Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Fractions of O. falcata on H9c2 Cell Injury
2.1.1. Effects of OFF1 on Cell Viability
2.1.2. Effects of OFF1 on Cardiomyocyte Damage
2.1.3. Effects of OFF1 on Cell Apoptosis
2.2. Identification of the Main Components of OFF1
2.3. Network Pharmacology Analysis
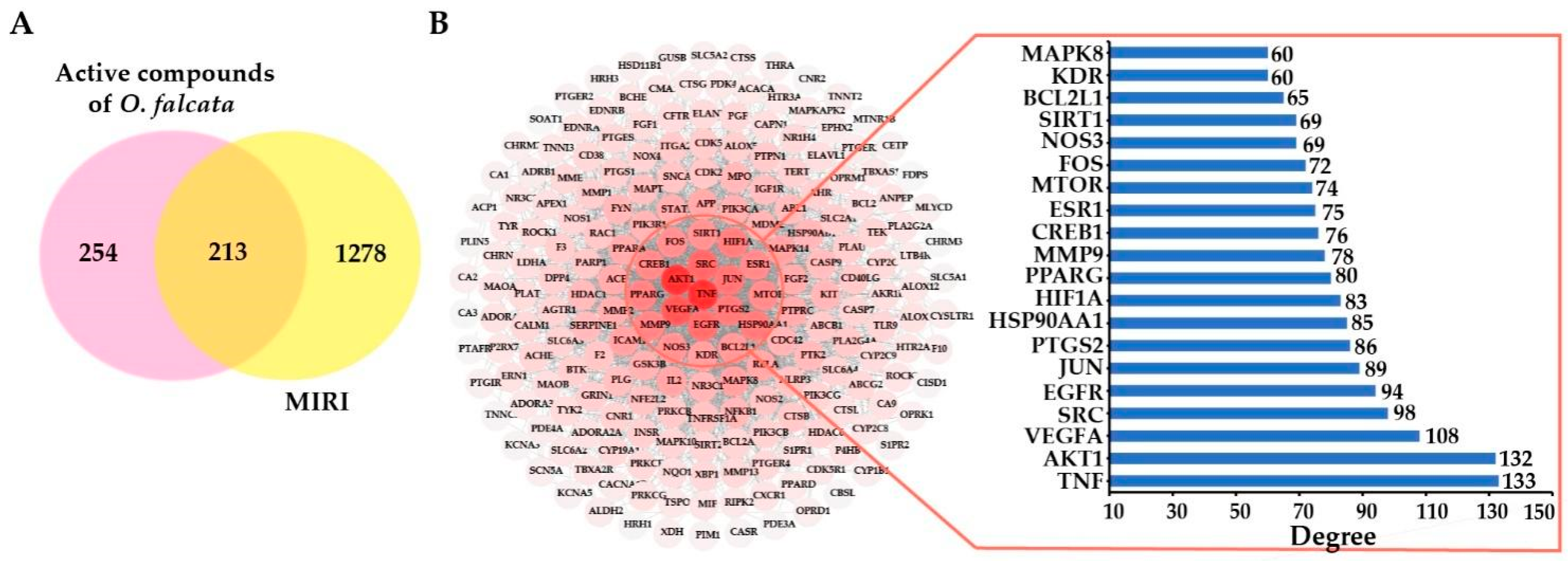
2.3.1. Selection of Active Compounds in O. falcata
2.3.2. Potential Targets and PPI Network Analysis
2.3.3. Compound-Disease-Target Network Analysis
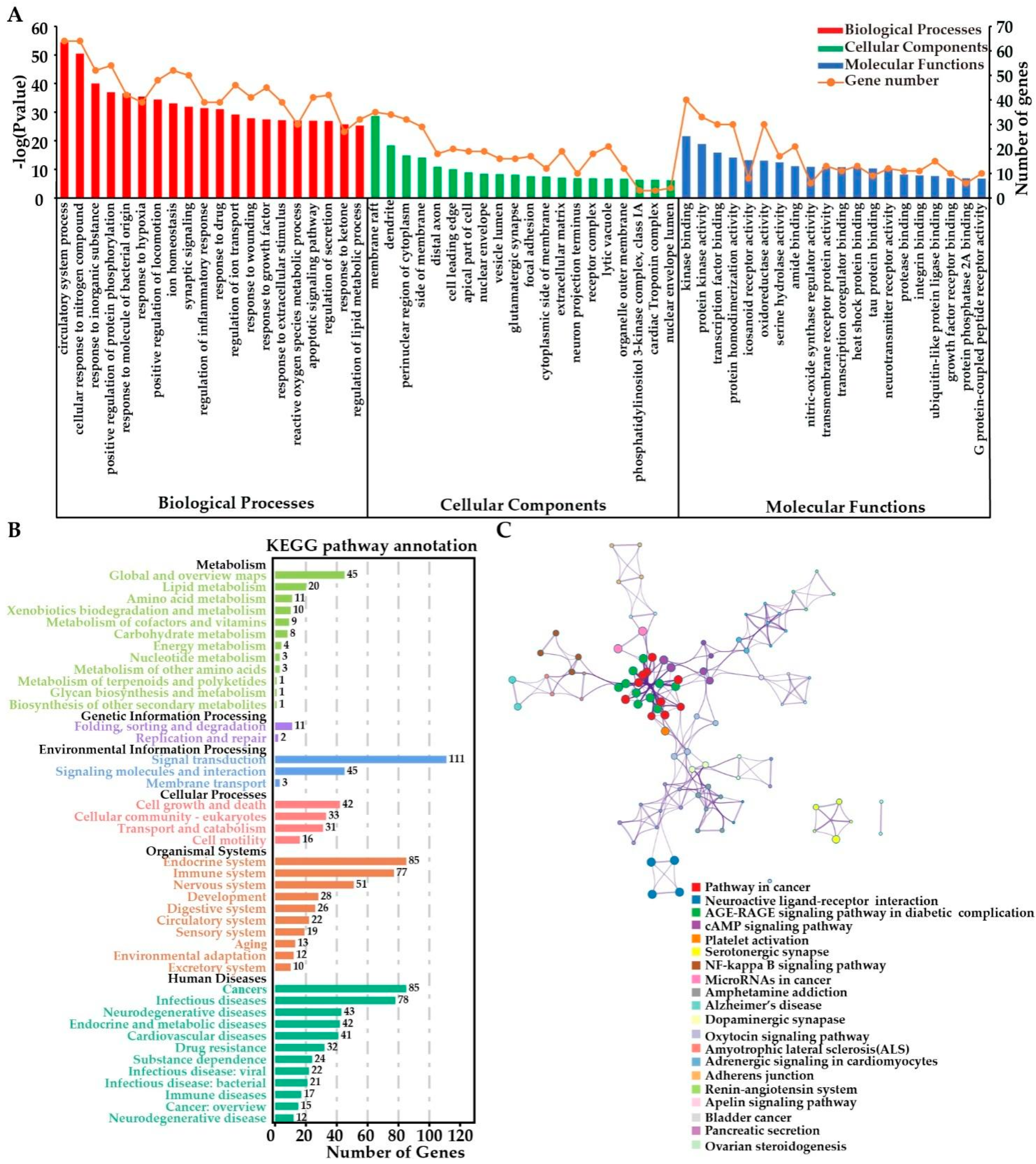
2.3.4. Functional Enrichment Analyses
2.4. Mechanism of Action of OFF1 in the Protection against MIRI In Vitro
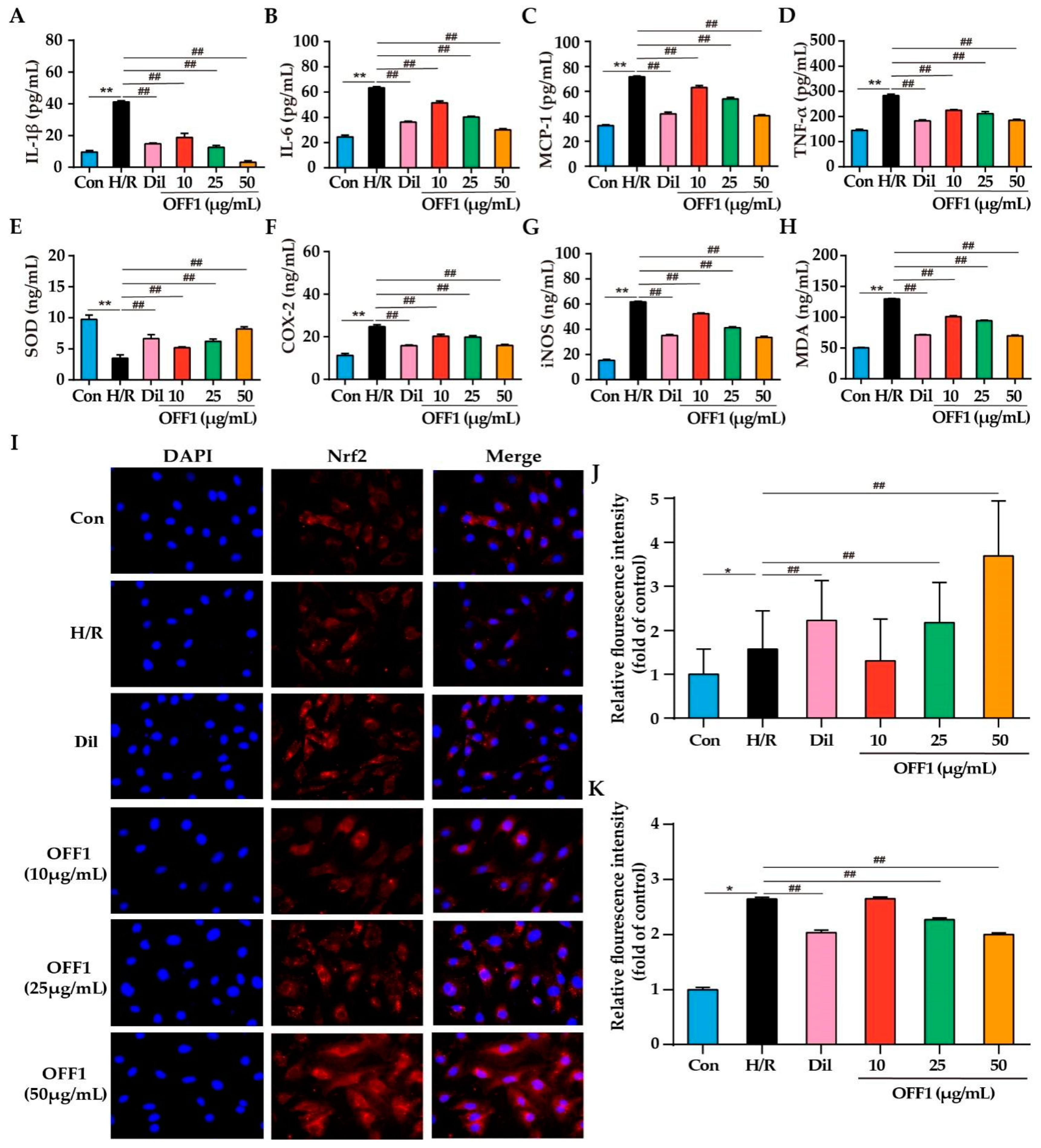
2.4.1. OFF1 Suppresses the Inflammatory Response
2.4.2. OFF1 Suppresses Oxidative Stress
2.4.3. OFF1 Activates the Nrf2 Signaling Pathway
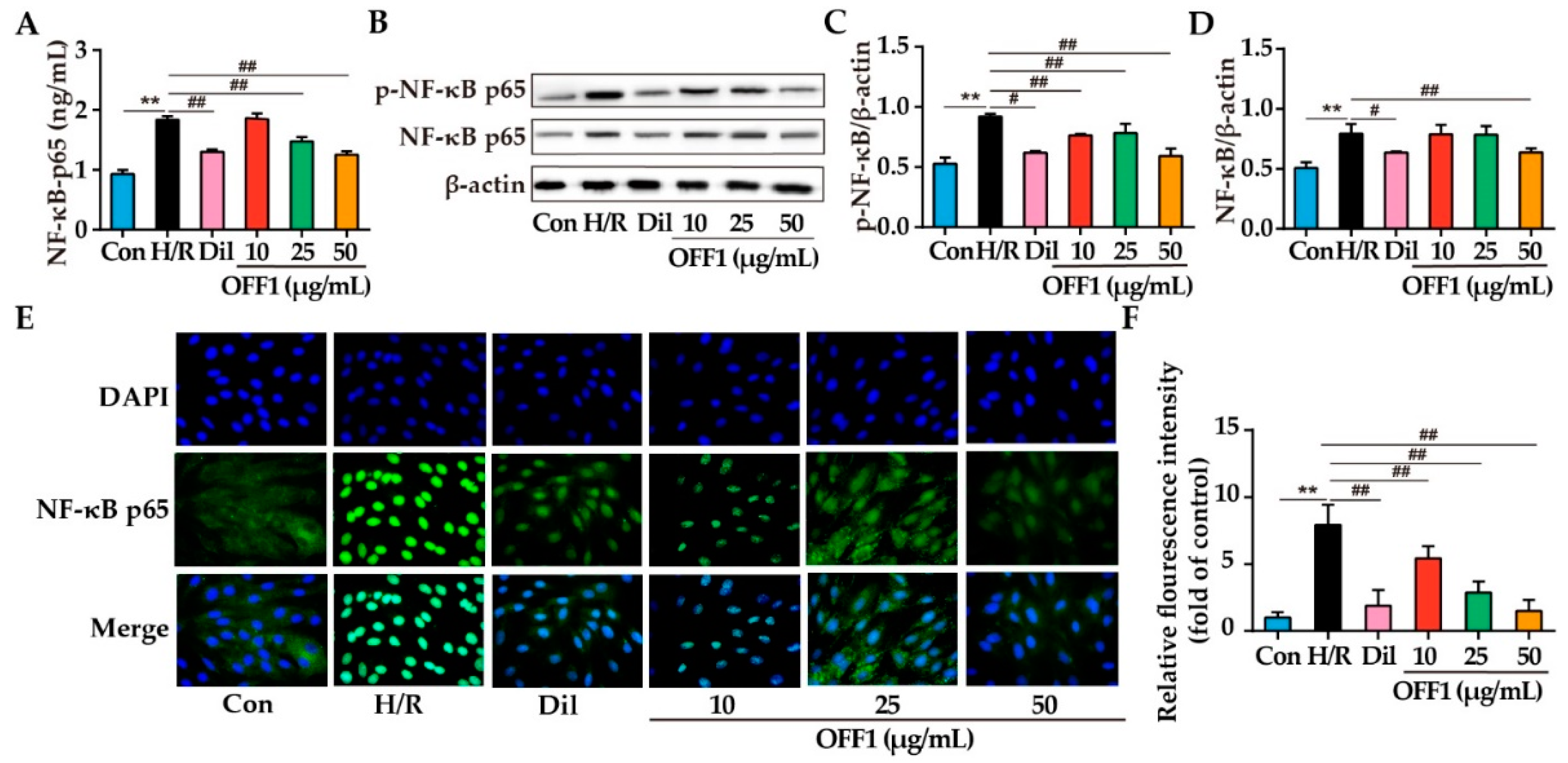
2.4.4. OFF1 Alleviates H/R Injury in Cardiomyocytes through the NF-κB Pathway
2.4.5. OFF1 Alleviates H/R Injury in Cardiomyocytes through the JNK/p38MAPK Pathway
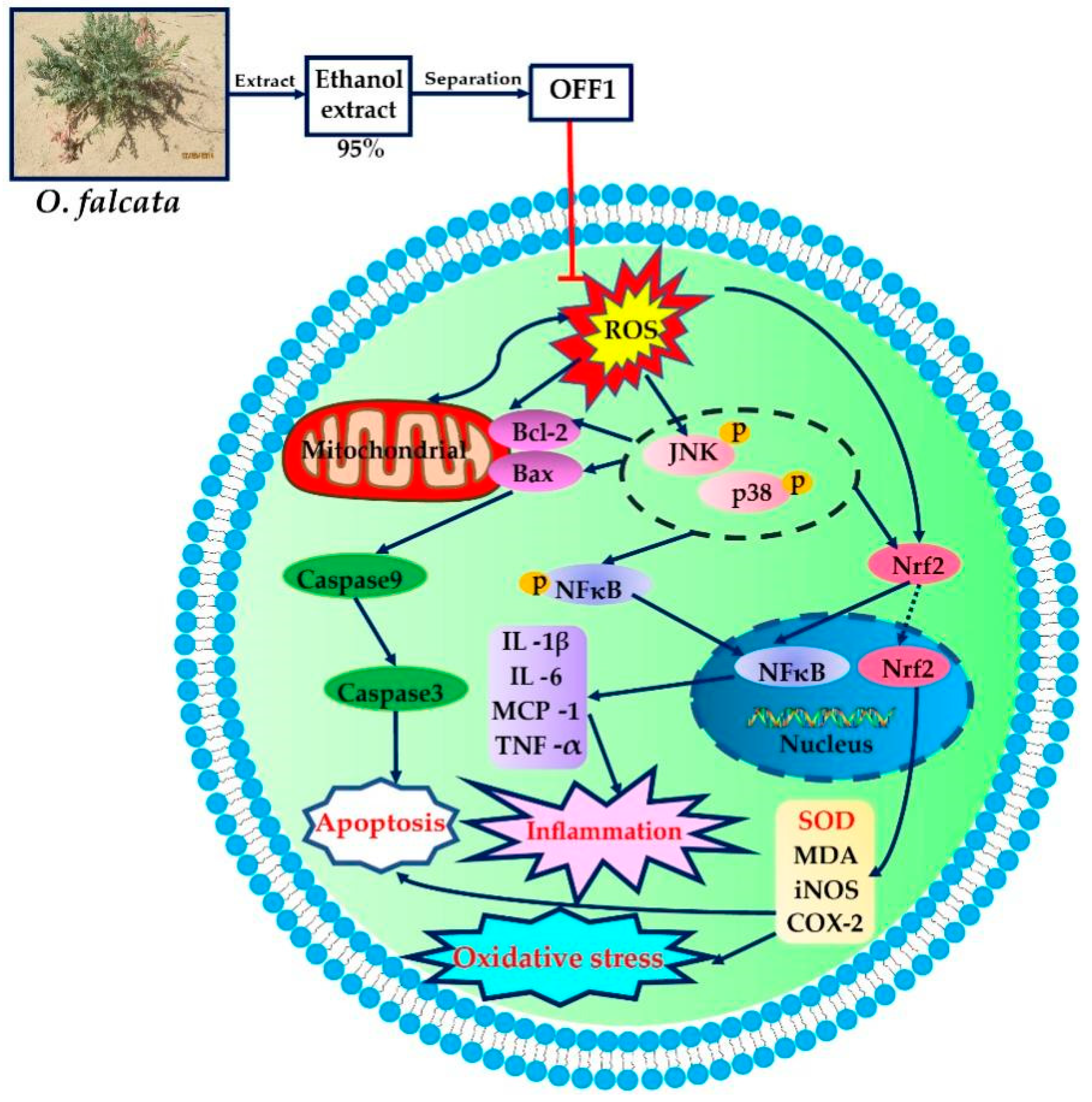
3. Discussion
4. Materials and Methods
4.1. Preparation of the Active Fraction of O. falcata
4.2. Identification of Main Compounds in Bioactive Fraction
4.3. Identification of Active Flavonoid Compounds in O. falcata
4.4. Prediction of Active Compound-Related and MIRI-Related Targets
4.5. Construction and Analysis of the PPI Network
4.6. Construction of Drug–Compound–Gene–Disease Network
4.7. GO and KEGG Pathway Enrichment Analysis
4.8. Cell Culture and H/R Modeling
4.9. Cell Viability Assay
4.10. Biochemical Assay
4.11. Measurement of ROS Levels
4.12. Western Blotting
4.13. Immunofluorescence
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kisa, A.; Kisa, S.; GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 26, 795–820. [Google Scholar]
- Bellis, A.; Di Gioia, G.; Mauro, C.; Mancusi, C.; Barbato, E.; Izzo, R.; Trimarco, B.; Morisco, C. Reducing Cardiac Injury during ST-Elevation Myocardial Infarction: A Reasoned Approach to a Multitarget Therapeutic Strategy. J. Clin. Med. 2021, 10, 2968. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Ekeloef, S.; Koyuncu, S.; Holst-Knudsen, J.; Gundel, O.; Meyhoff, C.S.; Homilius, M.; Stilling, M.; Ekeloef, P.; Münster, A.M.B.; Mathiesen, O.; et al. Cardiovascular events in patients undergoing hip fracture surgery treated with remote ischaemic preconditioning: 1-year follow-up of a randomised clinical trial. Anaesthesia 2021, 76, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.M.; Glinge, C.; Jabbari, R.; Nepper-Christensen, L.; Høfsten, D.E.; Tilsted, H.-H.; Holmvang, L.; Pedersen, F.; Joshi, F.R.; Sørensen, R.; et al. Comparison of Effect of Ischemic Postconditioning on Cardiovascular Mortality in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention with versus without Thrombectomy. Am. J. Cardiol. 2021, 166, 18–24. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Aimo, A.; Emdin, M.; Pico, F. Remote Ischemic Conditioning in Ischemic Stroke and Myocardial Infarction: Similarities and Differences. Front. Neurol. 2021, 12, 716316. [Google Scholar] [CrossRef]
- Shen, S.; Ye, J.; Wu, X.; Li, X. Association of N-terminal pro-brain natriuretic peptide level with adverse outcomes in patients with acute myocardial infarction: A meta-analysis. Heart Lung 2021, 50, 863–869. [Google Scholar] [CrossRef]
- Torregroza, C.; Yueksel, B.; Ruske, R.; Stroethoff, M.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Feige, K. Combination of Cyclosporine A and Levosimendan Induces Cardioprotection under Acute Hyperglycemia. Int. J. Mol. Sci. 2021, 22, 4517. [Google Scholar] [CrossRef]
