Abstract
Aesculus flowers and leaves are an excellent source of bioactive compounds, including flavanols, phenolic acids, and anthocyanins, and the leaves also contain antioxidant carotenoids and chlorophylls. The aim of this study was to analyse and compare the amounts of bioactive compounds present in Aesculus hippocastanum and Aesculus × carnea flowers and leaves over two years. These two species from six independent locations (parks and green areas) located in Warsaw were assessed in this study. The dry matter by the scale method and polyphenol, carotenoid, and chlorophyll content by the HPLC method of the flowers and leaves was evaluated. Red horse chestnut flowers contained significantly more total carotenoids (40.6 µg/g FW) and chlorophylls (36.9 µg/g FW) than horse chestnut flowers, and red horse chestnut flowers contained higher levels of anthocyanins (5.41 µg/g FW) than other species. We observed that horse chestnut flowers were characterized by a higher total polyphenols concentration (9.45 µg/g FW) compared to red horse chestnut flowers. In addition, the analysis of leaves showed that all quality parameters were higher in red horse chestnut species. Five individual anthocyanins were identified in both species’ flowers, but a higher concentration was found in red horse chestnut flowers, and pelargonidin-3-O-glucoside was the predominant form among a pool of total anthocyanins. In both experimental years, leaves (109.25 mMol/100 g FW and 112.0 mMol/100 g FW) were characterized by a higher antioxidant activity than flowers (27.0 mMol/100 g FW and 27.5 mMol/100 g FW).
1. Introduction
Polyphenols are common among plant secondary metabolites [1,2,3]. Plants produce polyphenols as a response to biotic and abiotic environmental stress conditions [4]. In addition, however, polyphenols are commonly called “natural pesticides” [5]. Pest attacks stimulate plants to produce and concentrate secondary metabolites, mostly phenolic acids and flavonoids. Plants develop different defence mechanisms, aided by biochemical bioactive compounds [6]. Unfortunately, plants cannot synthesize defence antibodies as animals do via their immune system. Therefore, plants evolved to produce numerous antimicrobial substances called phytoalexins. Many of these are polyphenols [7,8]. UV radiation is another environmental trigger for polyphenol production. In many dark flowers, a higher concentration of anthocyanins occurs as a protection from intense sunlight [9]. In other plant parts, including leaves, UV radiation stimulates flavonol synthesis [10]. Horse chestnut and red horse chestnut are two of the best-known ornamental and medicinal tree species. The decorative effects of trees are visible, especially in springtime when they flower. Flowers in spectacular inflorescences reach up to 20 cm in height. Aesculus hippocastanum is characterized by white flowers with light red spots, but Aesculus (×) carnea flowers are red. The presence of polyphenols in the leaves, seeds, flowers, and bark of horse chestnut has been reported in the literature but not in red horse chestnut because it is a less well-known species. According to the literature, the content of polyphenols is: in horse chestnuts leaves (7.81–24.48 mg/g FW) [11], seeds (24.24–70.40 mg/g FW) [12], flowers (8.17 mg/g) [13], and bark (363.58 mg/g FW) [14]. For medicinal purposes, fruits, seeds, and cortex are used. Extracts and concentrates are used for creams and ointments with antiinflammatory properties for use in treating phlebitis and different kinds of cancer. However, no culinary properties of chestnut or horse chestnut flowers have been reported. If vegetables and fruits are a useful source of bioactive compounds in our diet, flowers can also be used. There are many plants with edible flowers. Their use in foods could expand the culinary arts, as an old adage suggests: “What is healthy for your stomach should be nice for eyes as well”. However, even though it may not be possible to create a complete diet from leaves and flowers rich in bioactive compounds such as polyphenols, fruits, vegetables, and flower parts are good sources of polyphenols in our diet. Many experiments have shown that flowers can be consumed and used in infusions and tinctures [15,16]. Previous research with black and bristly locust flowers showed that flowers of this species are a reliable source of flavonoids and phenolic acids [17]. Only a limited number of experiments on polyphenol content in the flowers and leaves of red horse chestnut and horse chestnut have been reported. The aim of the present work was to assess the polyphenol content and antioxidant capacity in the flowers and leaves of red horse chestnut and horse chestnut over two annual growth cycles
2. Results
The flower and leaf dry matter content in both species is presented in Table 1. In the first year of the experiment, the flowers contained less dry matter than the leaves. In the second year, the flowers of both species contained comparatively more dry matter than in the first year, but the values between years were not statistically significant (p > 0.05). Red horse chestnut was characterized by a higher dry matter content in leaves in both years of the experiment but not the flowers, although the results were not statistically significant. Leaves of red and horse chestnut are a much better source of total carotenoids than the flowers. Carotenoids are connected mostly with chlorophyll content. Red horse chestnut leaves were characterized by a higher concentration of total carotenoids than white leaves (p < 0.0001). A comparable situation was noted with the flowers, but only in the first year (2018). In the case of chlorophylls, we observed that the two species varied significantly in the content of this pigment in both leaves and flowers (Table 1).

