The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy
Abstract
:1. Introduction
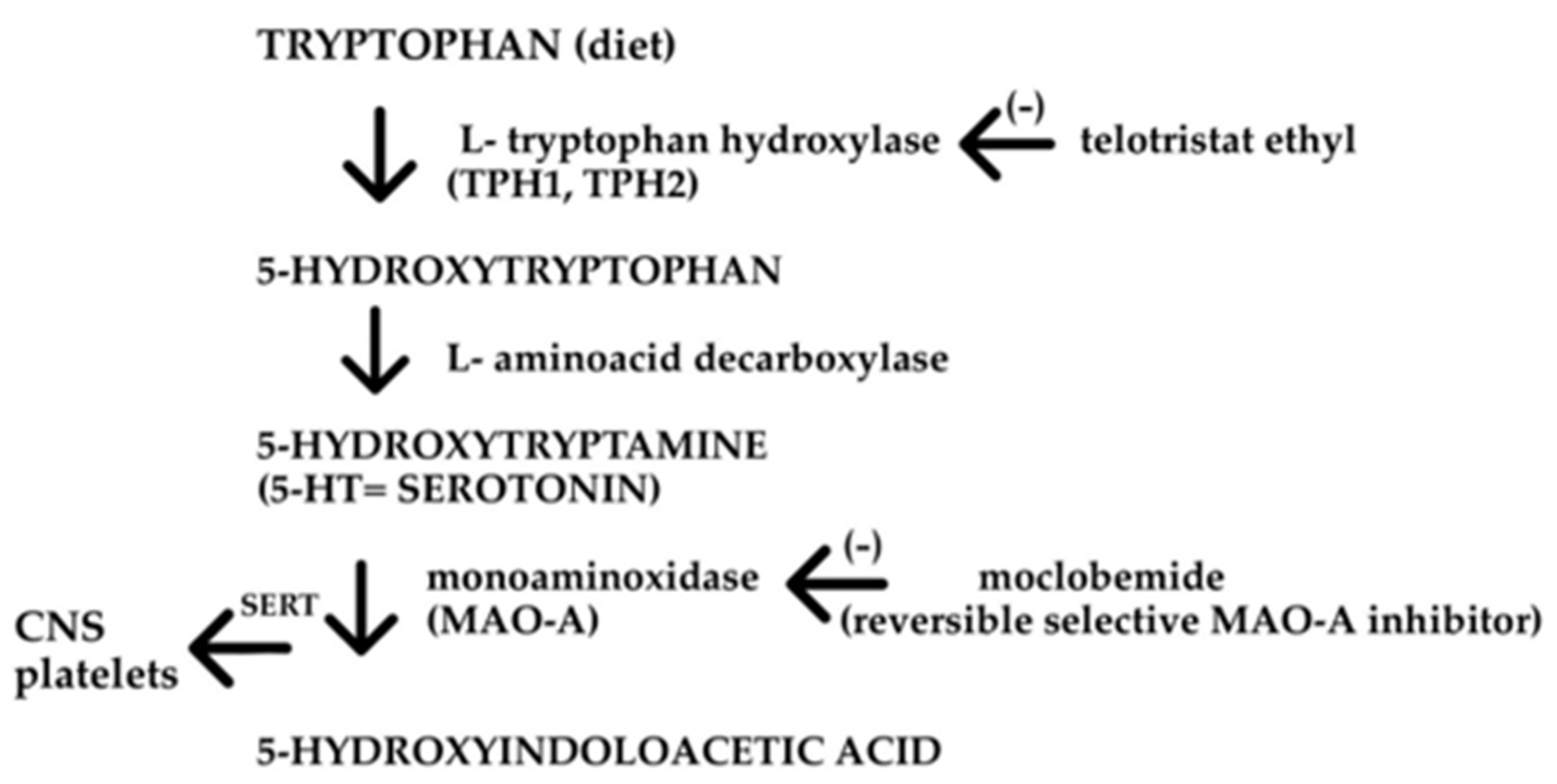
2. Serotonin in the GI Tract
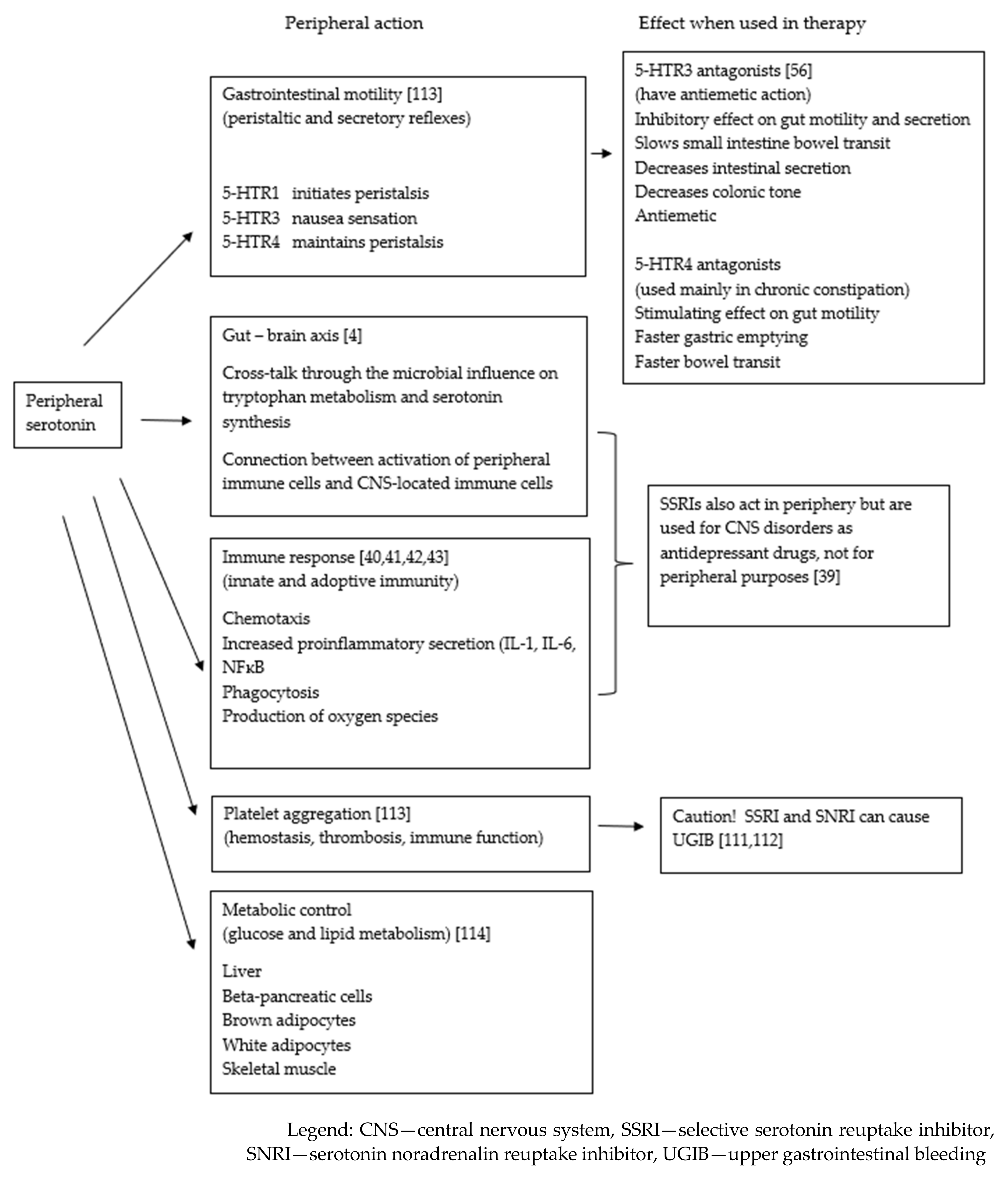
2.1. GI Serotonin—Linkage to Metabolism
2.2. Serotonin in GI Tract Disorders
2.2.1. Irritable Bowel Syndrome (IBS)
2.2.2. Inflammatory Bowel Disease (IBD)
2.2.3. Carcinoid
2.3. 5-HT Receptors and Drugs Acting in the GI Tract
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The mechanism of secretion and metabolism of gut-derived 5-hydrixytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.S.; Machado, J.D.; Camacho, M.; Diaz, J.; Morales, Y.G.; Alvarezde la Rosa, D.; Carmona, E.; Castaneyra, A.; Viveros, O.H.; O’Connor, D.T.; et al. The crucial role of chromogranins in storage and exocytosis revealed using chromaffin cells from chromogranin A null mouse. J. Neurosci. 2008, 28, 3350–3358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The diverse metabolic roles of peripheral serotonin. Endocrinology 2016, 158, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargova, V.; Mechirova, V.; Felsoci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Gershon, D.M. 5-hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 10, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Rapport, M.M.; Green, A.A.; Page, I.H. Serum vasoconstrictor (serotonin); isolation and characterization. J. Biol. Chem. 1948, 176, 1243–1251. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharm. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Ghia, J.E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Cote, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef] [Green Version]
- Manocha, M.; Khan, W.I. Serotonin and GI disorders: An update of clinical and experimental studies. Clin. Transl. Gastroenterol. 2012, 3, e13. [Google Scholar] [CrossRef]
- Gershon, M.D.; Liu, M.T. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol. Motil. 2007, 19, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Gonkowski, S. Bisphenol A (BPA)-induced changes in the number of serotonin-positive cells in the mucosal layer of porcine small intestine-the preliminary studies. Int. J. Mol. Sci. 2020, 21, 1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, M. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. 1999, 13, 15–30. [Google Scholar] [CrossRef]
- Racke, K.; Reimann, A.; Schworer, H.; Kilbinger, H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1995, 73, 83–87. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signalling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon monoxide and nitric oxide as examples of the youngest class of transmitters. Int. J. Mol. Sci. 2021, 22, 6029. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.T.; Smith, J.A.M. Serotonin pharmacology in the gastrointestinal tract: A review. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008, 377, 181–203. [Google Scholar] [CrossRef]