- Procopio, M.C.; Lauro, R.; Nasso, C.; Carerj, S.; Squadrito, F.; Bitto, A.; Di Bella, G.; Micari, A.; Irrera, N.; Costa, F. Role of Adenosine and Purinergic Receptors in Myocardial Infarction: Focus on Different Signal Transduction Pathways. Biomedicines 2021, 9, 204. [Google Scholar] [CrossRef]
- Zuo, J.; Park, C.; Kung, J.Y.C.; Bou-Chacra, N.A.; Doschak, M.; Löbenberg, R. Traditional Chinese Medicine “Pill”, an Ancient Dosage Form with Surprising Modern Pharmaceutical Characteristics. Pharm. Res. 2021, 38, 199–211. [Google Scholar] [CrossRef]
- Xie, F.; Wu, Y.-Y.; Duan, G.-J.; Wang, B.; Gao, F.; Wei, P.-F.; Chen, L.; Liu, A.P.; Li, M. Anti-Myocardial Ischemia Reperfusion Injury Mechanism of Dried Ginger-Aconite Decoction Based on Network Pharmacology. Front. Pharmacol. 2021, 12, 609702. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gao, Y.; Zhu, J.; Zhang, J.; Dong, M.; Mao, Y. Protective action of the ginsenoside Rh3 in a rat myocardial ischemia-reperfusion injury model by inhibition of apoptosis induced via p38 mitogen-activated protein kinase/caspase-3 signaling. J. Int. Med. Res. 2020, 48, 300060520969090. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Zhou, J.; Wu, D.; Ye, J.; Sun, G.; Sun, X. Saponins in Chinese Herbal Medicine Exerts Protection in Myocardial Ischemia-Reperfusion Injury: Possible Mechanism and Target Analysis. Front. Pharmacol. 2020, 11, 570867. [Google Scholar] [CrossRef] [PubMed]
- Vedarathinam, R.C.; Rajkumar, Y.; Vetriselvan, P.; Prem, P.N.; Ganapathy, A.; Kurian, G.A. Resveratrol-mediated cardioprotection against myocardial ischemia-reperfusion injury was revoked by statin-induced mitochondrial alterations. Drug Chem. Toxicol. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Kong, L.; Yuan, T.; Li, W.; Zhang, W.; Hou, B.; Lu, Y.; Du, G. The strategies and techniques of drug discovery from natural products. Pharmacol. Ther. 2020, 216, 107686. [Google Scholar] [CrossRef]
- Wu, Z.; Raven, P.H.; Hong, D. Flora of China; Science Press: St. Louis, MO, USA, 1998; Volume 49. [Google Scholar]
- Jiang, H.; Hu, J.R.; Zhan, W.Q.; Liu, X. Screening for fractions of Oxytropis falcata Bunge with antibacterial activity. Nat. Prod. Res. 2009, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.Y.; Gao, L.M. Study on the Chemical Constituents of Oxytropis falcata Bunge. Nat. Prod. Res. Dev. 2009, 21, 246–248. [Google Scholar]
- Yang, H.; Dong, W.; Li, T.; Cai, B. Flavonoid aglycones of Oxytropis falcata. Chem. Nat. Compd. 2009, 45, 239–241. [Google Scholar]
- Gong, X.; Xiong, H.; Liu, S.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Zhao, Z.; Chen, W.; Mei, Z. Qingpeng Ointment Ameliorates Inflammatory Responses and Dysregulation of Itch-Related Molecules for Its Antipruritic Effects in Experimental Allergic Contact Dermatitis. Front. Pharmacol. 2019, 10, 354. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Caifeng, L.; Na, Z.; Xiaorui, Z.; Jianzhi, C.; Shasha, G.; Junqi, W. A network pharmacological study on the potential mechanism of Shierwei Yishou San in the treatment of Nian Yu Nai. World Sci. Technol.—Mod. Tradit. Chin. Med. 2021, 23, 1978–1987. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Lin, X.; Wen, J.; Bai, X. Systematic review on clinical efficacy and safety of Cheezheng Pain Relieving Plaster for soft tissue injury. Chin. J. Tradit. Chin. Med. 2020, 45, 431–438. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, W.; Zhang, B.; Hong, E.K.; Shi, P.; Yang, Z.; Zhang, Y.; Wang, H. Analysis of chemical constituents in the extract and rat serum from the chloroform extract of Oxytropis falcata Bunge by HPLC-MS. Pak. J. Pharm. Sci. 2020, 33, 669–674. [Google Scholar] [PubMed]
