The Synthesis of 3-(R)- and 3-(S)-Hydroxyeicosapentaenoic Acid
Abstract
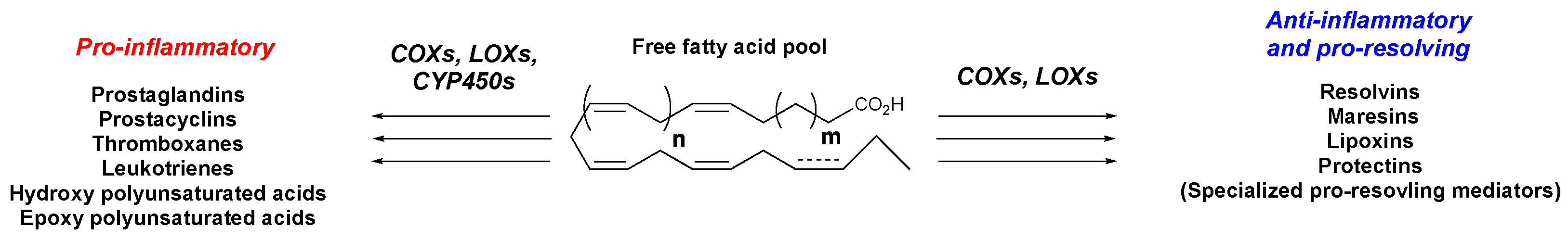
1. Introduction
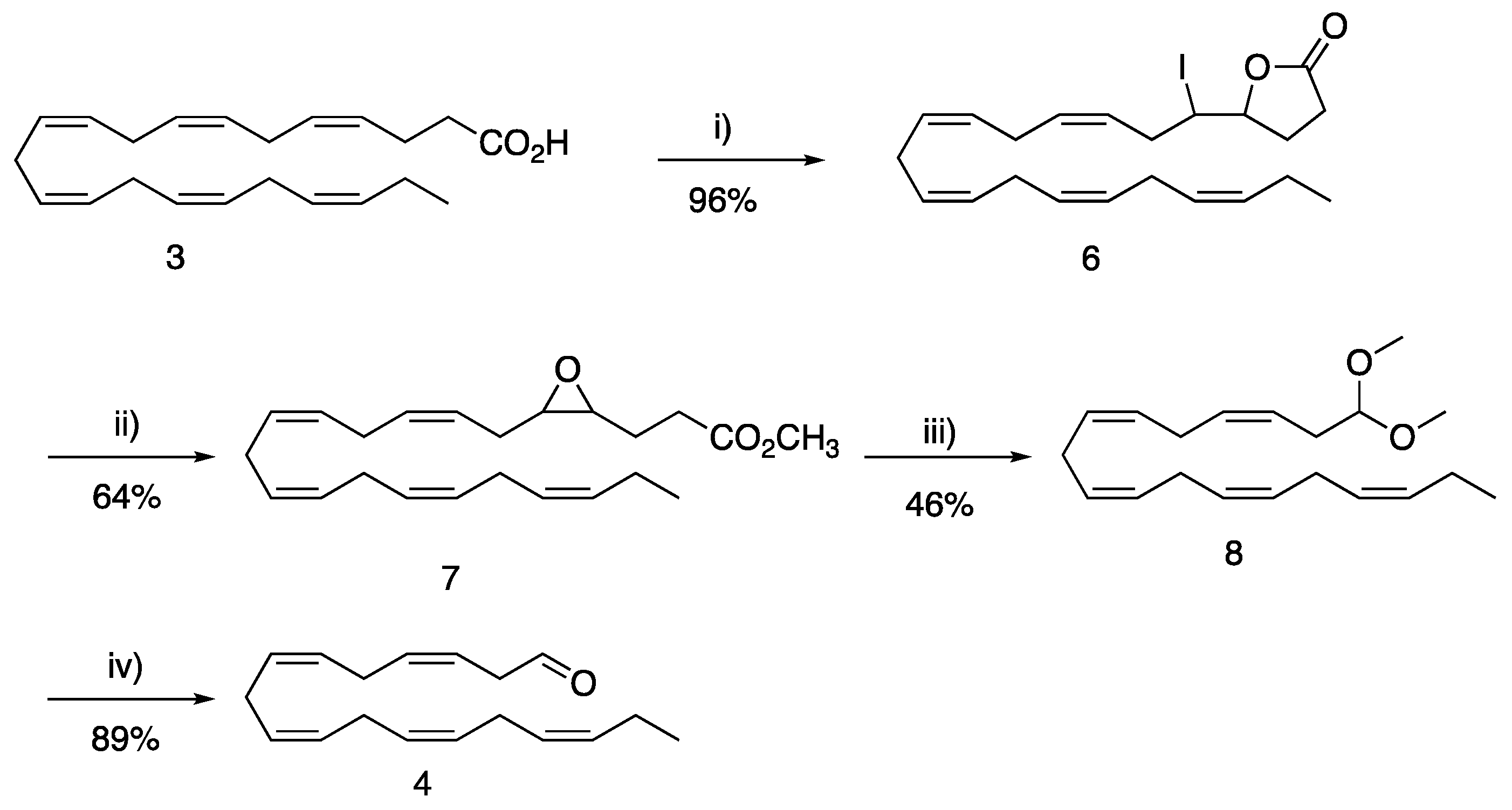
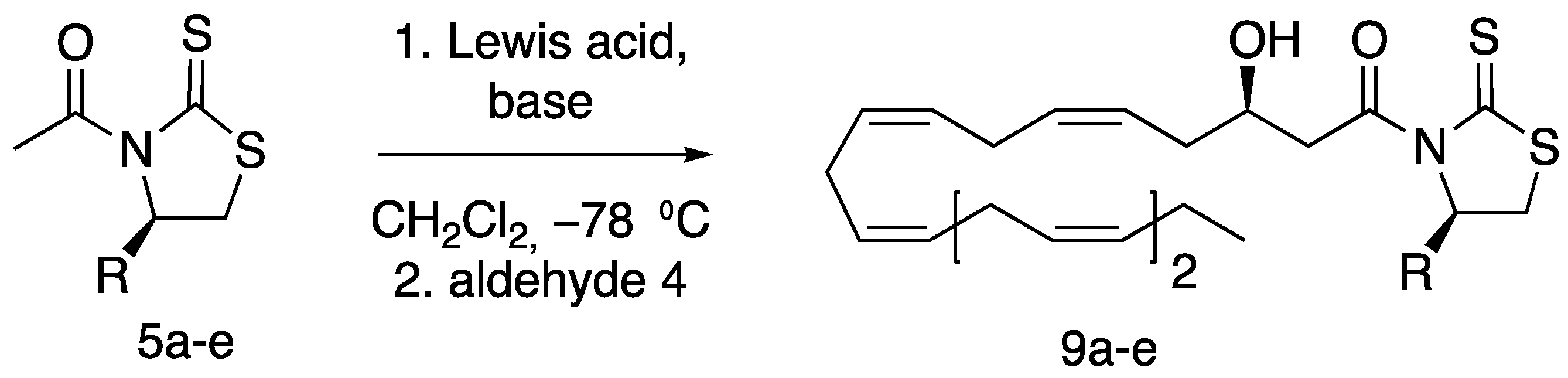
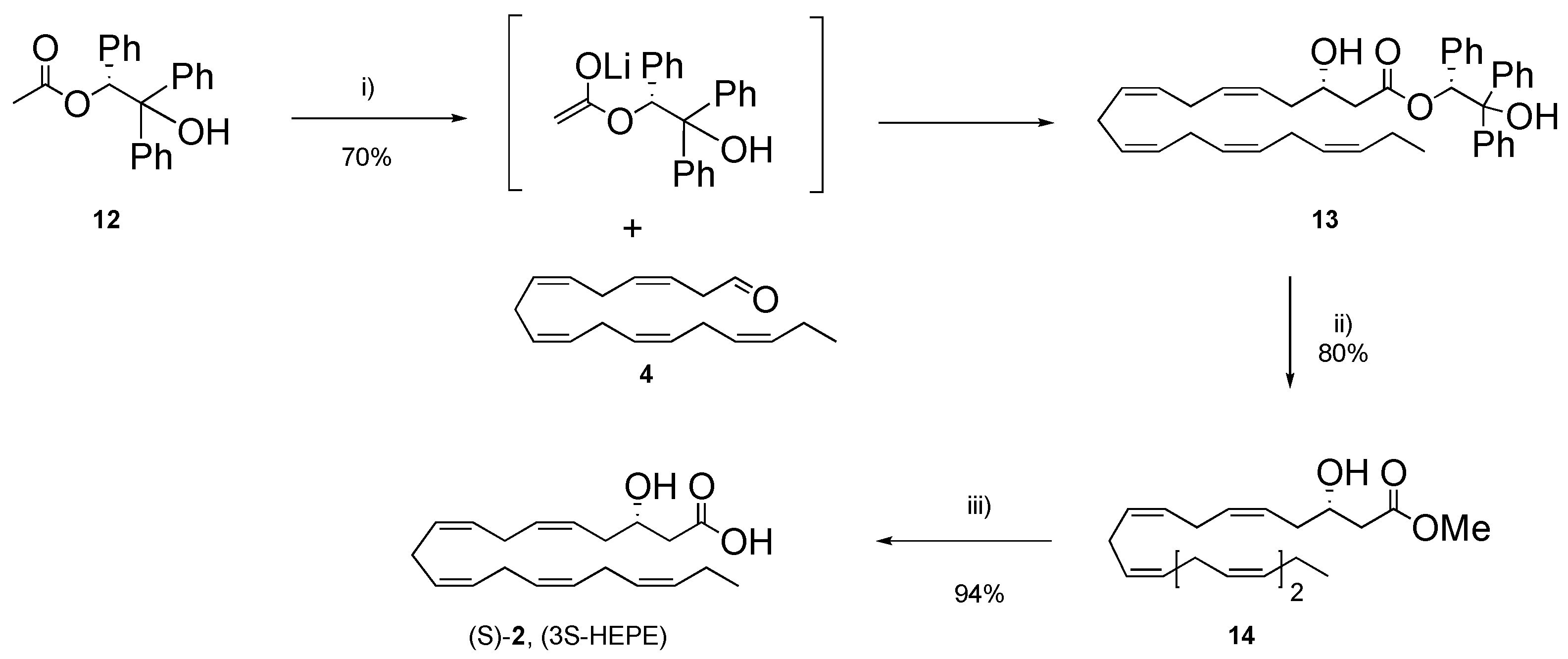
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.1.1. 5-((3Z,6Z,9Z,12Z,15Z)-1-Iodooctadeca-3,6,9,12,15-pentaenyl)dihydro-2(3H)-furanone (6)
3.1.2. Methyl 3-(3-((2Z,5Z,8Z,11Z,14Z)-heptadeca-2,5,8,11,14-pentaen-1-yl)oxiran-2-yl) Propanoate (7)
3.1.3. (3Z,6Z,9Z,12Z,15Z)-1,1-Dimethoxyoctadeca-3,6,9,12,15-pentaene (8)
3.1.4. (3Z,6Z,9Z,12Z,15Z)-Octadeca-3,6,9,12,15-pentaenal (4)
3.1.5. (R)-1-(4-Isopropyl-2-thioxothiazolidin-3-yl) Ethan-1-one (5b)
3.1.6. (R)-1-(4-Methyl-2-thioxothiazolidin-3-yl) Ethan-1-one (5a)
3.1.7. (R)-1-(4-Isobutyl-2-thioxothiazolidin-3-yl) Ethan-1-one (5c)
3.1.8. (R)-1-(4-Benzyl-2-thioxothiazolidin-3-yl) Ethan-1-one (5d)
3.1.9. (R)-1-(4-Phenyl-2-thioxothiazolidin-3-yl) Ethan-1-one (5e)
3.1.10. (R,5Z,8Z,11Z,14Z,17Z)-3-Hydroxy-1-((R)-4-isopropyl-2-thioxothiazolidin-3-yl) Icosa-5,8,11,14,17-pentaen-1-one (9b)
3.1.11. 9b Diastereomer (Minor Product)
3.1.12. (S)-1-((R,5Z,8Z,11Z,14Z,17Z)-3-((Tert-butyldimethylsilyl)oxy)icosa-5,8,11,14,17-pentaenoyl)-5-isopropylpyrrolidin-2-one (10)
3.1.13. Ethyl (R,5Z,8Z,11Z,14Z,17Z)-3-((Tert-butyldimethylsilyl)oxy)icosa-5,8,11,14,17-penta-enoate (11)
3.1.14. Synthesis of 3R-HEPE (2)
3.1.15. 3R-HEPE (2) from Aldol Product 9b
3.1.16. (R)-2-Hydroxy-1,2,2-triphenylethyl (S,5Z,8Z,11Z,14Z,17Z)-3-hydroxyicosa-5,8,11,14,17-pentaenoate (13)
3.1.17. Methyl (S,5Z,8Z,11Z,14Z,17Z)-3-Hydroxyicosa-5,8,11,14,17-pentaenoate (S-14)
3.1.18. 3S-HEPE (2)
