Anticancer Activity of Cordia dichotoma against a Panel of Human Cancer Cell Lines and Their Phytochemical Profiling via HPLC and GCMS
Abstract
:1. Introduction
2. Results
2.1. Screening for Cytotoxic Activity
2.2. Mechanistic Assays
2.2.1. Chloroform Fraction Triggered Chromatin Condensation
2.2.2. ROS Production by the Chloroform Fraction Causes Cell Death
2.2.3. Effect of Fraction on Colonies Development
2.2.4. Inhibition of Cell Migration by Chloroform Fraction
2.3. Identification of Anticancer Molecules
2.3.1. High-Performance Liquid Chromatography
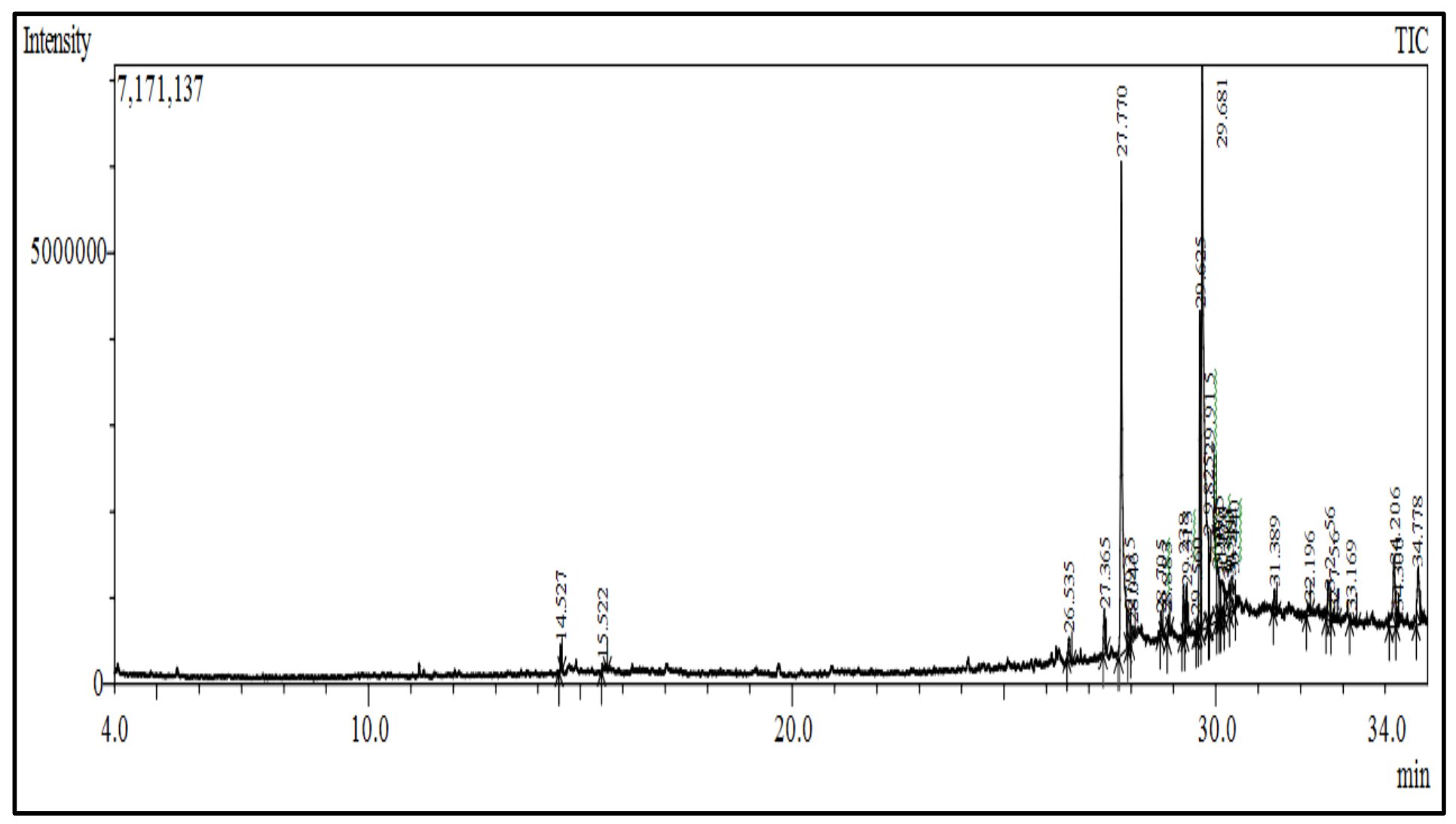
2.3.2. Gas Chromatography Mass Spectroscopy Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Plant Material and Preparation of Extract and Fractions
4.3. Sample Preparation and Culturing of Cells
4.4. SRB Assay for Cytotoxicity
4.5. Mechanistic Assays
4.5.1. Evaluation of Cell Apoptosis
4.5.2. Estimation of Reactive Oxygen Species Production
4.5.3. Colony Formation Assay
4.5.4. Wound Healing Assay
4.6. Analysis of Phytochemicals by HPLC and GCMS
4.7. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Greenwell, M.; Rahman, P.K.S.M. Medicinal plants: Their use in anticancer treatment. Int. J. Pharm. Sci. 2015, 6, 4103–4112. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [PubMed] [Green Version]
- Aslam, M.S.; Ahmad, M.S. Worldwide importance of medicinal plants: Current and historical perspectives. Recent. Adv. Biol. Med. 2016, 2, 88–93. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar]
- Rybak, S.M.; Pearson, J.W.; Fogler, W.E.; Volker, K.; Spence, S.E.; Newton, D.L.; Longo, D.L. Enhancement of vincristine cytotoxicity in drug-resistant cells by simultaneous treatment with onconase, an antitumor ribonuclease. J. Natl. Cancer Inst. 1996, 88, 747–753. [Google Scholar]
- Silvestri, R. New prospects for vinblastine analogues as anticancer agents. J. Med. Chem. 2013, 56, 625–627. [Google Scholar]
- Son, Y.O.; Choi, K.C.; Lee, J.C.; Kook, S.H.; Lee, S.K.; Takada, K.; Jang, Y.S. Involvement of caspase activation and mitochondrial stress in taxol-induced apoptosis of Epstein–Barr virus-infected Akata cells. Biochim. Biophys. Acta 2006, 1760, 1894–1902. [Google Scholar]