Table 1.
The content of bioactive compounds in total in flowers and leaves of horse chestnut and red horse chestnut (±standard error). N.S (statistically not significant).
We observed that in 2018 the total polyphenol content in the flowers of both species was similar. In 2019, both species contained more total polyphenols in the leaves than in the flowers, but in 2018, the situation was the opposite. Horse chestnut flowers and leaves were characterized by a higher concentration of total phenolic acids than those of red horse chestnut. It is worth noting that in 2019 the amounts of polyphenols were much higher than those in 2018 and the difference was statistically significant (p < 0.0001). However, we did not observe significant differences in the total flavonoid content between the flowers of the two species. However, in both years, the leaves contained significantly more (p < 0.0001) total flavonoids than the flowers. Purple colorants (anthocyanins) were detected only in red horse chestnut flowers. The leaves of both species are anthocyanin-free. Detailed carotenoid and chlorophyll analysis showed three individual carotenoids as well as two chlorophylls in both the flowers and leaves of both species (Figure 1).
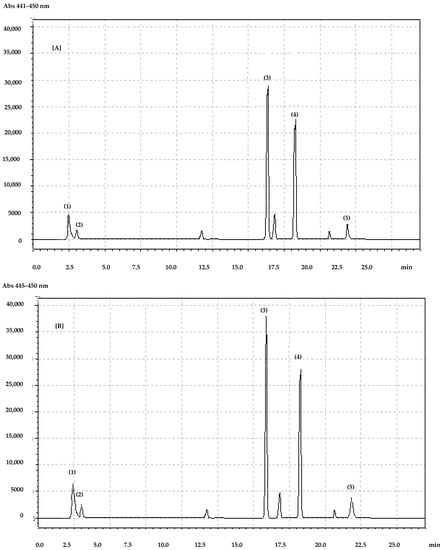
Figure 1.
Chromatogram of carotenoids and chlorophylls identified in (A) horse chestnut and (B) red horse chestnut leaves. (1) lutein, (2) zeaxanthin, (3) chlorophyll b, (4) chlorophyll a, (5) beta-carotene.
In both years, we observed a higher concentration of lutein in flowers than leaves. Moreover, red horse chestnut contained more lutein than horse chestnut and the results were statistically significant. The zeaxanthin content was higher in leaves than in the flowers in both years for both species. Red horse chestnuts contained significantly more zeaxanthin (p < 0.0001) in both leaves and flowers than horse chestnuts in both years (Table 2).

Table 2.
Content of identified carotenoids and chlorophylls (µg/g FW) in both species of horse chestnuts’ flowers and leaves (mean value ± standard error).
The main carotenoid characterized for both species is beta-carotene. In 2018 and 2019, red horse chestnuts contained significantly more (p < 0.0001) of this carotenoid than horse chestnut leaves and flowers. In both years, red horse chestnut contained significantly more chlorophyll a (p < 0.0001) in leaves than horse chestnuts. Although chlorophyll a was detected in the flowers of both species, red horse chestnut characteristically contained a higher concentration than occurred in chestnut (Table 2). Chlorophyll b was more abundant in red horse chestnut. Both flowers (p < 0.0001) and leaves (p < 0.0001) of red horse chestnut contained significantly more chlorophyll b than horse chestnut in 2018 and 2019. Six different anthocyanin compounds were identified in both species but only in flowers, not leaves (Table 3). In five of the six cases, red horse chestnut contained significantly more anthocyanins than horse chestnut. This is because of the intensive pink flower colour in the red horse chestnut (Figure 2).

Table 3.
Content of identified anthocyanins (mg/g FW) in both species of horse chestnuts’ flowers (mean value ± standard error). N.S (statistically not significant).

Figure 2.
Chromatogram of anthocyanins identified in (A) horse chestnut and (B) red horse chestnut flowers. (1) cyanidin-3-O-glucoside, (2) pelargonidin-3-O-glucoside, (3) delphinidin-3-O-glucoside, (4) malvidin-3-O-glucoside, (5) peonidin-3-O-glucoside, (6) petunin-3-O-glucoside.
