In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents
Abstract
1. Introduction
2. Results
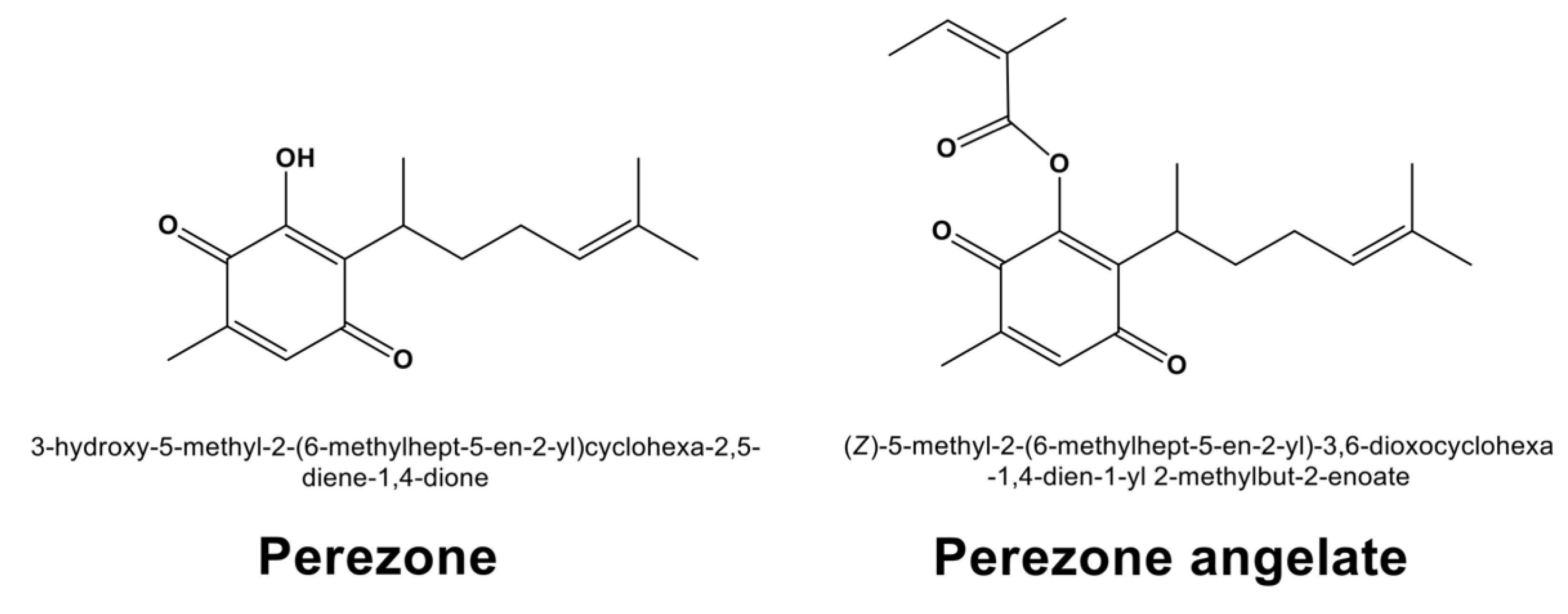
2.1. Extraction of Perezone and Perezone Angelate
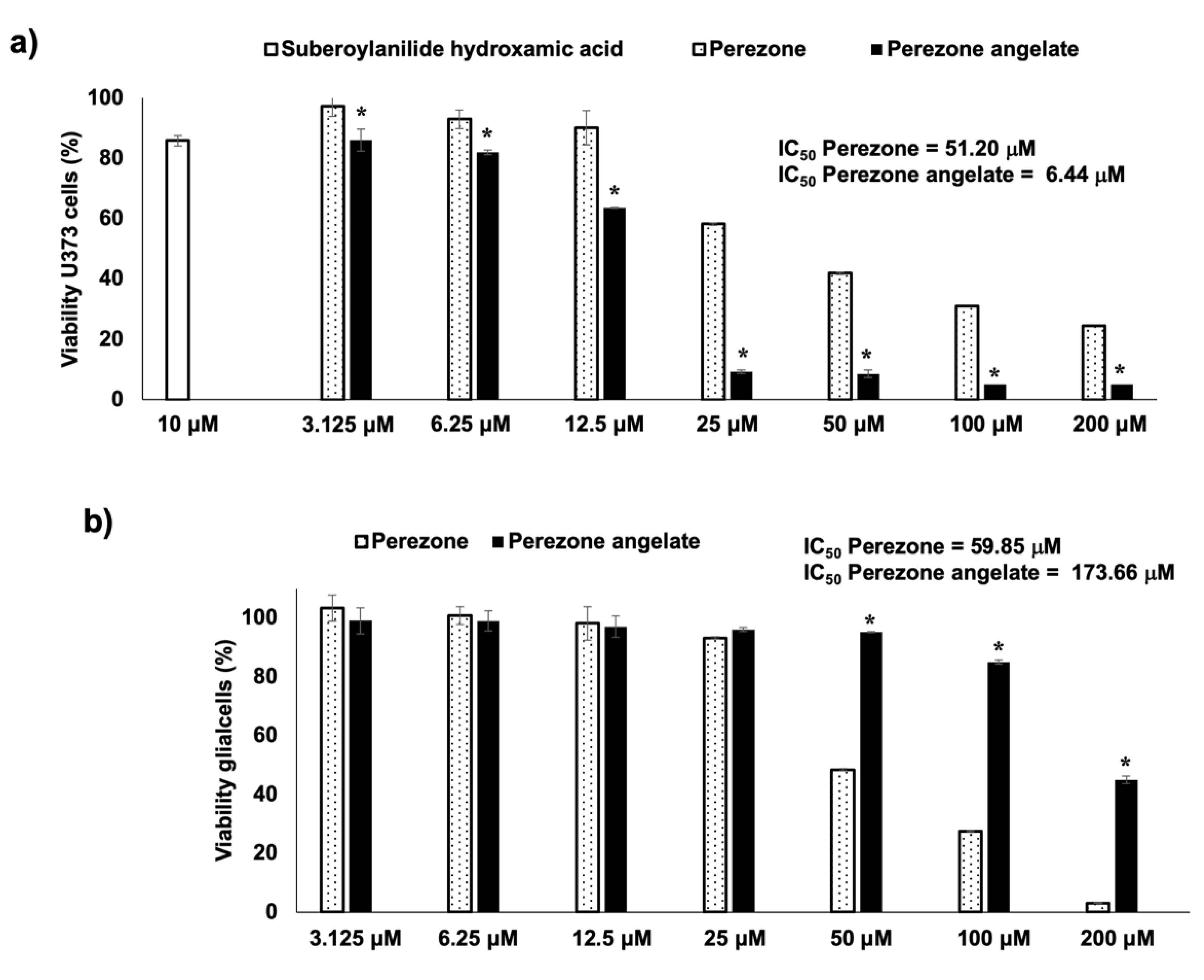
2.2. Perezone Angelate Showed Greater Cytotoxic Activity in U373 Cells than Normal Rat Glial Cells
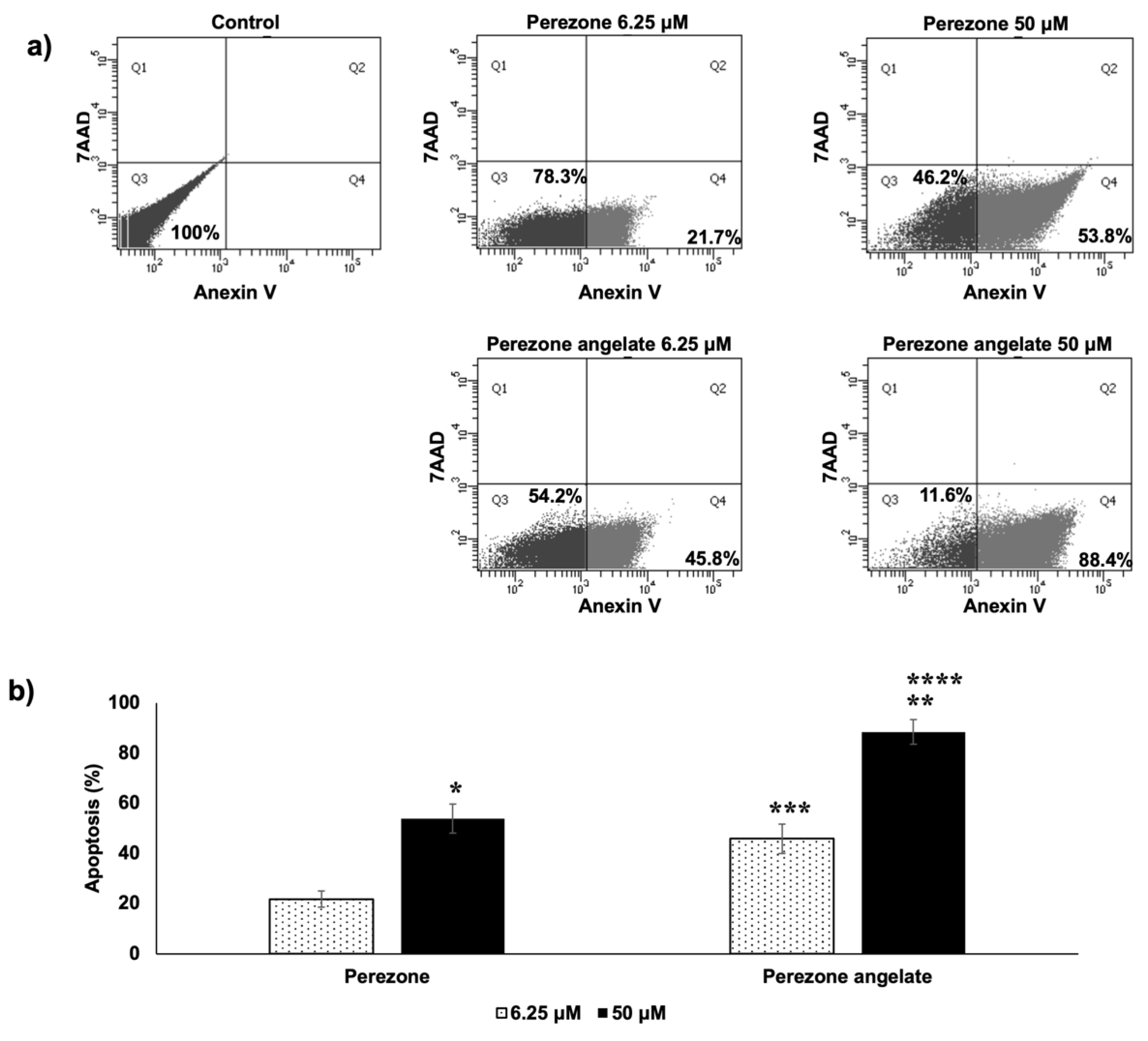
2.3. Perezone Angelate Induces Apoptosis in U373 Cells
2.4. Perezone and Perezone Angelate Inhibit U373 Cell Migration
2.5. Perezone Angelate Is a PARP-1 Inhibitor
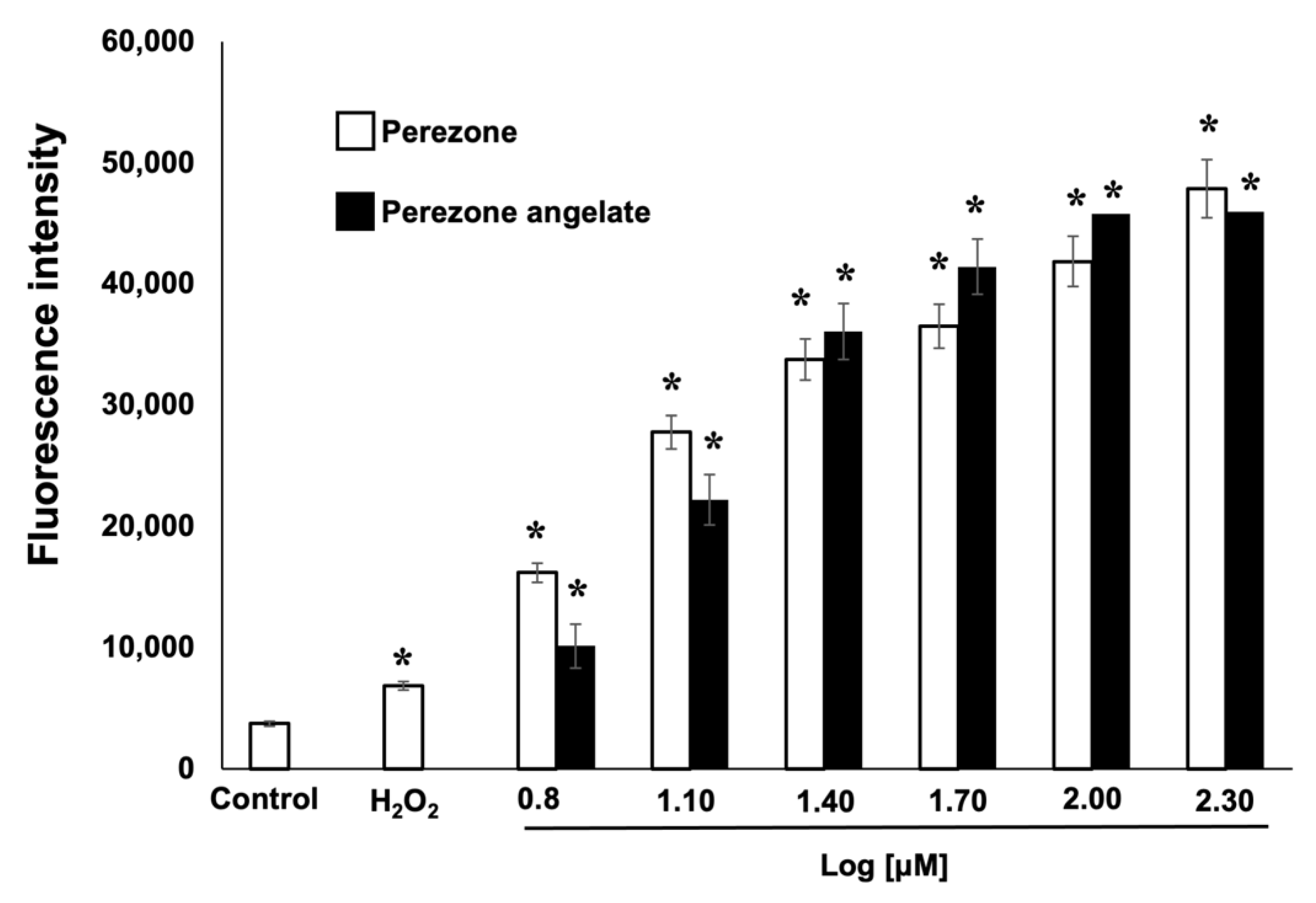
2.6. Perezone and Perezone Angelate Increases Reactive Oxygen Species (ROS) Production in U373 Cells
2.7. Perezone Angelate Interacts with the PARP-1 Active Site Revealed by Docking Studies
2.8. Perezone Angelate Showed a High Probability to Permeate Cells and Cross the BBB According to ADMET Predictions
3. Discussion
4. Materials and Methods
4.1. General
4.2. Anti-Proliferative Evaluation
4.3. Apoptosis Determination
4.4. Scratch Wound Assay
4.5. PARP-1 Inhibition Activity
4.6. ROS Production
4.7. Statistical Analysis
4.8. Docking Studies
4.9. ADMET Predictions Details
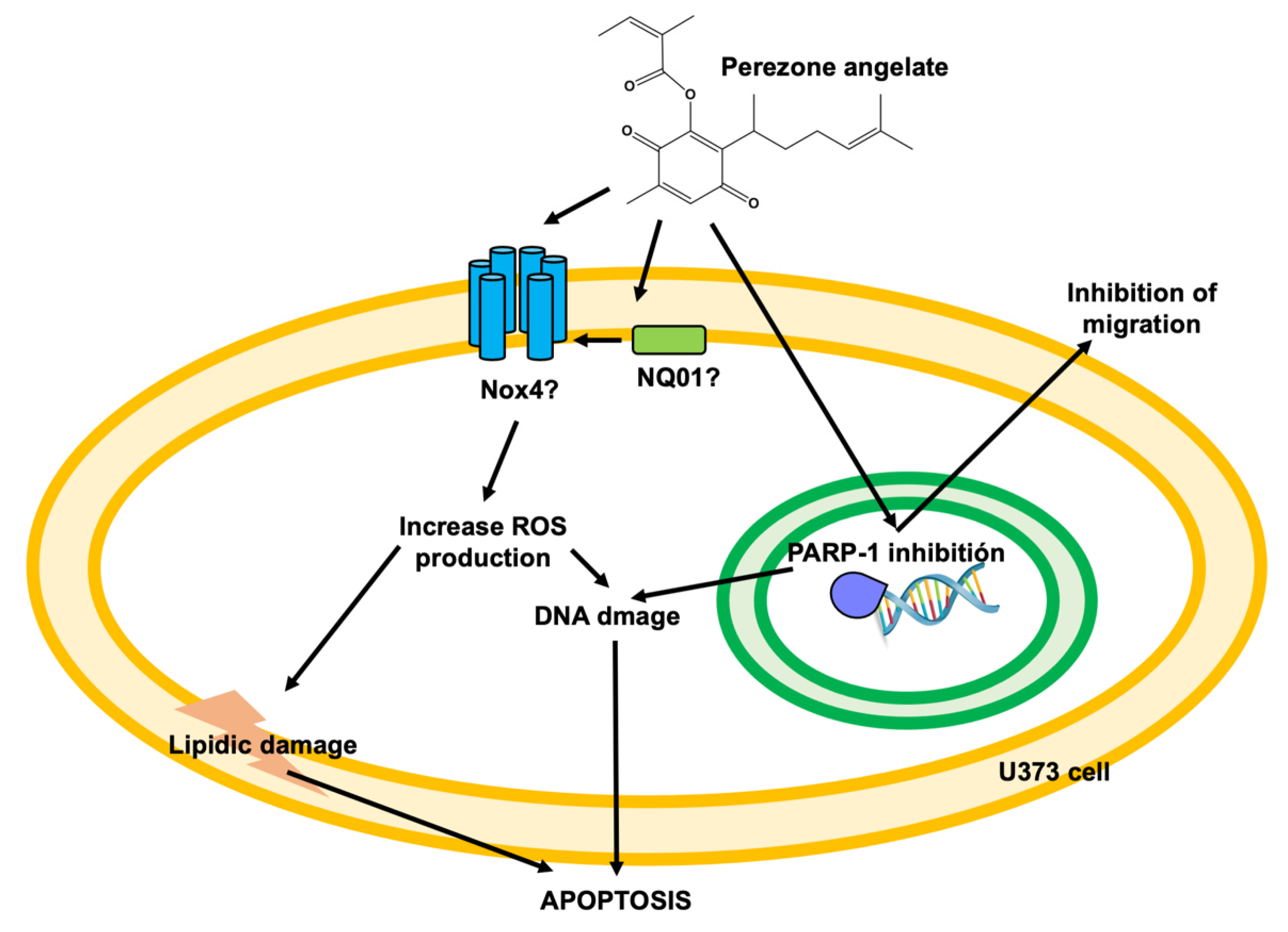
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodbelt, A.; Greenberg, D.; Winters, T.; Williams, M.; Vernon, S.; Collins, V.P.; Natl, N. Glioblastoma in England: 2007–2011. Eur. J. Cancer 2015, 51, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, C.J.; Altschuler, G.; Jeong, J.; Strømme, K.K.; Stangeland, B.; Murrell, W.; Grasmo-Wendler, U.H.; Myklebost, O.; Helseth, E.; Vik-Mo, E.O.; et al. Comparison of glioma stem cells to neural stem cells from the adult human brain identifies dysregulated Wnt- signaling and a fingerprint associated with clinical outcome. Exp. Cell Res. 2013, 319, 2230–2243. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.C. Temozolomide: Therapeutic limitations in the treatment of adult high-grade gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. [Google Scholar] [CrossRef]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Paw, I.; Carpenter, R.C.; Watabe, K.; Debinski, W.; Lo, H.W. Mechanisms regulating glioma invasion. Cancer Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Use of nanoparticles for drug delivery in glioblastoma multiforme. Expert Rev. Neurother 2007, 7, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Batash, R.; Asna, N.; Schaffer, P.; Francis, N.; Schaffer, M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr. Med. Chem. 2017, 24, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef] [PubMed]
- Galia, A.; Calogero, A.E.; Condorelli, R.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 2012, 56, e9. [Google Scholar] [CrossRef] [PubMed]
- Salinas, B.; Irwin, C.P.; Kossatz, S.; Bolaender, A.; Chiosis, G.; Pillarsetty, N.; Weber, W.A.; Reiner, T. Radioiodinated PARP1 tracers for glioblastoma imaging. EJNMMI Res. 2015, 5, 123. [Google Scholar] [CrossRef]
- Murnyák, B.; Kouhsari, M.C.; Hershkovitch, R.; Kálmán, B.; Marko-Varga, G.; Klekner, Á.; Hortobágyi, T. PARP1 expression and its correlation with survival is tumour molecular subtype dependent in glioblastoma. Oncotarget 2017, 11, 46348–46362. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Mendoza Sánchez, P.I.; Macias Perez, M.E.; Rosales Cruz, E.; Mera Jiménez, E.; Nicolás Vázquez, M.I.; Ruvalcaba, R.M. In vitro and computational studies showed that perezone inhibits PARP-1 and increase ROS levels in K562 cells. Arch. Biochem. Biophys. 