- Dallavalle, S.; Giannini, G.; Alloatti, D.; Casati, A.; Marastoni, E.; Musso, L.; Zunino, F. Synthesis and cytotoxic activity of polyamine analogues of camptothecin. J. Med. Chem. 2006, 49, 5177–5186. [Google Scholar]
- Benderoth, M.; Pfalz, M.; Kroymann, J. Methylthioalkylmalate synthases: Genetics, ecology and evolution. Phytochem. Rev. 2009, 8, 255–268. [Google Scholar]
- Ham, S.L.; Nasrollahi, S.; Shah, K.N.; Soltisz, A.; Paruchuri, S.; Yun, Y.H.; Tavana, H. Phytochemicals potently inhibit migration of metastatic breast cancer cells. Integr Biol. 2015, 7, 792–800. [Google Scholar]
- Tuominen, E.K.; Zhu, K.; Wallace, C.J.; Lewis, C.I.; Craig, D.B.; Rytoomaa, M.; Kinnunen, P.K. ATP induces a conformational change in lipid-bound cytochrome c. J. Biol. Chem. 2011, 276, 19356–19362. [Google Scholar]
- Chen, Q.; Gong, B.; Almasan, A. Distinct stages of cytochrome c release from mitochondria: Evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000, 7, 227–233. [Google Scholar]
- Haridas, V.; Higuchi, M.; Jayatilake, G.S.; Bailey, D.; Mujoo, K.; Blake, M.E.; Gutterman, J.U. Avicins: Triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc. Natl. Acad. Sci. USA 2001, 98, 5821–5826. [Google Scholar] [PubMed] [Green Version]
- Korsmeyer, S.J. Regulators of cell death. Trends Genet. 1995, 11, 101–105. [Google Scholar] [PubMed]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; Guyader, L.; Gao, G.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar]
- Jamkhande, P.G.; Barde, S.R.; Patwekar, S.L.; Tidke, P.S. Plant profile, phytochemistry and pharmacology of Cordia dichotoma (Indian cherry): A review. Asian Pac. J. Trop. Biomed. 2013, 3, 1009–1012. [Google Scholar]
- Hussain, N.; Kakoti, B.B. Review on ethnobotany and phytopharmacology of C. dichotoma. J. Drug. Deliv. Ther. 2013, 3. [Google Scholar]
- Nariya, P.B.; Bhalodia, N.R.; Shukla, V.J.; Acharya, R.N. Antimicrobial and antifungal activities of Cordia dichotoma (Forster F.) bark extracts. Ayu 2011, 32, 585. [Google Scholar]
- Kuppast, I.J.; Nayak, P.V. Wound healing activity of Cordia dichotoma Forst. f. fruits. Indian. J. Nat. Prod. Resour. 2006, 5, 99–102. [Google Scholar]
