Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis
Abstract
1. Introduction
2. Results
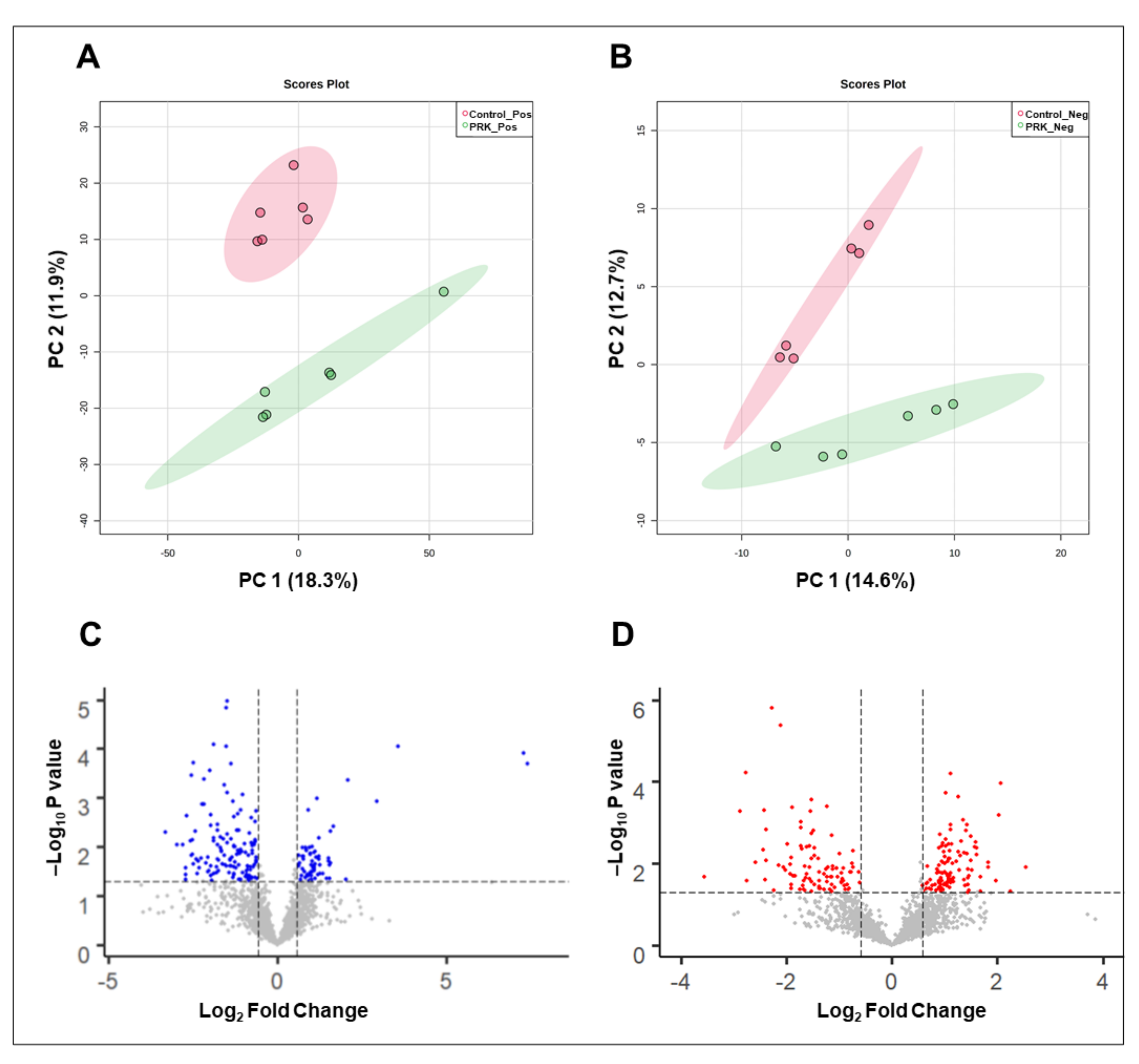
2.1. Mass Spectrometry Analysis of PRK-Treated Mtb H37Rv
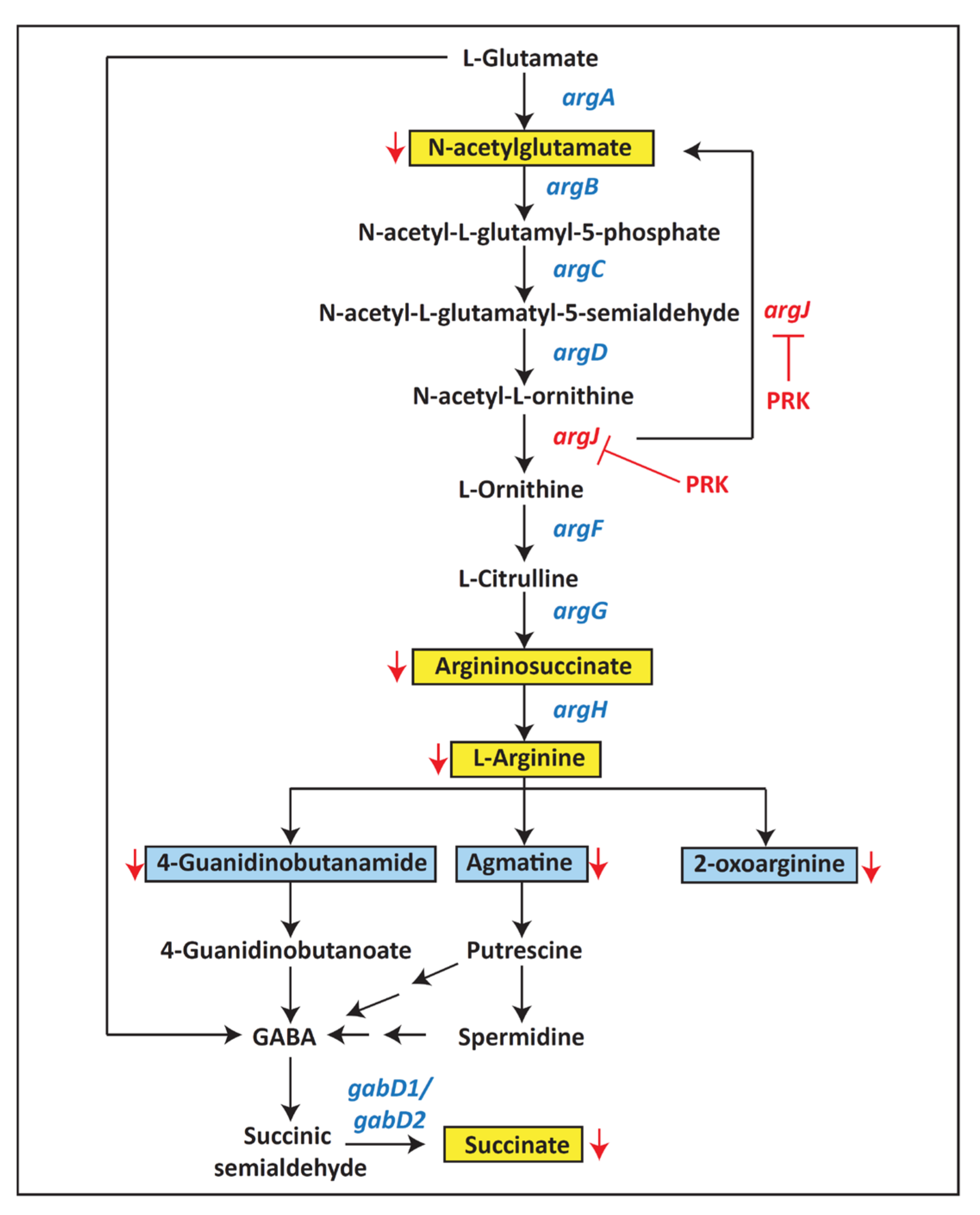
2.2. Differentially Expressed Mtb Metabolites by PRK
2.3. Pathway Analysis and Metabolite Classification
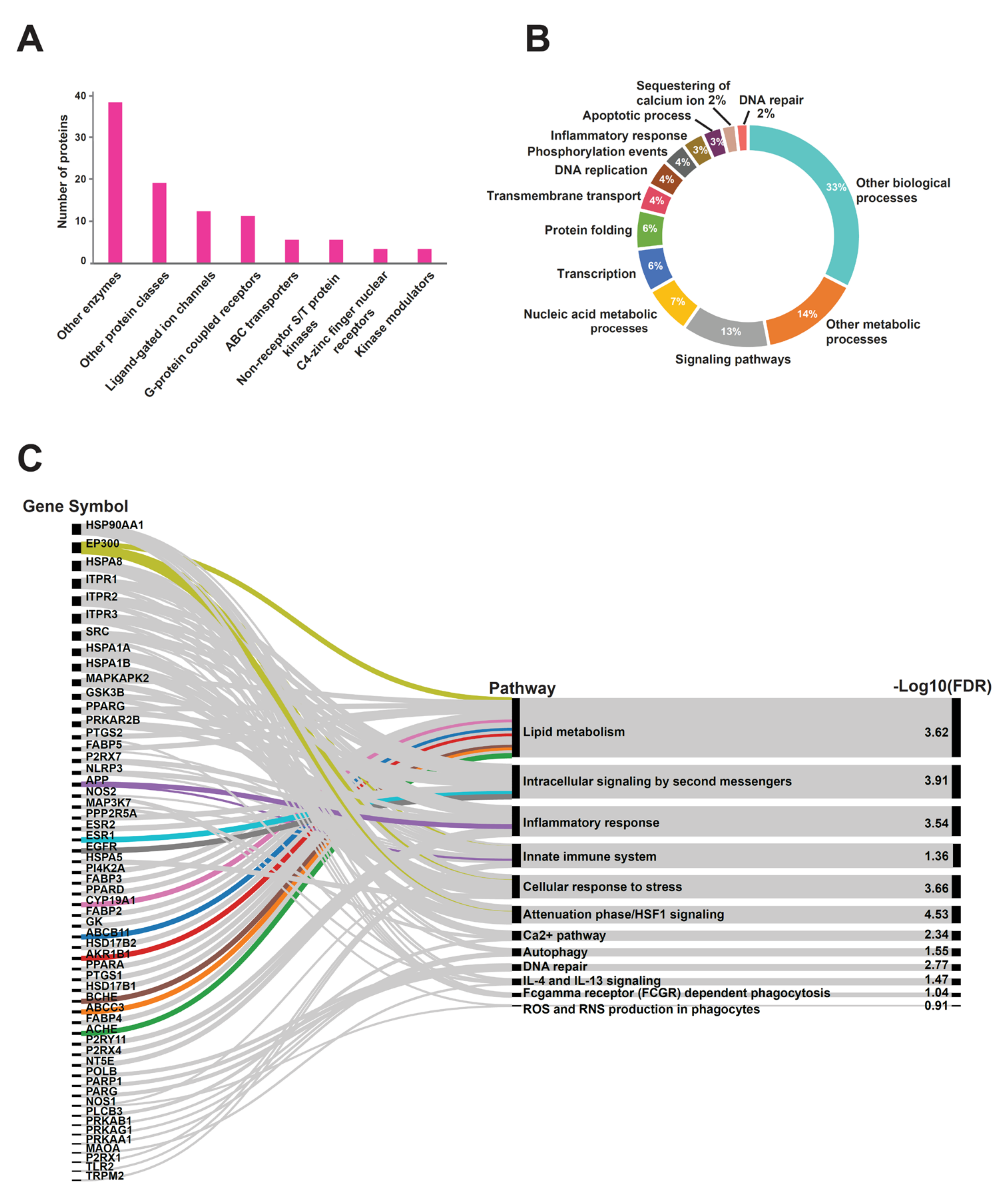
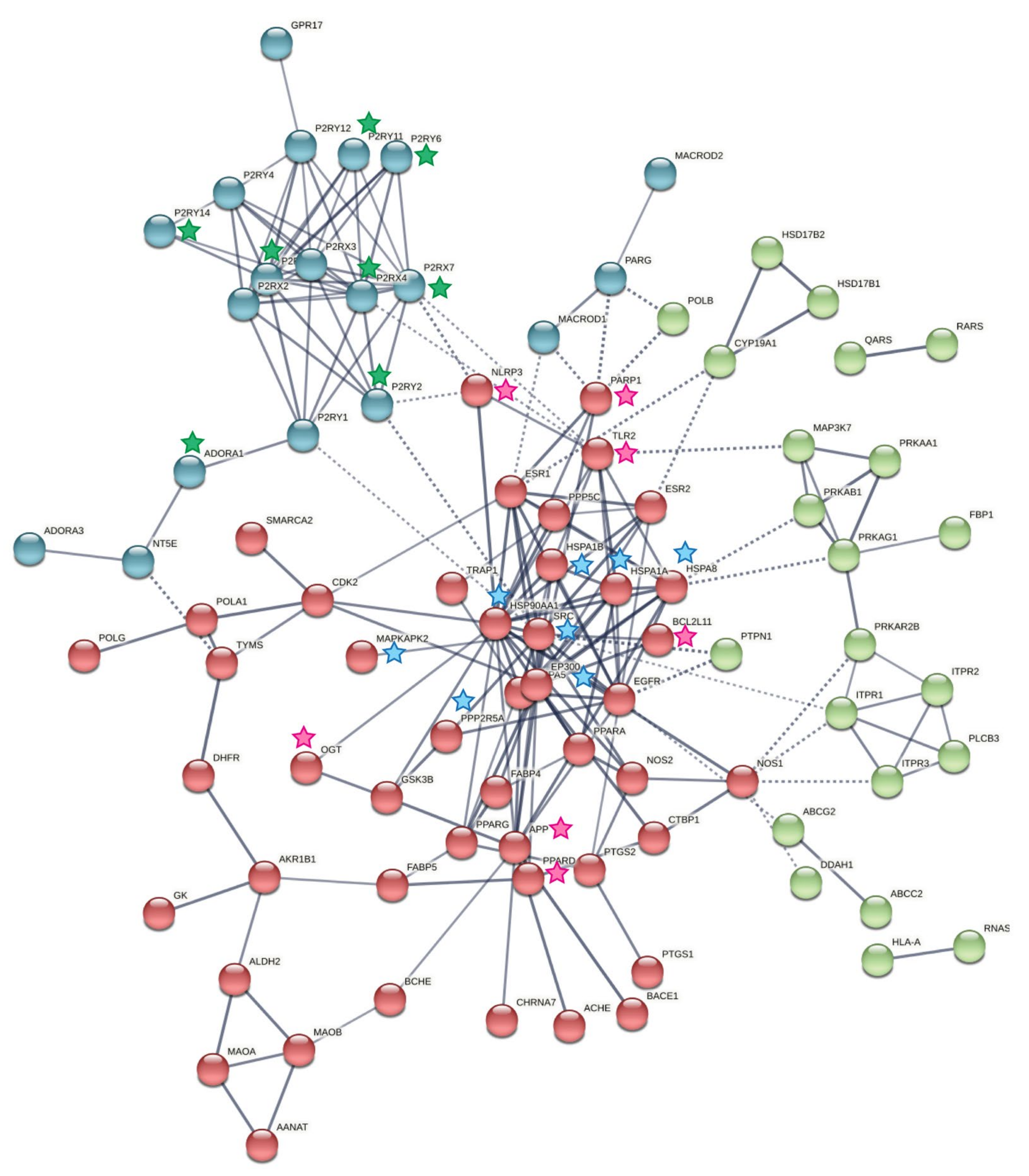
2.4. Host Protein Target Prediction against PRK-Treated Mtb-Dysregulated Metabolites
2.5. Validation of Metabolites
3. Discussion
4. Materials and Methods
4.1. Mtb H37Rv Culture and Treatment Conditions
4.2. Mtb H37Rv Cell Llysis and Metabolite Extraction
4.3. LC-MS/MS Analysis for Global Metabolomic Profiling
4.4. MZmine Data Analysis and Metabolite Assignment
4.5. Statistical and Functional Analysis
4.6. Targeted Analysis of Metabolites by Multiple Reaction Monitoring (MRM)
4.7. Data Availability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| TB | Tuberculosis |
| PRK | Pranlukast |
| SRB | Sorafenib |
| PCA | Principal component analysis |
| PLS-DA | PLS discriminant analysis |
| GO | Gene Ontology |
| ACN | Acetonitrile |
| ETC | Electron transport chain |
| LPS | Lipopolysaccharide |
| MRM | Multiple reaction monitoring |
| QC | Quality control |
| FC | Fold change |
| RT | Retention time |
| DP | Declustering potential |
| EP | Entry potential |
| CE | Collision energy |
| CXP | Cell exit potential |
References
- Chaves-Torres, N.M.; Fadul, S.; Patino, J.; Netto, E. Factors associated with unfavorable treatment outcomes in patients with rifampicin-resistant tuberculosis in Colombia 2013–2015: A retrospective cohort study. PLoS ONE 2021, 16, e0249565. [Google Scholar] [CrossRef] [PubMed]
