“Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry
Abstract
:1. Introduction
2. Application of Non-Conventional Energy Sources in Organoselenium Chemistry
2.1. Microwave-Assisted Reactions
2.2. Sonochemistry
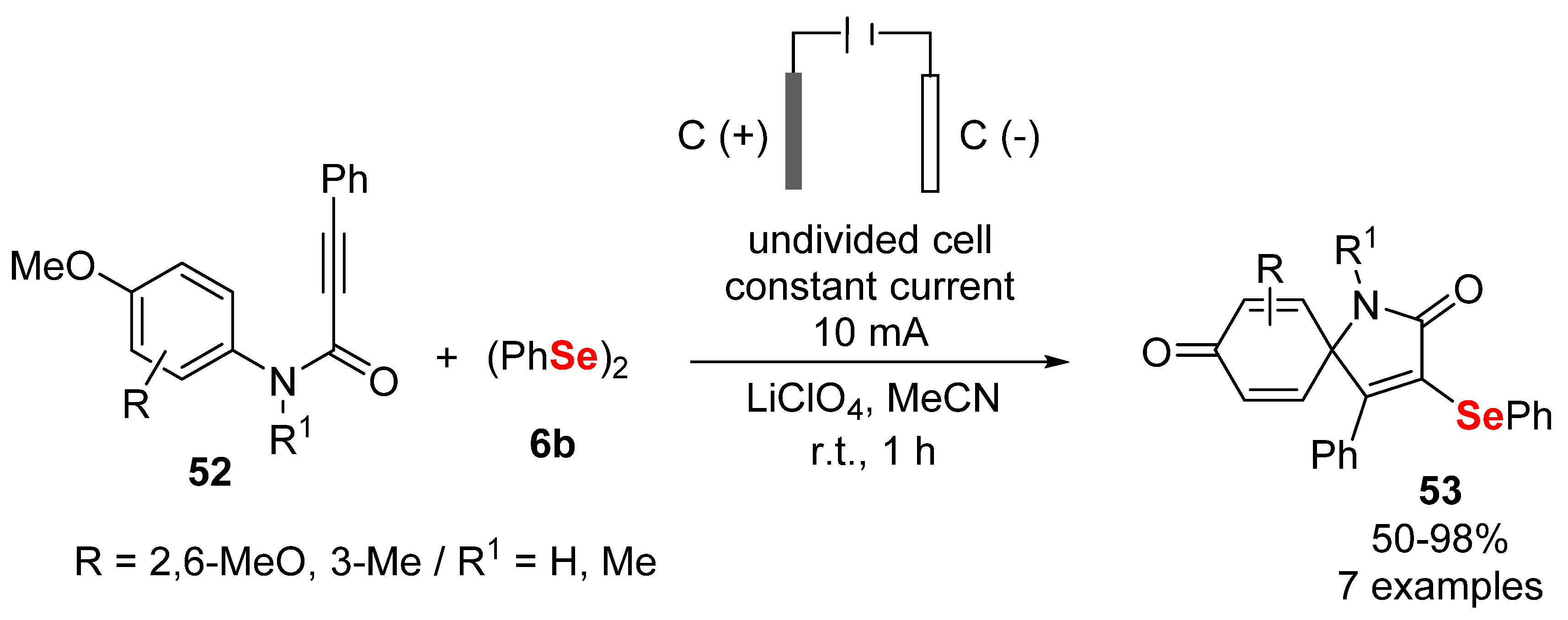
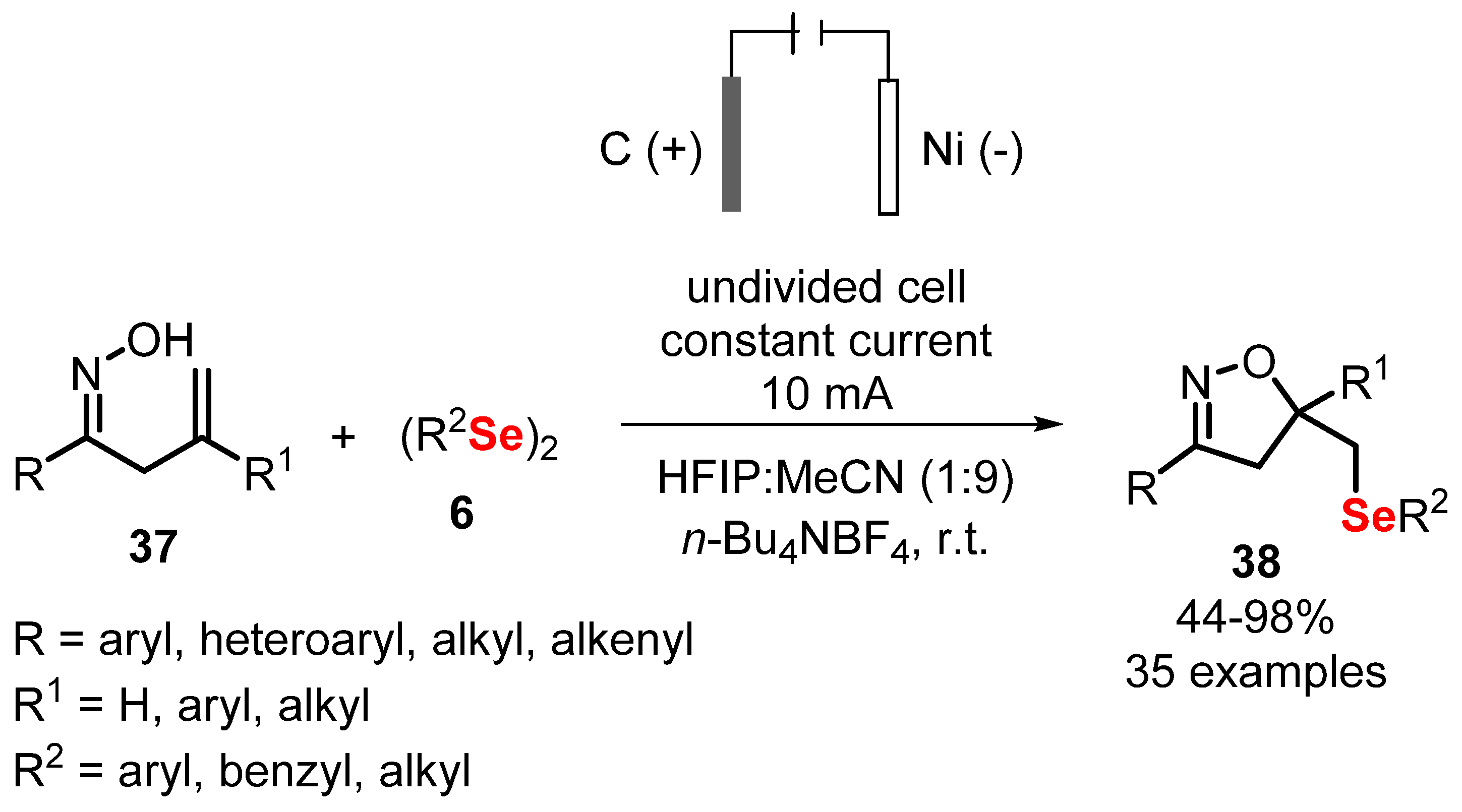
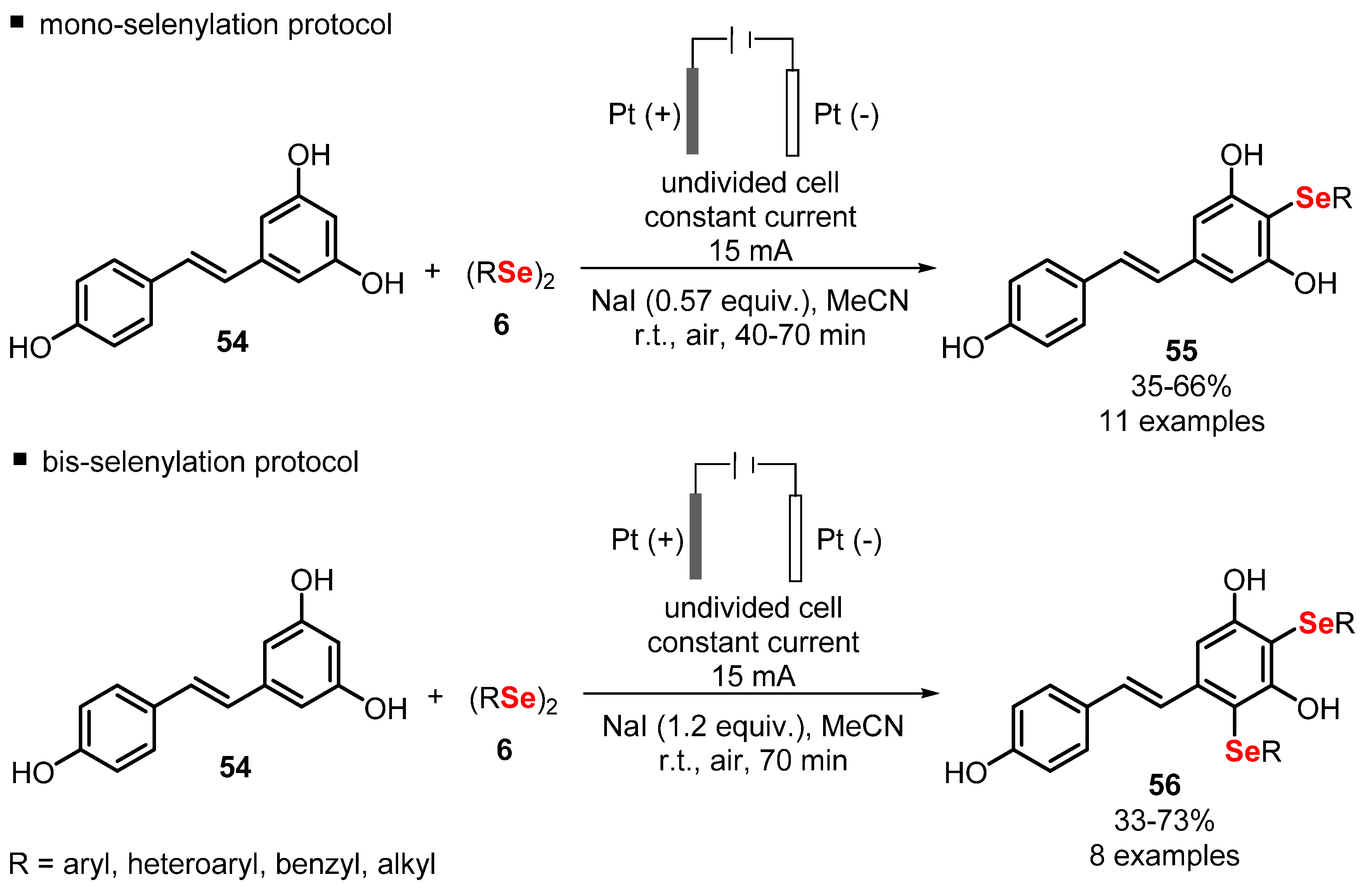
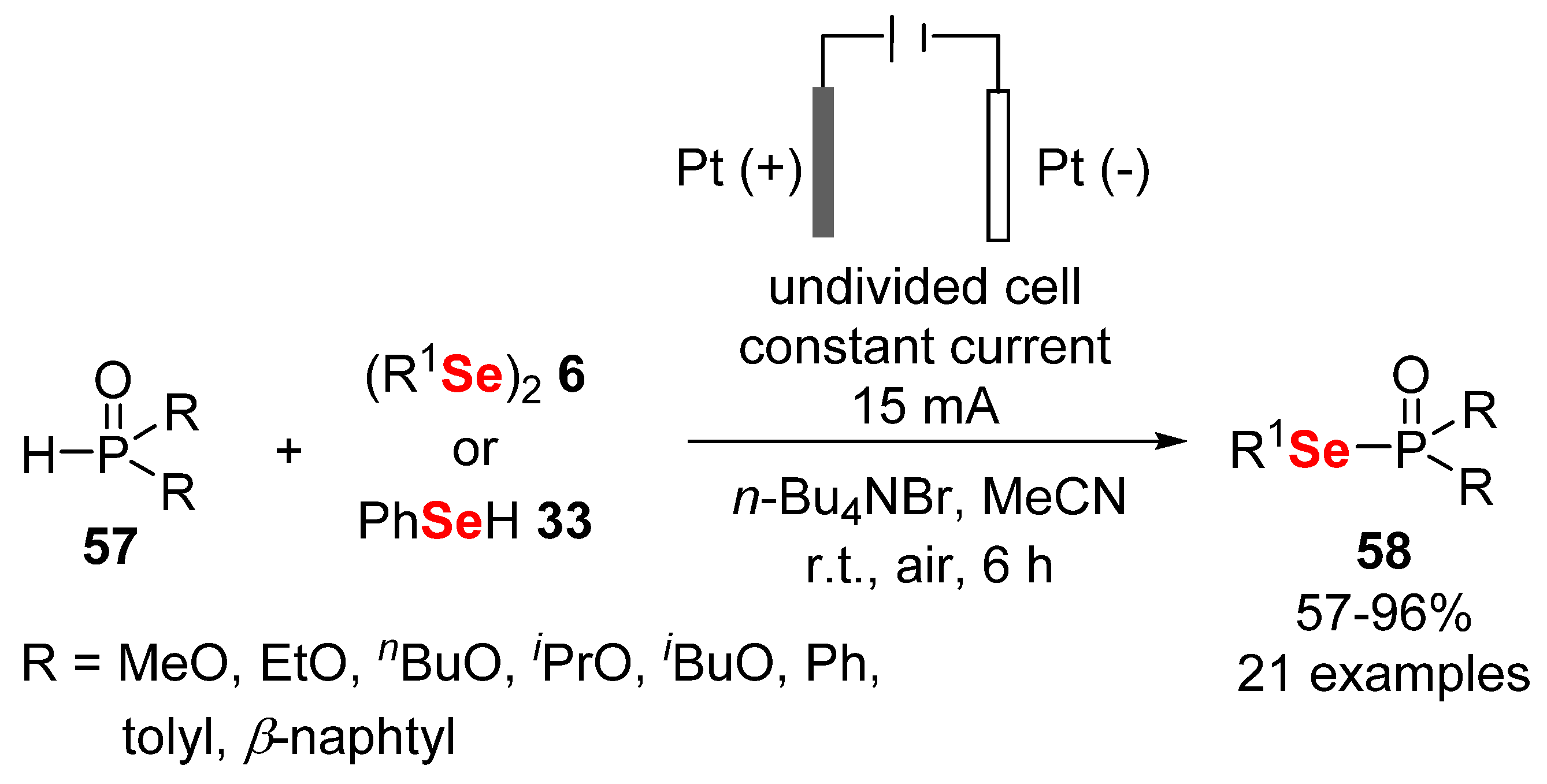
2.3. Electrochemically Promoted Reactions
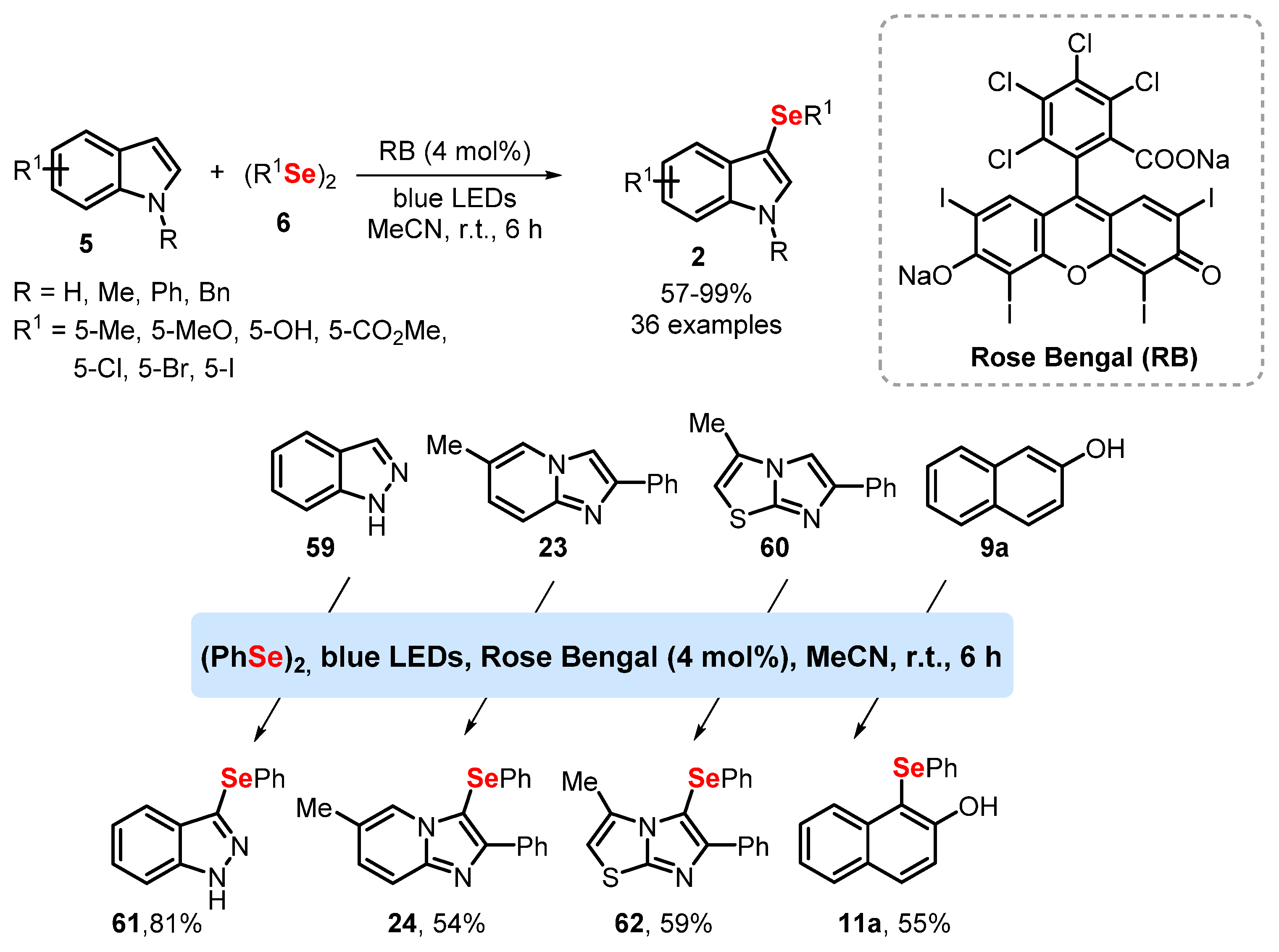
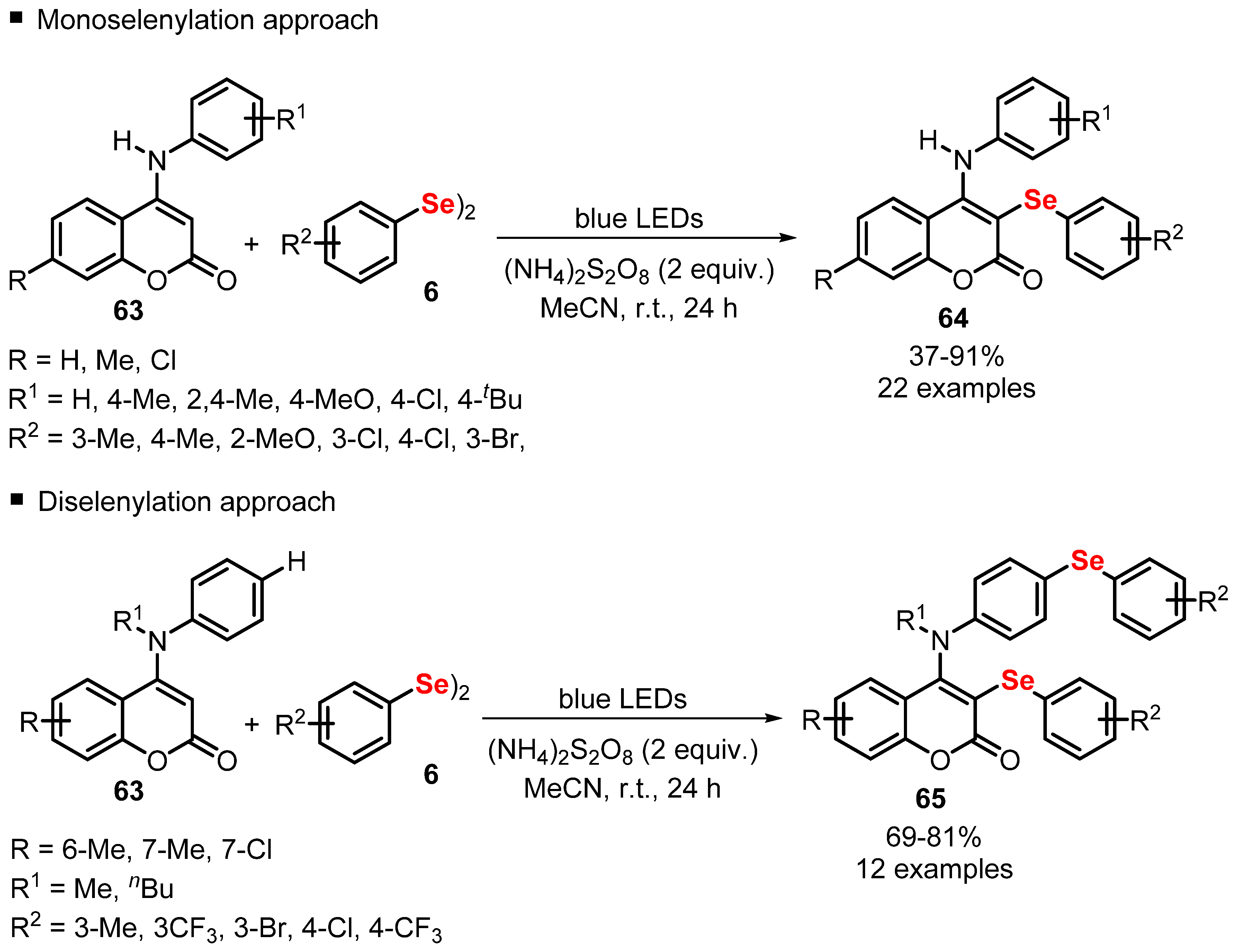
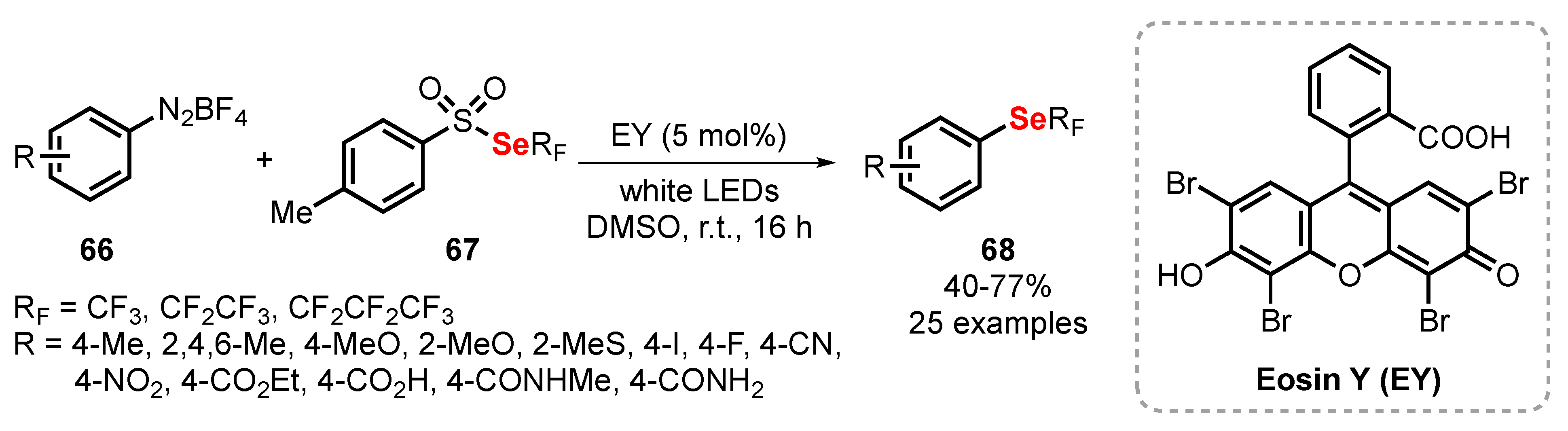
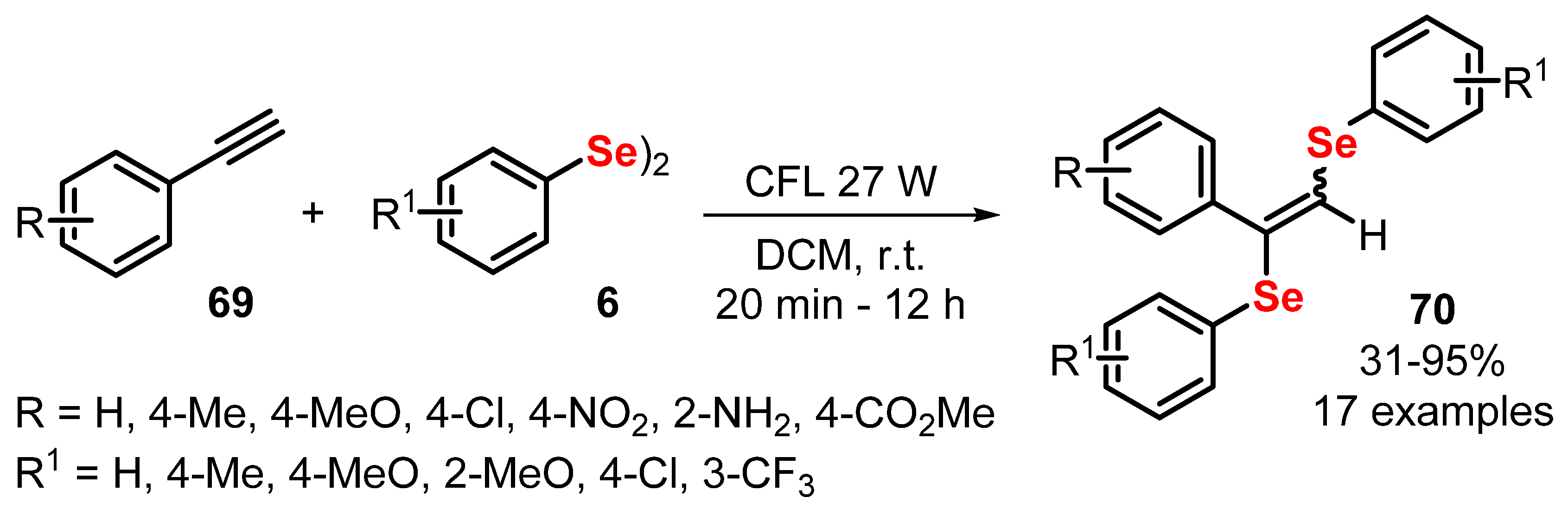
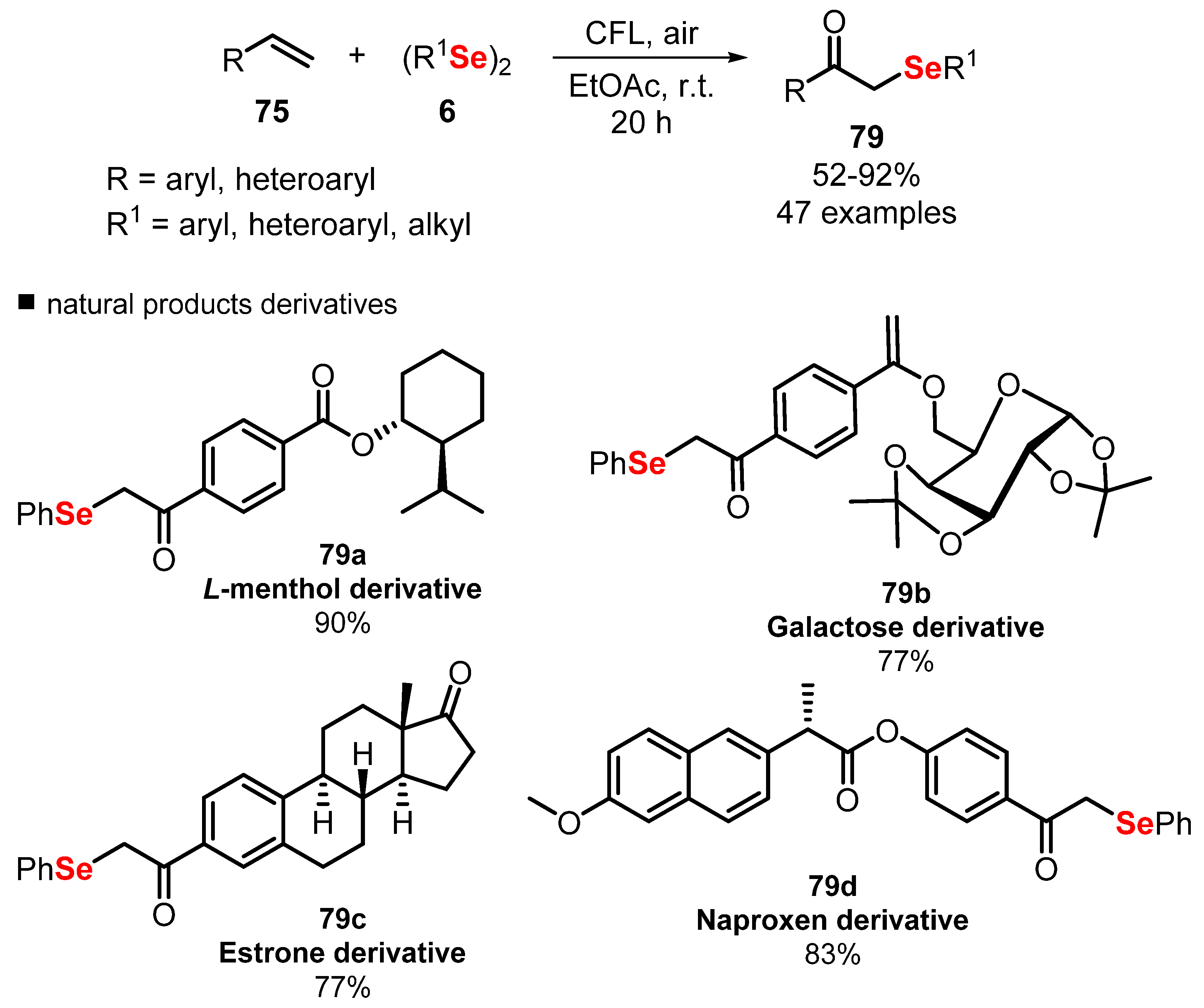
2.4. Photochemistry
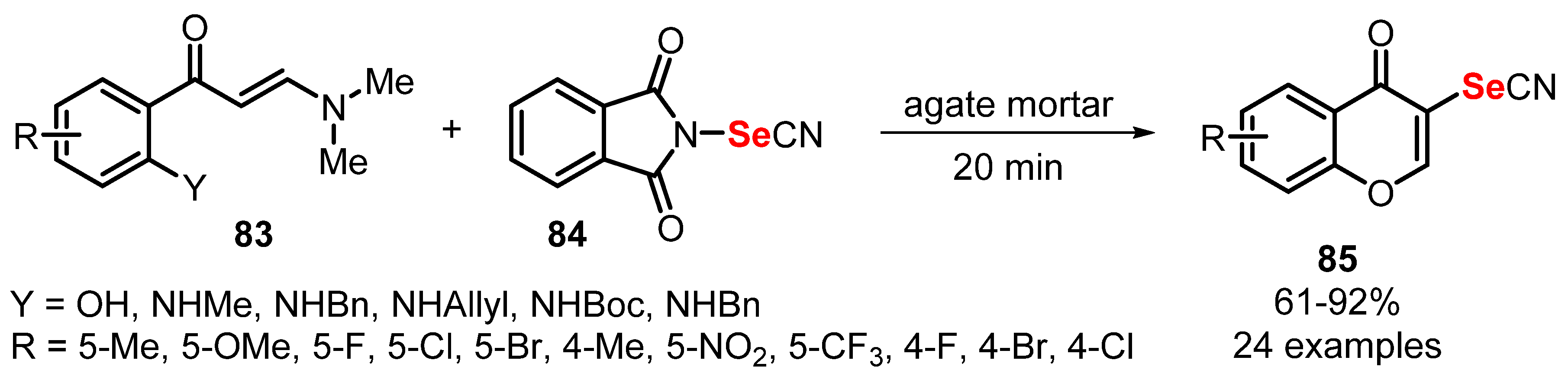
2.5. Mechanochemistry
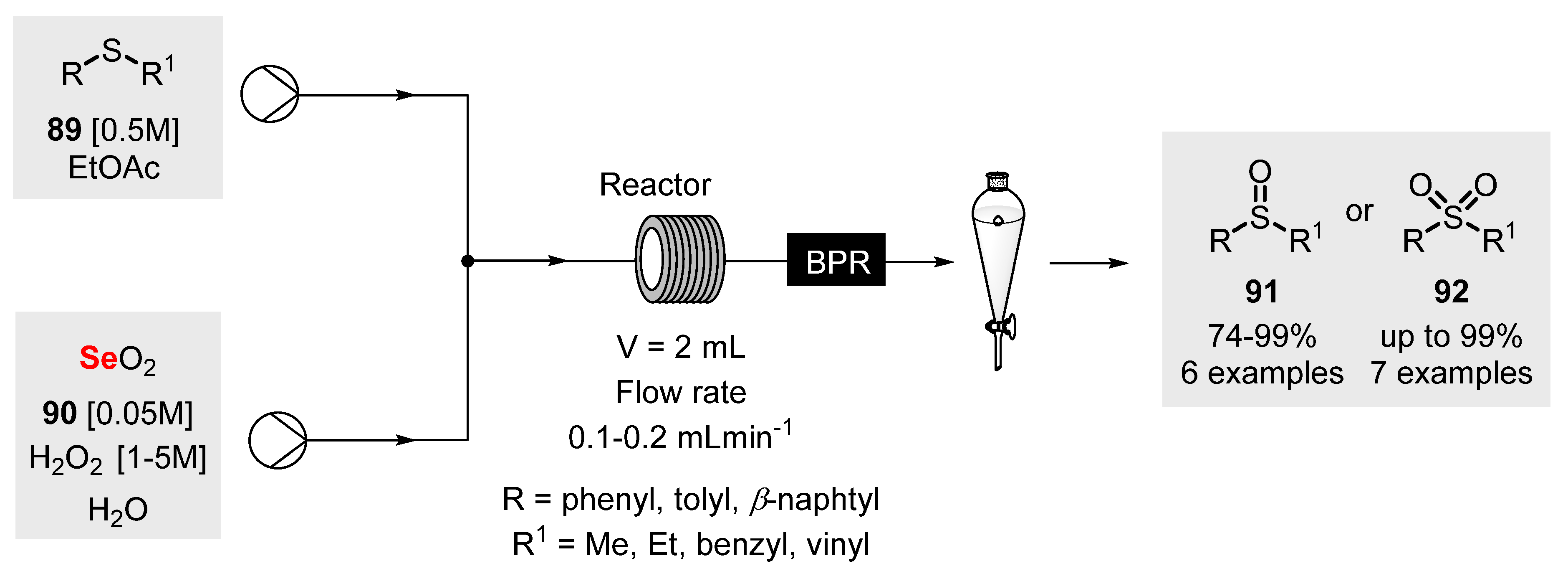
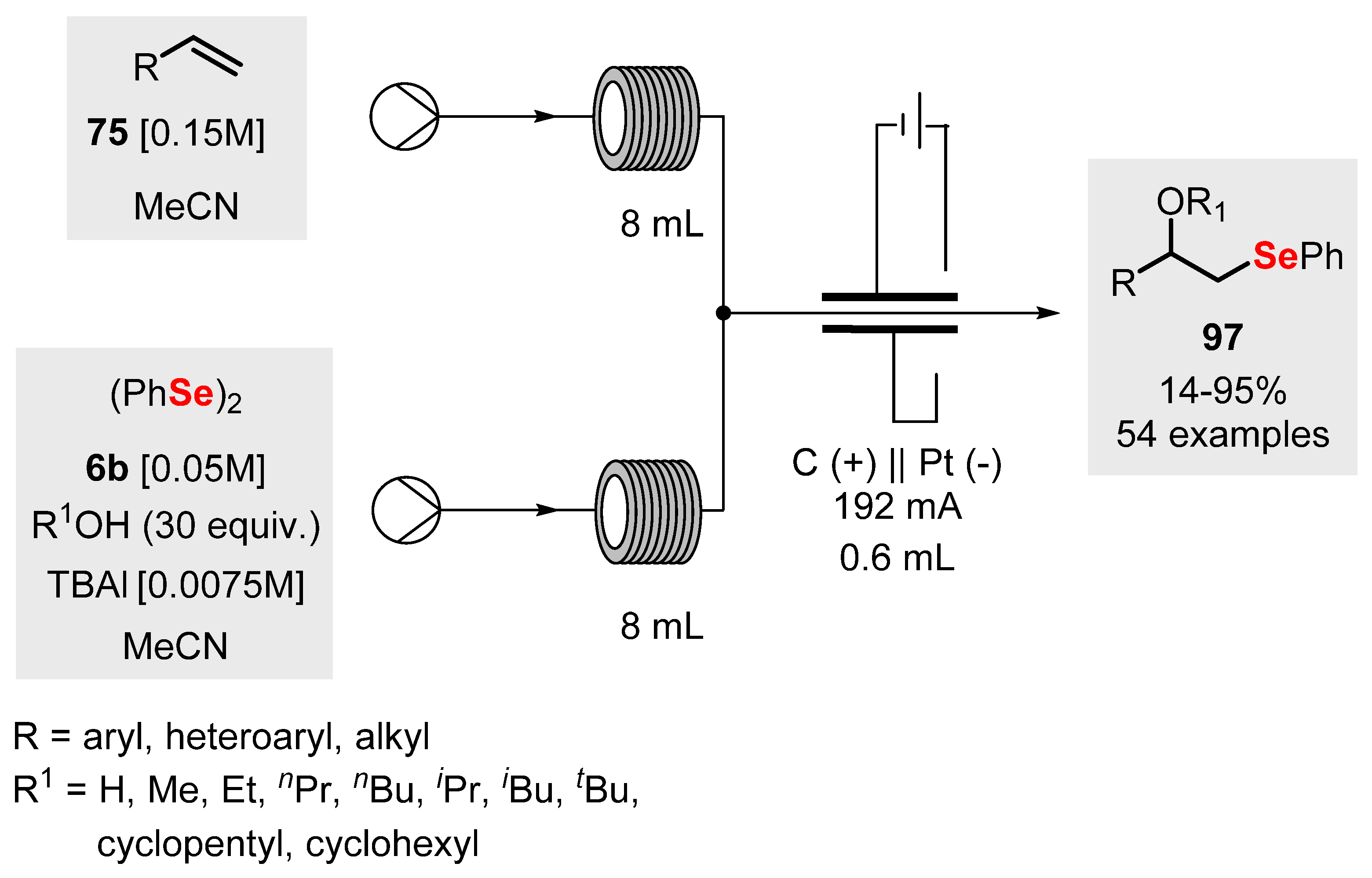
2.6. Flow Chemistry
3. Application of Selenium or Organoselenium Compounds as Catalysts in Oxidation Reactions
4. Organoselenium Chemistry in Non-Conventional Solvents
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lenardão, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-92404-5. [Google Scholar]
- Nogueira, C.W.; Rocha, J.B.T. Toxicology and pharmacology of selenium: Emphasis on synthetic organoselenium compounds. Arch. Toxicol. 2011, 85, 1313–1359. [Google Scholar] [CrossRef] [PubMed]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Di Leo, I.; Messina, F.; Nascimento, V.; Nacca, F.G.; Pietrella, D.; Lenardão, E.J.; Perin, G.; Sancineto, L. Synthetic Approaches to Organoselenium Derivatives with Antimicrobial and Anti-Biofilm Activity. Mini Rev. Org. Chem. 2019, 16, 589–601. [Google Scholar] [CrossRef]
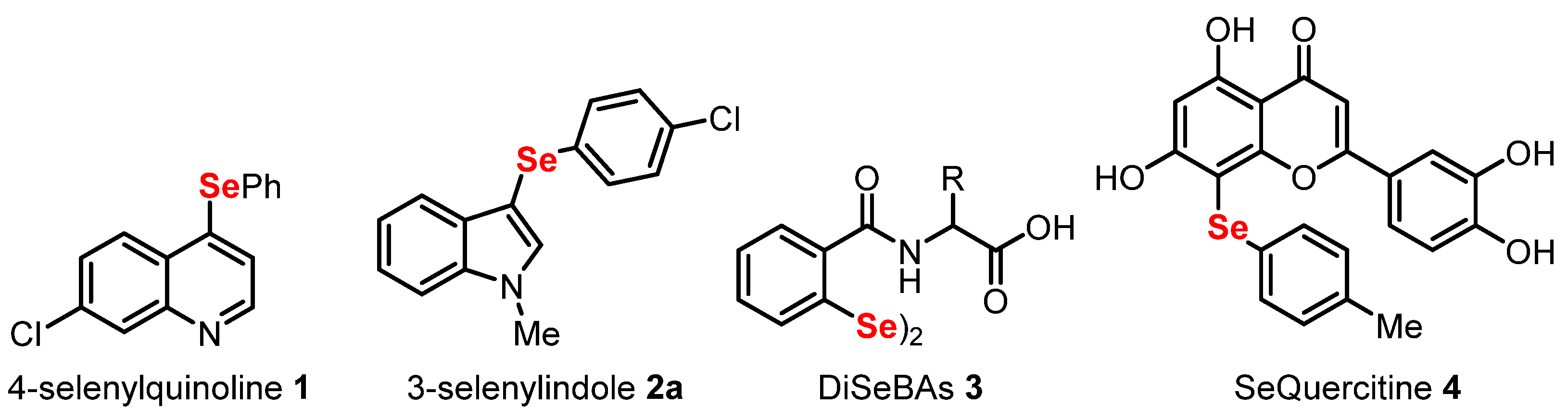
- Pinz, M.; Reis, A.S.; Duarte, V.; da Rocha, M.J.; Goldani, B.S.; Alves, D.; Savegnago, L.; Luchese, C.; Wilhelm, E.A. 4-Phenylselenyl-7-chloroquinoline, a new quinoline derivative containing selenium, has potential antinociceptive and anti-inflammatory actions. Eur. J. Pharmacol. 2016, 780, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Casaril, A.M.; de Lourenço, D.A.; Domingues, M.; Smaniotto, T.Â.; Birmann, P.T.; Vieira, B.; Sonego, M.S.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Anhedonic- and anxiogenic-like behaviors and neurochemical alterations are abolished by a single administration of a selenium-containing compound in chronically stressed mice. Compr. Psychoneuroendocrinol. 2021, 6, 100054. [Google Scholar] [CrossRef]
- Casaril, A.M.; Domingues, M.; Bampi, S.R.; de Lourenço, D.A.; Smaniotto, T.Â.; Segatto, N.; Vieira, B.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. The antioxidant and immunomodulatory compound 3-[(4-chlorophenyl)selanyl]-1-methyl-1H-indole attenuates depression-like behavior and cognitive impairment developed in a mouse model of breast tumor. Brain. Behav. Immun. 2020, 84, 229–241. [Google Scholar] [CrossRef]
- Sancineto, L.; Mariotti, A.; Bagnoli, L.; Marini, F.; Desantis, J.; Iraci, N.; Santi, C.; Pannecouque, C.; Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity. J. Med. Chem. 2015, 58, 9601–9614. [Google Scholar] [CrossRef]
- Sancineto, L.; Iraci, N.; Tabarrini, O.; Santi, C. NCp7: Targeting a multitasking protein for next-generation anti-HIV drug development part 1: Covalent inhibitors. Drug Discov. Today 2018, 23, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Iraci, N.; Tabarrini, O.; Santi, C.; Sancineto, L. NCp7: Targeting a multitask protein for next-generation anti-HIV drug development part 2. Noncovalent inhibitors and nucleic acid binders. Drug Discov. Today 2018, 23, 687–695. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Botwina, P.; Menichetti, E.; Bagnoli, L.; Rosati, O.; Marini, F.; Fonseca, S.F.; Abenante, L.; Alves, D.; Dabrowska, A.; et al. Seleno-Functionalization of Quercetin Improves the Non-Covalent Inhibition of Mpro and Its Antiviral Activity in Cells against SARS-CoV-2. Int. J. Mol. Sci. 2021, 22, 7048. [Google Scholar] [CrossRef]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, A.; Gieshoff, T.; Möhle, S.; Rodrigo, E.; Zirbes, M.; Waldvogel, S.R. Electrifying Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 5594–5619. [Google Scholar] [CrossRef] [PubMed]
- Freudendahl, D.M.; Santoro, S.; Shahzad, S.A.; Santi, C.; Wirth, T. Green Chemistry with Selenium Reagents: Development of Efficient Catalytic Reactions. Angew. Chem. Int. Ed. 2009, 48, 8409–8411. [Google Scholar] [CrossRef]