2019, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Burgueño-Tapia, E.; Castillo, L.; González- Coloma, A.; Joseph-Nathan, P. Antifeedant and phyto toxic activity of the sesquiterpene p-benzoquinone perezone and some of its derivatives. J. Chem. Ecol. 2008, 34, 766. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.Y.; Jeong, N.Y.; Lee, S.Y.; Yoon, Y.G.; Choi, Y.H.; Yan, C.; Chu, I.S.; Koh, H.; Park, H.T.; et al. Expression of αB-crystallin overrides the anti-apoptotic activity of XIAP. Neuro Oncol. 2012, 14, 1332–1345. [Google Scholar] [CrossRef][Green Version]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Shergalis, A.; Bankhead, A.; Luesakul, U.; Muangsin, N.; Neamati, N. Current challenges and opportunities in treating glioblastoma. Pharmacol. Rev. 2018, 70, 412–445. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, S.; Sun, Q.; Wang, N.; Li, D.; Miao, S.; Gao, C.; Chen, Y.; Tan, C.; Jiang, Y. Olaparib hydroxamic acid derivatives as dual PARP and HDAC inhibitors for cancer therapy. Bioorg. Med. Chem. 2017, 25, 4100–4109. [Google Scholar] [CrossRef]
- Malyuchenko, N.V.; Kotova, E.Y.; Kulaeva, O.I.; Kirpichnikov, M.P.; Studitskiy, V.M. PARP1 Inhibitors: Antitumor drug design. Acta Naturae 2015, 7, 27–37. [Google Scholar] [CrossRef]
- Baptista, S.J.; Silva, M.M.; Moroni, E.; Meli, M.; Colombo, G.; Dinis, T.C.; Salvador, J.A. Novel PARP-1 inhibitor scaffolds disclosed by a dynamic structure-based pharmacophore approach. PLoS ONE 2017, 12, e0170846. [Google Scholar] [CrossRef]
- Wills, P.; Warnery, A.; Phung-Ba, V.; Legrain, S.; Scherman, D. High lipophilicity decreases drug transport across intestinal epithelial cells. J. Pharmacol. Exp. Ther. 1994, 269, 654–658. [Google Scholar]