- Jiang, H.; Zhan, W.Q.; Liu, X.; Jiang, S.X. Antioxidant activities of extracts and flavonoid compounds from Oxytropis falcata Bunge. Nat. Prod. Res. 2008, 22, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Li, Y.; Wang, X.-L.; Li, X.-Z.; Chen, Y.-W.; Yang, L.-L.; Ming, H.-X. Effect of Total Flavonoids of Bunge on the Expression of p-JAK1-and p-STAT1-Related Proteins in Idiopathic Pulmonary Fibrosis. Evid. Based Complement. Alternat. Med. 2020, 2020, 2407239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cai, X.; Cao, Y.; Zhou, C.; Yu, L.; Chen, J. Preparation, characterization, and pharmacodynamic study on deep second degree burns of total flavonoids composite phospholipids liposome gel of Bunge. Drug Dev. Ind. Pharm. 2020, 46, 2000–2009. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z.; Jiang, L.; Sun, W.; Fan, Q.; Liu, T. Total Flavonoids Extracted from Bunge Improve Insulin Resistance through Regulation on the IKK/NF-B Inflammatory Pathway. Evid.-Based Complement. Alternat. Med. 2017, 2017, 2405124. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Ahmed, N.; Rungatscher, A.; Linardi, D.; Kulsoom, B.; Innamorati, G.; Meo, S.A.; Gebrie, M.A.; Mani, R.; Merigo, F.; et al. Cocoa Flavonoids Reduce Inflammation and Oxidative Stress in a Myocardial Ischemia-Reperfusion Experimental Model. Antioxidants 2020, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-J.; Chen, R.-C.; Sun, G.-B.; Yang, L.-P.; Zhu, Y.-d.; Xu, X.-D.; Sun, X.-B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Shen, W.; Ma, S.; Chen, W.; Qi, R. Rosa rugosa flavonoids alleviate myocardial ischemia reperfusion injury in mice by suppressing JNK and p38 MAPK. Microcirculation 2017, 24, e12385. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, J.; Lu, D.; Zheng, Q.; Li, Y.; Zhang, D. Protective Effects of Medicine Oxytropis falcata Bunge on Hypoxia/Reoxygen Injury in H9C2 Cardiomyocytes. Genom. Appl. Biol. 2017, 36, 1180–1183. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Li, F.; Ren, S.; Ma, J.; Zhang, D. The Antioxidative Effect of Oxytropis falcata Bunge Ethanol Extract on Rats’ Hearts Against Myocardial Ischemia and Reperfusion Injury. Nat. Prod. Res. Dev. 2014, 26, 423–427. [Google Scholar]
- Du, H.; Liu, M.; Li, F.; Ren, S.; Ma, J.; Zhang, D. Influence of Oxytropis falcata Bunge on expression of Bcl-2 and Bax of myocardial ischemia and reperfusion injury in Rats. J. Qinghai Med. Coll. 2013, 34, 203–206. [Google Scholar]
- Gao, L.; Cao, M.; Li, J.-Q.; Qin, X.-M.; Fang, J. Traditional Chinese Medicine Network Pharmacology in Cardiovascular Precision Medicine. Curr. Pharm. Des. 2021, 27, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Shawky, E. Prediction of potential cancer-related molecular targets of North African plants constituents using network pharmacology-based analysis. J. Ethnopharmacol. 2019, 238, 111826. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tian, S.; Zhao, J.; Zhang, W. Exploring the mechanism of TCM formulae in the treatment of different types of coronary heart disease by network pharmacology and machining learning. Pharmacol. Res. 2020, 159, 105034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Jiang, H.; Lan, T.; Qiu, Y.; Yang, K.; Chen, K.; Yao, X.; Yao, Z.; Lu, W. Chemical profile and potential mechanisms of Huo-Tan-Chu-Shi decoction in the treatment of coronary heart disease by UHPLC-Q/TOF-MS in combination with network pharmacology analysis and experimental verification. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1175, 122729. [Google Scholar] [CrossRef]