- Santoro, S.; Azeredo, J.B.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Santi, C. The green side of the moon: Ecofriendly aspects of organoselenium chemistry. RSC Adv. 2014, 4, 31521–31535. [Google Scholar] [CrossRef]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Obermayer, D.; Gutmann, B.; Kappe, C.O. Microwave Chemistry in Silicon Carbide Reaction Vials: Separating Thermal from Nonthermal Effects. Angew. Chem. Int. Ed. 2009, 48, 8321–8324. [Google Scholar] [CrossRef]
- Henary, M.; Kananda, C.; Rotolo, L.; Savino, B.; Owens, E.A.; Cravotto, G. Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry. RSC Adv. 2020, 10, 14170–14197. [Google Scholar] [CrossRef]
- Gude, V.G.; Martinez-Guerra, E. Green chemistry with process intensification for sustainable biodiesel production. Environ. Chem. Lett. 2018, 16, 327–341. [Google Scholar] [CrossRef]
- Cho, H.; Török, F.; Török, B. Energy efficiency of heterogeneous catalytic microwave-assisted organic reactions. Green Chem. 2014, 16, 3623–3634. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Godoi, M.; Schwab, R.S.; Botteselle, G.V.; Braga, A.L. Synthesis of Thiol Esters Using Nano CuO/Ionic Liquid as an Eco-Friendly Reductive System Under Microwave Irradiation. Eur. J. Org. Chem. 2013, 2013, 5188–5194. [Google Scholar] [CrossRef]
- Vieira, A.A.; Azeredo, J.B.; Godoi, M.; Santi, C.; da Silva Júnior, E.N.; Braga, A.L. Catalytic Chalcogenylation under Greener Conditions: A Solvent-Free Sulfur- and Seleno-functionalization of Olefins via I 2 /DMSO Oxidant System. J. Org. Chem. 2015, 80, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
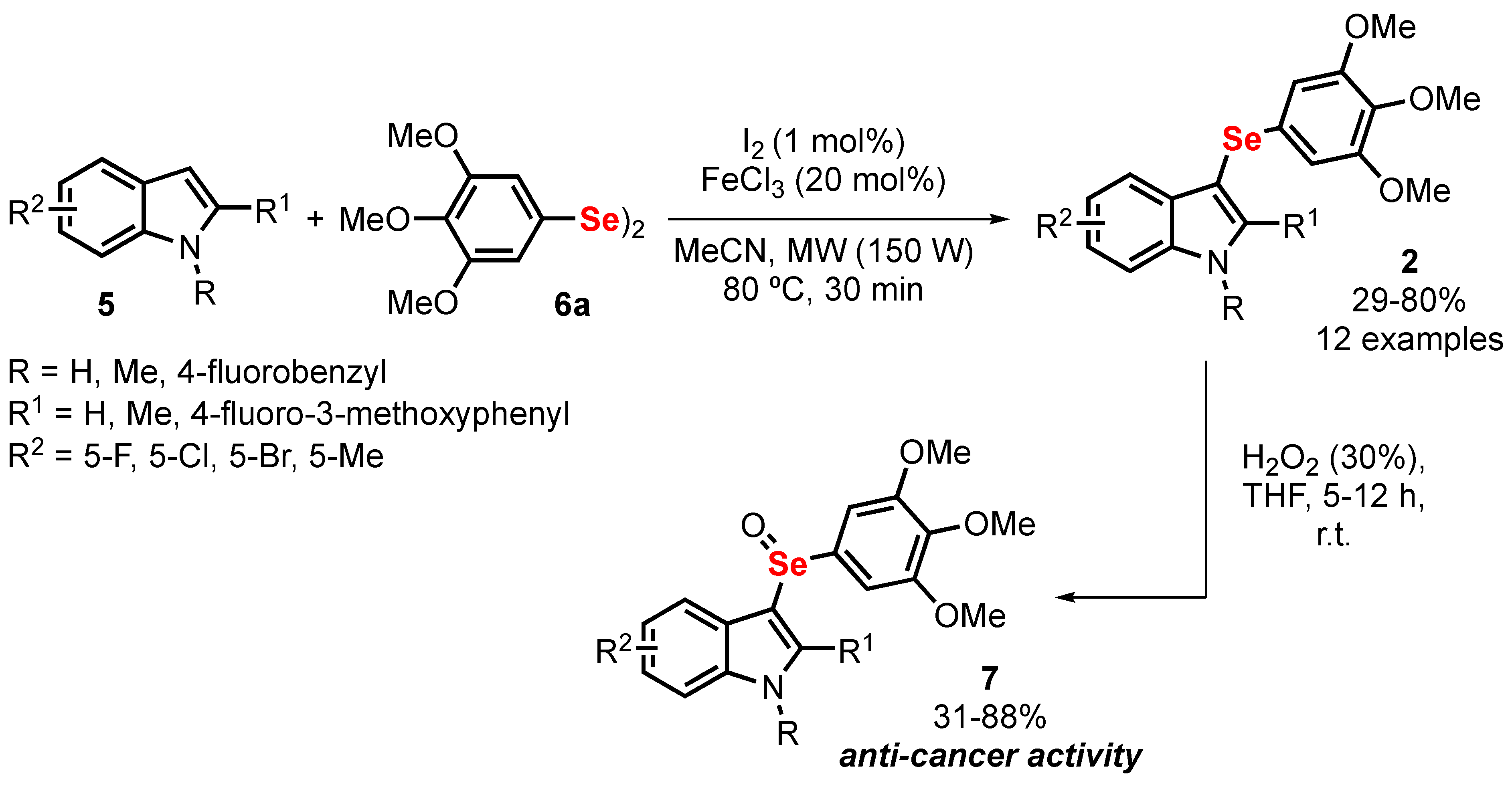
- Wen, Z.; Xu, J.; Wang, Z.; Qi, H.; Xu, Q.; Bai, Z.; Zhang, Q.; Bao, K.; Wu, Y.; Zhang, W. 3-(3,4,5-Trimethoxyphenylselenyl)-1 H -indoles and their selenoxides as combretastatin A-4 analogs: Microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef]
- Azeredo, J.B.; Godoi, M.; Martins, G.M.; Silveira, C.C.; Braga, A.L. A Solvent- and Metal-Free Synthesis of 3-Chacogenyl-indoles Employing DMSO/I 2 as an Eco-friendly Catalytic Oxidation System. J. Org. Chem. 2014, 79, 4125–4130. [Google Scholar] [CrossRef] [PubMed]
- Saba, S.; Rafique, J.; Braga, A.L. DMSO/iodine-catalyzed oxidative C–Se/C–S bond formation: A regioselective synthesis of unsymmetrical chalcogenides with nitrogen- or oxygen-containing arenes. Catal. Sci. Technol. 2016, 6, 3087–3098. [Google Scholar] [CrossRef]
- Saba, S.; Botteselle, G.; Godoi, M.; Frizon, T.; Galetto, F.; Rafique, J.; Braga, A. Copper-Catalyzed Synthesis of Unsymmetrical Diorganyl Chalcogenides (Te/Se/S) from Boronic Acids under Solvent-Free Conditions. Molecules 2017, 22, 1367. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, L.F.; Bettanin, L.; da Trindade, R.N.; da Silva, C.; Leitemberger, A.; Godoi, M.; Galetto, F.Z. One-pot modular synthesis of β-chalcogen amides via regioselective 2-oxazolines ring-opening reaction promoted by indium chalcogenolates under microwave irradiation. Tetrahedron Lett. 2020, 61, 152180. [Google Scholar] [CrossRef]
- Jadhav, A.A.; More, P.V.; Khanna, P.K. Microwave synthesis of bis(cycloalkeno)-1,4-diselenins: A novel source of Se for CdSe QDs. New J. Chem. 2017, 41, 7438–7446. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. Power ultrasound in organic synthesis: Moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006, 35, 180–196. [Google Scholar] [CrossRef]
- Martínez, R.F.; Cravotto, G.; Cintas, P. Organic Sonochemistry: A Chemist’s Timely Perspective on Mechanisms and Reactivity. J. Org. Chem. 2021, 86, 13833–13856. [Google Scholar] [CrossRef]
- Penteado, F.; Monti, B.; Sancineto, L.; Perin, G.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Ultrasound-Assisted Multicomponent Reactions, Organometallic and Organochalcogen Chemistry. Asian J. Org. Chem. 2018, 7, 2368–2385. [Google Scholar] [CrossRef]
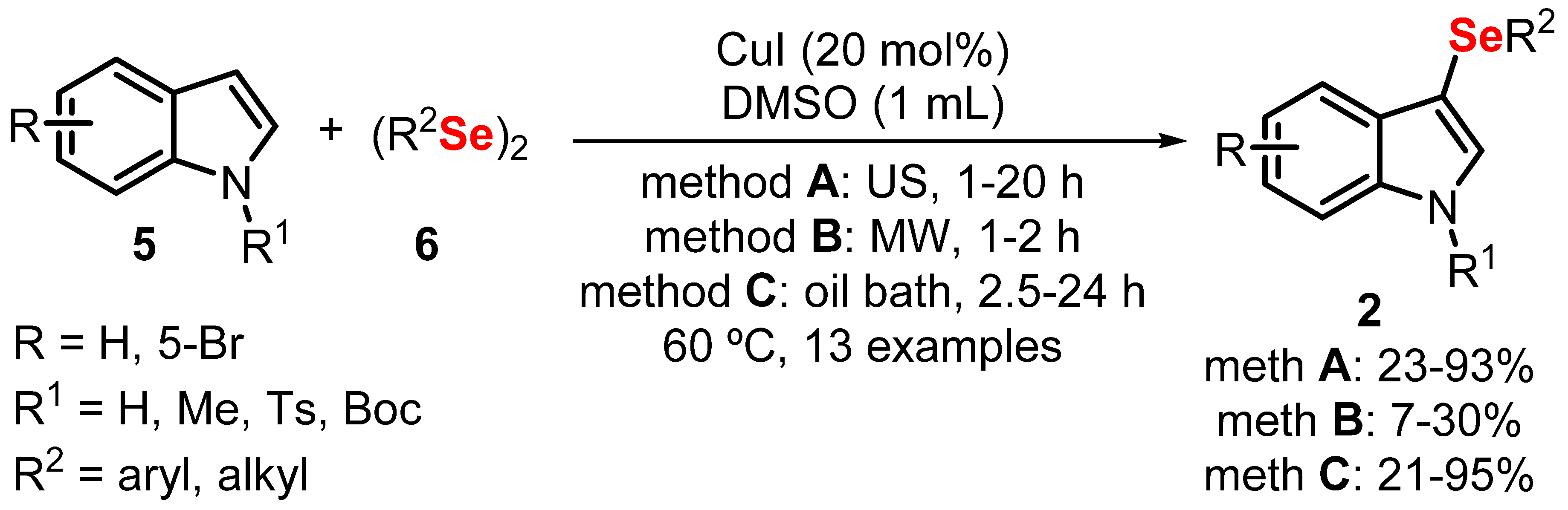
- Vieira, B.M.; Thurow, S.; Brito, J.S.; Perin, G.; Alves, D.; Jacob, R.G.; Santi, C.; Lenardão, E.J. Sonochemistry: An efficient alternative to the synthesis of 3-selanylindoles using CuI as catalyst. Ultrason. Sonochem. 2015, 27, 192–199. [Google Scholar] [CrossRef]
- Vieira, B.M.; Thurow, S.; da Costa, M.; Casaril, A.M.; Domingues, M.; Schumacher, R.F.; Perin, G.; Alves, D.; Savegnago, L.; Lenardão, E.J. Ultrasound-Assisted Synthesis and Antioxidant Activity of 3-Selanyl-1 H -indole and 3-Selanylimidazo [1,2-a] pyridine Derivatives. Asian J. Org. Chem. 2017, 6, 1635–1646. [Google Scholar] [CrossRef]
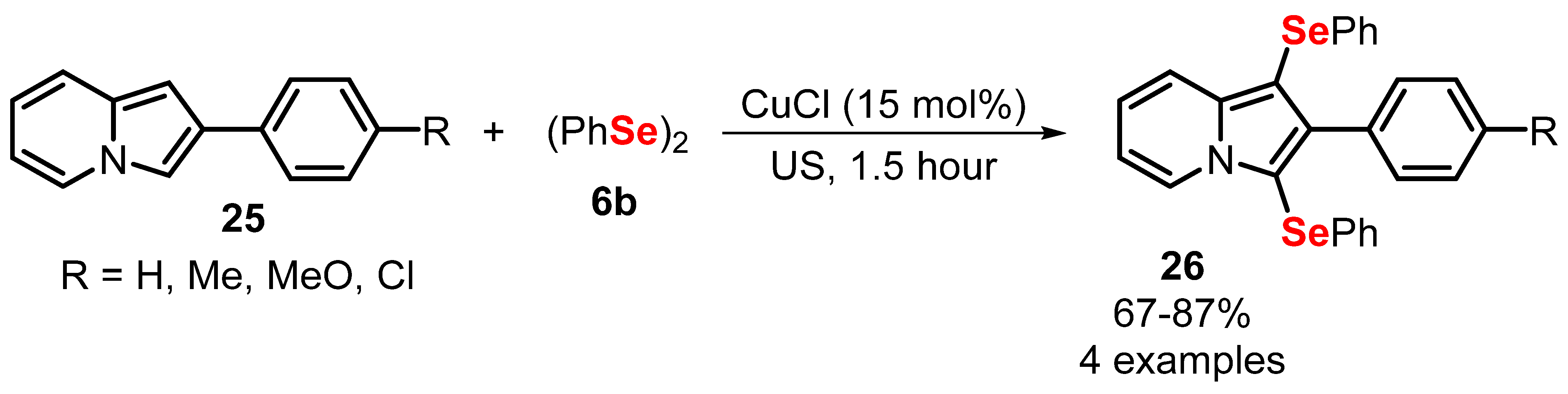
- Penteado, F.; Bettanin, L.; Machado, K.; Perin, G.; Alves, D.; Lenardão, E.J. Sonochemistry and Copper Catalysis: An Efficient Duo in the Synthesis of Chalcogenylindolizines. Asian J. Org. Chem. 2020, 9, 1631–1637. [Google Scholar] [CrossRef]