- Rode, H.B.; Lade, D.M.; Gree, R.; Mainkar, P.S.; Chandrasekhar, S. Strategies towards the synthesis of anti-tuberculosis drugs. Org. Biomol. Chem. 2019, 17, 5428–5459. [Google Scholar] [CrossRef] [PubMed]
- Dookie, N.; Rambaran, S.; Padayatchi, N.; Mahomed, S.; Naidoo, K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J. Antimicrob. Chemother. 2018, 73, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Sassetti, C.M.; Boyd, D.H.; Rubin, E.J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 2003, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sassetti, C.M.; Rubin, E.J. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 2003, 100, 12989–12994. [Google Scholar] [CrossRef]
- Gordhan, B.G.; Smith, D.A.; Alderton, H.; McAdam, R.A.; Bancroft, G.J.; Mizrahi, V. Construction and phenotypic characterization of an auxotrophic mutant of Mycobacterium tuberculosis defective in L-arginine biosynthesis. Infect. Immun. 2002, 70, 3080–3084. [Google Scholar] [CrossRef]
- Tiwari, S.; van Tonder, A.J.; Vilcheze, C.; Mendes, V.; Thomas, S.E.; Malek, A.; Chen, B.; Chen, M.; Kim, J.; Blundell, T.L.; et al. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2018, 115, 9779–9784. [Google Scholar] [CrossRef]
- Gupta, P.; Thomas, S.E.; Zaidan, S.A.; Pasillas, M.A.; Cory-Wright, J.; Sebastian-Perez, V.; Burgess, A.; Cattermole, E.; Meghir, C.; Abell, C.; et al. A fragment-based approach to assess the ligandability of ArgB, ArgC, ArgD and ArgF in the L-arginine biosynthetic pathway of Mycobacterium tuberculosis. Comput. Struct. Biotechnol. J. 2021, 19, 3491–3506. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Cherney, M.M.; Garen, C.; Garen, G.; Niu, C.; Yuan, M.; James, M.N. The molecular structure of ornithine acetyltransferase from Mycobacterium tuberculosis bound to ornithine, a competitive inhibitor. J. Mol. Biol. 2010, 397, 979–990. [Google Scholar] [CrossRef]