Cyanidin-3-O-glucoside occurred in only small amounts in horse chestnut flowers but was three times higher in red horse chestnut flowers in both years, and the differences were statistically significant (p-value < 0.0001). The highest concentration among anthocyanin pigments was of pelargonidin-3-O-glucoside. No differences between species were observed in delphinidin-3-O-glucoside content. Both species in both years contained similar amounts of anthocyanin. Malvidin-3-O-glucoside was the only anthocyanin that occurred at a higher concentration in horse chestnut flowers, measuring 0.54 mg/g in 2018 FW and 0.38 mg/g in 2019 FW, while red horse chestnut flowers contained only 0.25 mg/g (2018) FW and 0.26 mg/g (2019) FW, and the results were statistically significant (p = 0.0061). Peonidin-3-O-glucoside occurred at a higher concentration in red horse chestnut flowers in both years, while horse chestnut flowers contained 0.67 mg/g (2018) FW and 0.40 mg/g (2019) FW of peonidin-3-O-glucoside (Table 3).
The last anthocyanin pigment identified in the flowers of both species was petunin-3-O-glucoside with red horse chestnuts containing significantly more (p < 0.0001) than occurred in horse chestnut.
We identified five phenolic acids and seven flavonoids in the flowers of both species (Figure 3). The dominant phenolic compound was gallic acid of which horse chestnut leaves and flowers contained significantly more (p < 0.0001) in both years compared to red horse chestnut leaves and flowers (Table 4). Chlorogenic acid was detected at higher concentrations in horse chestnut and the results were statistically significant (p = 0.0072). There were no statistically significant differences between the leaves and flowers of both species in 2018 and 2019. In both years, caffeic acids were more abundant in red horse chestnut flowers (p < 0.0001) but higher in the leaves of horse chestnut (p < 0.0001). In both years, the flowers of horse chestnuts contained significantly more p-coumaric acid than those of red horse chestnut, while in leaves the higher concentration was in red horse chestnut. We observed that both the flowers and leaves of horse chestnuts contained significantly more ferulic acid in both years than red horse chestnut (p < 0.0001). Conversely, in both years we found higher concentrations of quercetin-3-O-rutinoside in red horse chestnut flowers and leaves than in horse chestnut flowers and leaves (p < 0.0001). The flowers of the horse chestnut contained significantly more kaempferol-3-O-glucoside than flowers of the red horse chestnut (p < 0.0001), while this was reversed in the leaves in which a greater amount was found in red horse chestnut leaves than horse chestnut leaves (Table 4).

Figure 3.
Chromatogram of polyphenols identified in (A) horse chestnut and (B) red horse chestnut flowers. (1) gallic acid, (2) chlorogenic acid, (3) caffeic acid, (4) quercetin-3-O-rutinoside, (5) p-coumaric, (6) ferulic acid, (7) kaempferol-3-O-glucoside, (8) myricetin, (9) luteolin, (10) quercetin, (11) quercetin-3-O-glucoside, (12) apigenin, (13) kaempferol.

Table 4.
Content of identified phenolic acids and flavonoids (mg/g FW) and antioxidant activity (mMol/100 g FW) in both species of horse chestnuts’ flowers and leaves (mean value ± standard error). N.S (statistically not significant).
The results showed that the flowers of both red and horse chestnut flowers contained significantly more quercetin-3-O-glucoside than the leaves, measuring at 0.39 mg/g FW in 2018 and 0.28 mg/g FW in 2019, respectively. In the leaves, the concentrations of quercetin-3-O-glucoside were 0.26 mg/g FW and 0.19 mg/g FW. The flowers of horse chestnuts contained significantly more quercetin-3-O-glucoside (p = 0.0052), but in the case of red horse chestnuts, it occurred more in the leaves than the flowers. In both years, red horse chestnuts contained significantly more myricetin (p = 0.0016) than horse chestnut. Luteolin was one of the phenolic compounds with a higher concentration in flowers and leaves, just after gallic acid. In 2018, the flowers and leaves of horse chestnut were characterized by a higher concentration of these phenolic compounds. This trend was not observed in 2019. The concentration of quercetin was significantly different only in the examined plant organs. In both years, we observed that leaves contained more (p = 0.0003) than flowers (Table 4). There were no differences between species in either year. Red horse chestnut contained more kaempferol in flowers, while horse chestnut contained more kaempferol in leaves. This situation was observed in both years. We observed that leaves were characterized by a higher total antioxidant activity than flowers in both years. In 2018, red horse chestnut had a higher total antioxidant status than horse chestnut (p < 0.0001). However, in 2019, a slightly higher antioxidant status was observed in the horse chestnut species.