- Sakaeda, T.; Okamura, N.; Nagata, S.; Yagami, T.; Horinouchi, M.; Okumura, K.; Yamashita, F.; Hashida, M. Molecular and pharmacokinetic properties of 222 commercially available oral drugs in humans. Biol. Pharm. Bull. 2001, 24, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Chen, C.; Yang, J. Predictive model of blood-brain barrier penetration of organic compounds. Acta Pharmacol. Sin. 2005, 26, 500–512. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharm. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The natural flavonoid galangin elicits apoptosis, pyroptosis, and autophagy in glioblastoma. Front. Oncol. 2019, 9, 942. [Google Scholar] [CrossRef]
- Mallick, S.; Gandhi, A.K.; Rath, G.K. Therapeutic approach beyond conventional temozolomide for newly diagnosed glioblastoma: Review of the present evidence and future direction. Indian J. Med. Paediatr. Oncol. 2015, 36, 229–237. [Google Scholar] [CrossRef]
- Venere, M.; Hamerlik, P.; Wu, Q.; Rasmussen, R.D.; Song, L.A.; Vasanji, A.; Tenley, N.; Flavahan, W.A.; Hjelmeland, A.B.; Bartek, J.; et al. Therapeutic targeting of constitutive PARP activation compromises stem cell phenotype and survival of glioblastoma-initiating cells. Cell Death Differ. 2014, 21, 258–269. [Google Scholar] [CrossRef]
- Bartkova, J.; Hamerlik, P.; Stockhausen, M.T.; Ehrmann, J.; Hlobilkova, A.; Laursen, H.; Kalita, O.; Kolar, Z.; Poulsen, H.S.; Broholm, H.; et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene 2010, 29, 5095–5102. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1-targeted radiotherapy in mouse models of glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef]