- Peglow, T.J.; da Costa, G.P.; Duarte, L.F.B.; Silva, M.S.; Barcellos, T.; Perin, G.; Alves, D. Ultrasound-Promoted One-Pot Synthesis of Mono- or Bis-Substituted Organylselanyl Pyrroles. J. Org. Chem. 2019, 84, 5471–5482. [Google Scholar] [CrossRef] [PubMed]
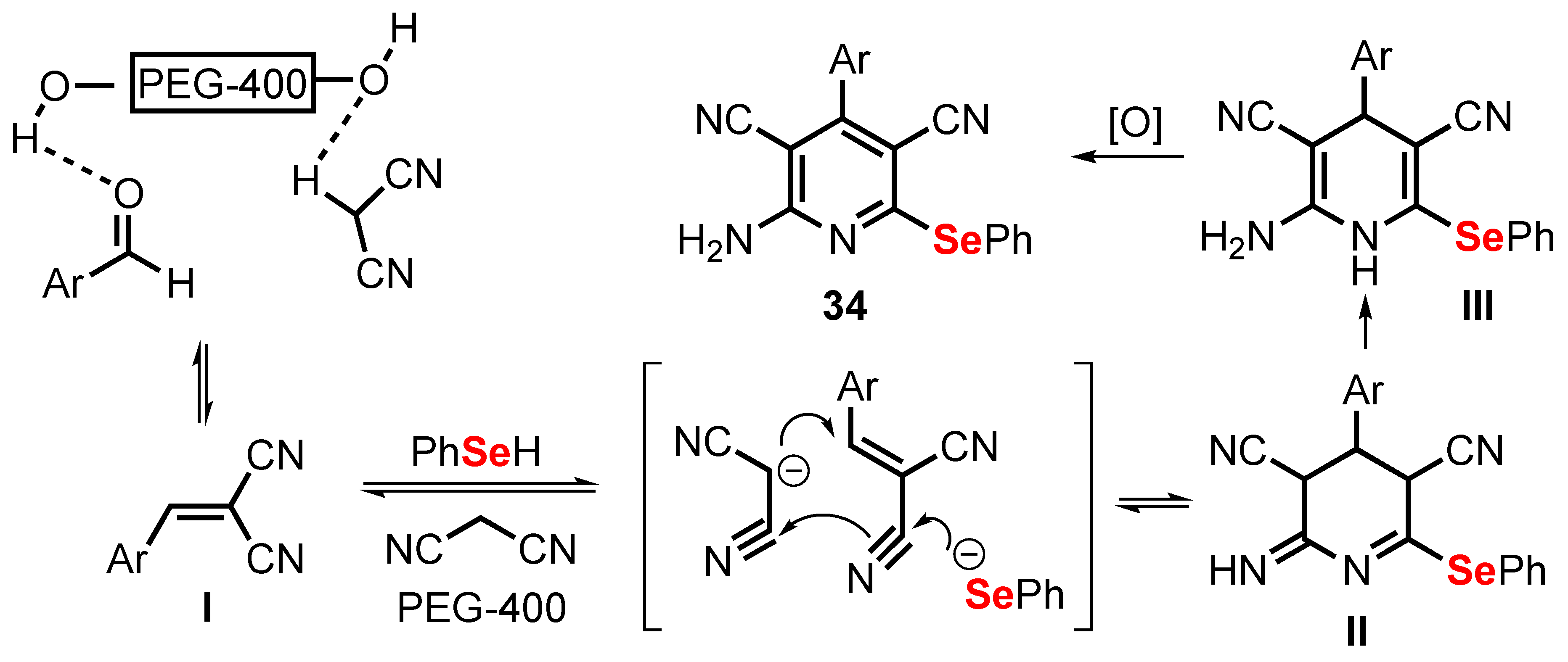
- Khan, M.N.; Karamthulla, S.; Choudhury, L.H.; Haque Faizi, M.S. Ultrasound assisted multicomponent reactions: A green method for the synthesis of highly functionalized selenopyridines using reusable polyethylene glycol as reaction medium. RSC Adv. 2015, 5, 22168–22172. [Google Scholar] [CrossRef]
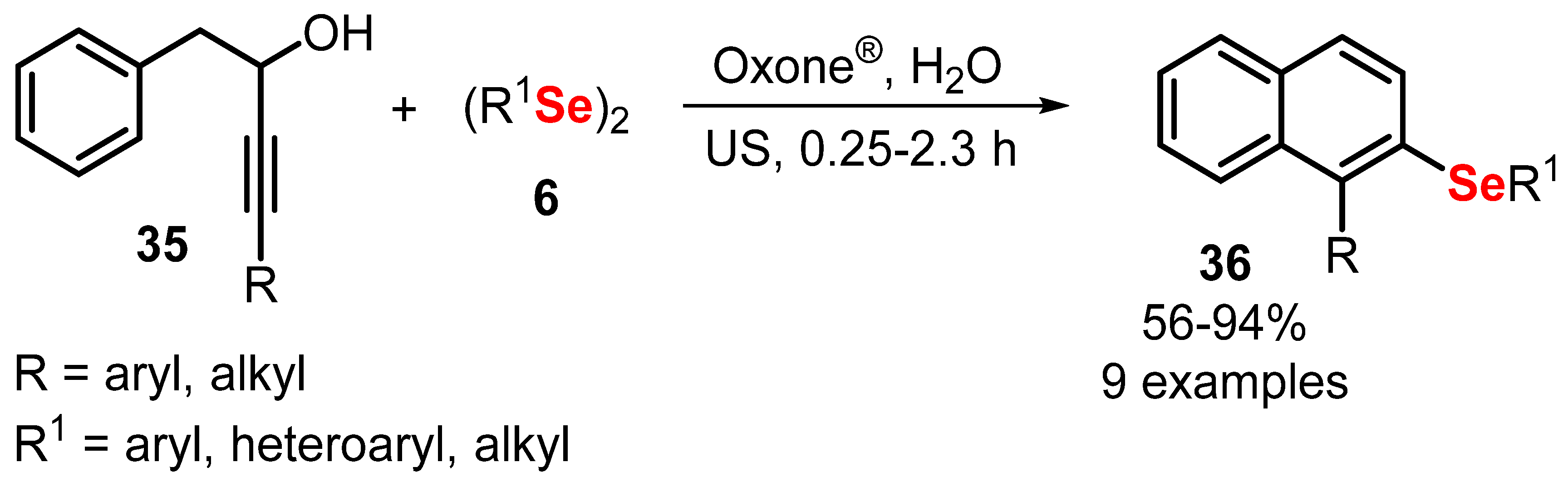
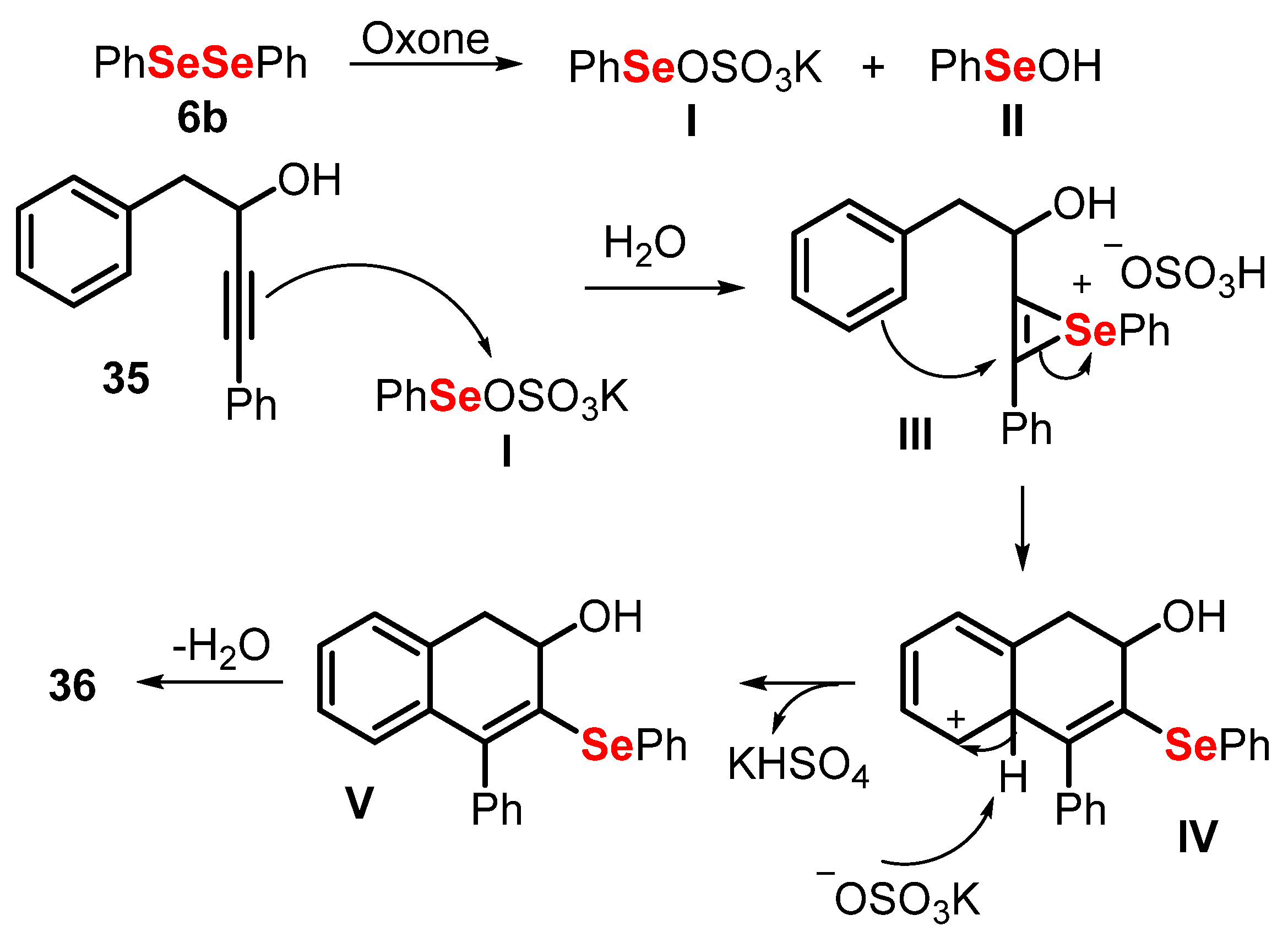
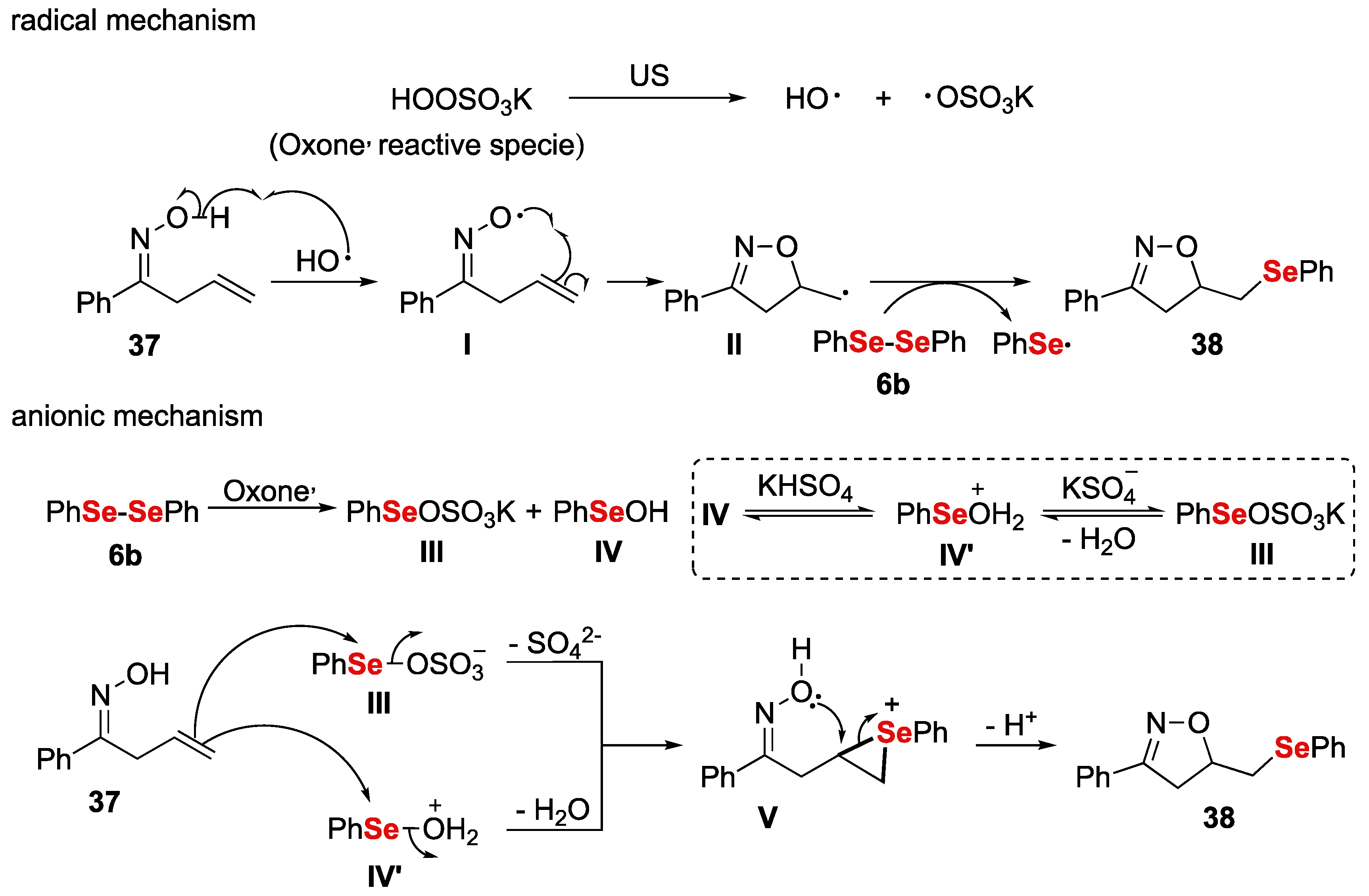
- Perin, G.; Araujo, D.R.; Nobre, P.C.; Lenardao, E.J.; Jacob, R.G.; Silva, M.S.; Roehrs, J.A. Ultrasound-promoted synthesis of 2-organoselanyl-naphthalenes using Oxone ® in aqueous medium as an oxidizing agent. PeerJ 2018, 6, e4706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, D.R.; Lima, Y.R.; Barcellos, A.M.; Silva, M.S.; Jacob, R.G.; Lenardão, E.J.; Bagnoli, L.; Santi, C.; Perin, G. Ultrasound-Promoted Radical Synthesis of 5-Methylselanyl-4,5-dihydroisoxazoles. Eur. J. Org. Chem. 2020, 2020, 586–592. [Google Scholar] [CrossRef]
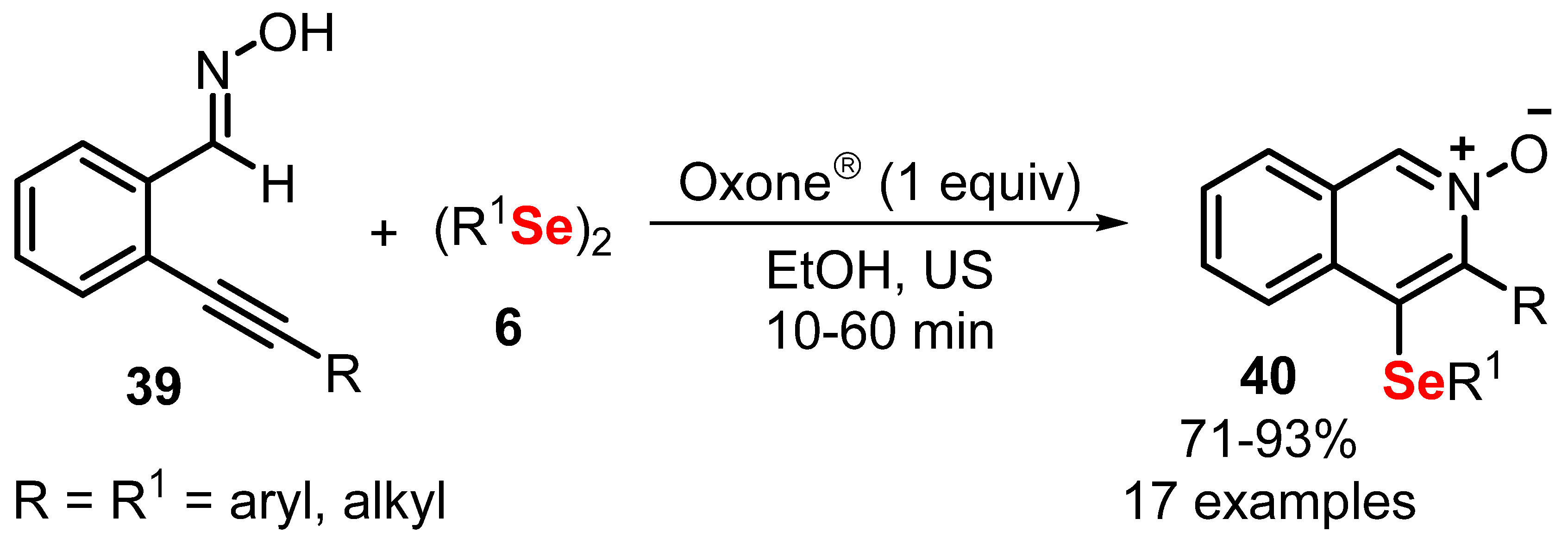
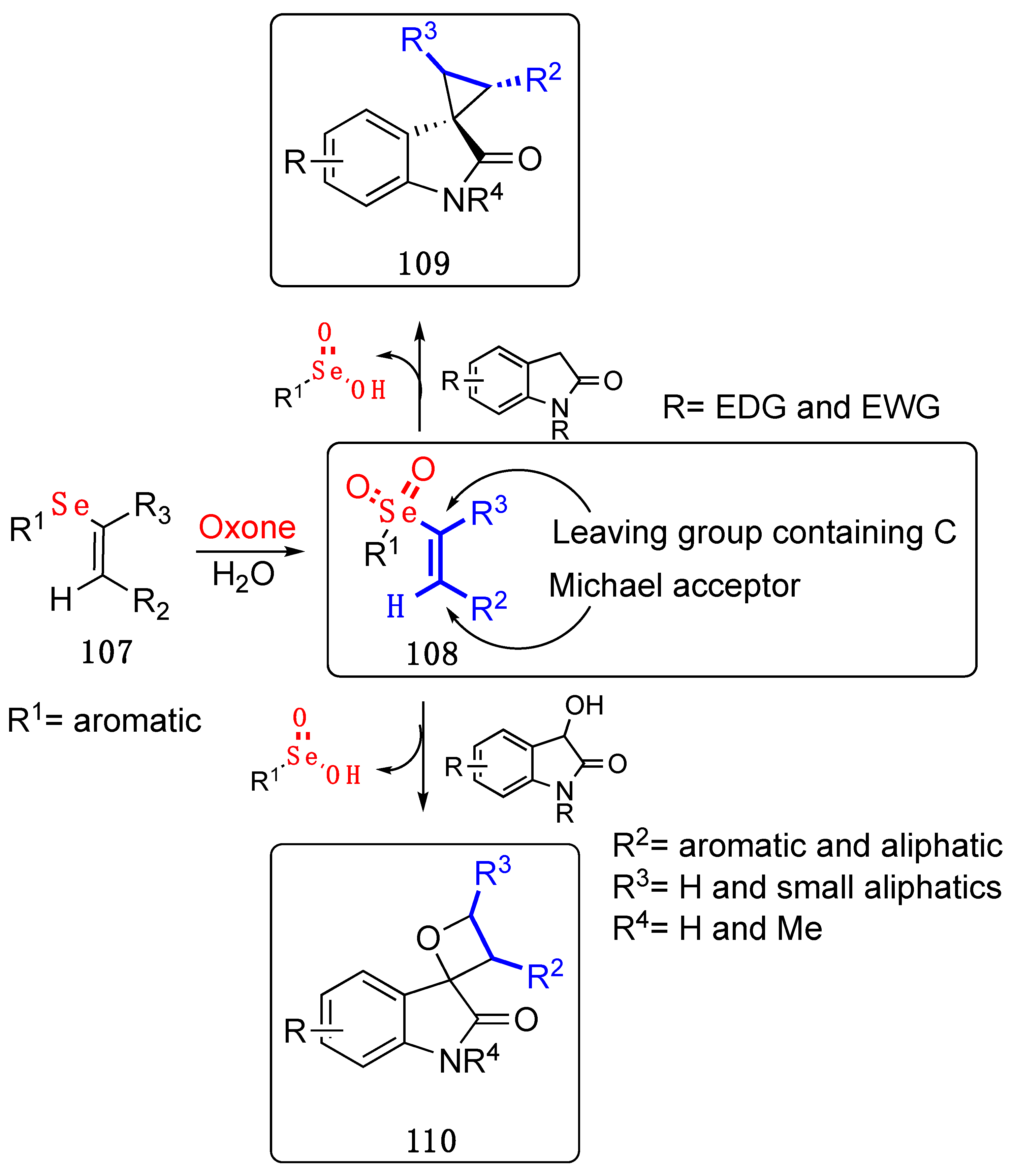
- Araujo, D.R.; Goulart, H.A.; Barcellos, A.M.; Cargnelutti, R.; Lenardão, E.J.; Perin, G. Oxone-Promoted Synthesis of 4-(Chalcogenyl)isoquinoline- N -oxides from Alkynylbenzaldoximes and Diorganyl Dichalcogenides. J. Org. Chem. 2021, 86, 1721–1729. [Google Scholar] [CrossRef]
- Yuan, Y.; Lei, A. Is electrosynthesis always green and advantageous compared to traditional methods? Nat. Commun. 2020, 11, 802. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic electrosynthesis: A promising green methodology in organic chemistry. Green Chem. 2010, 12, 2099–2119. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of Electrochemistry in the Synthesis of Heterocyclic Structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.S.P.; Šljukić, B.; Santos, D.M.F.; Sequeira, C.A.C. Organic Electrosynthesis: From Laboratorial Practice to Industrial Applications. Org. Process Res. Dev. 2017, 21, 1213–1226. [Google Scholar] [CrossRef]
- Martins, G.M.; Meirinho, A.G.; Ahmed, N.; Braga, A.L.; Mendes, S.R. Recent Advances in Electrochemical Chalcogen (S/Se)-Functionalization of Organic Molecules. ChemElectroChem 2019, 6, 5928–5940. [Google Scholar] [CrossRef]
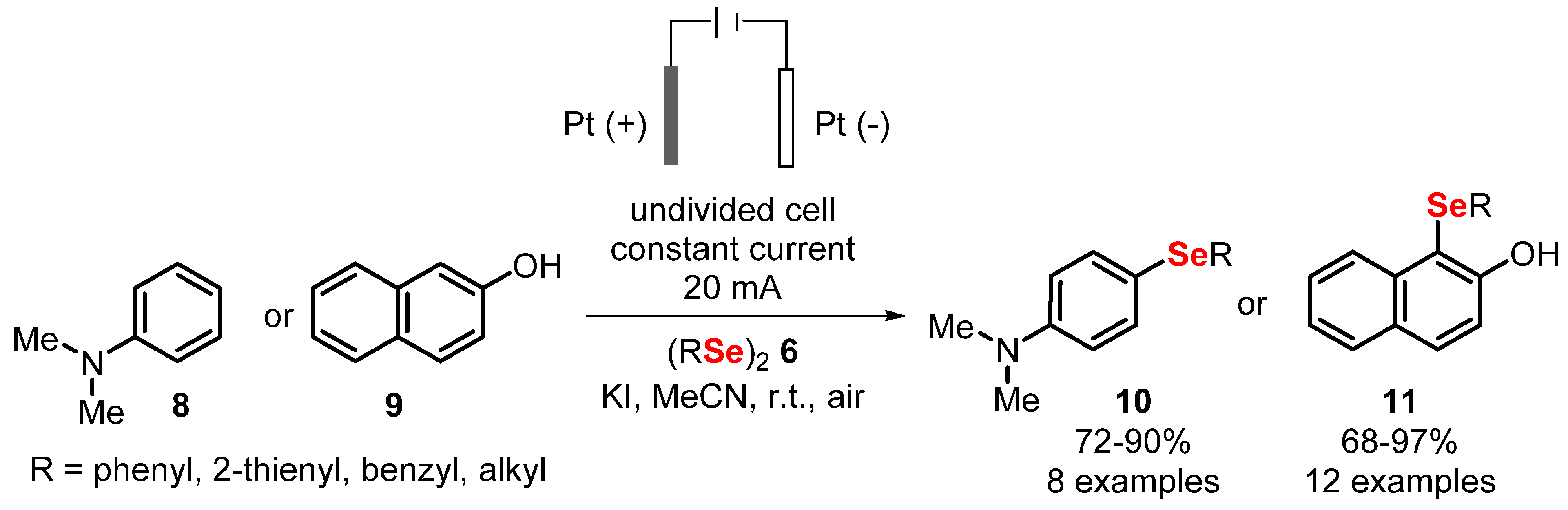
- Meirinho, A.G.; Pereira, V.F.; Martins, G.M.; Saba, S.; Rafique, J.; Braga, A.L.; Mendes, S.R. Electrochemical Oxidative C(sp 2)-H Bond Selenylation of Activated Arenes. Eur. J. Org. Chem. 2019, 2019, 6465–6469. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Deng, J.; Huang, Y.; Pan, Z.; Yu, Y.; Cao, H. Electrochemical diselenylation of indolizines via intermolecular C–Se formation with 2-methylpyridines, α-bromoketones and diselenides. Chem. Commun. 2020, 56, 735–738. [Google Scholar] [CrossRef]
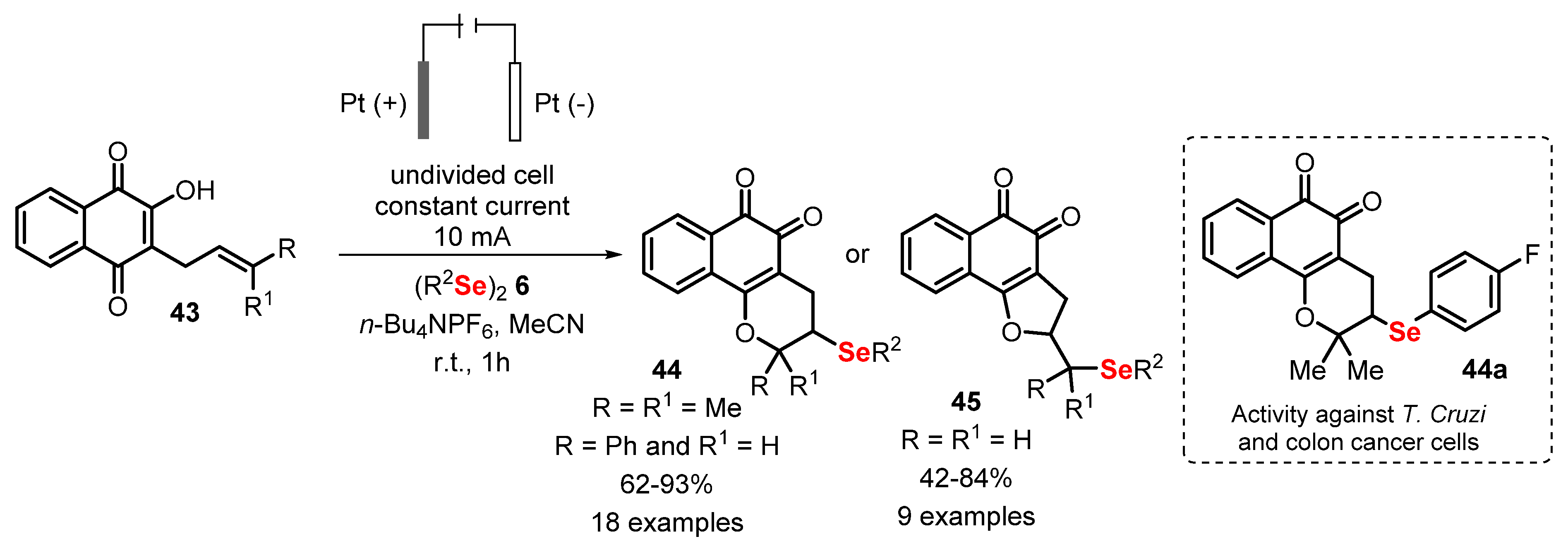
- Kharma, A.; Jacob, C.; Bozzi, Í.A.O.; Jardim, G.A.M.; Braga, A.L.; Salomão, K.; Gatto, C.C.; Silva, M.F.S.; Pessoa, C.; Stangier, M.; et al. Electrochemical Selenation/Cyclization of Quinones: A Rapid, Green and Efficient Access to Functionalized Trypanocidal and Antitumor Compounds. Eur. J. Org. Chem. 2020, 2020, 4474–4486. [Google Scholar] [CrossRef]
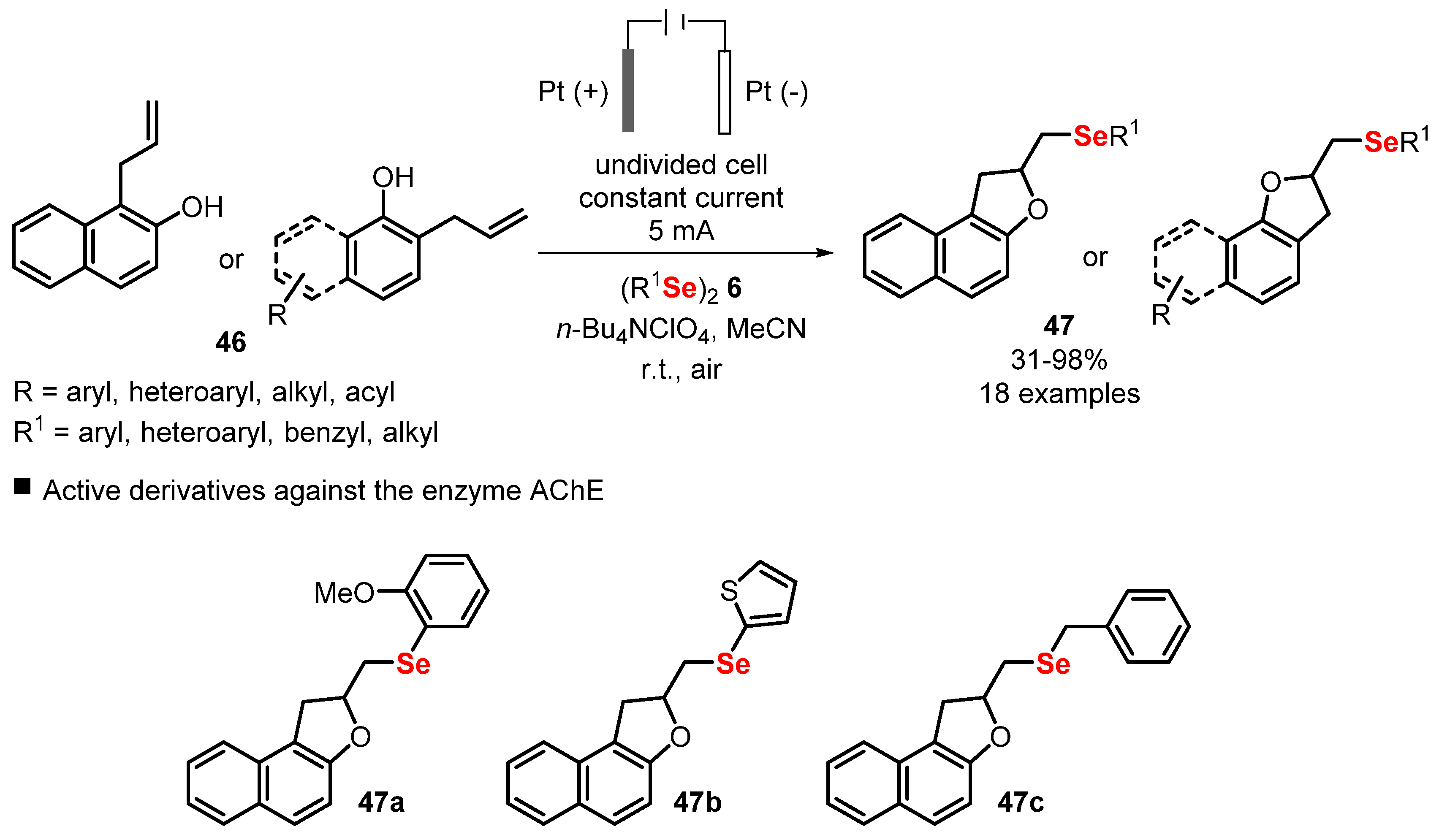
- Scheide, M.R.; Schneider, A.R.; Jardim, G.A.M.; Martins, G.M.; Durigon, D.C.; Saba, S.; Rafique, J.; Braga, A.L. Electrochemical synthesis of selenyl-dihydrofurans via anodic selenofunctionalization of allyl-naphthol/phenol derivatives and their anti-Alzheimer activity. Org. Biomol. Chem. 2020, 18, 4916–4921. [Google Scholar] [CrossRef]
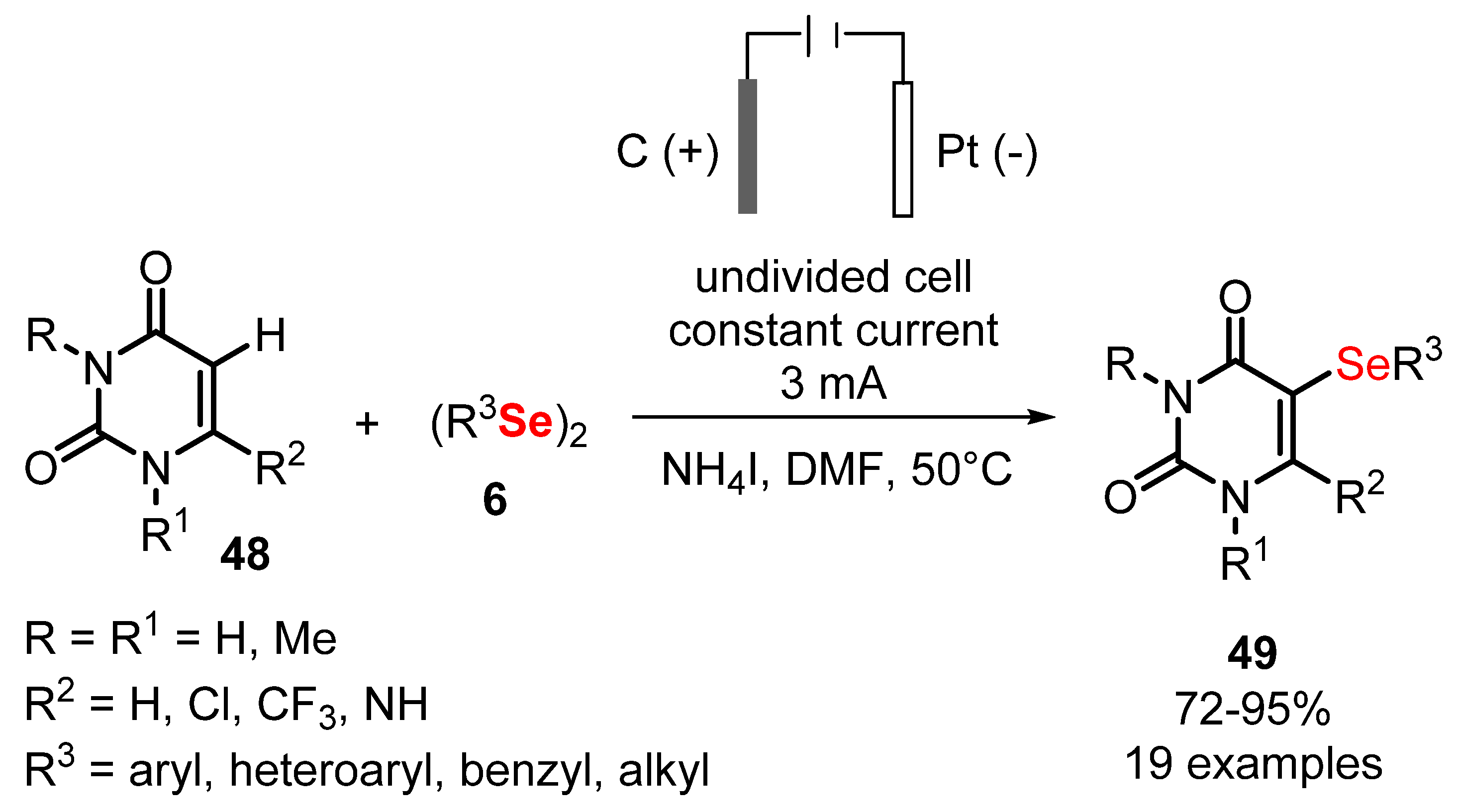
- Wang, Q.; Ma, X.-L.; Chen, Y.-Y.; Jiang, C.-N.; Xu, Y.-L. Electrochemical Synthesis of 5-Selenouracil Derivatives by Selenylation of Uracils. Eur. J. Org. Chem. 2020, 2020, 4384–4388. [Google Scholar] [CrossRef]
- Santi, C.; Tomassini, C.; Sancineto, L. Organic Diselenides: Versatile Reagents, Precursors, and Intriguing Biologically Active Compounds. Chim. Int. J. Chem. 2017, 71, 592–595. [Google Scholar] [CrossRef]
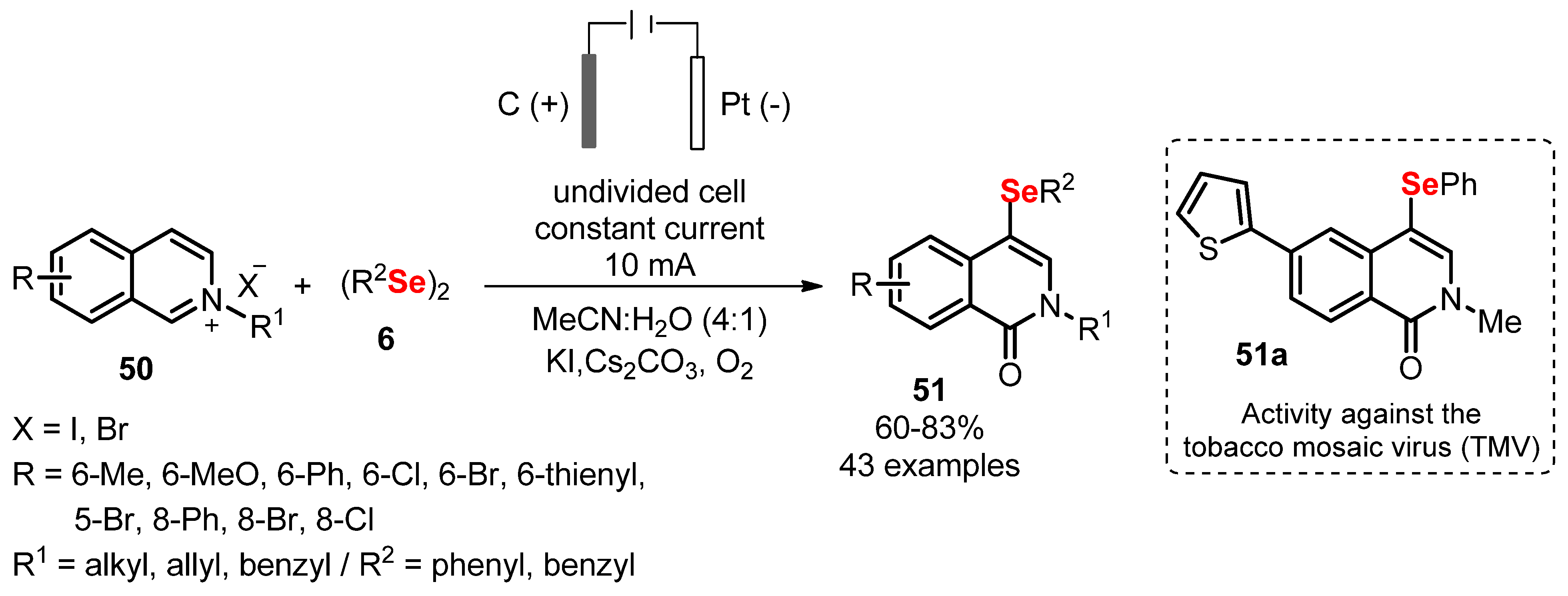