3.1.19. Methyl (S,5Z,8Z,11Z,14Z,17Z)-3-Hydroxyicosa-5,8,11,14,17-pentaenoate (R-14)
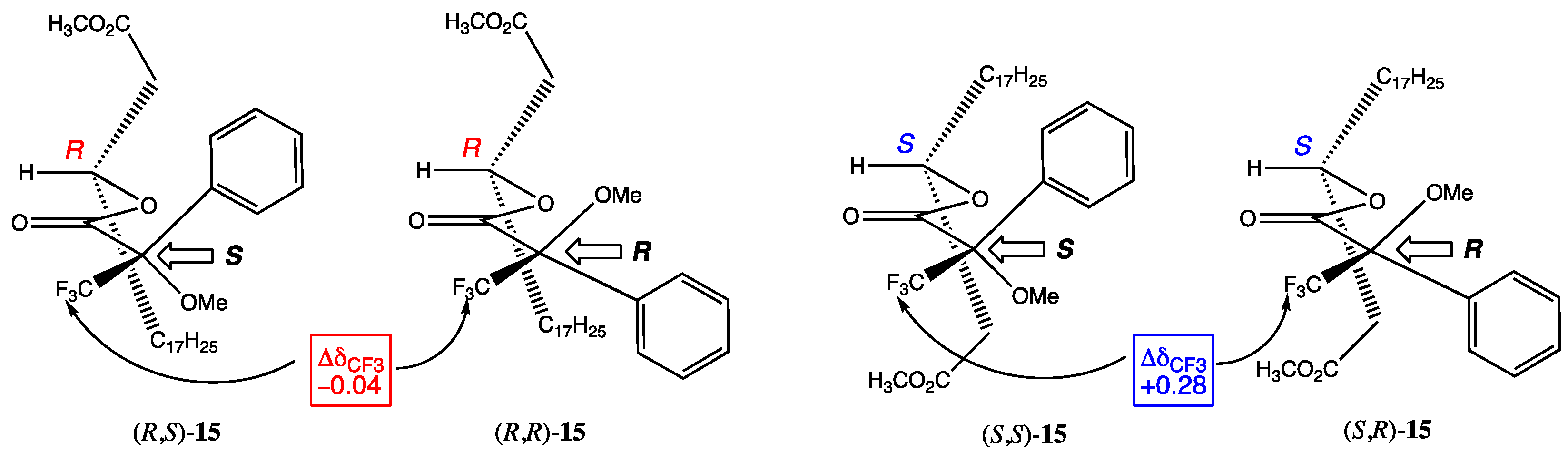
3.2. Mosher Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Nolsøe, J.M.N.; Aursnes, M.; Stenstrøm, Y.; Hansen, T.V. Some Biogenetic Considerations Regarding the Marine Natural Product (−)-Mucosin. Molecules 2019, 24, 4147. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Hansen, T.V. Fatty Acids and their Derivatives. In From Biosynthesis to Total Synthesis; Zografos, A.L., Ed.; Wiley: New York, NY, USA, 2016; pp. 130–161. [Google Scholar] [CrossRef]
- Samuelsson, B. Role of basic science in the development of new medicines: Examples from the eicosanoid field. J. Biol. Chem. 2012, 287, 10070–10080. [Google Scholar] [CrossRef] [PubMed]
- Haeggström, J.Z. Leukotriene biosynthetic enzymes as therapeutic targets. J. Clin. Investig. 2018, 128, 2680–2690. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Ramphal, J.Y.; Petasis, N.A.; Serhan, C.N. Lipoxins and Related Eicosanoids: Biosynthesis, Biological Properties, and Chemical Synthesis. Angew. Chem. Int. Ed. 1991, 30, 1100–1116. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.A.; Norris, A.W. Arachidonic acid cytochrome P450 epoxygenase pathway. Am. J. Physiol. Cell Physiol. 2007, 292, C996–C1012. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Singh, I.P. Structural Diversity of Marine Oxylipins. In Lipid Biotechnology; Kuo, T.M., Gardner, H.W., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 249–275. [Google Scholar] [CrossRef]
- Kock, J.L.F.; Strauss, C.J.; Pohl, C.H.; Nigam, S. The distribution of 3-hydroxy oxylipins in fungi. Prostaglandins Other Lipid Mediat. 2003, 71, 85–96. [Google Scholar] [CrossRef]