- Grider, J.R. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J. Pharm. Exp. 2003, 307, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.M.; Lumsden, A.L.; Young, R.L.; Jessup, C.F.; Spencer, N.J.; Keating, D.J. Regional differences in nutrient-induced secretion of gut serotonin. Physiol. Rep. 2017, 5, e13199. [Google Scholar] [CrossRef]
- Sumara, G.; Sumara, O.; Kim, J.K.; Karsenty, G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Met. 2012, 16, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Mishima, Y.; Ishihara, S. Molecular Mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int. J. Mol. Sci. 2020, 21, 8664. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnel, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells—A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef]
- Digestive diseases. National Center of Health Statistics. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/fastats/digestive-diseases.htm (accessed on 18 December 2021).
- Mishima, Y.; Ishihara, S. Enteric microbiota –mediated serotoninergic signalling in pathogenesis of irritable bowel syndrome. Int. J. Mol. Sci. 2021, 22, 10235. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 2017, 152, 327–339.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 3310345. [Google Scholar] [CrossRef] [PubMed]
- Waclawikova, B.; Bullock, A.; Schwalbe, M.; Aranzamendi, C.; Nelemans, S.A.; van Dijk, G.; El Aidy, S. Gut bacteria-derived 5-hydroxyidole is a potent stimulant of intestinal motility via its action on L-type calcium channels. PLoS Biol. 2021, 19, e3001070. [Google Scholar] [CrossRef]
- Golubeva, A.V.; Joyce, S.A.; Moloney, G.; Burokas, A.; Sherwin, E.; Arboleya, S.; Flynn, I.; Khochanskiy, D.; Moya-Perez, A.; Peterson, V.; et al. Microbiota-Related changes in bile acid & tryptophan metabolism are associated with gastrointestinal dysfunction in a mouse model of autism. EBioMedicine 2017, 24, 166–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolis, K.G.; Li, Z.; Stevanovic, K.; Saurman, V.; Israelyan, N.; Anderson, G.M.; Snyder, I.; Veenstra-VanderWeele, J.; Blakley, R.D.; Gershon, M.D. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J. Clin. Investig. 2016, 126, 2221–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, S. Role of serotonin 5-HT3 receptors in intestinal inflammation. Biol. Pharm. Bull. 2013, 36, 1406–1409. [Google Scholar] [CrossRef] [Green Version]
- Mawe, G.M.; Hoffman, J.M. Serotonin signalling in the gut-functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 473–486. [Google Scholar] [CrossRef] [Green Version]