- Liu, X.; Wang, Y.; Song, D.; Wang, Y.; Cao, H. Electrochemical regioselective selenylation/oxidation of N -alkylisoquinolinium salts via double C(sp 2)–H bond functionalization. Chem. Commun. 2020, 56, 15325–15328. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Kong, X.; Yang, J.; Li, G.; Xu, B.; Chen, Q. Electrochemical Oxidative Halogenation of N -Aryl Alkynamides for the Synthesis of Spiro[4.5]trienones. J. Org. Chem. 2021, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, B.; Zong, L.; Yu, L.; Li, X.; Li, Q.; Zhang, X.; Zhang, S.; Xu, K. Electrochemical Tandem Cyclization of Unsaturated Oximes with Diselenides: A General Approach to Seleno Isoxazolines Derivatives with Quaternary Carbon Center. Eur. J. Org. Chem. 2021, 2021, 2431–2435. [Google Scholar] [CrossRef]
- Lazzaris, M.J.; Martins, G.M.; Xavier, F.R.; Braga, A.L.; Mendes, S.R. Versatile Electrochemical Oxidative C(sp 2)−H Bond Selenylation of Resveratrol. Eur. J. Org. Chem. 2021, 2021, 4411–4416. [Google Scholar] [CrossRef]
- Lopin, C.; Gouhier, G.; Gautier, A.; Piettre, S.R. Phosphonyl, Phosphonothioyl, Phosphonodithioyl, and Phosphonotrithioyl Radicals: Generation and Study of Their Addition onto Alkenes. J. Org. Chem. 2003, 68, 9916–9923. [Google Scholar] [CrossRef]
- Guo, S.; Li, S.; Zhang, Z.; Yan, W.; Cai, H. Electrochemical selenation of phosphonates and phosphine oxides. Tetrahedron Lett. 2020, 61, 151566. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Cannalire, R.; Pelliccia, S.; Sancineto, L.; Novellino, E.; Tron, G.C.; Giustiniano, M. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 2021, 50, 766–897. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020, 11, 803. [Google Scholar] [CrossRef]
- Sun, K.; Lv, Q.-Y.; Chen, X.-L.; Qu, L.-B.; Yu, B. Recent advances in visible-light-mediated organic transformations in water. Green Chem. 2021, 23, 232–248. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Kappe, C.O. Recent advances toward sustainable flow photochemistry. Curr. Opin. Green Sustain. Chem. 2020, 25, 100351. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [Green Version]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Hari, D.P.; König, B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014, 50, 6688–6699. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-S.; Dixneuf, P.H.; Soulé, J.-F. Photoredox Catalysis for Building C–C Bonds from C(sp 2)–H Bonds. Chem. Rev. 2018, 118, 7532–7585. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Natl. Sci. Rev. 2017, 4, 359–380. [Google Scholar] [CrossRef] [Green Version]
- Parasram, M.; Gevorgyan, V. Visible light-induced transition metal-catalyzed transformations: Beyond conventional photosensitizers. Chem. Soc. Rev. 2017, 46, 6227–6240. [Google Scholar] [CrossRef]
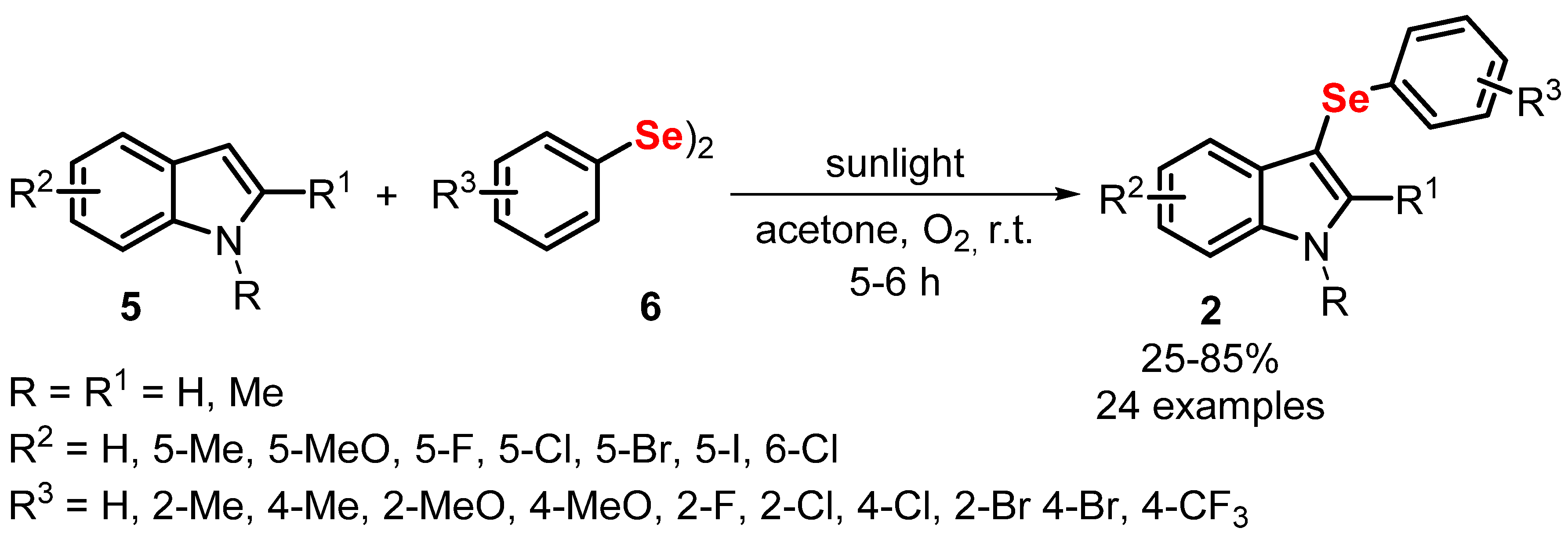
- Rafique, J.; Rampon, D.S.; Azeredo, J.B.; Coelho, F.L.; Schneider, P.H.; Braga, A.L. Light-Mediated Seleno-Functionalization of Organic Molecules: Recent Advances. Chem. Rec. 2021, 21, 2739–2761. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ramesh, V.; Gangadhar, M.; Vijaykumar, S. Catalyst and Sensitizer-Free Visible-Light-Induced C(sp 2)−H Chalcogenation of Arenes/ Heteroarenes with Dichalcogenides. Asian J. Org. Chem. 2018, 7, 1689–1697. [Google Scholar] [CrossRef]
- Lemir, I.D.; Castro-Godoy, W.D.; Heredia, A.A.; Schmidt, L.C.; Argüello, J.E. Metal- and photocatalyst-free synthesis of 3-selenylindoles and asymmetric diarylselenides promoted by visible light. RSC Adv. 2019, 9, 22685–22694. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-B.; Ban, Y.-L.; Yuan, P.-F.; Peng, S.-J.; Fang, J.-G.; Wu, L.-Z.; Liu, Q. Visible-light-mediated aerobic selenation of (hetero)arenes with diselenides. Green Chem. 2017, 19, 5559–5563. [Google Scholar] [CrossRef]
- Abe, T.; Yamada, K.; Satake, S. Biomimetic Synthesis of Iheyamine A from Spirocyclic Oxindoles. Heterocycles 2019, 99, 379. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Li, G.; Xing, C.; Cui, W.; Li, K.; Wei, W. Metal- and photocatalyst-free visible-light-promoted regioselective selenylation of coumarin derivatives via oxidation-induced C–H functionalization. Org. Chem. Front. 2018, 5, 2974–2979. [Google Scholar] [CrossRef]
- Gandeepan, P.; Mo, J.; Ackermann, L. Photo-induced copper-catalyzed C–H chalcogenation of azoles at room temperature. Chem. Commun. 2017, 53, 5906–5909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, T.; Das, S.; De Sarkar, S. Nickel(II) Tetraphenylporphyrin as an Efficient Photocatalyst Featuring Visible Light Promoted Dual Redox Activities. Adv. Synth. Catal. 2019, 361, 3200–3209. [Google Scholar] [CrossRef]
- Saba, S.; Rafique, J.; Franco, M.S.; Schneider, A.R.; Espíndola, L.; Silva, D.O.; Braga, A.L. Rose Bengal catalysed photo-induced selenylation of indoles, imidazoles and arenes: A metal free approach. Org. Biomol. Chem. 2018, 16, 880–885. [Google Scholar] [CrossRef]