- Sharma, U.S.; Sharma, U.K.; Sutar, N.; Singh, A.; Shukla, D.K. Anti-inflammatory activity of Cordia dichotoma forst f. seeds extracts. Int. J. Pharm. Anal. 2010, 2, 1. [Google Scholar]
- Bharathi, D.; Vasantharaj, S.; Bhuvaneshwari, V. Green synthesis of silver nanoparticles using Cordia dichotoma fruit extract and its enhanced antibacterial, anti-biofilm and photo catalytic activity. Mater. Res. Express. 2018, 5, 055404. [Google Scholar]
- Del-Toro-Sanchez, C.L.; Rodríguez-Felix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Karpuz, M.; Silindir, G.M.; Ozer, A.Y. Current and future approaches for effective cancer imaging and treatment. Cancer Biother. Radiopharm. 2018, 33, 39–51. [Google Scholar]
- Nobili, S.; Lippi, D.; Witort, E.; Donnini, M.; Bausi, L.; Mini, E.; Capaccioli, S. Natural compounds for cancer treatment and prevention. Pharmacol. Res. Commun. 2009, 59, 365–378. [Google Scholar]
- Hussain, N.; Kakoti, B.B.; Rudrapal, M.; Sarwa, S.S. Anticancer and antioxidant activities of Cordia dichotoma Forst. Biomed. Pharmacol. J. 2020, 13, 2093–2099. [Google Scholar]
- Rahman, M.A.; Akhtar, J. Evaluation of anticancer activity of Cordia dichotoma leaves against a human prostate carcinoma cell line, PC3. J. Tradit. Complement. Med. 2017, 7, 315–321. [Google Scholar]
- Ibrahim, A.Y.; El-Newary, S.A.; Ibrahim, G.E. Antioxidant, cytotoxicity and anti-tumor activity of Cordia dichotoma fruits accompanied with its volatile and sugar composition. Ann. Agric. Sci. 2019, 64, 29–37. [Google Scholar]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview of the role of dietary phenolics for the treatment of cancers. J. Nutr. 2016, 15, 99–106. [Google Scholar]
- Usmani, S.; Ahmad, M.; Hussain, A.; Arshad, M.; Ali, M. Cellular oxidative stress and antiproliferative effects of Cordia dichotoma (Linn.) seeds extract and their fractions on human cervix epitheloid (HeLa) and human lung (A549) carcinoma cells. Eur. J. Integr. Med. 2018, 21, 1–10. [Google Scholar]
- Massry, K.F.; Farouk, A.; Mahmoud, K.F.; Ghorab, A.H.; Musa, A.; Mostafa, E.M.; Abdelgawad, M.A. Chemical characteristics and targeted encapsulated Cordia myxa fruits extracts nanoparticles for antioxidant and cytotoxicity potentials. Saudi J. Biol. Sci. 2021, 28, 5349–5358. [Google Scholar] [PubMed]