- Khaw, A.K.; Sameni, S.; Venkatesan, S.; Kalthur, G.; Hande, M.P. Plumbagin alters telomere dynamics, induces DNA damage and cell death in human brain tumour cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2015, 793, 86–95. [Google Scholar] [CrossRef]
- Carr, C.; Ng, J.; Wigmore, T. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. Care 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Ray, A.; Manjila, S.; Hdeib, A.M.; Radhakrishnan, A.; Nock, C.J.; Cohen, M.L.; Sloan, A.E. Extracranial metastasis of gliobastoma: Three illustrative cases and current review of the molecular pathology and management strategies. Mol. Clin. Oncol 2015, 3, 479–486. [Google Scholar] [CrossRef]
- Shi, M.M.; Kugelman, A.; Iwamoto, T.; Tian, L.; Forman, H.J. Quinone-induced oxidative stress elevates glutathione and induces gamma-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J. Biol. Chem. 1994, 269, 26512–26517. [Google Scholar] [CrossRef]
- Nguyen, M.V.; Lardy, B.; Rousset, F.; Hazane-Puch, F.; Zhang, L.; Trocmé, C.; Serrander, L.; Krause, K.H.; Morel, F. Quinone compounds regulate the level of ROS production by the NADPH oxidase Nox4. Biochem. Pharmacol. 2013, 85, 1644–1654. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Hong, Y.; Sengupta, S.; Hur, W.; Sim, T. Identification of Novel ROS Inducers: Quinone Derivatives Tethered to Long Hydrocarbon Chains. J. Med. Chem. 2015, 58, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torres, L.E.; Torres-Martínez, J.A.; Godínez-Victoria, M.; Omar, J.M.; Velasco-Bejarano, B. Perezone and its isomer isoperezone induce caspase-dependent and caspase-independent cell death. Phytomedicine 2010, 17, 614–620. [Google Scholar] [CrossRef]