- Jia, L.-Y.; Cao, G.-Y.; Li, J.; Gan, L.; Li, J.-X.; Lan, X.-Y.; Meng, Z.-Q.; He, X.; Zhang, C.-F.; Wang, C.-Z.; et al. Investigating the Pharmacological Mechanisms of SheXiang XinTongNing Against Coronary Heart Disease Based on Network Pharmacology and Experimental Evaluation. Front. Pharmacol. 2021, 12, 698981. [Google Scholar] [CrossRef]
- Jin, Y.; Yin, X.; Li, Z.; Xu, J. Mechanism of Baihe Decoction in the treatment of coronary heart disease based on network pharmacology and molecular docking. Ann. Palliat. Med. 2021, 10, 3205–3218. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, R.; Wang, L.; Yang, B. Effects and mechanisms of Danshen-Shanzha herb-pair for atherosclerosis treatment using network pharmacology and experimental pharmacology. J. Ethnopharmacol. 2019, 229, 104–114. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Wu, Q.; Chen, T.; Li, S. A network pharmacology approach to investigate the anti-inflammatory mechanism of effective ingredients from Salvia miltiorrhiza. Int. Immunopharmacol. 2020, 81, 106040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Liu, Y.-M.; Chen, Y.-Y.; Yang, X.-C.; Duan, L. The Synergistic Effects of and on Coronary Heart Disease Identified by Network Pharmacology and Experiment. Drug Des. Dev. Ther. 2021, 15, 4053–4069. [Google Scholar] [CrossRef] [PubMed]
- Umme, H.; Kandagalla, S.; Sharath, B.S.; Jyothsna, K.; Manjunatha, H. Network Pharmacology Approach Uncovering Pathways Involved in Targeting Hsp90 Through Curcumin and Epigallocatechin to Control Inflammation. Curr. Drug Discov. Technol. 2021, 18, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, L.; Hong, Z.; Yu, X.; Wang, Z.; Feng, J. Network pharmacology-based identification of the key mechanism of quercetin acting on hemochromatosis. Metallomics 2021, 13, mfab025. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Liang, H.; Du, K.; Guo, Z.; Meng, D. Isolation of triterpenoids and phytosterones from Bl. to treat breast cancer based on network pharmacology. Nat. Prod. Res. 2021, 35, 5939–5942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-R.; Jiang, K.; Yang, J.-L.; Shi, Y.-P. Flavonoids as key bioactive components of bunge, a traditional anti-inflammatory and analgesic Tibetan medicine. Nat. Prod. Res. 2020, 34, 3335–3352. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Tian, L.; Li, Q.; Luo, J.; Zhang, Y. Analysis of the Physicochemical Properties of Acaricides Based on Lipinski’s Rule of Five. J. Comput. Biol. 2020, 27, 1397–1406. [Google Scholar] [CrossRef]
- Tsiklauri, L.; Švík, K.; Chrastina, M.; Poništ, S.; Dráfi, F.; Slovák, L.; Alania, M.; Kemertelidze, E.; Bauerova, K. Bioflavonoid Robinin from Lam. Mildly Improves the Effect of Metothrexate in Rats with Adjuvant Arthritis. Nutrients 2021, 13, 1268. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Y.; Zhang, M.; Zhang, Y.; Ji, H.; Shen, L. Recent advances in research on vine tea, a potential and functional herbal tea with dihydromyricetin and myricetin as major bioactive compounds. J. Pharm. Anal. 2021, 11, 555–563. [Google Scholar] [CrossRef]