- Vandyk, M.S.; Kock, J.L.F.; Coetzee, D.J.; Augustyn, O.P.H.; Nigam, S. Isolation of a novel arachidonic acid metabolite 3-hydroxy-5,8,11,14-eicosatetraenoic acid (3-HETE) from the yeast Dipodascopsis uninucleata UOFs-Y128. Febs. Lett. 1991, 283, 195–198. [Google Scholar] [CrossRef]
- Ells, R.; Kock, J.L.F.; Albertyn, J.; Pohl, C.H. Arachidonic acid metabolites in pathogenic yeast. Lipids Health Dis. 2012, 11, 100. [Google Scholar] [CrossRef]
- Venter, P.; Kock, J.L.; Kumar, A.; Botha, D.J.; Coetzee, D.J.; Botes, P.J.; Bhatt, R.K.; Falck, J.R.; Schewe, T.; Nigam, S. Production of 3R-hydroxy-polyenoic fatty acids by the yeast Dipodascopsis uninucleata. Lipids 1997, 32, 1277–1283. [Google Scholar] [CrossRef]
- Sebolai, O.M.; Pohl, C.H.; Kock, L.J.F.; Chaturvedi, V.; del Poeta, M. The presence of 3-hydroxy oxylipins in pathogenic microbes. Prostaglandins Other Lipid Mediat. 2012, 97, 17–21. [Google Scholar] [CrossRef][Green Version]
- Bhatt, R.K.; Falck, J.R.; Nigam, S. Enantiospecific total synthesis of a novel arachidonic acid metabolite 3-hydroxy eicosatetraenoic acid. Tetrahedron Lett. 1998, 39, 249–252. [Google Scholar] [CrossRef]
- Groza, N.V.; Ivanov, I.V.; Romanov, S.G.; Myagkova, G.I.; Nigam, S. A novel synthesis of 3(R)-ETE, 3(R)-HTDE and enzymatic synthesis of 3(R), 15(S)-DiHETE. Tetrahedron 2002, 58, 9859–9863. [Google Scholar] [CrossRef]
- Jones, P.M.; Bennett, M.J. Clinical applications of 3-hydroxy fatty acids analysis by gas chromatography-mass spectrometry. Biochem. Biophys. Acta 2011, 1811, 657–662. [Google Scholar] [CrossRef]