- Moheb, A.; Grondin, M.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Selective anticancer potential of several methylated phenolic compounds. J. Nat. Pharm. 2013, 4, 44. [Google Scholar]
- Zhang, Y.X.; Yu, P.F.; Gao, Z.M.; Yuan, J.; Zhang, Z. Caffeic acid n-butyl ester-triggered necrosis-like cell death in lung cancer cell line A549 is prompted by ROS mediated alterations in mitochondrial membrane potential. Eur. Rev. Med. Pharm. Sci. 2017, 21, 1665–1671. [Google Scholar]
- Maurya, D.K.; Nandakumar, N.; Devasagayam, T.P.A. Anticancer property of gallic acid in A549, a human lung adenocarcinoma cell line, and possible mechanisms. J. Clin. Biochem. Nutr. 2010, 48, 85–90. [Google Scholar]
- Gheena, S.; Ezhilarasan, D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum. Exp. Toxicol. 2019, 38, 694–702. [Google Scholar]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytoscience. 2018, 4, 1–6. [Google Scholar]
- Bharath, B.; Perinbam, K.; Devanesan, S.; AlSalhi, M.S.; Saravanan, M. Evaluation of the anticancer potential of Hexadecanoic acid from brown algae Turbinaria ornata on HT–29 colon cancer cells. J. Mol. Struct. 2021, 1235, 130229. [Google Scholar]
- Field, C.J.; Blewett, H.H.; Proctor, S.; Vine, D. Human health benefits of vaccenic acid. Appl. Physiol. Nutr. Metab. 2009, 34, 979–991. [Google Scholar]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [PubMed]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Boyd, M. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [PubMed]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [PubMed] [Green Version]
- Chashoo, G.; Singh, S.K.; Sharma, P.R.; Mondhe, D.M.; Hamid, A.; Saxena, A.; Andotra, S.S.; Shah, B.A.; Qazi, N.A.; Taneja, S.C.; et al. Apropionyloxy derivative of 11-keto -boswellic acid induces apoptosis in HL-60 cells mediated through topoisomerase I & II inhibition. Chem. Biol. Interact. 2011, 189, 60–71. [Google Scholar]
- Banskota, S.; Regmi, S.C.; Kim, J.A. NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Mol. Cancer 2015, 14, 1–14. [Google Scholar]