- Papatheodorou, I.; Galatou, E.; Panagiotidis, G.-D.; Ravingerová, T.; Lazou, A. Cardioprotective Effects of PPARβ/δ Activation against Ischemia/Reperfusion Injury in Rat Heart Are Associated with ALDH2 Upregulation, Amelioration of Oxidative Stress and Preservation of Mitochondrial Energy Production. Int. J. Mol. Sci. 2021, 22, 6399. [Google Scholar] [CrossRef]
- Vashi, R.; Patel, B.M. NRF2 in Cardiovascular Diseases: A Ray of Hope! J. Cardiovasc. Transl. Res. 2021, 14, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Rahim, I.; Sayed, R.K.; Fernández-Ortiz, M.; Aranda-Martínez, P.; Guerra-Librero, A.; Fernández-Martínez, J.; Rusanova, I.; Escames, G.; Djerdjouri, B.; Acuña-Castroviejo, D. Melatonin alleviates sepsis-induced heart injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, F.; Wang, S.; Li, W.-N.; Ye, Q. Mangiferin Attenuates Myocardial Ischemia-Reperfusion Injury via MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid. Med. Cell. Longev. 2019, 2019, 7285434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Cai, D. Midazolam suppresses ischemia/reperfusion-induced cardiomyocyte apoptosis by inhibiting the JNK/p38 MAPK signaling pathway. Can. J. Physiol. Pharmacol. 2021, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Qiang, T.; Chen, J.; Wang, X. Role of epigenetic regulation in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2021, 170, 105743. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Wang, X. Cardioprotection of pharmacological postconditioning on myocardial ischemia/reperfusion injury. Life Sci. 2021, 264, 118628. [Google Scholar] [CrossRef] [PubMed]
- Gunata, M.; Parlakpinar, H. A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem. Funct. 2021, 39, 190–217. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Mou, X.; Luan, M.; Zhang, X.; He, X.; Zhao, F.; Meng, Q. The Advances on the Protective Effects of Ginsenosides on Myocardial Ischemia and Ischemia-Reperfusion Injury. Mini Rev. Med. Chem. 2020, 20, 1610–1618. [Google Scholar] [CrossRef]
- Ma, H.; Hao, J.; Liu, H.; Yin, J.; Qiang, M.; Liu, M.; He, S.; Zeng, D.; Liu, X.; Lian, C.; et al. Peoniflorin Preconditioning Protects Against Myocardial Ischemia/Reperfusion Injury Through Inhibiting Myocardial Apoptosis: RISK Pathway Involved. Appl. Biochem. Biotechnol. 2021, 194, 1149–1165. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, P.; Xiao, Q.-X.; Liu, Q.; Zhang, Y. Protective effect of Shengmai injection on myocardial endothelial cell glycoprotein detachment after myocardial ischemia-reperfusion injury in isolated rat hearts. Perfusion 2021, 36, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.J.; Borthwick, G.M.; Arthur, H.M. The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Cong, N.; Liang, M.; Wang, Y.; Wang, J. Systems Pharmacology Dissection of the Protective Effect of Myricetin Against Acute Ischemia/Reperfusion-Induced Myocardial Injury in Isolated Rat Heart. Cardiovasc. Toxicol. 2017, 17, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Janeesh, P.A.; Abraham, A. Robinin modulates doxorubicin-induced cardiac apoptosis by TGF-β1 signaling pathway in Sprague Dawley rats. Biomed. Pharmacother. 2014, 68, 989–998. [Google Scholar] [CrossRef]
- Kim, J.W.; Jin, Y.C.; Kim, Y.M.; Rhie, S.; Kim, H.J.; Seo, H.G.; Lee, J.H.; Ha, Y.L.; Chang, K.C. Daidzein administration in vivo reduces myocardial injury in a rat ischemia/reperfusion model by inhibiting NF-kappaB activation. Life Sci. 2009, 84, 227–234. [Google Scholar] [CrossRef]
- Yu, D.; Li, M.; Tian, Y.; Liu, J.; Shang, J. Luteolin inhibits ROS-activated MAPK pathway in myocardial ischemia/reperfusion injury. Life Sci. 2015, 122, 15–25. [Google Scholar] [CrossRef]