- Stewart, A.G.; Paterson, D.L. How urgent is the need for new antifungals? Expert Opin. Pharmacother. 2021, 22, 1857–1870. [Google Scholar] [CrossRef]
- Vik, A.; Hansen, T.V. Synthetic manipulations of polyunsaturated fatty acids as a convenient strategy for the synthesis of bioactive compounds. Org. Biomol. Chem. 2018, 16, 9319–9333. [Google Scholar] [CrossRef]
- Pangopoulos, M.K.; Nolsøe, J.M.N.; Antonsen, S.G.; Colas, R.A.; Dalli, J.; Aursnes, M.; Stenstrøm, Y.; Hansen, T.V. Enzymatic studies with 3-oxa n-3 DPA. Bioorg. Chem. 2020, 96, 103653. [Google Scholar] [CrossRef]
- Nagao, Y.; Hagiwara, Y.; Kumagai, T.; Ochiai, M.; Inoue, T.; Hashimoto, K.; Fujita, E. New C-4-chiral 1,3-thiazolidine-2-thiones: Excellent chiral auxiliaries for highly diastereo-controlled aldol-type reactions of acetic acid and α,β-unsaturated aldehydes. J. Org. Chem. 1986, 51, 2391–2393. [Google Scholar] [CrossRef]
- Crimmins, M.T.; King, B.W.; Tabet, E.A.; Chaudhary, K. Asymmetric aldol additions: Use of titanium tetrachloride and (−)-sparteine for the soft enolization of N-acyl oxazolidinones, oxazolidinethiones, and thiazolidinethiones. J. Org. Chem. 2001, 66, 894–902. [Google Scholar] [CrossRef]
- González, A.; Aiguadé, J.; Urpí, F.; Vilarrasa, J. Asymmetric acetate aldol reactions in connection with an enantioselective total synthesis of macrolactin A. Tetrahedron Lett. 1996, 37, 8949–8952. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Dechert, A.-M.R. Enantioselective Total Synthesis of (−)-Pironetin: Iterative Aldol Reactions of Thiazolidinethiones. Org. Lett. 2009, 11, 1635–1638. [Google Scholar] [CrossRef]