- Franken, N.A.P.; Rhodermond, H.M.; Stap, J.; Haveman, J.; Bree, C.V. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar]
- Tarnawski, M.; Depta, K.; Grejciun, D.; Szelepin, B. HPLC determination of phenolic acids and antioxidant activity in concentrated peat extract—A natural immunomodulator. J. Pharm. Biomed. Anal. 2006, 41, 182–188. [Google Scholar]
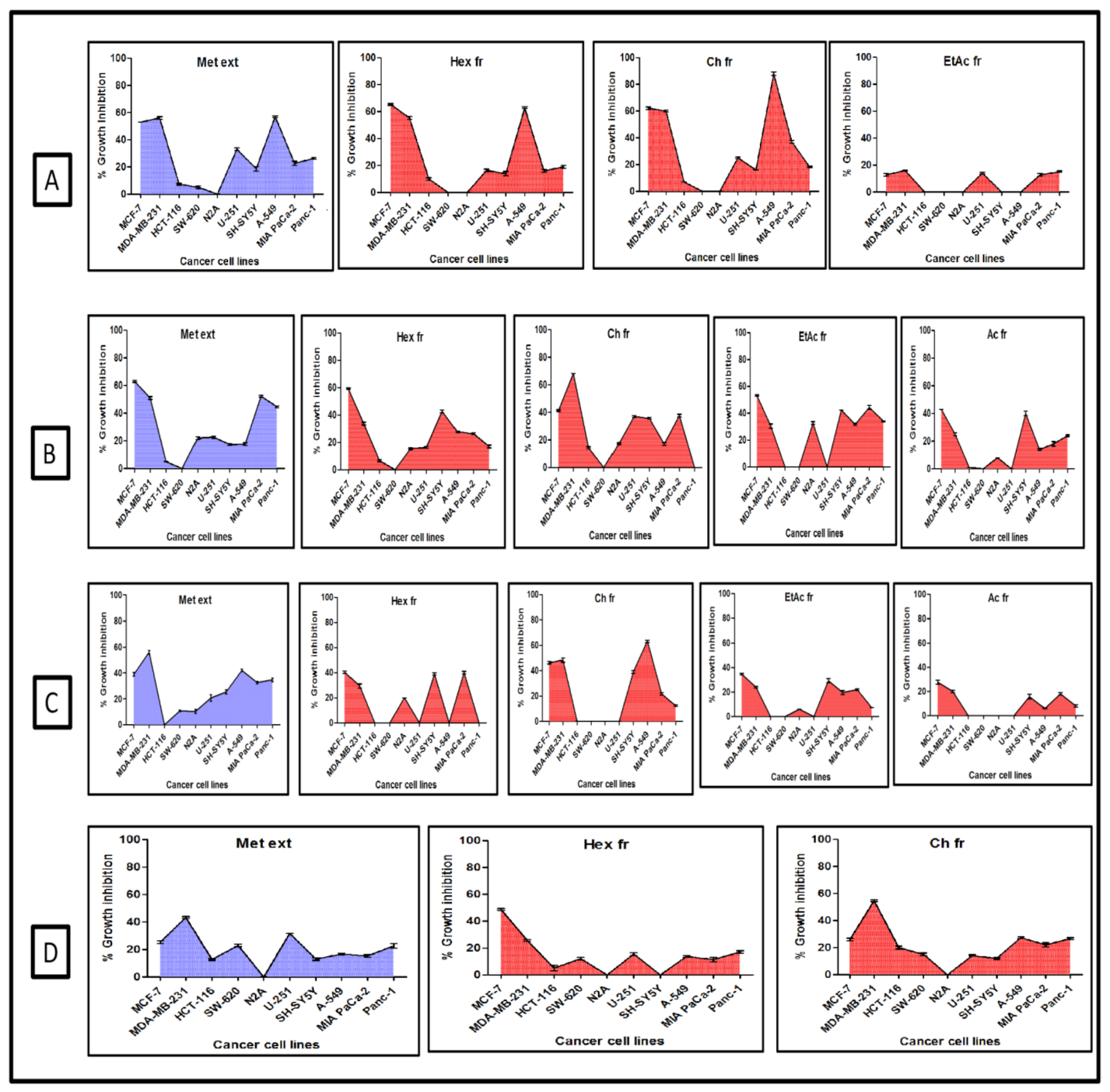


| Cell Line | Chloroform Fraction of Bark IC50 (µg/mL) | Standard Drug (Paclitaxel) IC50 (nM) |
|---|---|---|
| A-549 | 37.978 | 4.8 |
| RT | Area (uV*sec) | Structure | Name |
|---|---|---|---|
| 1.988 | 144,969.86 | 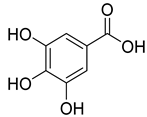 | Gallic acid |
| 4.842 | 2497.31 | 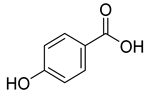 | p-hydroxy benzoic acid |
| 5.908 | 104.26 | 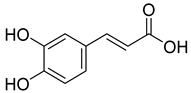 | Caffeic acid |
| 6.093 | 13,775.39 | 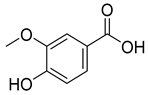 | Vanillic acid |
| 7.130 | 2724.56 | 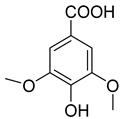 | Syringic acid |
| 10.909 | 275.75 | 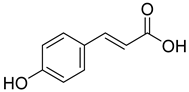 | p-Counaric acid |
| 14.218 | 472.87 | 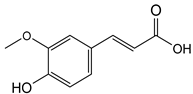 | Ferulic acid |
| Compounds | Class of Compound | Area % | m/z | R. Time |
|---|---|---|---|---|
| 1,3-Di-tert-butyl benzene | Phenyl propanes | 0.68 | 175.10 | 14.527 |
| 4-Vinylguaiacol | Phenol | 0.51 | 150.15 | 15.522 |
| Diisobutyl phthalate | Ester | 0.83 | 149.05 | 26.535 |
| Methyl palmitate | Ester | 1.29 | 74.00 | 27.365 |
| Palmitic acid | Fatty acid | 18.34 | 73 | 27.770 |
| Benzylamine, 2-hydroxy-N,N-di[2-aminoethyl)amino]methyl)phenol | Phenol | 1.73 | 71.05 | 27.935 |
| 16-Hydroxyhexadecanoic acid | Fatty acid | 1.43 | 60.10 | 28.046 |
| Methyl linoleate | Fatty acid | 1.23 | 81.00 | 29.238 |
| Methyl cis-9,10-epoxystearate | Ester | 1.41 | 55.00 | 29.313 |
| Octadecadienoic acid | Fatty acids | 9.46 | 67.00 | 29.625 |
| cis-Vaccenic acid | Fatty acids | 28.81 | 55.00 | 29.681 |
| 1-Dimethyl(pentafluorophenyl)silyloxydodecane | NC | 1.82 | 79.05 | 29.825 |
| Palmitoleic acid | Fatty acids | 12.54 | 73.00 | 29.915 |
| 1-(beta.-d-Ribofuranosyl)-4-difluormethoxy-uracil | Dihydropyridine | 1.94 | 69.00 | 30.065 |
| Methylallyl Trifluoroacetate | Allyl acyls | 1.89 | 55.05 | 30.170 |