- Li, J.P.; Yan, R.; Ma, P.L.; Fu, P.; Tian, H.T.; Wang, L.L. Effects of luteolin in different doses on the cardiomyocyte apoptosis in rats with myocardial ischemia reperfusion. J. Biol. Regul. Homeost. Agents 2020, 34, 2311–2315. [Google Scholar] [CrossRef]
- Yu, L.-M.; Dong, X.; Zhang, J.; Li, Z.; Xue, X.-D.; Wu, H.-J.; Yang, Z.-L.; Yang, Y.; Wang, H.-S. Naringenin Attenuates Myocardial Ischemia-Reperfusion Injury via cGMP-PKGI Signaling and In Vivo and In Vitro Studies. Oxid. Med. Cell. Longev. 2019, 2019, 7670854. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Wu, B.; Zhong, B.; Lin, L.; Ding, Y.; Jin, X.; Huang, Z.; Lin, M.; Wu, H.; Xu, D. Naringenin alleviates myocardial ischemia/reperfusion injury by regulating the nuclear factor-erythroid factor 2-related factor 2 (Nrf2)/System xc-/glutathione peroxidase 4 (GPX4) axis to inhibit ferroptosis. Bioengineered 2021, 12, 10924–10934. [Google Scholar] [CrossRef]
- Gu, X.; Shi, Y.; Chen, X.; Sun, Z.; Luo, W.; Hu, X.; Jin, G.; You, S.; Qian, Y.; Wu, W.; et al. Isoliquiritigenin attenuates diabetic cardiomyopathy via inhibition of hyperglycemia-induced inflammatory response and oxidative stress. Phytomedicine 2020, 78, 153319. [Google Scholar] [CrossRef]
- Sengupta, B.; Sahihi, M.; Dehkhodaei, M.; Kelly, D.; Arany, I. Differential roles of 3-Hydroxyflavone and 7-Hydroxyflavone against nicotine-induced oxidative stress in rat renal proximal tubule cells. PLoS ONE 2017, 12, e0179777. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Tan, R.-Z.; Li, J.-C.; Liu, T.-T.; Zhong, X.; Yan, Y.; Yang, J.-K.; Lin, X.; Fan, J.-M.; Wang, L. Isoliquiritigenin Attenuates UUO-Induced Renal Inflammation and Fibrosis by Inhibiting Mincle/Syk/NF-Kappa B Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1455–1468. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.I.; Malhotra, R.K.; Rani, N.; Sahu, A.K.; Tomar, A.; Garg, S.; Nag, T.C.; Ray, R.; Ojha, S.; Arya, D.S.; et al. Febuxostat Modulates MAPK/NF-Bp65/TNF- Signaling in Cardiac Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2017, 2017, 8095825. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Liu, L.; Chen, S.; Niu, J.; Li, S.; Xie, W.; Cheng, X. Azathioprine pretreatment ameliorates myocardial ischaemia reperfusion injury in diabetic rats by reducing oxidative stress, apoptosis, and inflammation. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1621–1632. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, L.; Ying, H.; Zhang, W.; Li, D.; Zhu, X.; Xue, W.; Wu, S.; Cao, M.; Fu, C.; et al. Endothelial CDS2 deficiency causes VEGFA-mediated vascular regression and tumor inhibition. Cell Res. 2019, 29, 895–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusinkevich, V.; Huang, Y.; Chen, Z.-Y.; Qiang, W.; Wang, Y.-G.; Shi, Y.-F.; Yang, H.-T. Temporal dynamics of immune response following prolonged myocardial ischemia/reperfusion with and without cyclosporine A. Acta Pharmacol. Sin. 2019, 40, 1168–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Nong, Y.; Tukaye, D.N.; Rokosh, G.; Du, J.; Zhu, X.; Book, M.; Tomlin, A.; Li, Q.; Bolli, R. Inducible cardiac-specific overexpression of cyclooxygenase-2 (COX-2) confers resistance to ischemia/reperfusion injury. Basic Res. Cardiol. 2019, 114, 32. [Google Scholar] [CrossRef] [PubMed]