- Crimmins, M.T.; O’Bryan, E.A. Enantioselective Total Synthesis of Spirofungins A and B. Org. Lett. 2010, 12, 4416–4419. [Google Scholar] [CrossRef]
- Hodge, M.B.; Olivo, H.F. Stereoselective aldol additions of titanium enolates of N-acetyl-4-isopropyl-thiazolidinethione. Tetrahedron 2004, 60, 9397–9403. [Google Scholar] [CrossRef]
- Braun, M.; Graef, S. Stereoselective aldol reaction of doubly deprotonated (R)-(+)-2-hydroxy-1,2,2-triphenylethyl acetate (HYTRA): (R)-3-hydroxy-4-methylpentanoic acid. Org. Synth. 1995, 72, 38–47. [Google Scholar] [CrossRef]
- Corey, E.J.; Albright, J.O.; Barton, A.E.; Hashimoto, S. Chemical and enzymatic syntheses of 5-HPETE, a key biological precursor of slow-reacting substance of anaphylaxis (SRS), and 5-HETE. J. Am. Chem. Soc. 1980, 102, 1435–1437. [Google Scholar] [CrossRef]
- Langseter, A.M.; Skattebøl, L.; Stenstrøm, Y. Synthesis of All-Z-1,6,9,12,15-Octadecapenten-3-one, A Vinyl Ketone Polyunsaturated Marine Natural Product Isolated from Callysponga sp. Tetrahedron Lett. 2012, 53, 940–941. [Google Scholar] [CrossRef]
- Dikstra, A.J.; Christie, W.W.; Knothe, G. Analysis. In The Lipid Handbook, 3rd ed.; Gunstone, F.D., Harwood, J.L., Dikstra, A.J., Eds.; CRC Press: London, UK, 2007; pp. 459–464. [Google Scholar] [CrossRef]
- Tungen, J.E.; Aursnes, M.; Hansen, T.V. Stereoselective total synthesis of ieodomycin C. Tetrahedron 2014, 70, 3793–3797. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Shao, F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat. Protoc. 2007, 2, 2451–2458. [Google Scholar] [CrossRef]