- Strober, W.; Fuss, I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology 2011, 140, 1756–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, C.; Cho, J.H. Inflammatory bowel disease. N. Engl. J. Med. 2009, 361, 2066–2078. [Google Scholar] [CrossRef]
- Wang, H.; Foong, J.P.P.; Harris, N.L.; Bornstein, J.C. Enteric neuroimmune interactions coordinate intestinal responses in health and disease. Mucosal Immunol. 2021, 15, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fointes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of gut microbiota composition n by serotonin signalling influences intestinal immune response and susceptibility to colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nzakaizwanayo, J.; Dedi, C.; Standen, G.; Mac Farlane, W.M.; Patel, B.A.; Jones, B.V. Escherihia coli Nissle 1917 enhances bioavailability of serotonin in gut tissues through modulation of synthesis and clearance. Sci. Rep. 2015, 5, 17324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Denna, T.H.; Storkersen, J.N.; Gerriets, V.A. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharm. Res. 2019, 140, 100–114. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front Cardiovasc Med 2017, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Shaijb, M.S.; Khan, I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 2113, 561–574. [Google Scholar] [CrossRef]
- Deuerschmied, D.; Siudan, G.L.; Demers, M.; Herr, N.; Carb, C.; Brill, C.H.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Regmi, S.C.; Park, S.; Ku, S.K.; Kim, J. Serotonin regulates innate immune responses of colonic epithelial cells through Nox-2-dervied reactive oxygen species. Free Radic. Biol. Med. 2014, 69, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Lee, L.; Jensen, R.T. Carcinoid-syndrome: Recent advances, current status and controversies. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A. Telotristat ethyl: A review in carcinoid syndrome diarrhoea. Drugs 2018, 78, 941–950. [Google Scholar] [CrossRef]
- Chan, D.L.; Singh, S. Developments in the treatment of carcinoid syndrome: Impact of telotristat. Clin. Risk Manag. 2018, 14, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xermelo. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/xermelo-epar-product-information_en.pdf (accessed on 18 December 2021).
- Medicines under Additional Monitoring. European Medicines Agency. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medicines-under-additional-monitoring (accessed on 18 December 2021).
- Boeckxstaens, G.E.; Tytgant, G.M.N.; Wajs, E.; Van Nueten, L.; De Ridder, F.; Meulemans, A.; Tack, J. The influence of the novel 5-HT1A agonist R137696 on the proximal stomach function in healthy volunteers. Neurogastroenterol. Motil. 2006, 18, 919–926. [Google Scholar] [CrossRef]