- Nakamura, Y.; Hoshino, M.; Sim, J.J.; Ishii, K.; Hosaka, K.; Sakamoto, T. Effect of the leukotriene receptor antagonist pranlukast on cellular infiltration in the bronchial mucosa of patients with asthma. Thorax 1998, 53, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Campos Alberto, E.; Suzuki, S.; Sato, Y.; Hoshioka, A.; Abe, H.; Saito, K.; Tsubaki, T.; Haraki, M.; Sawa, A.; et al. Pranlukast reduces asthma exacerbations during autumn especially in 1- to 5-year-old boys. Asia Pac. Allergy 2017, 7, 10–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mishra, A.; Mamidi, A.S.; Rajmani, R.S.; Ray, A.; Roy, R.; Surolia, A. An allosteric inhibitor of Mycobacterium tuberculosis ArgJ: Implications to a novel combinatorial therapy. EMBO Mol. Med. 2018, 10, e8038. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Li, X.; Gianoulis, T.A.; Yip, K.Y.; Gerstein, M.; Snyder, M. Extensive in vivo metabolite-protein interactions revealed by large-scale systematic analyses. Cell 2010, 143, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Li, Q.H.; Xu, Z.D.; Dou, J.J. Mass spectrometry-based metabolomics in health and medical science: A systematic review. RSC Adv. 2020, 10, 3092–3104. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, A.H.; Miao, J.H.; Sun, H.; Han, Y.; Yan, G.L.; Wua, F.F.; Wang, X.J. Metabolomics biotechnology, applications, and future trends: A systematic review. RSC Adv. 2019, 9, 37245–37257. [Google Scholar] [CrossRef]