- Dietlein, F.; Thelen, L.; Reinhardt, H.C. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends. Genet. 2014, 30, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Cavone, L.; Aldinucci, A.; Ballerini, C.; Biagioli, T.; Moroni, F.; Chiarugi, A. PARP-1 inhibition prevents CNS migration of dendritic cells during EAE, suppressing th.he encephalitogenic response and relapse severity. Mult. Scler. J. 2011, 17, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Correa-Basurto, J.; Gutiérrez, A.; Vitorica, J.; Rosales-Hernández, M.C. Asp32 and Asp228 determine the selective inhibition of BACE1 as shown by docking and molecular dynamics simulations. Eur. J. Med. Chem. 2016, 124, 1142–1154. [Google Scholar] [CrossRef]
- Davis, A.M.; Riley, R.J. Predictive ADMET studies, the challenges and the opportunities. Curr. Opin. Chem. Biol. 2004, 8, 378–386. [Google Scholar] [CrossRef]
- Orlando, R.A.; Gonzales, A.M.; Royer, R.E.; Deck, L.M.; Vander Jagt, D.L. A chemical analog of curcumin as an improved inhibitor of amyloid abeta oligomerization. PLoS ONE 2012, 7, e31869. [Google Scholar] [CrossRef] [PubMed]
- Turkez, H.; Nóbrega, F.R.D.; Ozdemir, O.; Bezerra Filho, C.D.S.M.; Almeida, R.N.; Tejera, E.; Perez-Castillo, Y.; Sousa, D.P. NFBTA: A Potent Cytotoxic Agent against Glioblastoma. Molecules 2019, 24, 2411. [Google Scholar] [CrossRef] [PubMed]
- Fann, L.Y.; Shih, J.H.; Tseng, J.H.; Huang, H.S.; Hsiao, S.H. CC12 Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Lines and Mouse Xenograft Model. Molecules 2020, 25, 1793. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, M.; Mendoza Sánchez, P.I.; Macías Perez, M.E.; Rosales Cruz, E.; Mera Jiménez, E.; Aceves-Hernández, J.M.; Nicolás-Vázquez, M.I.; Miranda Ruvalcaba, R. In vitro and computational studies of natural products related to perezone as anti-neoplastic agents. Biochimie 2020, 171–172, 158–169. [Google Scholar] [CrossRef]