- Coulie, B.; Tack, J.; Maes, B.; Geypens, B.; De Roo, M.; Jenssens, J. Sumatriptan, a selective 5-HT1 receptor agonist, induces a lag phase for gastric emptying of liquids in humans. Am. J. Physiol. 1997, 272, G902–G908. [Google Scholar] [CrossRef] [PubMed]
- Sifrim, D.; Holloway, R.H.; Tack, J.; Zelter, A.; Misotten, T.; Coulie, B.; Janssens, J. Effect on sumatriptan, a 5-HT1 agonist, on the frequency of transient lower oesophageal sphincter relaxations and gastroesophageal reflux in healthy subjects. Am. J. Gastroenterol. 1999, 94, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Morelli, N.; Gori, S.; Choub, A.; Maluccio, M.R.; Orlandi, G.; Guazelli, M.; Murri, L. Do 5HT1B/1D receptor agonists have an effect on mood and anxiety disorders? Cephalagia 2007, 27, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Borman, R.A.; Burleigh, D.E. 5-HT1D and 5-HT2B receptors mediate contraction of smooth muscle in human small intestine. Ann. N. Y. Acad. Sci. 1997, 812, 222–223. [Google Scholar] [CrossRef]
- Kirchgessner, A.L.; Liu, M.T.; Tamir, H.; Gershon, M.D. Identyfication and localization of 5-HT1P receptors in the guinea pig pancreas. Am. J. Physiol. 1992, 262, 553–566. [Google Scholar] [CrossRef]
- Kirchgessner, A.L.; Liu, M.T.; Howard, M.J.; Gershon, M.D. Detection of the 5-HT1A receptor and 5-HT1A receptor mRNA in the rat bowel and pancreas: Comparison with 5-HT1P receptors. J. Comp. Neurol. 1993, 327, 33–250. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. Review article: Serotonin receptors and transporters—Roles in normal and abnormal gastrointestinal motility. Aliment. Pharm. 2004, 20, 3–14. [Google Scholar] [CrossRef]
- Branchek, T.A.; Mawe, G.M.; Gershon, M.D. Characterization and localization of a peripheral neural 5-hydroksytryptamine receptor subtype (5-HT1P) with a selective agonist, 3H-5-hydroxyindalpine. J. Neurosci. 1998, 8, 2582–2595. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Beltran, E.; Labastida-Ramirez, A.; Haanes, K.A.; van den Bogaerdt, A.; Bogers, A.; Zanelli, E.; Meeus, L.; Danser, A.H.J.; Gralinski, M.R.; Senese, P.B.; et al. Characterization of binding, functional activity, and contractile responses of the selective 5-HT1F receptor agonist Lasmiditan. Br. J. Pharm. 2019, 176, 4681–4695. [Google Scholar] [CrossRef] [Green Version]
- Clemow, D.B.; Johnson, K.W.; Hochstetler, H.M.; Ossipov, M.H.; Hake, A.M.; Blumenfeld, A.M. Lasmiditan mechanism of action—Review of a selective 5-HT1F agonist. J. Headache Pain 2020, 21, 71. [Google Scholar] [CrossRef]
- International Union of Basic and Clinical Pharmacology IUPHAR. Available online: https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=1 (accessed on 18 October 2021).