- Martinez-Sena, T.; Luongo, G.; Sanjuan-Herraez, D.; Castell, J.V.; Vento, M.; Quintas, G.; Kuligowski, J. Monitoring of system conditioning after blank injections in untargeted UPLC-MS metabolomic analysis. Sci. Rep. 2019, 9, 9822. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, M.; Ramesha, K.P.; Hallur, S.; Karthikkeyan, G.; Rana, E.; Azharuddin, N.; Raj, S.R.; Jeyakumar, S.; Kumaresan, A.; Kataktalware, M.A.; et al. Differences in milk metabolites in Malnad Gidda (Bos indicus) cows reared under pasture-based feeding system. Sci. Rep. 2021, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, D1045–D1053. [Google Scholar] [CrossRef]
- Yelamanchi, S.D.; Surolia, A. Targeting amino acid metabolism of Mycobacterium tuberculosis for developing inhibitors to curtail its survival. IUBMB Life 2021, 73, 643–658. [Google Scholar] [CrossRef]
- Cumming, B.M.; Chinta, K.C.; Reddy, V.P.; Steyn, A.J.C. Role of ergothioneine in microbial physiology and pathogenesis. Antioxid. Redox Signal. 2018, 28, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.P.S.; Guan, X.L. Metabolic versatility of Mycobacterium tuberculosis during infection and dormancy. Metabolites 2021, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Pecsi, I.; Hards, K.; Ekanayaka, N.; Berney, M.; Hartman, T.; Jacobs, W.R., Jr.; Cook, G.M. Essentiality of succinate dehydrogenase in Mycobacterium smegmatis and its role in the generation of the membrane potential under hypoxia. mBio 2014, 5, e01093-14. [Google Scholar] [CrossRef]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Gohlmann, H.W.; Neefs, J.M.; Winkler, H.; van Gestel, J.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef]