- Safaee, S.; Fardi, M.; Hemmat, N.; Khosravi, N.; Derakhshani, A.; Silvestris, N.; Baradaran, B. Silencing ZEB2 Induces Apoptosis and Reduces Viability in Glioblastoma Cell Lines. Molecules 2021, 26, 901. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, 2009, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.; Pople, J. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Henre, W.; Ditchfield, R.; Pople, J. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar]
- Hariharan, P.; Pople, J. Accuracy of AH n equilibrium geometries by single determinant molecular orbital theory. Mol. Phys. 1973, 27, 209–214. [Google Scholar] [CrossRef]
- Gordon, M. The isomers of silacyclopropane. Chem. Phys. Lett. 1980, 76, 63–168. [Google Scholar] [CrossRef]
- Durán, L.A.; Hernández, M.C.; Hernández Rodríguez, M.; Mendieta, J.E.; Trujillo, J.; Correa, J. Mapping myeloperoxidase to identify its promiscuity properties using docking and molecular dynamics simulations. Curr. Pharm. Des. 2012, 19, 2204–2215. [Google Scholar] [CrossRef] [PubMed]



| Compound | MWt | Log P | Log D | HBD | HBA | TPSA | Sw |
|---|---|---|---|---|---|---|---|
| Expected Values | |||||||
| (≤450 g/mol) | (≤5) | (≤4) | (≤3) | (≤7) | (140 Å2) | (≥0.010 mg/mL) | |
| Perezone | 248.32 | 3.336 | 0.883 | 1 | 3 | 54.37 | 0.118 |
| Perezone angelate | 330.43 | 4.478 | 4.478 | 0 | 4 | 60.44 | 0.010 |
| Compound | Vd | Peff | MDCK | %Unbnd | RBP | BBB Filter | logBB a | P-gp Substrate/ Inhibitor |
|---|---|---|---|---|---|---|---|---|