- Szabó, M.R.; Pipicz, M.; Csont, T.; Csonka, C. Modulatory Effect of Myokines on Reactive Oxygen Species in Ischemia/Reperfusion. Int. J. Mol. Sci. 2020, 21, 9382. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, Y.; Lai, W.; Guan, X.; Sun, X.; Han, Q.; Wang, F.; Pan, X.; Ji, Y.; Luo, H.; et al. Maternal inflammation activated ROS-p38 MAPK predisposes offspring to heart damages caused by isoproterenol via augmenting ROS generation. Sci. Rep. 2016, 6, 30146. [Google Scholar] [CrossRef]
- Pei, Y.; Cui, F.; Du, X.; Shang, G.; Xiao, W.; Yang, X.; Cui, Q. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int. J. Nanomed. 2019, 14, 4145–4155. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, A.-D.; Li, T.-S.; Tang, Q.; Wang, X.-C.; Chen, X.-B. Ghrelin ameliorates cardiac fibrosis after myocardial infarction by regulating the Nrf2/NADPH/ROS pathway. Peptides 2021, 144, 170613. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.; Zhong, S.; Gao, F.; Chen, Y.; Wang, B.; Cai, W.; Zhang, Z.; Li, W.; Lu, S.; et al. N-n-Butyl Haloperidol Iodide, a Derivative of the Anti-psychotic Haloperidol, Antagonizes Hypoxia/Reoxygenation Injury by Inhibiting an Egr-1/ROS Positive Feedback Loop in H9c2 Cells. Front. Pharmacol. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Lei, Y.-S.; Meng, X.-W.; Liu, H.-Y.; Li, L.-G.; Zhang, J.; Zhang, J.-X.; Tao, W.-H.; Peng, K.; Lin, J.; et al. Iguratimod Alleviates Myocardial Ischemia/Reperfusion Injury through Inhibiting Inflammatory Response Induced by Cardiac Fibroblast Pyroptosis via COX2/NLRP3 Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 746317. [Google Scholar] [CrossRef] [PubMed]
- Valikeserlis, I.; Athanasiou, A.-A.; Stakos, D. Cellular mechanisms and pathways in myocardial reperfusion injury. Coron. Artery Dis. 2021, 32, 567–577. [Google Scholar] [CrossRef]
- Peng, K.; Liu, H.; Yan, B.; Meng, X.-W.; Song, S.-Y.; Ji, F.-H.; Xia, Z. Inhibition of cathepsin S attenuates myocardial ischemia/reperfusion injury by suppressing inflammation and apoptosis. J. Cell. Physiol. 2021, 236, 1309–1320. [Google Scholar] [CrossRef] [PubMed]
- Woodman, O.L.; Chin, K.Y.; Thomas, C.J.; Ng, D.C.H.; May, C.N. Flavonols and Flavones—Protecting Against Myocardial Ischemia/Reperfusion Injury by Targeting Protein Kinases. Curr. Med. Chem. 2018, 25, 4402–4415. [Google Scholar] [CrossRef]
- Du, X.-J.; Wei, J.; Tian, D.; Yan, C.; Hu, P.; Wu, X.; Yang, W.; Hu, X. NEAT1 promotes myocardial ischemia-reperfusion injury via activating the MAPK signaling pathway. J. Cell. Physiol. 2019, 234, 18773–18780. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Li, C.; Hu, H.; Wang, S. The Activation of ROS/NF-κB/MMP-9 Pathway Promotes Calcium-Induced Kidney Crystal Deposition. Oxid. Med. Cell. Longev. 2021, 2021, 8836355. [Google Scholar] [CrossRef]



| No. | Name | Molecular Formula | Error (ppm) | MW (Measured) | ESI+/− (m/z) | MS/MS Fragment Ions |
|---|---|---|---|---|---|---|
| 1 | Daidzein | C15H10O4 | 1.77 | 254.0584 | 255.1022/253.1437 | 186.9758, 204.9867, 219.2112, 237.2218 |
| 2 | Pinocembrin | C15H12O4 | 1.08 | 256.0738 | 257.0046/ | 215.9778, 256.2641, 210.9722, 233.0177 |
| 3 | 7-Hydroxyflavone | C15H10O3 | 2.16 | 238.06351 | 239.1283/238.8211 | 195.1230, 239.1073 |
| 4 | Isoliquiritigenin | C15H12O4 | 1.44 | 256.07393 | 257.0046/– | 192.9624, 210.9722, 215.9778, 233.0177 |
| 5 | 2,4,4′-Trihydroxydihydrochalcone | C15H14O4 | −3.4 | 258.08833 | –/257.1943 | 219.8445, 256.1910 |
| 6 | Strobopinin | C16H14O4 | −2.57 | 270.0885 | –/269.2114 | 225.1019, 228.9878, 221.5644, 232.3254, |
| 7 | Sakuranetin | C16H14O5 | 2.24 | 286.08476 | 287.0556/– | 121.0292, 153.0187, 165.0190 |