3. Discussion
In the present study, the species with a white flower colour contained more dry matter compared to those with red flowers. The dry matter content in plants was genetically diverse. The range of dry matter content in different Aesculus species was 11.0–17.3 g/100 g FW. This value is similar to those presented by others for different species with white- and pink-coloured flowers [17]. The obtained results are the opposite to those presented in the literature. Pink and red flowers contained more dry matter compared to white flowers [18]. Carotenoids are bioactive compounds that play a key role as pro-health compounds. In the present study, the leaves of both species contained a high concentration of carotenoids. The obtained value of 723.9 µg/g FW for horse chestnut leaves and 923.5 µg/g for red horse chestnuts leaves is similar to popular leafy vegetables such as green lettuce (727.2 µg/g FW) and much higher than that in spinach (125.4 µg/g FW) [19,20]. Healthy properties of leaves can provide chlorophylls as well polyphenols. According to the literature, chlorophylls are molecules that play a vital role as chelators of heavy metals [21,22]. In our study, the concentration of chlorophylls in horse chestnut and red horse chestnut leaves was much higher than that of leafy vegetables such as ice-head lettuce (52 mg/100 g FW), butter-head lettuce (41 mg/100 g FW), and spinach (112 mg/100 g FW). For horse chestnuts, it was 118.5 mg/100 g FW, and for red horse chestnuts, it was 170.5 mg/100 g FW [23]. Polyphenols with a high antioxidant status play a significant role in maintaining good health [24,25]. Many diseases, such as cancer and cardiovascular diseases, are associated with low antioxidant status and polyphenol intake with foods such as fruits and vegetables [26,27]. Flowers and leaves are potentially good sources of dietary polyphenol compounds [28]. The health effects of eating flowers have been examined for many years [29,30,31,32]. In the case of horse chestnuts and red horse chestnuts, any therapeutic effects have mostly been connected and examined with fruits and seeds, not flowers and leaves. In our experiment, we showed that the flowers and leaves of both species are a useful source of polyphenol compounds. To approximate the concentrations of polyphenolic compounds in the flowers and leaves of horse chestnuts, the obtained values should be compared with those of fruits and vegetables. One of the important fruits recommended as a good source of polyphenols is apples. The range of total polyphenol concentrations in five cultivars was shown to be 148–190 mg/100 g FW [33]. In our experiment, we showed that horse chestnut flowers contained total polyphenols in a range of 445–945 mg/100 g FW, much higher than occurs in apples. Citrus fruits are considered to be among the best sources of flavonoids. Different citrus fruits contain total flavonoids in the range of 14.2–70.6 mg/100 g FW [34]. However, our experiment focused on the flowers of horse chestnuts, which are a much better source of total flavonoids with a range of these compounds in flowers of 249–398 mg/100 g FW. Berries are a group of fruits with a high anthocyanin content. Flowers of red horse chestnuts contain a satisfactory level of anthocyanin compounds compared with berry fruits such as highbush blueberry, strawberry, and raspberry [35]. In addition to diminishing disease risk, other functions of plant flavonoids connected with horse chestnut flowers, fruits, leaves, and seeds include vessel strength and wound healing [36]. Therefore, it may be worth combining the health-promoting and therapeutic effects of horse chestnut flowers and leaves due to their high content of polyphenolic compounds, particularly flavonoids. Skin disorders including wounds are common indispositions. Various risk factors accompany wound healing, such as microbial infection and inflammation with high ROS (reactive oxygen species) generation. Applying food with a high concentration of bioactive compounds can reduce these problems [37]. Determining the content of antioxidant agents in edible flowers is one of the most important aims of such experiments. In our experiment, we showed that both red horse chestnuts and horse chestnuts are excellent sources for bioactive compound content. Flowers can be used not only for decorative dish purposes but also as food. A previous experiment with black and bristly locust flowers showed that not only typical edible flowers, such as pansy, rose or daisy flowers, can be used for consumption purposes [17]. The concentration of bioactive compounds in flowers is connected with their colour. The main colorant of flowers is anthocyanins, which are characterized by a high antioxidant status. In our experiment, as found in previous experiments, we show that colourful flowers contain more phenolic compounds than similar species with white flowers. Red horse chestnut flowers with a pink colour also contained more kaempferol and quercetin-3-O-glucoside than a closely related horse chestnut species with white flowers (Table 4). Comparable results were obtained with black and bristly locust flowers and daisy flowers [17,18].
4. Materials and Methods
4.1. Flowers’ and Leaves’ Origin
Our experiment focused on two Aesculus species: Aesculus hippocastanum (horse chestnut) and Aesculus (×) carnea (red horse chestnut). The first is characterized by white flowers with small red spots (Figure 4), and the second species has pink flowers (Figure 5). Our experiment was conducted over two years, 2018–2019. Flowers of both species were collected in the morning from six independent trees located in Warsaw parks and green areas between 1 and 4 of May each year and the samples were quickly transported to the laboratory. The location of trees was: Lazienki Park (52°22″ N; 21°03″ E), Saski Park (52°14″ N; 21°0″ E), Królikarnia Park (52°41″ N; 21°42″ E), Mokotowskie Pole Park (52°21″ N; 20°99″ E), Sowiński Park (52°23″ N; 20°95″ E), Szczęśliwicki Park (52°21″ N; 20°96″ E).