| Expected Values | ||||||||
| (≤3.7 L/kg) | (≥0.5 cm/s·104) | (≥30 cm/s·107) | (>10%) | (<1) | (High/ Low) | |||
| Perezone | 0.188 | 6.445 | 399.055 | 7.67 | 0.601 | High | −1.057 | No/No |
| Perezone angelate | 1.560 | 4.547 | 1106.031 | 11.45 | 0.787 | High | 0.459 | No/Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rodríguez, M.; Mendoza Sánchez, P.I.; Martínez, J.; Macías Pérez, M.E.; Rosales Cruz, E.; Żołek, T.; Maciejewska, D.; Miranda Ruvalcaba, R.; Mera Jiménez, E.; Nicolás-Vázquez, M.I. In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents. Molecules 2022, 27, 1565. https://doi.org/10.3390/molecules27051565
Hernández-Rodríguez M, Mendoza Sánchez PI, Martínez J, Macías Pérez ME, Rosales Cruz E, Żołek T, Maciejewska D, Miranda Ruvalcaba R, Mera Jiménez E, Nicolás-Vázquez MI. In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents. Molecules. 2022; 27(5):1565. https://doi.org/10.3390/molecules27051565
Chicago/Turabian StyleHernández-Rodríguez, Maricarmen, Pablo I. Mendoza Sánchez, Joel Martínez, Martha E. Macías Pérez, Erika Rosales Cruz, Teresa Żołek, Dorota Maciejewska, René Miranda Ruvalcaba, Elvia Mera Jiménez, and María I. Nicolás-Vázquez. 2022. "In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents" Molecules 27, no. 5: 1565. https://doi.org/10.3390/molecules27051565
APA StyleHernández-Rodríguez, M., Mendoza Sánchez, P. I., Martínez, J., Macías Pérez, M. E., Rosales Cruz, E., Żołek, T., Maciejewska, D., Miranda Ruvalcaba, R., Mera Jiménez, E., & Nicolás-Vázquez, M. I. (2022). In Vitro and Computational Studies of Perezone and Perezone Angelate as Potential Anti-Glioblastoma Multiforme Agents. Molecules, 27(5), 1565. https://doi.org/10.3390/molecules27051565