- Rao, M.; Streur, T.L.; Aldwell, F.E.; Cook, G.M. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 2001, 147, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Morii, H.; Ogawa, M.; Fukuda, K.; Taniguchi, H.; Koga, Y. A revised biosynthetic pathway for phosphatidylinositol in Mycobacteria. J. Biochem. 2010, 148, 593–602. [Google Scholar] [CrossRef]
- Eoh, H.; Brown, A.C.; Buetow, L.; Hunter, W.N.; Parish, T.; Kaur, D.; Brennan, P.J.; Crick, D.C. Characterization of the Mycobacterium tuberculosis 4-diphosphocytidyl-2-C-methyl-D-erythritol synthase: Potential for drug development. J. Bacteriol. 2007, 189, 8922–8927. [Google Scholar] [CrossRef]
- Buetow, L.; Brown, A.C.; Parish, T.; Hunter, W.N. The structure of Mycobacteria 2C-methyl-D-erythritol-2,4-cyclodiphosphate synthase, an essential enzyme, provides a platform for drug discovery. BMC Struct. Biol. 2007, 7, 68. [Google Scholar] [CrossRef]
- Masini, T.; Kroezen, B.S.; Hirsch, A.K. Druggability of the enzymes of the non-mevalonate-pathway. Drug. Discov. Today 2013, 18, 1256–1262. [Google Scholar] [CrossRef]
- Wang, X.; Dowd, C.S. The methylerythritol phosphate pathway: Promising drug targets in the fight against tuberculosis. ACS Infect. Dis. 2018, 4, 278–290. [Google Scholar] [CrossRef]
- Ostrovsky, D.; Diomina, G.; Lysak, E.; Matveeva, E.; Ogrel, O.; Trutko, S. Effect of oxidative stress on the biosynthesis of 2-C-methyl-D-erythritol-2,4-cyclopyrophosphate and isoprenoids by several bacterial strains. Arch. Microbiol. 1998, 171, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Teufel, R.; Mascaraque, V.; Ismail, W.; Voss, M.; Perera, J.; Eisenreich, W.; Haehnel, W.; Fuchs, G. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc. Natl. Acad. Sci. USA 2010, 107, 14390–14395. [Google Scholar] [CrossRef]