Figure 4.
Flowers of Aesculus hippocastanum (horse chestnut flowers).

Figure 5.
Flowers of Aesculus (×) carnea (red horse chestnut flowers).
4.2. Plant Material Preparation
Inflorescences, similar to leaves of both species, were gently divided into single flowers. From each tree, 200–300 individual flowers and 50 leaves were collected. Each tree was treated as a single replication. The fresh weight of the samples was between 250 and 350 g per tree for flowers and 150–170 g per tree for leaves. Each species samples were divided into two parts. The first part was used for dry matter evaluation, and the second part was freeze-dried using a Labconco (2.5) freeze-dryer (Warsaw, Poland, −40 °C, pressure 0.100 mbar). After the freeze-drying process, the experimental material was ground in a laboratory mill (A-11). Then, the ground samples were stored at −80 °C in small scyntylic tubes.
4.3. Chemical Analysis
4.3.1. Dry Matter Analysis
The dry matter content of the Aesculus flowers and leaves was measured before the freeze-drying process. The dry matter content was determined using a scale as described by Polish Norm PN-R-04013:1988 [38]. Flower samples were dried at 105 °C for 48 h using an FP-25 W Farma Play dryer (Bytom, Poland). The dry matter content was calculated for the Robinia flower samples based on their mass differences and is given in units of g/100 g FW (fresh weight).
4.3.2. Polyphenols—Extraction Parameters
Polyphenols (flavonols and phenolic acids) were measured using HPLC [39]. One hundred milligrams of freeze-dried flower or leaf powder was mixed with 5 mL of 80% methanol (HPLC grade) and mixed on a Vortex 326 M (Marki, Poland). Then, all samples were extracted in an ultrasonic bath (10 min, 30 °C, 5500 Hz). After 10 min of extraction, the flower and leaf samples were moved to a centrifuge (10 min, 6000 rpm, 5 °C). After centrifugation, each supernatant was collected in a clean Eppendorf tube and centrifuged again (5 min, 12,000 rpm, 0 °C). A total of 500 μL of supernatant was transferred to HPLC vials and analysed. Polyphenols (anthocyanins) were measured by HPLC [19]. The samples were extracted with a mixture of methanol and ultrapure water (80:20). After the first centrifugation (see previous section), 2.5 mL of the supernatant was collected into a new plastic tube, and then 2.5 mL of 10 M hydrochloric acid (HCl) and 5 mL of pure methanol were added. The samples were gently shaken and placed in a refrigerator (5 °C, 10 min). Then, 1 mL of each extract was transferred to an HPLC vial and analysed.
4.3.3. Polyphenols—Equipment Description
For polyphenol separation and identification, a Synergi Fusion-RP 80i Column 250 × 4.60 mm (Phenomenex, Warsaw, Poland) was used. Analysis was conducted with the use of Shimadzu equipment (Chicago, IL, USA): two LC-20AD pumps, a CBM-20A controller, an SIL-20AC column oven, and a UV/Vis SPD-20 AV spectrometer. For separation of phenolic compounds (flavonols and phenolic acids), gradient conditions with a flow rate of 1 mL/min were used. Two gradient phases were used: 10% (v:v) acetonitrile and ultrapure water (phase A) and 55% (v:v) acetonitrile and ultrapure water (phase B). The phases were acidified with ortho-phosphoric acid (pH 3.0). The total time of the analysis was 38 min. The phase-time program was as follows: 1.00–22.99 min, 95% phase A and 5% phase B; 23.00–27.99 min, 50% phase A and 50% phase B; 28.00–28.99 min, 80% phase A and 20% phase B; 29.00–38.00 min, 95% phase A and 5% phase B. The wavelengths of detection were 250 nm for phenolic acids and 370 nm for flavonols.
Anthocyanins were separated under isocratic conditions with a flow rate of 1.5 mL/min. One mobile phase was used containing: acetic acid (5%), methanol (HPLC pure), and acetonitrile (HPLC pure) (70:10:20). The analysis time was 10 min with detection at 570 nm. The anthocyanins were identified by using 99.9% pure standards (Sigma-Aldrich, Warsaw, Poland) and the analysis times for the standards [39].