- Chen, J.; Chen, R.; Mei, L.; Yan, S.; Wu, Y.; Li, Q.; Yuan, B. Visible-Light-Induced Difunctionalization of Styrenes: An Efficient and Green Protocol for the Synthesis of β-Acyloxyselenides. Asian J. Org. Chem. 2020, 9, 181–184. [Google Scholar] [CrossRef]
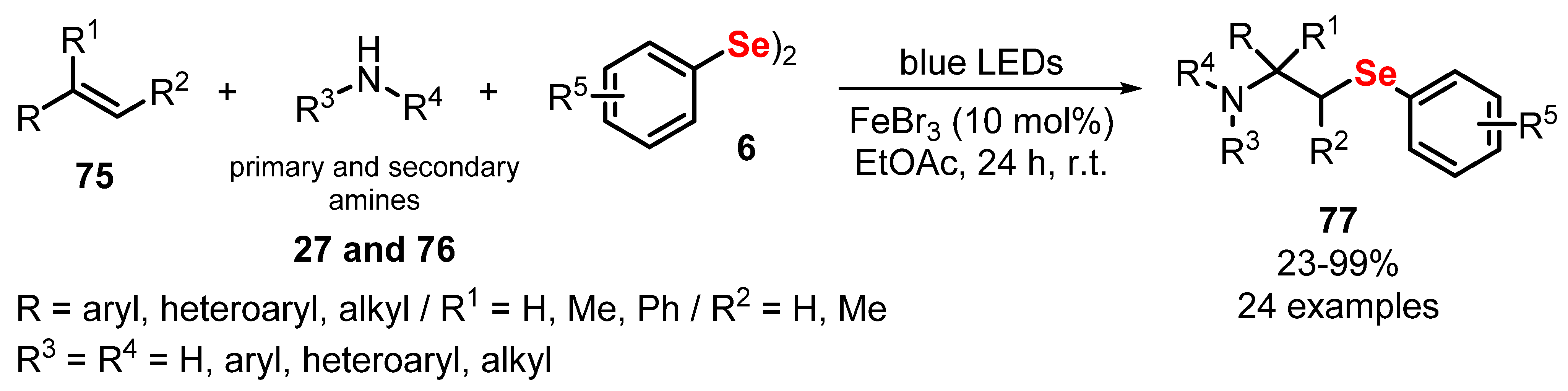
- Huang, B.; Li, Y.; Yang, C.; Xia, W. Three-component aminoselenation of alkenes via visible-light enabled Fe-catalysis. Green Chem. 2020, 22, 2804–2809. [Google Scholar] [CrossRef]
- Kobiki, Y.; Kawaguchi, S.; Ogawa, A. Highly regioselective hydroselenation of inactivated terminal alkynes using diselenide–Ph2P(O)H mixed systems under visible-light irradiation. Tetrahedron Lett. 2013, 54, 5453–5456. [Google Scholar] [CrossRef]
- Chen, H.; Ding, R.; Tang, H.; Pan, Y.; Xu, Y.; Chen, Y. Simultaneous Construction of C−Se And C−S Bonds via the Visible-Light-Mediated Multicomponent Cascade Reaction of Diselenides, Alkynes, and SO2. Chem. Asian J. 2019, 14, 3264–3268. [Google Scholar] [CrossRef]
- Ghiazza, C.; Khrouz, L.; Billard, T.; Monnereau, C.; Tlili, A. Fluoroalkylselenolation of Alkyl Silanes/Trifluoroborates under Metal-Free Visible-Light Photoredox Catalysis. Eur. J. Org. Chem. 2020, 2020, 1559–1566. [Google Scholar] [CrossRef] [Green Version]
- Anand, D.; He, Y.; Li, L.; Zhou, L. A photocatalytic sp 3 C–S, C–Se and C–B bond formation through C–C bond cleavage of cycloketone oxime esters. Org. Biomol. Chem. 2019, 17, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, H.; Mandal, A.; Dana, S.; Baidya, M. Visible Light-Induced Synthetic Approach for Selenylative Spirocyclization of N -Aryl Alkynamides with Molecular Oxygen as Oxidant. Adv. Synth. Catal. 2018, 360, 1099–1103. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.; Mo, Z.; Ma, X.; Chen, Y.; Tang, H.; Pan, Y.; Xu, Y. Visible-Light-Promoted Selenylative Spirocyclization of Indolyl-ynones toward the Formation of 3-Selenospiroindolenine Anticancer Agents. Chem. Asian J. 2020, 15, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Mampuys, P.; McElroy, C.R.; Clark, J.H.; Orru, R.V.A.; Maes, B.U.W. Thiosulfonates as Emerging Reactants: Synthesis and Applications. Adv. Synth. Catal. 2020, 362, 3–64. [Google Scholar] [CrossRef] [Green Version]
- Ghiazza, C.; Debrauwer, V.; Monnereau, C.; Khrouz, L.; Médebielle, M.; Billard, T.; Tlili, A. Visible-Light-Mediated Metal-Free Synthesis of Trifluoromethylselenolated Arenes. Angew. Chem. Int. Ed. 2018, 57, 11781–11785. [Google Scholar] [CrossRef]
- Weber, A.C.H.; Coelho, F.L.; Affeldt, R.F.; Schneider, P.H. Visible-Light Promoted Stereoselective Arylselanyl Functionalization of Alkynes. Eur. J. Org. Chem. 2018, 2018, 6738–6742. [Google Scholar] [CrossRef]
- Rathore, V.; Kumar, S. Visible-light-induced metal and reagent-free oxidative coupling of sp 2 C–H bonds with organo-dichalcogenides: Synthesis of 3-organochalcogenyl indoles. Green Chem. 2019, 21, 2670–2676. [Google Scholar] [CrossRef]
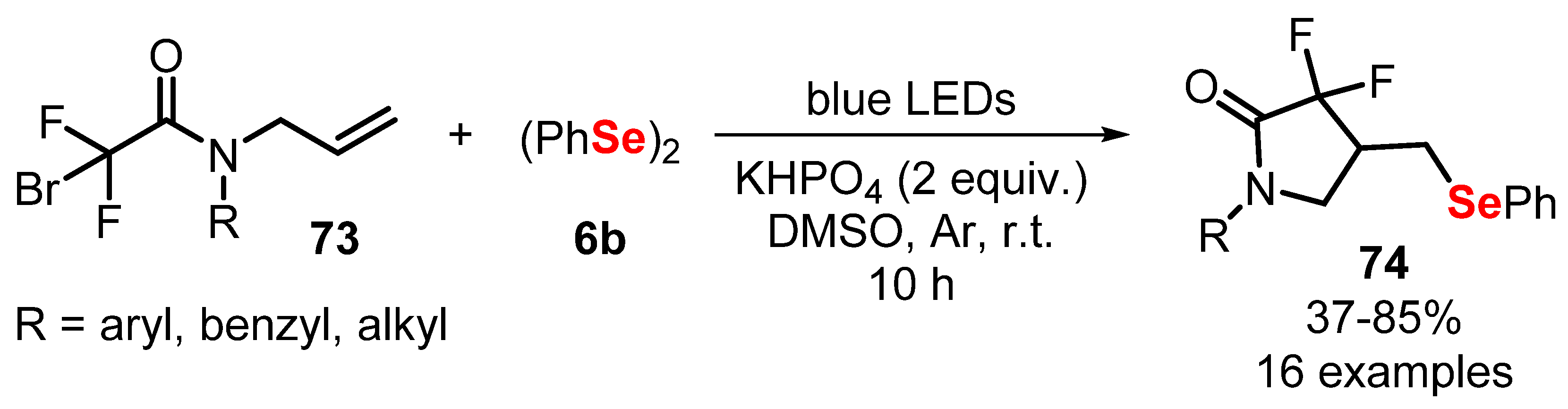
- Ye, Z.-P.; Xia, P.-J.; Liu, F.; Hu, Y.-Z.; Song, D.; Xiao, J.-A.; Huang, P.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. Visible-Light-Induced, Catalyst-Free Radical Cross-Coupling Cyclization of N -Allylbromodifluoroacetamides with Disulfides or Diselenides. J. Org. Chem. 2020, 85, 5670–5682. [Google Scholar] [CrossRef]
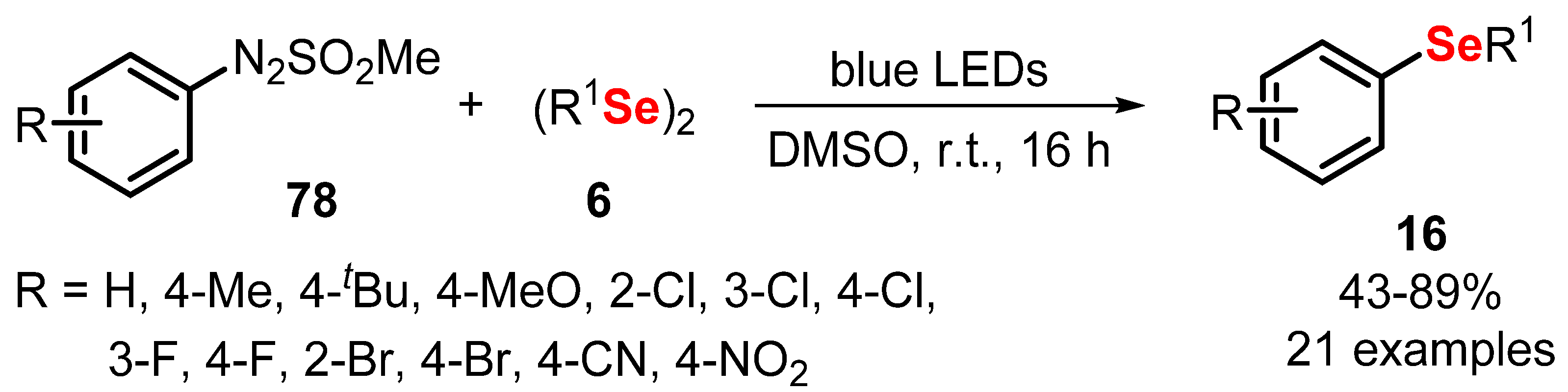
- Jang, J.; Kim, R.; Kim, D.Y. Photocatalyst-free photoredox synthesis of diaryl selenides by reaction of diselenides with aryldiazo sulfones. Synth. Commun. 2021, 51, 720–726. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Yi, W.; Wang, P.-F.; Liu, J.; Ma, M.; Hao, D.-Y.; Ming, L.; Ling, Y. Visible-light-induced oxidative coupling of vinylarenes with diselenides leading to α-aryl and α-alkyl selenomethyl ketones. Green Chem. 2021, 23, 1840–1846. [Google Scholar] [CrossRef]
- Li, W.; Zhou, L. Visible-light-driven radical 1,3-addition of selenosulfonates to vinyldiazo compounds. Green Chem. 2021, 23, 6652–6658. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.; Mack, J. Mechanochemistry and organic synthesis: From mystical to practical. Green Chem. 2018, 20, 1435–1443. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, T.; Ranu, B.C. Reaction under Ball-Milling: Solvent-, Ligand-, and Metal-Free Synthesis of Unsymmetrical Diaryl Chalcogenides. J. Org. Chem. 2013, 78, 11110–11114. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chatterjee, T.; Ranu, B.C. Transition metal- and solvent-free synthesis of unsymmetrical diaryl sulfides and selenides under ball-milling. Arkivoc 2015, 2016, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Panja, S.; Paul, B.; Jalal, S.; Ghosh, T.; Ranu, B.C. Mechanochemically Induced Chalcogenation of Bicyclic Arenes under Solvent-, Ligand-, Metal-, and Oxidant-Free Conditions. ChemistrySelect 2020, 5, 14198–14202. [Google Scholar] [CrossRef]
