes |
| 25 | 8.84 | SE3, SE4 | Betulin | 425.4 | 442.4 | C30H50O2 | Triterpenes |
| 26 | 8.89 | SE4 | Ergosterol peroxide | 429.4 | 428.3 | C28H44O3 | Ergostane steroids |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, V.Q.; Anh, L.H.; Quan, N.V.; Xuan, T.D.; Hanamura, I.; Uchino, K.; Karnan, S.; Takami, A. Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines. Molecules 2022, 27, 2322. https://doi.org/10.3390/molecules27072322
Lam VQ, Anh LH, Quan NV, Xuan TD, Hanamura I, Uchino K, Karnan S, Takami A. Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines. Molecules. 2022; 27(7):2322. https://doi.org/10.3390/molecules27072322
Chicago/Turabian StyleLam, Vu Quang, La Hoang Anh, Nguyen Van Quan, Tran Dang Xuan, Ichiro Hanamura, Kaori Uchino, Sivasundaram Karnan, and Akiyoshi Takami. 2022. "Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines" Molecules 27, no. 7: 2322. https://doi.org/10.3390/molecules27072322
APA StyleLam, V. Q., Anh, L. H., Quan, N. V., Xuan, T. D., Hanamura, I., Uchino, K., Karnan, S., & Takami, A. (2022). Cytotoxicity of Callerya speciosa Fractions against Myeloma and Lymphoma Cell Lines. Molecules, 27(7), 2322. https://doi.org/10.3390/molecules27072322