- Almeciga-Diaz, C.J.; Hidalgo, O.A.; Olarte-Avellaneda, S.; Rodriguez-Lopez, A.; Guzman, E.; Garzon, R.; Pimentel-Vera, L.N.; Puentes-Tellez, M.A.; Rojas-Rodriguez, A.F.; Gorshkov, K.; et al. Identification of ezetimibe and pranlukast as pharmacological chaperones for the treatment of the rare disease mucopolysaccharidosis type IVA. J. Med. Chem. 2019, 62, 6175–6189. [Google Scholar] [CrossRef]
- Theron, A.J.; Steel, H.C.; Tintinger, G.R.; Gravett, C.M.; Anderson, R.; Feldman, C. Cysteinyl leukotriene receptor-1 antagonists as modulators of innate immune cell function. J. Immunol. Res. 2014, 2014, 608930. [Google Scholar] [CrossRef]
- Singh, R.K.; Tandon, R.; Dastidar, S.G.; Ray, A. A review on leukotrienes and their receptors with reference to asthma. J. Asthma 2013, 50, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Amaral, E.P.; Ribeiro, S.C.; Lanes, V.R.; Almeida, F.M.; de Andrade, M.R.; Bomfim, C.C.; Salles, E.M.; Bortoluci, K.R.; Coutinho-Silva, R.; Hirata, M.H.; et al. Pulmonary infection with hypervirulent Mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog. 2014, 10, e1004188. [Google Scholar] [CrossRef] [PubMed]
- Warny, M.; Aboudola, S.; Robson, S.C.; Sevigny, J.; Communi, D.; Soltoff, S.P.; Kelly, C.P. P2Y(6) nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 2001, 276, 26051–26056. [Google Scholar] [CrossRef]