- Xiao, J.-A.; Cheng, X.-L.; Meng, R.-F.; Qin, X.-S.; Peng, H.; Ren, J.-W.; Xie, Z.-Z.; Cui, J.-G.; Huang, Y.-M. Straightforward Synthesis of 3-Selenocyanato-Substituted Chromones through Electrophilic Selenocyanation of Enaminones under Grinding Conditions. Synthesis 2021, 53, 954–960. [Google Scholar] [CrossRef]
- Guidi, M.; Seeberger, P.H.; Gilmore, K. How to approach flow chemistry. Chem. Soc. Rev. 2020, 49, 8910–8932. [Google Scholar] [CrossRef]
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef] [Green Version]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberger, P.H. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef] [PubMed]
- Reizman, B.J.; Jensen, K.F. Feedback in Flow for Accelerated Reaction Development. Acc. Chem. Res. 2016, 49, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science (80-.) 2016, 352, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Gemoets, H.P.L.; Su, Y.; Shang, M.; Hessel, V.; Luque, R.; Noël, T. Liquid phase oxidation chemistry in continuous-flow microreactors. Chem. Soc. Rev. 2016, 45, 83–117. [Google Scholar] [CrossRef] [Green Version]
- Gioiello, A.; Mancino, V.; Filipponi, P.; Mostarda, S.; Cerra, B. Concepts and optimization strategies of experimental design in continuous-flow processing. J. Flow Chem. 2016, 6, 167–180. [Google Scholar] [CrossRef]
- Mándity, I.M.; Ötvös, S.B.; Fülöp, F. Strategic Application of Residence-Time Control in Continuous-Flow Reactors. ChemistryOpen 2015, 4, 212–223. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology-A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.J.; Battilocchio, C.; Fitzpatrick, D.E.; Sliwinski, E.; Hawkins, J.M.; Ley, S.V. A Systems Approach towards an Intelligent and Self-Controlling Platform for Integrated Continuous Reaction Sequences. Angew. Chem. Int. Ed. 2015, 54, 144–148. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Straathof, N.J.W.; Hessel, V.; Noël, T. Photochemical Transformations Accelerated in Continuous-Flow Reactors: Basic Concepts and Applications. Chem. A Eur. J. 2014, 20, 10562–10589. [Google Scholar] [CrossRef] [PubMed]
- Maljuric, S.; Jud, W.; Kappe, C.O.; Cantillo, D. Translating batch electrochemistry to single-pass continuous flow conditions: An organic chemist’s guide. J. Flow Chem. 2020, 10, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Cambié, D.; Noël, T. Solar Photochemistry in Flow. Top. Curr. Chem. 2018, 376, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, M.; Sancineto, L.; Nascimento, V.; Braun Azeredo, J.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow Biocatalysis: A Challenging Alternative for the Synthesis of APIs and Natural Compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef]
- Cerra, B.; Mangiavacchi, F.; Santi, C.; Lozza, A.M.; Gioiello, A. Selective continuous flow synthesis of hydroxy lactones from alkenoic acids. React. Chem. Eng. 2017, 2, 467–471. [Google Scholar] [CrossRef]
- Mangiavacchi, F.; Crociani, L.; Sancineto, L.; Marini, F.; Santi, C. Continuous Bioinspired Oxidation of Sulfides. Molecules 2020, 25, 2711. [Google Scholar] [CrossRef]
- Begini, F.; Krasowska, D.; Jasiak, A.; Drabowicz, J.; Santi, C.; Sancineto, L. Continuous flow synthesis of 2,2′-diselenobis(benzoic acid) and derivatives. React. Chem. Eng. 2020, 5, 641–644. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, C.A.; Kulkarni, A.A. Automating multistep flow synthesis: Approach and challenges in integrating chemistry, machines and logic. Beilstein J. Org. Chem. 2017, 13, 960–987. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.; Jamison, T.F. Continuous flow multi-step organic synthesis. Chem. Sci. 2010, 1, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Heredia, A.A.; Soria-Castro, S.M.; Castro-Godoy, W.D.; Lemir, I.D.; López-Vidal, M.; Bisogno, F.R.; Argüello, J.E.; Oksdath-Mansilla, G. Multistep Synthesis of Organic Selenides under Visible Light Irradiation: A Continuous-Flow Approach. Org. Process Res. Dev. 2020, 24, 540–545. [Google Scholar] [CrossRef]
- Amri, N.; Wirth, T. Automated Electrochemical Selenenylations. Synthesis 2020, 52, 1751–1761. [Google Scholar] [CrossRef]
- Amri, N.; Wirth, T. Accelerating Electrochemical Synthesis through Automated Flow: Efficient Synthesis of Chalcogenophosphites. Synlett 2020, 31, 1894–1898. [Google Scholar] [CrossRef]
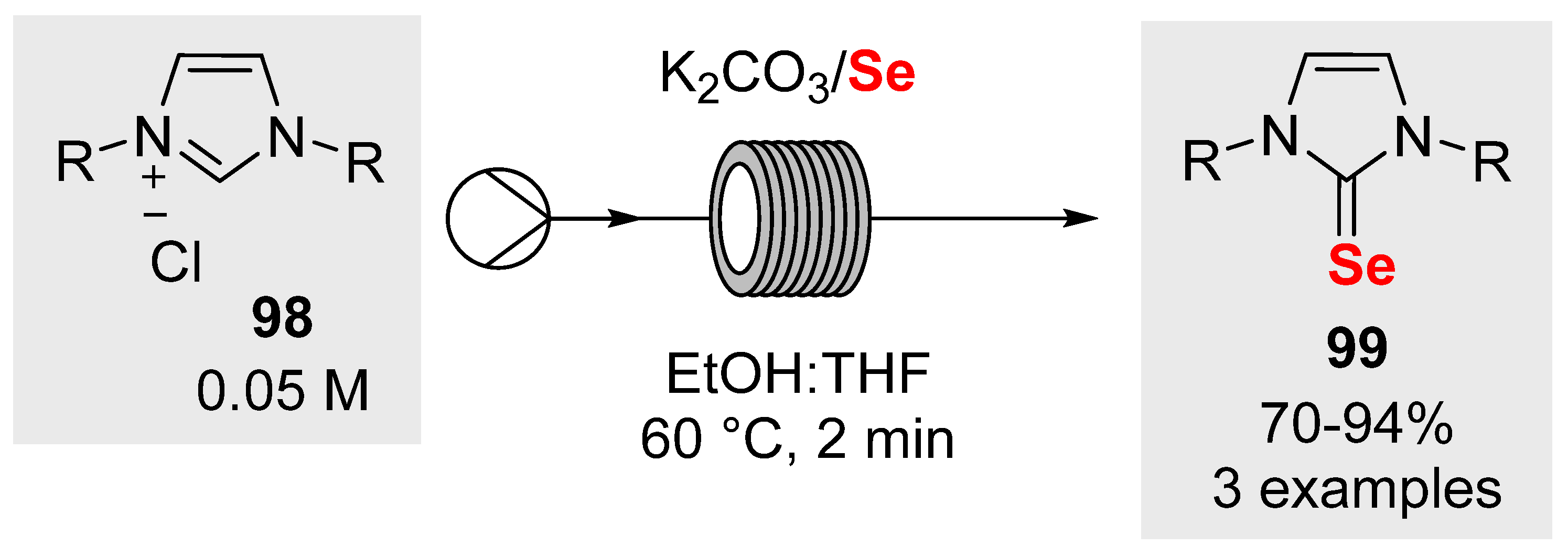