4.3.4. Polyphenols—Results Calculation
All polyphenols were identified by using pure standards (Sigma-Aldrich, Warsaw, Poland) and the retention times for the internal standards. Standard curves prepared for all phenolic compounds are presented in Figure 1 and Figure 2. Pure phenolic standards were used for standard solution preparation. From each standard solution, five injections were made. Each time, the chromatographic pick area was determined and calculated based on the standard solution concentration. From standard curves, a mathematical equation was prepared. On the basis of the dilution coefficient and equation, the concentration of individual compounds was calculated.
4.3.5. Carotenoids and Chlorophylls—Extraction Parameters
Carotenoids and chlorophylls were measured by HPLC [40]. One hundred and fifty-five milligrams of freeze-dried flower or leaf powder was mixed with 5 mL of 100% acetone (HPLC grade) and mixed on a Vortex 326 M (Marki, Poland). Then, all samples were extracted in a cold ultrasonic bath (15 min, 0 °C, 5500 Hz). After 15 min of extraction, the flower and leaf samples were moved to a centrifuge (15 min, 5000 rpm, 0 °C). After centrifugation, each supernatant was collected in a clean Eppendorf tube and centrifuged again (5 min, 12,000 rpm, 0 °C). A total of 900 μL of supernatant was transferred to HPLC vials and analysed, using a column injection of 50 µL.
4.3.6. Carotenoids—Equipment Description
For carotenoid and chlorophyll separation and identification, a Max-RP 80i Column 250 × 4.60 mm (Phenomenex, Warsaw, Poland) was used. Analysis was conducted with Shimadzu equipment, as described above. For separation of carotenoids and chlorophyll compounds, gradient conditions with a flow rate of 1 mL/min were used. Two gradient phases were used: acetonitrile with methanol 90:10 (phase A) and methanol with ethyl acetate (68:32) (phase B). The total time of the analysis was 25 min. The phase-time program was as follows: 1.00–14.99 min, 100% phase A, 15.00–22.99 min, 40% phase A and 60% phase B; 23.00–27.99 min, 100% phase B. The wavelengths for detection were 445 nm for xanthophylls and 450 nm for carotenes and chlorophylls [40].
4.3.7. Carotenoids—Results Calculation
All carotenoids and chlorophylls were identified by using pure standards (Sigma-Aldrich, Warsaw, Poland) and the retention times for the internal standards. Pure carotenoid (lutein, zeaxanthin, beta-carotene) and chlorophyll (a and b) standards were used for standard solution preparation. From each standard solution, five injections were made. Each time, the chromatographic pick area was determined and calculated based on the standard solution concentration. From standard curves, a mathematical equation was prepared. On the basis of the dilution coefficient and equation, the concentration of individual compounds was calculated.
4.3.8. Antioxidant Activity Measurement and Calculation
ABTS Reagent Preparation
Twenty millilitres of distilled water was added to 0.0265 g of potassium persulfate (K2S2O8). Five millilitres of distilled water followed by 5 mL of a previously prepared aqueous solution of potassium persulfate was added to 0.0384 g of ABTS·+ (2′2-azinebis-3-ethylbenzothiazolin-6-sulfonic acid) reagent. The solution was prepared a minimum of 12 h before the planned assay and stored in a dark place. A total of 250 milligrams of the freeze-dried plant material was weighed into a plastic tube with a cap (50 mL), and 25 mL of distilled water was added. It was placed onto a vortex shaker (LP shaker Vortex, Labo Plus, Warsaw, Poland) for 60 s at 2000 rpm for complete mixing. Subsequently, the sample was incubated in a shaker incubator (IKA KS 4000 Control, IKA, Staufen im Breisgau, Germany) for 60 min (temperature 30 °C, 6000 rpm). After incubation, the sample was again shaken on a vortex shaker for 60 s for complete mixing and then centrifuged (centrifuge, MPW-380 R, Warsaw, Poland) at 5 °C and 8000 rpm for 20 min. After centrifugation, the supernatant was used for determinations. In 10 mL glass tubes, test extract solution, measured with a predetermined dilution scheme (0.5–1.5 mL), was then added to 3.0 mL of ABTS•+ cationic solution in PBS (phosphate-buffered saline). Absorbance measurements were taken exactly 6 min after incubation at room temperature. Absorbance was measured at a wavelength λ = 734 nm using a spectrophotometer (Helios, Thermo Scientific, Warsaw, Poland). The obtained measurements were calculated using a special formula including the dilution factor. The results were expressed as mmol of TE (Trolox equivalents per 100 g FW (fresh weight of flowers and leaves)) [41].
4.4. Statistical Analysis
All results were statistically assessed. For experimental purposes, Statgraphics Centurion 15.2.11.0 software (StatPoint Technologies, Inc., Warrenton, VA, USA) was used. The statistical calculations were based on two-way analysis of variance with the use of Tukey’s test (p = 0.05). A lack of statistically significant differences between the examined groups is indicated by similar letters. The standard error (SE) is provided with each mean value reported in the tables.