- Rieser, M.J.; Hui, Y.H.; Rupprecht, J.K.; Kozlowski, J.F.; Wood, K.V.; McLaughlin, J.L.; Hanson, P.R.; Zhuang, Z.; Hoye, T.R. Determination of absolute configuration of stereogenic carbinol centers in annonaceous acetogenins by proton and fluorine 19-NMR analysis of Mosher ester derivatives. J. Am. Chem. Soc. 1992, 114, 10203–10213. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H.J. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Tyagi, R.; Shimpukade, B.; Blaettermann, S.; Kostenis, E.; Ulven, T. A concise synthesis of the potent inflammatory mediator 5-oxo-ETE. MedChemComm 2012, 3, 195–198. [Google Scholar] [CrossRef]
- Flock, S.; Lundquist, M.; Skattebol, L. Syntheses of some polyunsaturated sulfur-and oxygen-containing fatty acids related to eicosapentaenoic and docosahexaenoic acids. Acta Chem. Scand. 1999, 53, 436–445. [Google Scholar] [CrossRef] [PubMed]
- McNulty, J.; McLeod, D. Total Enantioselective Synthesis of the Endophytic Fungal Polyketide Phomolide H and Its Structural Revision. Eur. J. Org. Chem. 2017, 1, 29–33. [Google Scholar] [CrossRef]
- Gallantree-Smith, H.C.; Antonsen, S.G.; Görbitz, C.H.; Hansen, T.V.; Nolsøe, J.M.J.; Stenstrøm, Y.H. Total synthesis based on the originally claimed structure of mucosin. Org. Biomol. Chem. 2016, 14, 8433–8437. [Google Scholar] [CrossRef]
- Mendoza, R.M.; Zamith-Miranda, D.; Takács, T.; Gascer, A.; Nosanchuk, J.D.; Guimaraes, A.J.J. Complex and Controversial Roles of Eicosanoids in Fungal Pathogenesis. Fungi 2021, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Bamboo, P.; Bera, S.; Mondal, S. TiCl4-Promoted Asymmetric Aldol Reaction of Oxazolidinones and its Sulphur-Congeners for Natural Product Synthesis. Asian J. Org. Chem. 2021, 10, 2763–2819. [Google Scholar] [CrossRef]