- Tack, J.; Caenepeel, P.; Corsetti, M.; Janssens, J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distension. Gastroenteroloigy 2004, 127, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, K.D.; Lu, Z.; Rudd, J.A. Looking beyond 5-HT(3) receptors: A review of the wider role of serotonin in the pharmacology of nausea and vomiting. Eur. J. Pharm. 2014, 722, 13–25. [Google Scholar] [CrossRef]
- De Clerck, F.; David, J.L.; Janssen, P.A.J. Inhibition of 5-hydrxytryptamine-induced and -amplified human platelet aggregation by ketanserin (R 41 468) a selective 5-HT2-receptor antagonist. Agents Actions 1982, 12, 388–397. [Google Scholar] [CrossRef]
- Engel, G.; Hoyer, D.; Kalkman, H.; Wick, M.B. Pharmacological similarity between 5-HT-D receptor on the guinea pig ileum and the 5-HT2 binding site. Br. J. Pharm. 1985, 84, 106. [Google Scholar]
- Muneta-Arrate, I.; Diez-Alarcia, R.; Horrillo, I.; Meana, J.J. Pimavanserin exhibits serotonin 5-HT2A receptor inverse agonism for Gαi1- and neutral antagonism for Gαq/11- proteins in human brain cortex. Eur. Neuropsychopharmacol. 2020, 36, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Launay, J.M.; Birraux, G.; Bondoux, D.; Callebert, J.; Choi, D.S.; Loric, S.; Maroteaux, L. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J. Biol. Chem. 1996, 271, 3141–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, N.; Oka, M.; Yamada-Okabe, H.; Hamada, K.; Nakayama, H.; Mori, N.; Tamesa, T.; Okada, T.; Takemoto, N.; Matoba, K.; et al. Molecular signature in three types of hepatocellular carcinoma with different viral origin by oligonucleotide microarray. Int. J. Oncol. 2004, 24, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, Y.; Zhou, W.; Gao, L.; Yuan, L.; Han, X. Serotonin receptor 2C and insulin secretion. PLoS ONE 2013, 8, e54250. [Google Scholar] [CrossRef]
- Stunes, A.K.; Reseland, J.E.; Hauso, O.; Kidd, M.; Tommeras, K.; Waldum, H.L.; Syversen, U.; Gustafsson, B.I. Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes. Metab. 2011, 13, 551–558. [Google Scholar] [CrossRef]
- Kappeler, J.; Möller, D.; Lasitschka, F.; Autschbach, F.; Hovius, R.; Rappold, D.; Brüss, M.; Gershon, M.D.; Niesler, B. Serotonin receptor diversity in the human colon: Expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J. Comp. Neurol. 2011, 519, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Niesler, B.; Frank, B.; Kapeller, J.; Rappold, G.A. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 2003, 310, 101–111. [Google Scholar] [CrossRef]
- Glatzle, J.; Sternini, C.; Robin, C.; Zittel, T.T.; Wong, H.; Reeve, J.R.; Raybould, H.E. Expression of 5-HT 3 receptors in the rat gastrointestinal tract. Gastroenterology 2002, 123, 217–226. [Google Scholar] [CrossRef]
- Bertrand, P.P.; Kunze, W.A.; Furness, J.B.; Bornstein, J.C. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydrokxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 2000, 101, 459–469. [Google Scholar] [CrossRef]
- Hansen, M.B.; Skadhauge, E. Signal transduction pathways for serotonin as an intestinal secretagogue. Comp. Biochem. Physil. A Physiol. 1997, 118, 283–290. [Google Scholar] [CrossRef]
- Shworer, H.; Ramadori, G. Autoreceptors can modulate 5-hydroxytryptamine release from porcine and human small intestine in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1998, 357, 548–552. [Google Scholar] [CrossRef]
- Aikiyo, S.; Kishi, K.; Kaji, N.; Mikawa, S.; Kondo, M.; Shimada, S.; Hori, M. Contribution of serotonin 3A receptor to motor function and its expression in the gastrointestinal tract. Digest 2021, 102, 516–526. [Google Scholar] [CrossRef]