5. Conclusions
The conducted experiment confirms that not only fruits and seeds but also the leaves and flowers of two species of Aesculus (horse chestnut and red horse chestnut) contain different qualities and quantities of bioactive compounds, such as carotenoids, chlorophylls, and polyphenols. Furthermore, a two-year-long experiment provided more accurate data regarding the specific profiles and concentrations of bioactive compounds that occurred in the flowers and leaves of both species. Our results bring important information to science regarding the chemical composition of a less well-known species, the red horse chestnut. Due to the high concentrations of various bioactive compounds that they contain, horse chestnut and red horse chestnut flowers and leaves offer high biological value as food additives with strong bioactive and antioxidant capacity.
Author Contributions
Conceptualization, E.H, A.M.B. and R.K.; methodology, E.H.; software, E.H.; validation, E.H.; formal analysis, A.M.B.; investigation, J.W.J.; resources, J.W.J.; data curation, E.H. and R.K.; writing—original draft preparation, A.M.B. and E.H.; writing—review and editing, E.H. and R.K.; visualization, A.M.B. and E.H.; supervision, E.H.; project administration, E.H.; funding acquisition, E.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education with funds of the Institute of Human Nutrition Sciences, Warsaw University of Life Sciences (WULS), for scientific research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the phenolic compounds (analytical standards) are available from the authors.
References
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Song, Y.; Desta, K.T.; Kim, G.-S.; Lee, S.J.; Lee, W.S.; Kim, Y.-H.; Jin, J.S.; El-Aty, A.; Shin, H.-C.; Shim, J.-H.; et al. Polyphenolic profile and antioxidant effects of various parts of Artemisia annua L. Biomed. Chromatogr. 2015, 30, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Bunea, C.-I.; Pop, N.; Babeş, A.C.; Matea, C.; Dulf, F.V.; Bunea, A. Carotenoids, total polyphenols and antioxidant activity of grapes (Vitis vinifera) cultivated in organic and conventional systems. Chem. Centr. J. 2012, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Oszmiański, J.; Kalisz, S.; Wojdyło, A. The content of phenolic compounds in leaf tissues of white (Aesculus hippocastanum L.) and red horse chestnut (Aesculus carea H.) colonized by the horse chestnut leaf miner (Cameraria ohridella Deschka & Dimić). Molecules 2014, 19, 14625–14636. [Google Scholar]
- Percival, G.C.; Holmes, S.P. The influence of systemic inducing agents on horse chestnut leaf miner (Cameraria ohridella) severity in white flowering horse chestnut (Aesculus hoppicastanum L.). Urban For. Urban Green. 2016, 20, 97–102. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Lim, S.; Lee, J.G.; Lee, E.J. VcBBX, VcMYB21, and VcR2R3MYB Transcription factors are involved in UV–B-induced anthocyanin biosynthesis in the peel of harvested blueberry fruit. J. Agric. Food Chem. 2017, 65, 2066–2073. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosche, M.; Keinanen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Phys. 2010, 30, 923–934. [Google Scholar] [CrossRef] [Green Version]
- Gagić, T.; Knez, Ž.; Škerget, M. Subcritical water extraction of horse chestnut (Aesculus hippocastanum) tree parts. J. Serb. Chem. Soc. 2021, 86, 603–613. [Google Scholar] [CrossRef]
- Kędzierski, B.; Kukula-Koch, W.; Widelski, J.; Głowniak, K. Impact of harvest time of Aesculus hippocastanum seeds on the composition, antioxidant capacity and total phenolic content. Ind. Crops Prod. 2016, 86, 68–72. [Google Scholar] [CrossRef]
- Owczarek, A.; Kołodziejczyk-Czepas, J.; Marczuk, P.; Siwek, J.; Wąsowicz, K.; Olszewska, M.A. Bioactivity potential of Aesculus hippocastanum L. flower: Phytochemical profile, antiradical capacity and protective effects on human plasma components under oxidative/nitrative stress in vitro. Pharmaceuticals 2021, 14, 1301. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, A.; Kolodziejczyk-Czepas, J.; Woźniak-Serwata, J.; Magiera, A.; Kobiela, N.; Wąsowicz, K.; Olszewska, M.A. Potential activity mechanisms of Aesculus hippocastanum bark: Antioxidant effects in chemical and biological in vitro models. Antioxidants 2021, 10, 995. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xie, Y.-Q.; Chen, F.; Zhao, Y.-Y.; Luo, C.-X.; Gao, Y.-Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Hallmann, E. Quantitative and qualitative identification of bioactive compounds inedible flowers of black and bristly locust and their antioxidant activity. Biomolecules 2020, 10, 1603. [Google Scholar] [CrossRef]