| 8 | Formononetin | C16H12O4 | 1.02 | 268.0738 | 269.2116/267.0655 | 123.1166, 155.1437, 251.2016, 2215.1795 |
| 9 | Bryaflavan | C17H18O6 | 0.58 | 318.1105 | 319.2024/– | 163.1125, 219.1749 |
| 10 | Luteolin | C15H10O6 | −2.7 | 286.0470 | –/285.9279 | 157.8609, 198.8836, 213.9066, 219.8449 |
| 11 | Genistein | C15H10O5 | 2.36 | 270.0535 | 271.0971/269.2114 | 144.0830, 217.1947, 225.1028, 235.2047 |
| 12 | Naringenin | C15H12O5 | 2.45 | 272.0691 | 273.0887/271.2271 | 200.9438, 205.9934, 215.1287, 245.0923, |
| 13 | Hispidulin | C16H12O6 | −3.07 | 300.0625 | –/299.2583 | 123.0438, 134.8927, 160.8404, 180.5528, 253.9687, 262.5875 |
| 14 | Glycitein | C16H12O5 | 0.52 | 284.0686 | 285.0764/283.2633 | 166.9462, 192.9622, 214.9700, 210.9728 |
| 15 | 2′,4′-Dihydroxychalcone | C15H12O3 | −3.4 | 240.0235 | –/241.1943 | 219.8445, 256.1910 |
| 16 | Miquelianin | C21H18O13 | 3.03 | 478.0762 | 479.0735/477.0154 | 303.0505, 229.0504, 153.0190 |
| 17 | Isorhamnetin 3-glucuronide | C22H20O13 | 2.4 | 492.0916 | 493.3905/491.2122 | 153.0189, 302.0428, 317.0661 |
| 18 | Lilaline | C20H17NO7 | 2.34 | 383.1014 | 384.1614/– | 206.0816, 118.0656, 160.0769, 188.0707, 248.0921 |
| 19 | Kaempferol 3-glucuronide | C21H18O12 | −2.2 | 462.0788 | –/461.0716 | 2253.0503, 261.9223, 320.8129, 357.7877 |
| 20 | Maackiain | C16H12O5 | 0.42 | 284.0686 | 285.0764/– | 214.9700, 192.9622, 210.9729, 184.9571 |
| 21 | 4’’’’-Acetylsagittatin A | C34H40O19 | 2.57 | 752.2183 | –/751.2076 | 298.0475, 607.1785, 426.9629, 395.3199 |
| 22 | Brosimacutin C | C20H22O5 | −2.89 | 342.1457 | –/341.1079 | 216.9878, 254.9855, 270.5942, 337.8138 |
| 23 | Phloretin | C15H14O5 | −2.51 | 274.0834 | –/273.0159 | 134.4794, 146.6030, 169.0068, 179.8389 |
| 24 | (S)-Equol | C15H14O3 | 1.78 | 242.0947 | 243.1915/– | 111.0926, 180.1755, 228.1808, 107.0865 |
| 25 | Isovolubilin | C23H24O9 | −2.24 | 444.1410 | –/443.1184 | 193.0493, 249.0610, 267.0711, 149.0591 |
| 26 | Astragalin | C21H20O11 | −2.37 | 448.0995 | –/447.0060 | 78.9576, 148.9995, 179.0102, 96.9678 |
| 27 | Rhamnetin | C16H12O7 | 1.65 | 316.0588 | 317.6330/– | 167.0349, 243.0658,274.0481, 302.0421 |
| 28 | Scrophulein | C17H14O6 | 1.71 | 314.07958 | 315.0506/– | 287.0554, 231.0657, 259.0604, 203.0712 |
| 29 | Isoquercetin | C21H20O12 | −2.18 | 464.0945 | –/463.0872 | 301.1073, 405.6862, 135.0440, 160.8422, |
| 30 | Isomucronulatol | C17H18O5 | −2.03 | 302.0258 | –/301.2122 | 113.0277, 120.0007, 181.0848 |
| 31 | Rutin | C27H30O16 | 2.07 | 610.1547 | 611.1619/– | 303.0504, 97.0289 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, B.-Y.; Peng, Y.-F.; Chang, L.C.; Li, Z.-Q.; Zhang, X.-X.; Zhang, D.-J. Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury. Molecules 2022, 27, 1706. https://doi.org/10.3390/molecules27051706
Guo Y, Zhang B-Y, Peng Y-F, Chang LC, Li Z-Q, Zhang X-X, Zhang D-J. Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury. Molecules. 2022; 27(5):1706. https://doi.org/10.3390/molecules27051706
Chicago/Turabian StyleGuo, Yang, Ben-Yin Zhang, Yan-Feng Peng, Leng Chee Chang, Zhan-Qiang Li, Xin-Xin Zhang, and De-Jun Zhang. 2022. "Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury" Molecules 27, no. 5: 1706. https://doi.org/10.3390/molecules27051706
APA StyleGuo, Y., Zhang, B.-Y., Peng, Y.-F., Chang, L. C., Li, Z.-Q., Zhang, X.-X., & Zhang, D.-J. (2022). Mechanism of Action of Flavonoids of Oxytropis falcata on the Alleviation of Myocardial Ischemia–Reperfusion Injury. Molecules, 27(5), 1706. https://doi.org/10.3390/molecules27051706