- Hagbom, M.; Hellysaz, A.; Istrate, C.; Nordgren, J.; Sharma, S.; de-Faria, F.M.; Magnusson, K.E.; Svensson, L. The 5-HT3 receptor affects rotavirus-induced motility. J. Virol. 2021, 95, e0075121. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, P.J. Understanding the pathobiology of chemotherapy induced nausea and vomiting. Providing a basis for therapeutic progress. Oncology 2004, 18, 9–14. [Google Scholar]
- Cubeddu, L.X.; Hoffman, I.S.; FuenMayor, N.T.; Finn, A.L. Efficacy of ondansetron (GR 38032F) and the role of serotonin in cisplatin-induced nausea and vomiting. N. Eng. J. Med. 1990, 322, 810–816. [Google Scholar] [CrossRef]
- Roiola, F.; Fatigoni, S. New antiemetic drugs. Ann. Oncol. 2006, 17, 96–100. [Google Scholar] [CrossRef]
- Clemens, C.H.M.; Samson, M.; Van Berge Henegouwen, G.P.; Fabri, M.; Smout, A.J.P.M. Effect on allosteron on left colonic motility in non-constipated patients with irritable bowel syndrome and healthy volunteers. Aliment. Pharm. 2002, 16, 993–1002. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Drug Safety Communication: New Information Regarding QT Prolongation with Ondansetron (Zofran). Available online: https://www.fda.gov/Drugs/DrugSafety/ucm310190.html (accessed on 17 October 2021).
- Damkier, P.; Caplan, Y.C.; Shechtman, S.; Diav-Citrin, O.; Cassina, M.; Weber-Schoendorfer, C. Ondansetron in pregnancy revisited: Assessment and pregnancy labelling by the European Medicines Agency (EMA) & Pharmacovigilance Risk Assessment Committee (PRAC). Basic Clin. Toxicol. Pharm. 2021, 128, 579–582. [Google Scholar] [CrossRef]
- European Medicines Agency Recommends Changes to the Use of Metoclopramide Changes Aim Mainly to Reduce the Risk of Neurological Side Effects. Available online: https://www.ema.europa.eu/en/documents/press-release/european-medicines-agency-recommends-changes-use-metoclopramide_en.pdf (accessed on 17 October 2021).
- Tanyiama, K.; Makimoto, N.; Furuichi, A.; Sakurai-Yamashita, Y.; Nagase, Y.; Kaibara, M.; Kanematsu, T. Functions of peripheral 5-hydroxytryptamine receptors, especially 5-hydroksytryptamie 4 receptor, in gastrointestinal motility. J. Gastroenterol. 2000, 35, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, H.; Kuniyasu, H.; Okumura, M.; Misawa, H.; Katsui, R.; Zhang, G.X.; Obata, K.; Takaki, M. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol. Motil. 2010, 22, 806–813.e226. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, I.; Kuniyasu, H.; Matsuyoshi, H.; Goto, K.; Obata, K.; Misawa, H.; Fujii, H.; Takaki, M. Comparison of effects of a selective 5-HT reuptake inhibitor versus a 5-HT4 receptor agonist on in vivo neurogenesis at the rectal anastomosis in rats. Am. J. Physiol. Gastrointest Liver Physiol. 2012, 302, G588–G597. [Google Scholar] [CrossRef] [Green Version]
- Kaumann, A.J.; Levy, F.O. 5-hydroksytryptamine receptors in the human cardiovascular system. Pharmacol. Ther. 2006, 111, 674–706. [Google Scholar] [CrossRef]
- Brattelid, T.; Kvingedal, A.M.; Krobert, K.A.; Andressen, K.W.; Bach, T.; Hystad, M.E.; Kaumann, A.J.; Levy, F.O. Cloning, pharmacological characterisation and tissue distribution of a novel 5-HT4 receptor splice variant, 5-HT4(i). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Brattelid, T.; Qvigstad, E.; Lynham, J.A.; Molenaaa, T.; Aasschgeiran, O.; Skomedal, T.; Osnes, J.B.; Levy, F.O.; Kaumann, A.J. Functional serotonin 5-HT4 receptors in porcine and human ventricular myocardium with increased 5-HT4 mRNA in heart failure. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Kaumann, A.J. Do human atrial 5-HT4 receptors mediate arrhythmias? Trends Pharm. Sci 1994, 15, 451–455. [Google Scholar] [CrossRef]