- Cauwenbergh, T.; Scattolin, T.; Simoens, A.; Tzouras, N.V.; Stevens, C.V.; Nolan, S.P. Continuous Flow Synthesis of Sulfur- and Selenium-NHC Compounds (NHC = N-heterocyclic carbene). Eur. J. Org. Chem. 2022, e202101296. [Google Scholar] [CrossRef]
- Müller, A.; Cadenas, E.; Graf, P.; Sies, H. A novel biologically active seleno-organic compound-1. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 1984, 33, 3235–3239. [Google Scholar] [CrossRef]
- Parnham, M.J.; Sies, H. The early research and development of ebselen. Biochem. Pharmacol. 2013, 86, 1248–1253. [Google Scholar] [CrossRef]
- Santi, C.; Scimmi, C.; Sancineto, L. Ebselen and Analogues: Pharmacological Properties and Synthetic Strategies for Their Preparation. Molecules 2021, 26, 4230. [Google Scholar] [CrossRef]
- Pacuła, A.J.; Mangiavacchi, F.; Sancineto, L.; Lenardão, E.J.; Ścianowski, J.; Santi, C. An Update on “Selenium Containing Compounds from Poison to Drug Candidates: A Review on the GPx-like Activity”. Curr. Chem. Biol. 2016, 9, 97–112. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
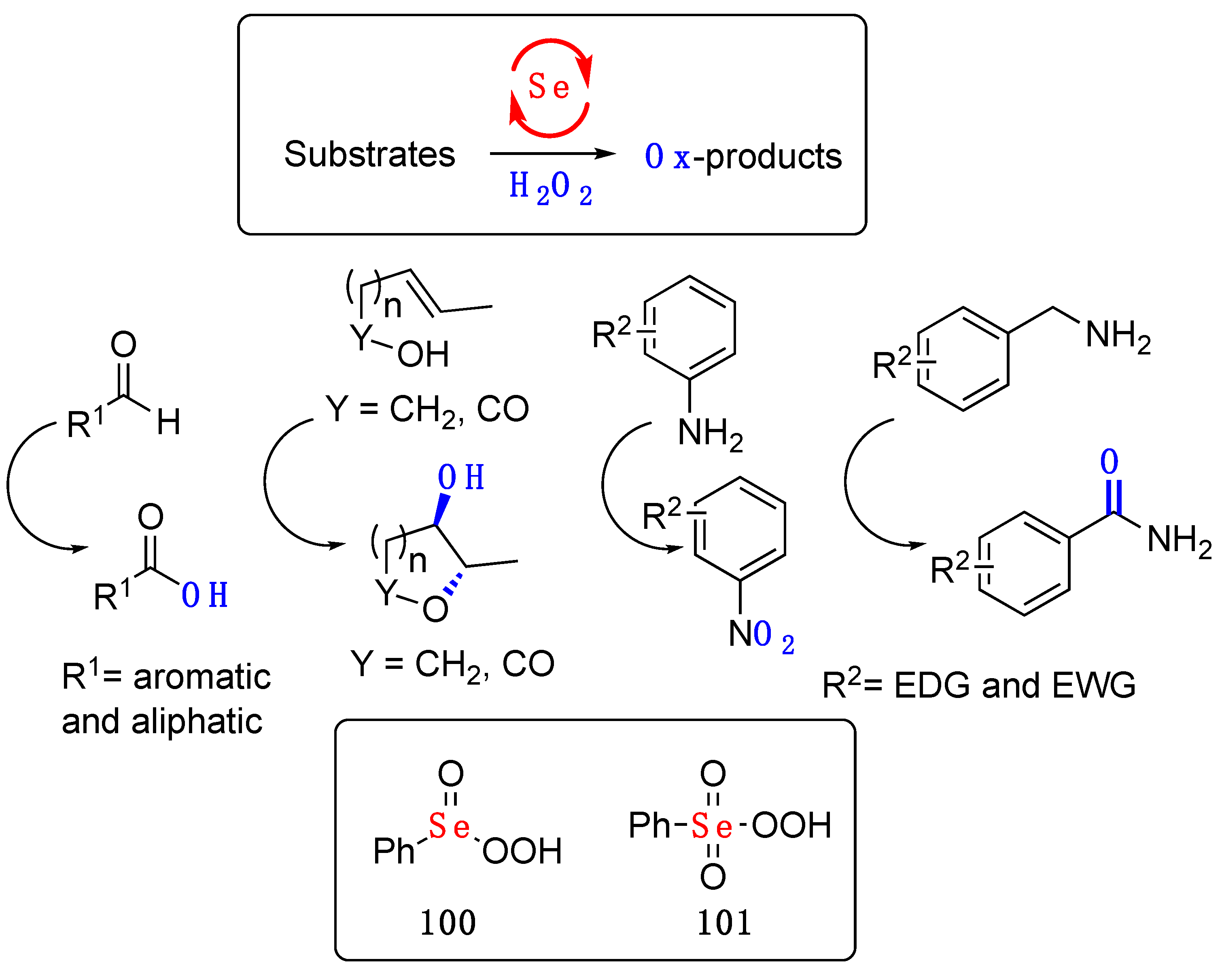
- Sancineto, L.; Tidei, C.; Bagnoli, L.; Marini, F.; Lenardão, E.; Santi, C. Selenium Catalyzed Oxidation of Aldehydes: Green Synthesis of Carboxylic Acids and Esters. Molecules 2015, 20, 10496–10510. [Google Scholar] [CrossRef] [PubMed]
- Sancineto, L.; Mangiavacchi, F.; Tidei, C.; Bagnoli, L.; Marini, F.; Gioiello, A.; Scianowski, J.; Santi, C. Selenium-Catalyzed Oxacyclization of Alkenoic Acids and Alkenols. Asian J. Org. Chem. 2017, 6, 988–992. [Google Scholar] [CrossRef]
- Tanini, D.; Dalia, C.; Capperucci, A. The polyhedral nature of selenium-catalysed reactions: Se(iv) species instead of Se(vi) species make the difference in the on water selenium-mediated oxidation of arylamines. Green Chem. 2021, 23, 5680–5686. [Google Scholar] [CrossRef]
- Sands, K.N.; Mendoza Rengifo, E.; George, G.N.; Pickering, I.J.; Gelfand, B.S.; Back, T.G. The Unexpected Role of Se VI Species in Epoxidations with Benzeneseleninic Acid and Hydrogen Peroxide. Angew. Chem. Int. Ed. 2020, 59, 4283–4287. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yang, T.; Lin, Z.; Jiang, X. Selenium-catalyzed intramolecular atom- and redox-economical transformation of o -nitrotoluenes into anthranilic acids. Green Chem. 2021, 23, 2986–2991. [Google Scholar] [CrossRef]
- Santi, C.; Jacob, R.; Monti, B.; Bagnoli, L.; Sancineto, L.; Lenardão, E. Water and Aqueous Mixtures as Convenient Alternative Media for Organoselenium Chemistry. Molecules 2016, 21, 1482. [Google Scholar] [CrossRef] [Green Version]
- Mangiavacchi, F.; Coelho Dias, I.F.; Di Lorenzo, I.; Grzes, P.; Palomba, M.; Rosati, O.; Bagnoli, L.; Marini, F.; Santi, C.; Lenardao, E.J.; et al. Sweet Selenium: Synthesis and Properties of Selenium-Containing Sugars and Derivatives. Pharmaceuticals 2020, 13, 211. [Google Scholar] [CrossRef]
- Rampon, D.S.; Rodembusch, F.S.; Gonçalves, P.F.B.; Lourega, R.V.; Merlo, A.A.; Schneider, P.H. An evaluation of the chalcogen atom effect on the mesomorphic and electronic properties in a new homologous series of chalcogeno esters. J. Braz. Chem. Soc. 2010, 21, 2100–2107. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Álvarez, E.; Plano, D.; Font, M.; Calvo, A.; Prior, C.; Jacob, C.; Palop, J.A.; Sanmartín, C. Synthesis and antiproliferative activity of novel selenoester derivatives. Eur. J. Med. Chem. 2014, 73, 153–166. [Google Scholar] [CrossRef]
- Spengler; Kincses; Mosolygó; Marć; Nové; Gajdács; Sanmartín; McNeil; Blair; Domínguez-Álvarez Antiviral, Antimicrobial and Antibiofilm Activity of Selenoesters and Selenoanhydrides. Molecules 2019, 24, 4264. [CrossRef] [PubMed] [Green Version]
- Makriyannis, A.; Guenther, W.H.H.; Mautner, H.G. Selenol esters as specific reagents of the acylation of thiol groups. J. Am. Chem. Soc. 1973, 95, 8403–8406. [Google Scholar] [CrossRef]
- Reinerth, W.A.; Tour, J.M. Protecting Groups for Organoselenium Compounds. J. Org. Chem. 1998, 63, 2397–2400. [Google Scholar] [CrossRef]
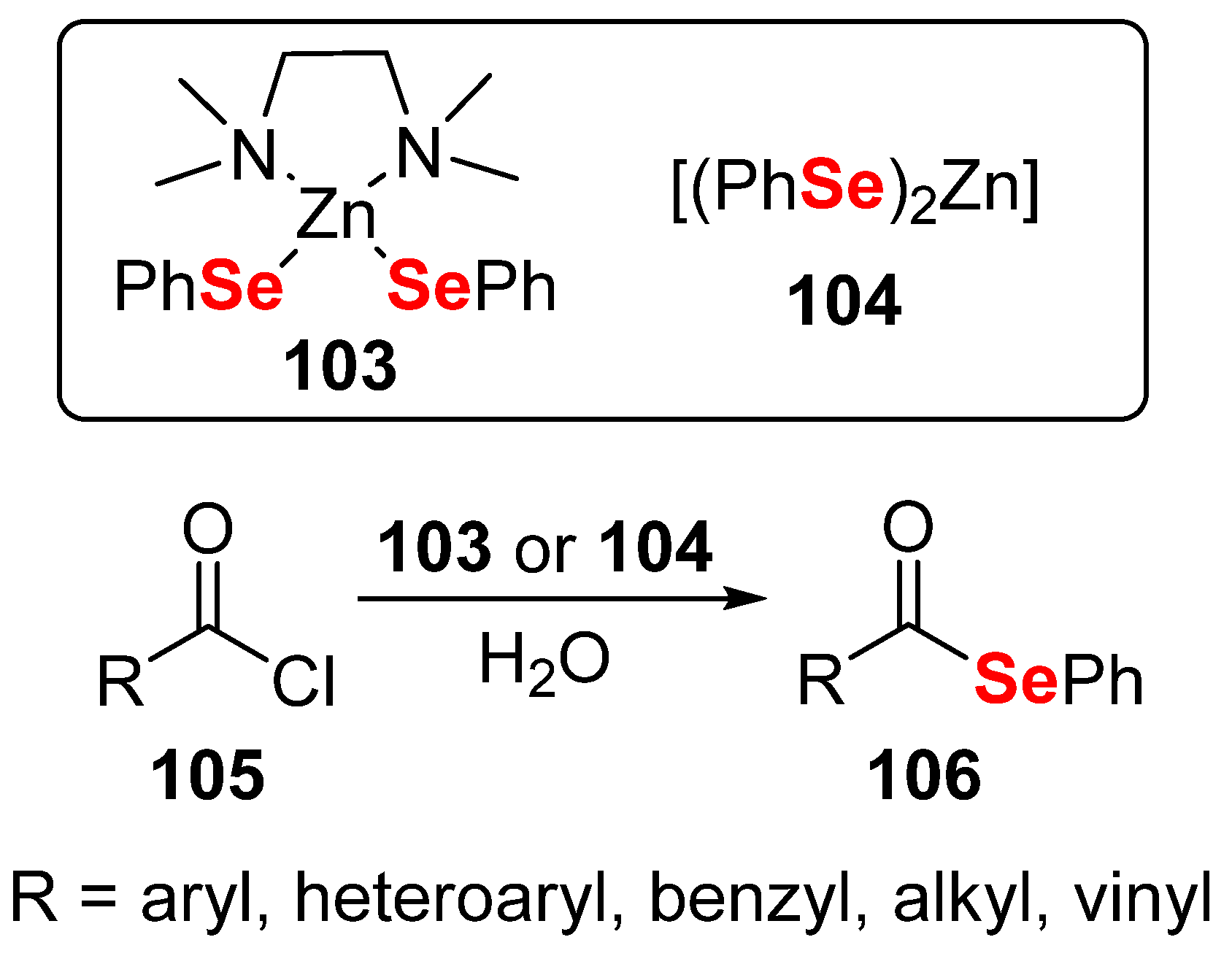
- Sancineto, L.; Vargas, J.P.; Monti, B.; Arca, M.; Lippolis, V.; Perin, G.; Lenardao, E.J.; Santi, C. Atom efficient preparation of zinc selenates for the synthesis of selenol esters under “On Water” conditions. Molecules 2017, 22, 953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monti, B.; Santi, C.; Bagnoli, L.; Marini, F.; Sancineto, L. Zinc Chalcogenolates As Green Reagents. Curr. Green Chem. 2016, 3, 68–75. [Google Scholar] [CrossRef]
- Santi, C.; Battistelli, B.; Testaferri, L.; Tiecco, M. On water preparation of phenylselenoesters. Green Chem. 2012, 14, 1277–1280. [Google Scholar] [CrossRef]