| Entry | R | Lewis Acid | Base | dr a (R,R:R,S) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | a: Me | TiCl4 | DIPEA c | 2.6:1 | 22 |
| 2 | b: i-Pr | TiCl4 | DIPEA c | 10:1 | 46 |
| 3 | b: i-Pr | TiCl4 | NMP d | 1.2:1 | 56 |
| 4 | c: i-Bu | TiCl4 | DIPEA c | 1.9:1 | 25 |
| 5 | d: Bn | TiCl4 | DIPEA c | 5.3:1 | 30 |
| 6 | e: Ph | TiCl4 | DIPEA c | nr e | N/A |
| 7 | b: i-Pr | TiCl4 | (−)-sparteine | 5.8:1 | 35 |
| Assumed Configuration of Carbinol | MTPA Configuration | 19F NMR Shifts | ΔδSR (= δS − δR) | Found Configuration of Carbinol |
|---|---|---|---|---|
| R | S R | −72.42 −72.38 | −0.04 | R |
| S | S R | −72.37 −72.65 | +0.28 | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjessing, G.; Johnsen, L.-I.G.; Antonsen, S.G.; Nolsøe, J.M.J.; Stenstrøm, Y.; Hansen, T.V. The Synthesis of 3-(R)- and 3-(S)-Hydroxyeicosapentaenoic Acid. Molecules 2022, 27, 2295. https://doi.org/10.3390/molecules27072295
Gjessing G, Johnsen L-IG, Antonsen SG, Nolsøe JMJ, Stenstrøm Y, Hansen TV. The Synthesis of 3-(R)- and 3-(S)-Hydroxyeicosapentaenoic Acid. Molecules. 2022; 27(7):2295. https://doi.org/10.3390/molecules27072295
Chicago/Turabian StyleGjessing, Gard, Lars-Inge Gammelsæter Johnsen, Simen Gjelseth Antonsen, Jens M. J. Nolsøe, Yngve Stenstrøm, and Trond Vidar Hansen. 2022. "The Synthesis of 3-(R)- and 3-(S)-Hydroxyeicosapentaenoic Acid" Molecules 27, no. 7: 2295. https://doi.org/10.3390/molecules27072295
APA StyleGjessing, G., Johnsen, L.-I. G., Antonsen, S. G., Nolsøe, J. M. J., Stenstrøm, Y., & Hansen, T. V. (2022). The Synthesis of 3-(R)- and 3-(S)-Hydroxyeicosapentaenoic Acid. Molecules, 27(7), 2295. https://doi.org/10.3390/molecules27072295