- Rahme, M.M.; Cotter, B.; Leistad, E.; Wadhwa, M.K.; Mohabir, R.; Ford, A.P.; Eglen, R.M.; Feld, G.K. Electrophysiological and antiarrhythmic effects of the atrial selective 5-HT(4) receptor antagonist RS-100302 in experimental atrial flutter and fibrillation. Circulation 1999, 100, 2010–2017. [Google Scholar] [CrossRef] [Green Version]
- Lezoualc’h, F.; Steplewski, K.; Sartiani, L.; Mugelli, A.; Fischmeister, R.; Bril, A. Quantitative mRNA analysis of serotonin 5-HT4 receptor isoforms, calcium handling proteins and ion channels in human atrial fibrillation. Biochem. Biophys. Res. Commun. 2007, 357, 218–224. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, F.; Crema, F. Treatment functional GI disease: The complex pharmacology of serotonergic drugs. Br. J. Clin. 2002, 54, 680–681. [Google Scholar] [CrossRef]
- Tack, J.; Camilleri, M.; Chang, L.; Chey, W.D.; Galligan, J.J.; Lacy, B.E.; Müller-Lissner, S.; Quigley, E.M.M.; Schuurkes, J.; De Maeyer, J.H.; et al. Systematic review: Cardiovascular safety profile of 5-HT4 agonists developed for gastrointestinal disorders. Aliment. Pharm. 2012, 35, 745–767. [Google Scholar] [CrossRef]
- Cisapride Withdrawal Requires Alternative Therapy. Clevland Clinic Center for Continuing Education. Available online: https://www.clevelandclinicmeded.com/medicalpubs/pharmacy/mayjune2000/cisapride.htm (accessed on 15 October 2021).
- Cisapride. European Medicine Agency. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/cisapride (accessed on 15 October 2021).
- Sanger, G.J. Translating 5-HT receptor pharmacology. Neurogastroenterol. Motil. 2009, 21, 1235–1238. [Google Scholar] [CrossRef]
- Resolor. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/resolor-epar-product-information_en.pdf (accessed on 2 February 2022).
- Carbone, F.; Van den Houte, K.; Clevers, E.; Andrews, C.N.; Papathanasopoulos, A.; Holvoet, L.; Van Oudenhove, L.V.; Caenepeel, P.; Arts, J.; Vanuytsel, T.; et al. Prucalopride in Gastroparesis: A Randomized Placebo-Controlled Crossover Study. Am. J. Gastroenterol. 2019, 114, 1265–1274. [Google Scholar] [CrossRef]
- Tack, J.; Rotondo, A.; Meulemans, A.; Thielemans, L.; Cools, M. Randomized clinical trial: A controlled pilot trial of the 5-HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis. Neurogastroenterol. Motil. 2016, 28, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Bianco, F.; Scott, R.B.; Keenan, C.M.; Linton, A.A.; O’Neill, C.H.; Bonora, E.; Dicay, M.; Lavoie, B.; Eilcox, R.L.; et al. Protective actions of epithelial 5-hydrokxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology 2016, 151, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguesz-Stanley, S.; Zubaidi, S.; Proskin, H.M.; Klarstein, J.R.; Shetzline, M.A.; Miner, P.B.; Philip, B. Effect of tegaserod on esophageal pain threshold, regurgitation, and symptom relief in patients with functional heartburn and mechanical sensitivity. Clin. Gastroenterol. Hepatol. 2006, 4, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Quigley, E.M.M.; Castell, D.O.; Specjhler, S.J. The effects of tegaserod (HTF919) on esophageal acid exposure in gastroesophageal reflux disease. Aliment. Pharm. 2000, 14, 1503–1509. [Google Scholar] [CrossRef]
- Grailhe, R.; Grabtree, G.W.; Hen, R. Human 5-HT(5) receptors: The 5-HT(5A) receptor is functional but 5-HT(5B) receptor was lost during mammalian evolution. Eur. J. Pharmacol. 2001, 418, 157–167. [Google Scholar] [CrossRef]