| Difluoroacetic acid | Monocarboxylic acid | 2.22 | 69.05 | 30.310 |
| Linoleic acid trimethylsilyl ester | Ester | 1.71 | 80.00 | 30.381 |
| (2-phenyl-1,3-dioxolan-4-yl)methyl (z)-octadec-9-enoate | NC | 0.85 | 73.00 | 30.440 |
| Tridecyl 2-Methoxyacetate | Ester | 0.70 | 57.00 | 31.389 |
| 6,9,12,15-Docosatetraenoic acid, methyl ester | Ester | 0.57 | 69.10 | 32.196 |
| Dotriacontane | Alkanes | 1.58 | 57.00 | 32.656 |
| (4-Allyloxy-3-methoxy-phenyl)-methanol | NC | 0.58 | 97.15 | 32.756 |
| 1-Propyltridecyl 4-bromobutanoate | Ester | 0.72 | 69.05 | 33.169 |
| 9-octylheptadecane | Alkanes | 3.39 | 57.00 | 34.206 |
| 1-[(1-Ethylundecyl)oxy]-1-methylsilinane | Phenylpyrazoles | 0.51 | 55.00 | 34.300 |
| Dioctyl phthalate | Benzoic acid | 2.66 | 148.95 | 34.778 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raina, S.; Sharma, V.; Sheikh, Z.N.; Kour, N.; Singh, S.K.; Zari, A.; Zari, T.A.; Alharby, H.F.; Hakeem, K.R. Anticancer Activity of Cordia dichotoma against a Panel of Human Cancer Cell Lines and Their Phytochemical Profiling via HPLC and GCMS. Molecules 2022, 27, 2185. https://doi.org/10.3390/molecules27072185
Raina S, Sharma V, Sheikh ZN, Kour N, Singh SK, Zari A, Zari TA, Alharby HF, Hakeem KR. Anticancer Activity of Cordia dichotoma against a Panel of Human Cancer Cell Lines and Their Phytochemical Profiling via HPLC and GCMS. Molecules. 2022; 27(7):2185. https://doi.org/10.3390/molecules27072185
Chicago/Turabian StyleRaina, Shilpa, Vikas Sharma, Zahid Nabi Sheikh, Navneet Kour, Shashank K. Singh, Ali Zari, Talal A. Zari, Hesham F. Alharby, and Khalid Rehman Hakeem. 2022. "Anticancer Activity of Cordia dichotoma against a Panel of Human Cancer Cell Lines and Their Phytochemical Profiling via HPLC and GCMS" Molecules 27, no. 7: 2185. https://doi.org/10.3390/molecules27072185
APA StyleRaina, S., Sharma, V., Sheikh, Z. N., Kour, N., Singh, S. K., Zari, A., Zari, T. A., Alharby, H. F., & Hakeem, K. R. (2022). Anticancer Activity of Cordia dichotoma against a Panel of Human Cancer Cell Lines and Their Phytochemical Profiling via HPLC and GCMS. Molecules, 27(7), 2185. https://doi.org/10.3390/molecules27072185