- Grzeszczuk, M.; Stefaniak, A.; Pachlewska, A. Biological value of various edible flower species. Acta Sci. Pol. Hortorum Cultus 2016, 15, 109–119. [Google Scholar]
- Cruz, R.; Baptista, P.; Cunha, S.; Pereira, J.A.; Casal, S. Carotenoids of lettuce (Lactuca sativa L.) grown on soil enriched with spent coffee grounds. Molecules 2012, 17, 1535–1547. [Google Scholar] [CrossRef] [Green Version]
- Bunea, A.; Andjelkovic, M.; Socaciu, C.; Bobis, O.; Neacsu, M.; Verhé, R.; Camp, J.V. Total and individual carotenoids and phenolic acids content in fresh, refrigerated and processed spinach (Spinacia oleracea L.). Food Chem. 2008, 108, 649–656. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chao, P.-Y.; Hu, S.-P.; Yang, C.-M. The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Sci. Nutr. 2013, 4, 35234. [Google Scholar] [CrossRef] [Green Version]
- Nihal, A.; Mithun, P.R.; Praveen, N. Effect of heavy metals (Hg, As and La) on biochemical constituents of Spinacia oleracea. J. Pharmacogn. Phytochem. 2019, 8, 669–674. [Google Scholar]
- Spinardi, A.; Cocetta, G.; Baldassarre, V.; Ferrante, A.; Mignani, I. Quality changes during storage of spinach and lettuce baby leaf. Acta Hortic. 2010, 877, 571–576. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 1700–1741. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Cicero, A.F.G.; Lippi, G.; Rizzo, M.; Toth, P.P.; Banach, M. Polyphenols: Potential use in the prevention and treatment of cardiovascular diseases. Curr. Pharm. Des. 2018, 24, 239–258. [Google Scholar] [CrossRef]
- Chensom, S.; Okumura, H.; Mishima, T. Primary screening of antioxidant activity, total polyphenol content, carotenoid content, and nutritional composition of 13 edible flowers from Japan. Prev. Nutr. Food Sci. 2019, 24, 171–178. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Cierniak, A. Antioxidant and genoprotective properties of extracts from edible flowers. J. Food Nutr. Res. 2019, 58, 42–50. [Google Scholar]
- Zeng, C.; Luo, S.; Feng, S.; Chen, T.; Zhou, L.; Yuan, M.; Huang, Y.; Liao, Y.; Ding, C. Phenolic composition, antioxidant and anticancer potentials of extracts from Rosa banksiae Ait. Flower. Molecules 2020, 58, 3068. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. Fruits and flowers: Chemical composition and related bioactivities. Food Rev. Int. 2020, 1–29. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic profile and antioxidant activity of five apple cultivars grown under organic and conventional agricultural practices. Int. J. Food Sci. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, C.; Bucheli, P.; Wei, D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Foods Hum. Nut. 2006, 61, 55–63. [Google Scholar] [CrossRef]
- Veberic, R.; Slatnar, A.; Bizjak, J.; Stampar, F.; Mikulic-Petkovsek, M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci. Techol. 2015, 60, 509–517. [Google Scholar] [CrossRef]
- Sharipov, A.; Tursunov, K.; Fazliev, S.; Azimova, B.; Razzokov, J. Hypoglycemic and anti-inflammatory effects of triterpene glycoside fractions from Aeculus hippocastanum seeds. Molecules 2021, 26, 3784. [Google Scholar] [CrossRef]
- Dudek-Makuch, M.; Studzińska-Sroka, E.; Korybalska, K.; Czepulis, N.; Łuczak, J.; Rutkowski, R.; Witowski, J. Biological activity of Aesculus hippocastanum flower extracts on vascular endothelial cells cultured in vitro. Phytochem. Lett. 2019, 30, 367–375. [Google Scholar] [CrossRef]
- Polish Standard Committee. Polish Norm PN-R-04013: The Estimation of Dry Matter in Fruits and Vegetables; Polish Standard Committee: Warszawa, Poland, 1988; pp. 1–5. [Google Scholar]
- Średnicka-Tober, D.; Ponder, A.; Hallmann, E.; Głowacka, A.; Rozpara, E. The profile and content of polyphenols and carotenoids in local and commercial sweet cherry fruits (Prunus avium L.) and their antioxidant activity in vitro. Antioxidants 2019, 8, 534. [Google Scholar] [CrossRef] [Green Version]
- Ponder, A.; Hallmann, E. Phenolics and carotenoid contents in the leaves of different organic and conventional raspberry (Rubus idaeus L.) cultivars and their in vitro activity. Antioxidants 2019, 8, 458. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Nala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).