- Thomas, D.R. 5-HT5A receptor as a therapeutic targets. Pharmacol. Ther. 2006, 111, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Metcalf, M.A.; Khan, M.; Druck, T.; Huebner, K.; Lachowicz, J.E.; Meltzer, H.Y.; Sibley, D.R.; Roth, B.L.; Hamblin, M.W. Cloning, characterization, and chromosomal localization of a human 5-HT6 serotonin receptor. J. Neurochem. 1996, 66, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Ahmed, M.; Miller, M.D. 5-HT6 receptor antagonists: Prospects for the treatment of cognitive disorders including dementia. Curr. Opin. Drug Discov. Devel 2008, 11, 642–654. [Google Scholar]
- Monro, R.L.; Bornstein, J.C.; Bertarnd, P.P. Slow excitatory postsynaptic potentials in myenteric AH neurons of the guinea pig ileum are reduced by the 5-hydroxytryptamine (7) receptor antagonist SB 269970. Neuroscience 2005, 134, 975–986. [Google Scholar] [CrossRef]
- Tonini, M. 5-hydroxytryptamine effects in the gut: The 3, 4, and 7 receptors. Neurogastroenterol. Motil. 2005, 17, 637642. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, R.; Connemann, B.J.; Schönfeldt-Lecuona, C.; Gahr, M. Risk of Bleeding Associated With Antidepressants: Impact of Causality Assessment and Competition Bias on Signal Detection. Front. Psychiatry 2021, 12, 727687. [Google Scholar] [CrossRef] [PubMed]
- Zeiss, R.; Hiemke, C.; Schönfeldt-Lecuona, C.; Connemann, B.J.; Gahr, M. Risk of bleeding associated with antidepressant drugs: The competitive impact of antithrombotics in quantative signal detection. Drugs-Real World Outcomes 2021, 8, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kanova, M.; Kohout, P. Serotonin—Its synthesis and roles in the healthy and the critically ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Moon, J.H.; Kim, H. Serotoninergic regulation of Energy metabolism in peripheral tissues. J. Endocrinol. 2020, 245, R1–R10. [Google Scholar] [CrossRef]
| Receptor Family | Receptor or Subtype | Function |
|---|---|---|
| 5-HT1 | 5-HT1A, 5HT1D | Gastric fundus relaxation |
| 5-HT1B/1D | Prokinetic intestinal stimulation | |
| 5-HT1D | Contraction of intestinal circular muscle | |
| 5-HT1B | Contraction of intestinal longitudinal muscle | |
| 5-HT1P | Peristaltic and secretory reflexes | |
| 5-HT2 | 5-HT2A | Contraction of smooth muscles |
| 5-HT2B | Contraction of smooth muscles in stomach fundus, relaxation of longitudinal muscle in the intestine | |
| 5-HT3 | 5-HT3 | Chloride secretion and serotonin release from EC cells |
| 5-HT3A | Increase intestinal motility | |
| 5-HT4 | 7 splice variants | Increase intestinal motility, contraction of esophagus, relaxation of colon, chloride secretion |
| 5-HT5 | - | Not known in gastrointestinal tract (essential solely in CNS) |
| 5-HT6 | - | Not known in gastrointestinal tract (essential solely in CNS) |
| 5-HT7 | 5 splice variants | Excitatory effect, anti-inflammatory activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. https://doi.org/10.3390/molecules27051680
Guzel T, Mirowska-Guzel D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules. 2022; 27(5):1680. https://doi.org/10.3390/molecules27051680
Chicago/Turabian StyleGuzel, Tomasz, and Dagmara Mirowska-Guzel. 2022. "The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy" Molecules 27, no. 5: 1680. https://doi.org/10.3390/molecules27051680
APA StyleGuzel, T., & Mirowska-Guzel, D. (2022). The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules, 27(5), 1680. https://doi.org/10.3390/molecules27051680