- Sancineto, L.; Tidei, C.; Bagnoli, L.; Marini, F.; Lippolis, V.; Arca, M.; Lenardão, E.J.; Santi, C. Synthesis of Thiol Esters Using PhSZnBr as Sulfenylating Agent: A DFT-Guided Optimization of Reaction Conditions. Eur. J. Org. Chem. 2016, 2016, 2999–3005. [Google Scholar] [CrossRef]
- Bellino, G.; Scisciani, M.; Vargas, J.P.; Sancineto, L.; Bagnoli, L.; Marini, F.; Lüdtke, D.S.; Lenardao, E.J.; Santi, C. Reaction of Acyl Chlorides with in Situ Formed Zinc Selenolates: Synthesis of Selenoesters versus Ring-Opening Reaction of Tetrahydrofuran. J. Chem. 2016, 2016, 2849140. [Google Scholar] [CrossRef]
- Palomba, M.; Bagnoli, L.; Marini, F.; Santi, C.; Sancineto, L. Recent advances in the chemistry of vinylchalcogenides. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 235–244. [Google Scholar] [CrossRef]
- Perin, G.; Lenardão, E.J.; Jacob, R.G.; Panatieri, R.B. Synthesis of Vinyl Selenides. Chem. Rev. 2009, 109, 1277–1301. [Google Scholar] [CrossRef] [PubMed]
- Tidei, C.; Sancineto, L.; Bagnoli, L.; Battistelli, B.; Marini, F.; Santi, C. A Recyclable Biphasic System for Stereoselective and Easily Handled Hydrochalcogenations. Eur. J. Org. Chem. 2014, 2014, 5968–5975. [Google Scholar] [CrossRef]
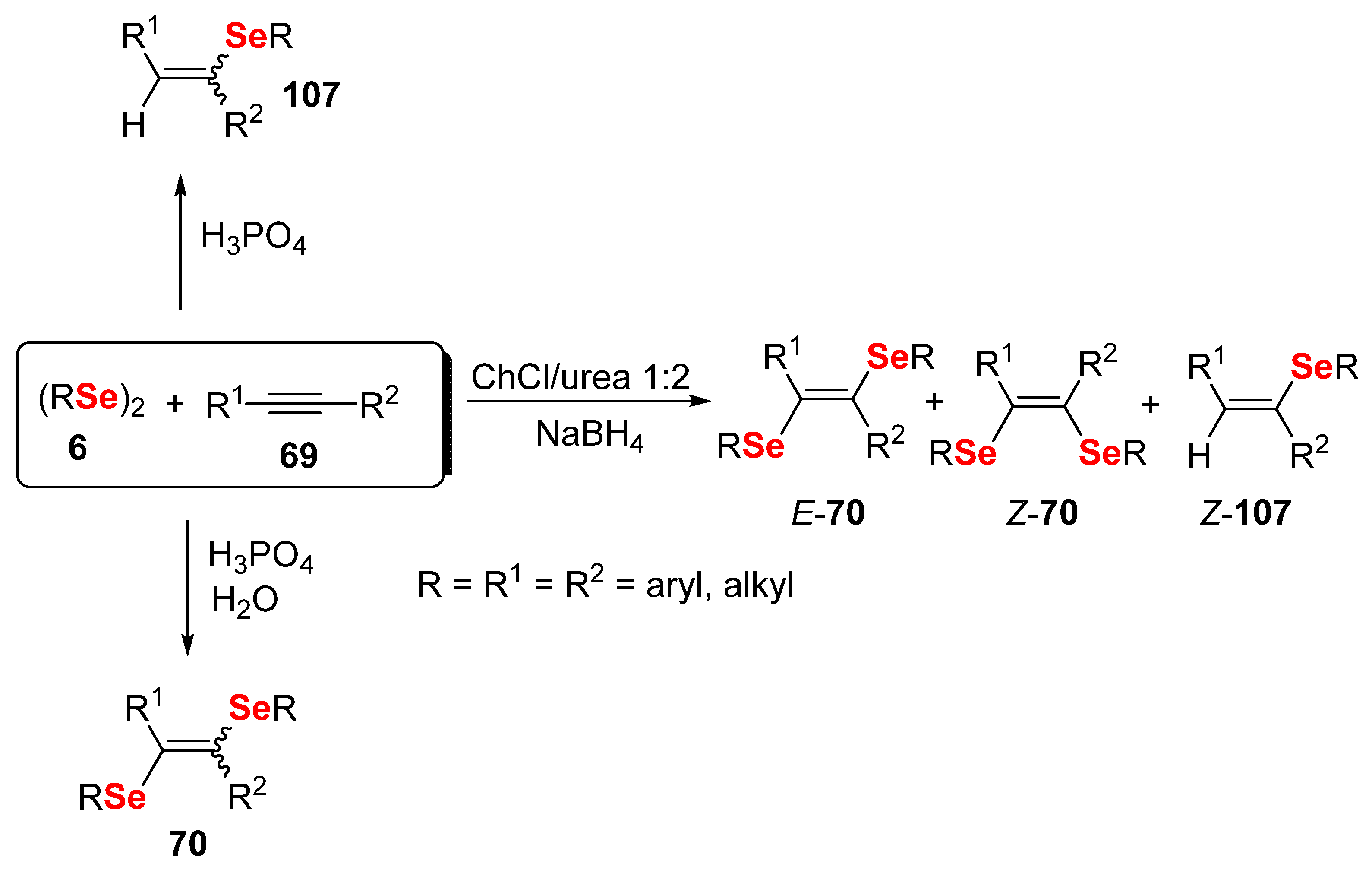
- Lopes, E.F.; Gonçalves, L.C.; Vinueza, J.C.G.; Jacob, R.G.; Perin, G.; Santi, C.; Lenardão, E.J. DES as a green solvent to prepare 1,2-bis-organylseleno alkenes. Scope and limitations. Tetrahedron Lett. 2015, 56, 6890–6895. [Google Scholar] [CrossRef]
- Luz, E.Q.; Lopes, E.F.; Ricordi, V.G.; Santi, C.; Barcellos, T.; Lenardão, E.J.; Perin, G.; Alves, D. Water-Dependent Selective Synthesis of Mono- or Bis-Selanyl Alkenes from Terminal Alkynes. ChemistrySelect 2016, 1, 4289–4294. [Google Scholar] [CrossRef]
- Perin, G.; Barcellos, A.M.; Luz, E.Q.; Borges, E.L.; Jacob, R.G.; Lenardão, E.J.; Sancineto, L.; Santi, C. Green hydroselenation of aryl alkynes: Divinyl selenides as a precursor of resveratrol. Molecules 2017, 22, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomba, M.; Franco Coelho Dias, I.; Rosati, O.; Marini, F. Modern Synthetic Strategies with Organoselenium Reagents: A Focus on Vinyl Selenones. Molecules 2021, 26, 3148. [Google Scholar] [CrossRef]
- Palomba, M.; Trappetti, F.; Bagnoli, L.; Santi, C.; Marini, F. Oxone-Mediated Oxidation of Vinyl Selenides in Water. Eur. J. Org. Chem. 2018, 2018, 3914–3919. [Google Scholar] [CrossRef]
- Palomba, M.; Rossi, L.; Sancineto, L.; Tramontano, E.; Corona, A.; Bagnoli, L.; Santi, C.; Pannecouque, C.; Tabarrini, O.; Marini, F. A new vinyl selenone-based domino approach to spirocyclopropyl oxindoles endowed with anti-HIV RT activity. Org. Biomol. Chem. 2016, 14, 2015–2024. [Google Scholar] [CrossRef]
- Palomba, M.; Scarcella, E.; Sancineto, L.; Bagnoli, L.; Santi, C.; Marini, F. Synthesis of Spirooxindole Oxetanes Through a Domino Reaction of 3-Hydroxyoxindoles and Phenyl Vinyl Selenone. Eur. J. Org. Chem. 2019, 2019, 5396–5401. [Google Scholar] [CrossRef]
- Penteado, F.; Peglow, T.J.; Silva, M.S.; Perin, G.; Lenardão, E.J. Greening the Synthesis of Selenium-Containing Heterocycles: Recent Efforts and Advances. Curr. Opin. Green Sustain. Chem. 2020, 26, 100372. [Google Scholar] [CrossRef]
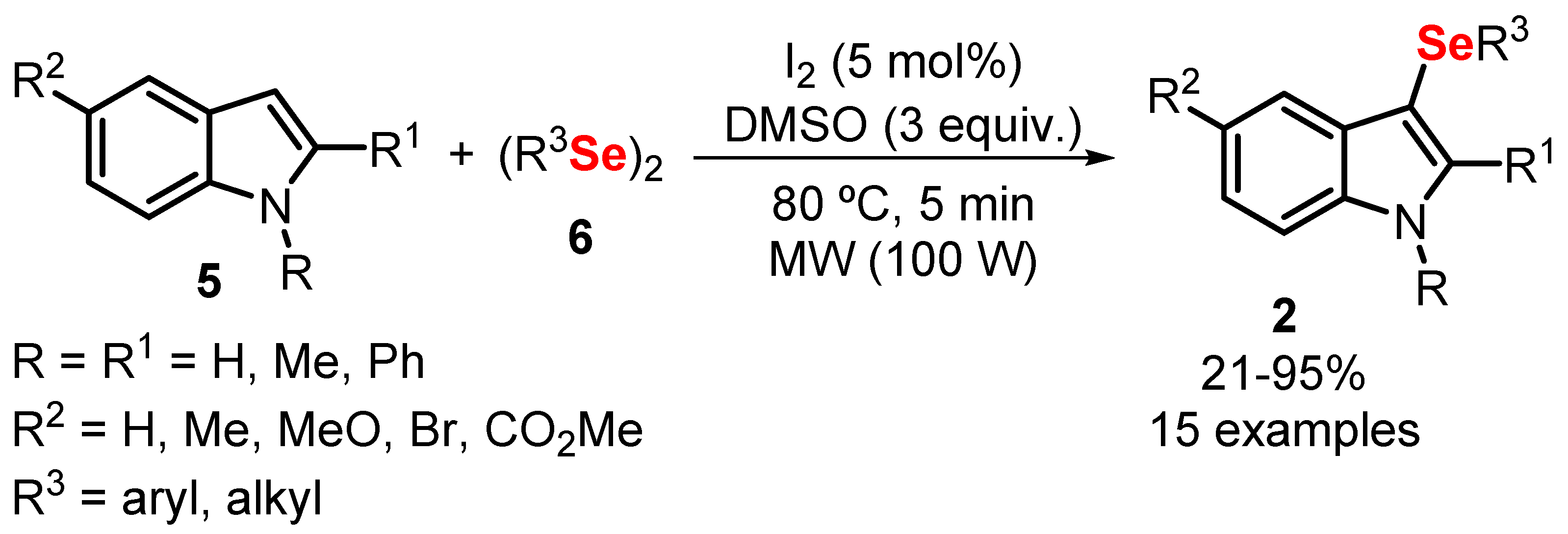

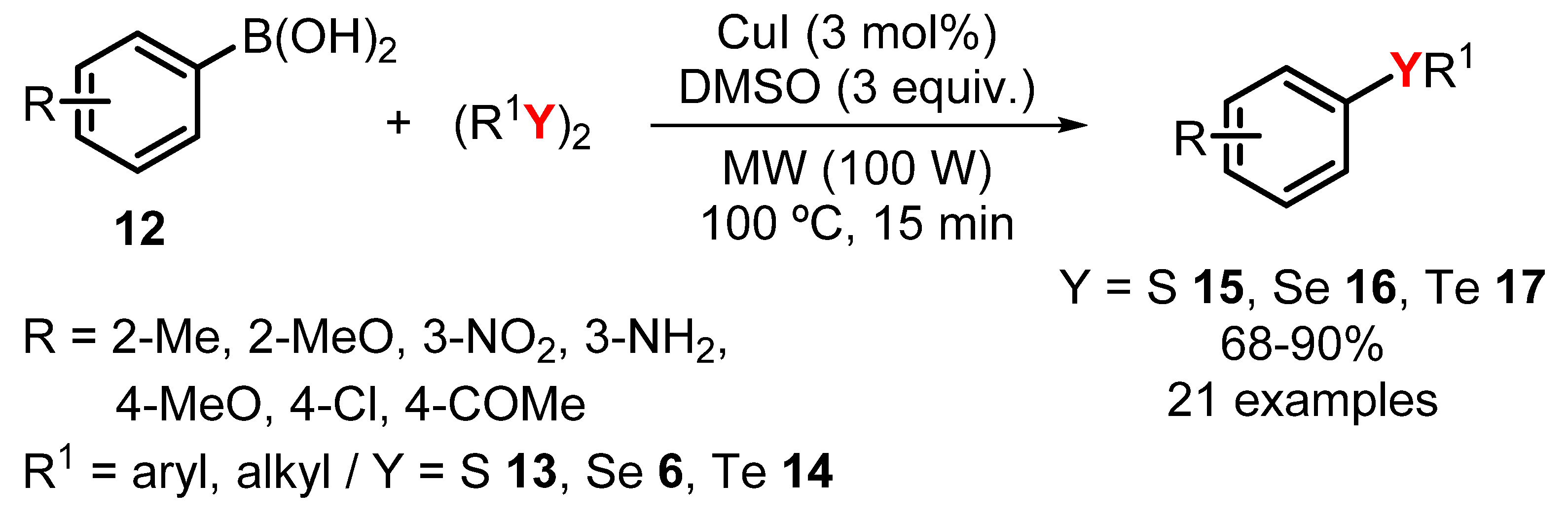
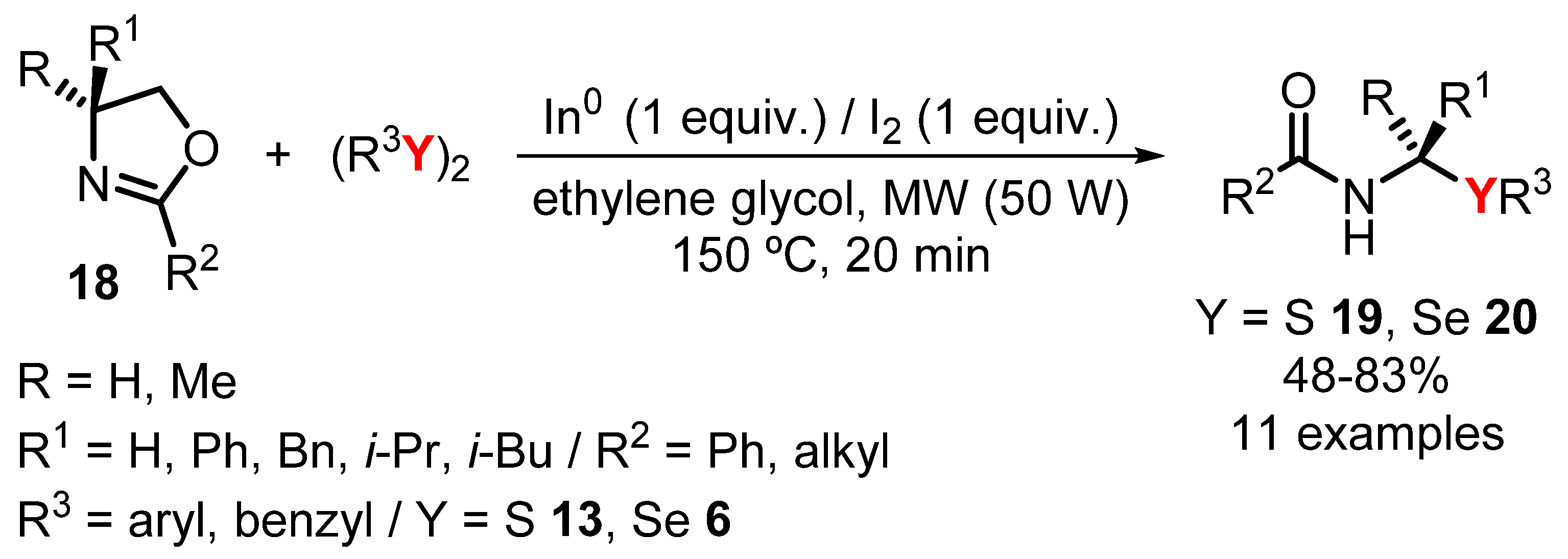

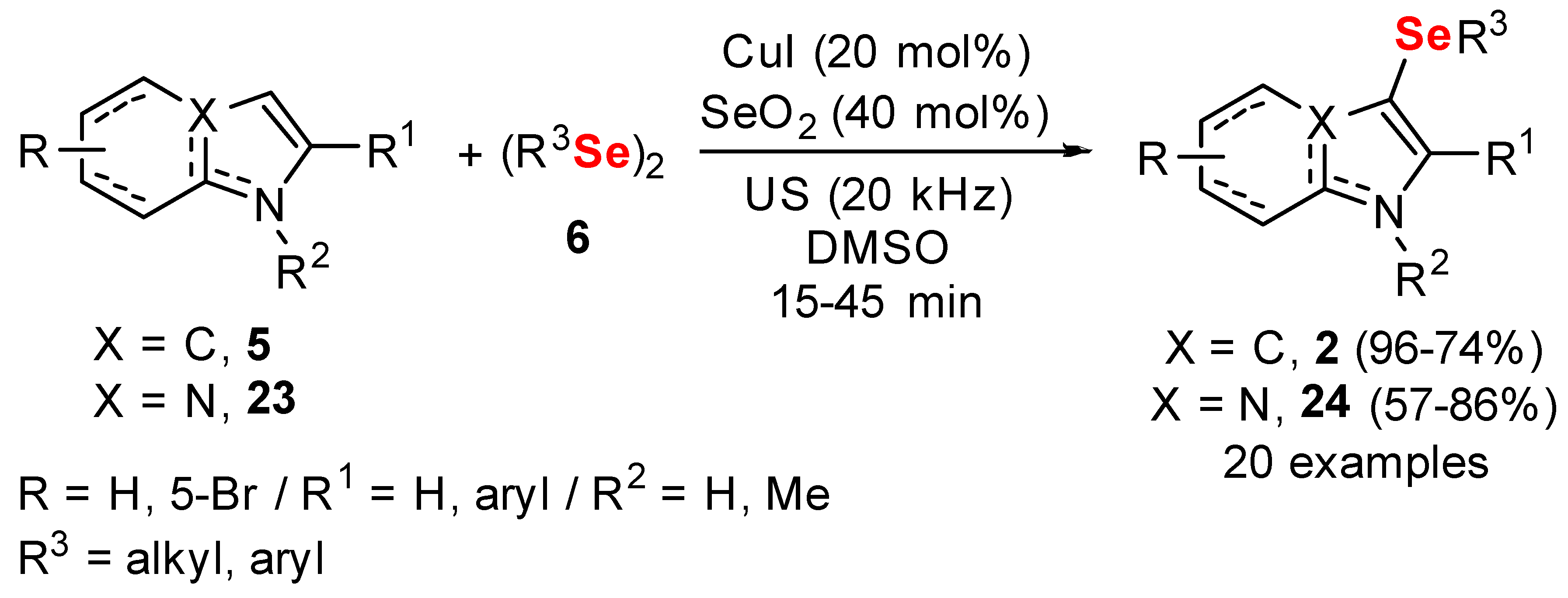
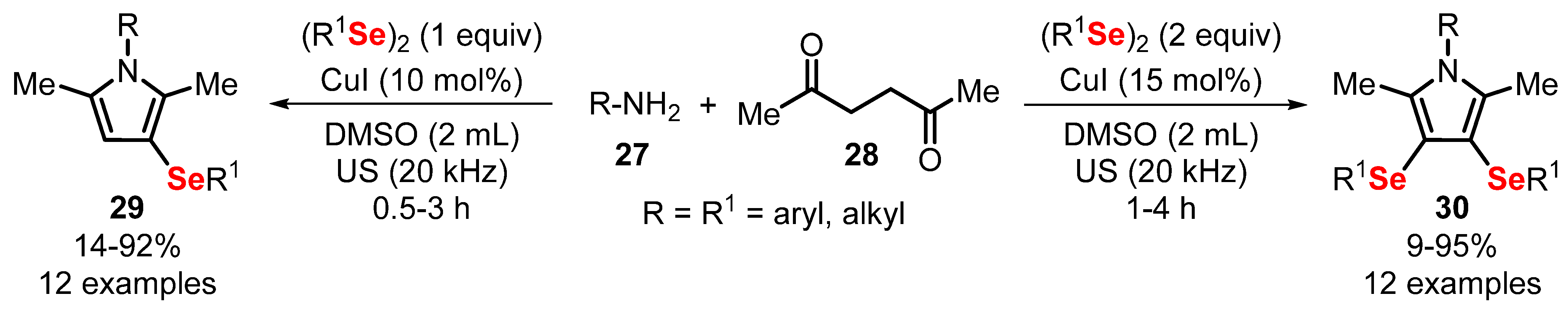

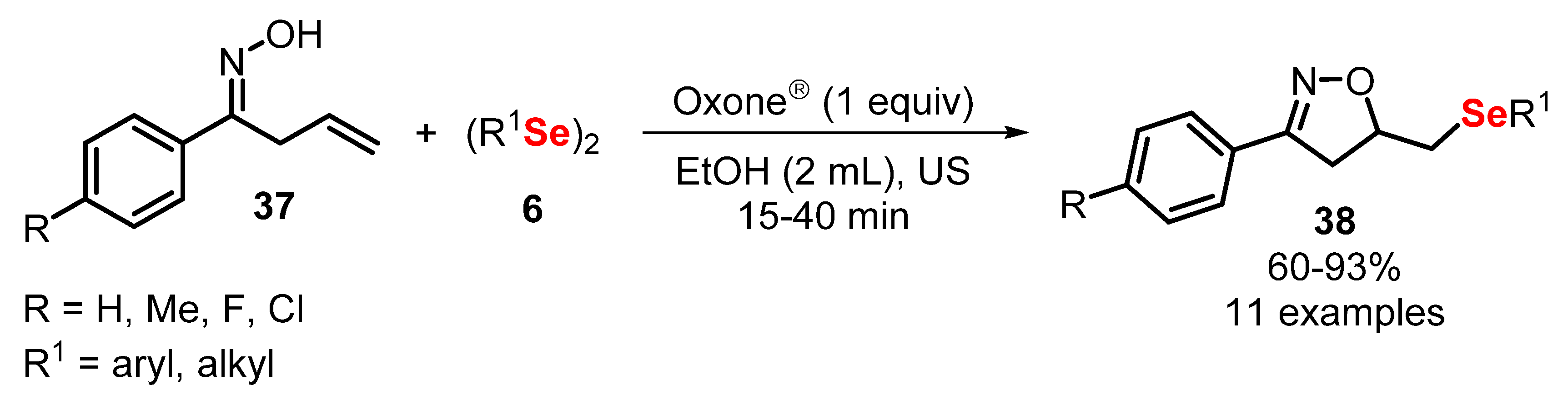










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azeredo, J.B.; Penteado, F.; Nascimento, V.; Sancineto, L.; Braga, A.L.; Lenardao, E.J.; Santi, C. “Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry. Molecules 2022, 27, 1597. https://doi.org/10.3390/molecules27051597
Azeredo JB, Penteado F, Nascimento V, Sancineto L, Braga AL, Lenardao EJ, Santi C. “Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry. Molecules. 2022; 27(5):1597. https://doi.org/10.3390/molecules27051597
Chicago/Turabian StyleAzeredo, Juliano B., Filipe Penteado, Vanessa Nascimento, Luca Sancineto, Antonio L. Braga, Eder João Lenardao, and Claudio Santi. 2022. "“Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry" Molecules 27, no. 5: 1597. https://doi.org/10.3390/molecules27051597
APA StyleAzeredo, J. B., Penteado, F., Nascimento, V., Sancineto, L., Braga, A. L., Lenardao, E. J., & Santi, C. (2022). “Green Is the Color”: An Update on Ecofriendly Aspects of Organoselenium Chemistry. Molecules, 27(5), 1597. https://doi.org/10.3390/molecules27051597