- Obba, S.; Hizir, Z.; Boyer, L.; Selimoglu-Buet, D.; Pfeifer, A.; Michel, G.; Hamouda, M.A.; Goncalves, D.; Cerezo, M.; Marchetti, S.; et al. The PRKAA1/AMPKalpha1 pathway triggers autophagy during CSF1-induced human monocyte differentiation and is a potential target in CMML. Autophagy 2015, 11, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Klaver, D.; Thurnher, M. Control of macrophage Inflammation by P2Y purinergic receptors. Cells 2021, 10, 98. [Google Scholar] [CrossRef]
- Aguilo, N.; Uranga, S.; Marinova, D.; Martin, C.; Pardo, J. Bim is a crucial regulator of apoptosis induced by Mycobacterium tuberculosis. Cell Death Dis. 2014, 5, e1343. [Google Scholar] [CrossRef]
- Su, H.; Zhu, S.; Zhu, L.; Huang, W.; Wang, H.; Zhang, Z.; Xu, Y. Recombinant lipoprotein Rv1016c derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC II antigen processing. Front. Cell Infect. Microbiol. 2016, 6, 147. [Google Scholar] [CrossRef]
- Qu, Z.; Zhou, J.; Zhou, Y.; Xie, Y.; Jiang, Y.; Wu, J.; Luo, Z.; Liu, G.; Yin, L.; Zhang, X.L. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1beta immune pathway. Sci. Adv. 2020, 6, eaba4733. [Google Scholar] [CrossRef]
- Shi, Q.J.; Wang, H.; Liu, Z.X.; Fang, S.H.; Song, X.M.; Lu, Y.B.; Zhang, W.P.; Sa, X.Y.; Ying, H.Z.; Wei, E.Q. HAMI 3379, a CysLT2R antagonist, dose- and time-dependently attenuates brain injury and inhibits microglial inflammation after focal cerebral ischemia in rats. Neuroscience 2015, 291, 53–69. [Google Scholar] [CrossRef]
- Ye, X.L.; Lu, L.Q.; Li, W.; Lou, Q.; Guo, H.G.; Shi, Q.J. Oral administration of ampelopsin protects against acute brain injury in rats following focal cerebral ischemia. Exp. Ther. Med. 2017, 13, 1725–1734. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Behera, S.K.; Kasaragod, S.; Karthikkeyan, G.; Narayana Kotimoole, C.; Raju, R.; Prasad, T.S.K.; Subbannayya, Y. MS2Compound: A user-friendly compound identification tool for LC-MS/MS-based metabolomics data. OMICS 2021, 25, 389–399. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Pon, A.; Karu, N.; Zheng, J.; Li, C.; Arndt, D.; Gautam, M.; Allen, F.; Wishart, D.S. CFM-ID 3.0: Significantly improved ESI-MS/MS prediction and compound identification. Metabolites 2019, 9, 72. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Lopez-Ibanez, J.; Pazos, F.; Chagoyen, M. MBROLE 2.0-functional enrichment of chemical compounds. Nucleic Acids Res. 2016, 44, W201–W204. [Google Scholar] [CrossRef]
- Adams, K.J.; Pratt, B.; Bose, N.; Dubois, L.G.; St John-Williams, L.; Perrott, K.M.; Ky, K.; Kapahi, P.; Sharma, V.; MacCoss, M.J.; et al. Skyline for small molecules: A unifying software package for quantitative metabolomics. J. Proteome Res. 2020, 19, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Haug, K.; Cochrane, K.; Nainala, V.C.; Williams, M.; Chang, J.; Jayaseelan, K.V.; O’Donovan, C. MetaboLights: A resource evolving in response to the needs of its scientific community. Nucleic Acids Res. 2020, 48, D440–D444. [Google Scholar] [CrossRef] [PubMed]


| S. No | Metabolite | Mode of Acquisition | Fold Change | p-Value |
|---|---|---|---|---|
| 1 | L-Arginine | Positive | 0.15 | 0.03 |
| 2 | Agmatine | Negative | 0.47 | 0.04 |
| 3 | 4-Guanidinobutanamide | Positive | 0.25 | 0.04 |
| 4 | 2-Oxoarginine | Positive | 0.15 | 0.04 |
| 5 | 5-Amino-6-(5′-phospho-D-ribitylamino) uracil | Positive | 1.92 | 0.05 |
| 6 | 2-Methylmalate | Negative | 1.59 | 0.03 |
| 7 | S-methyl-5-thio-D-ribose | Positive | 0.59 | 0.04 |
| 8 | Oxalosuccinate | Positive | 1.92 | 0.04 |
| 9 | 3-Hydroxypropionyl-CoA | Positive | 0.61 | 0.03 |
| 10 | Menaquinol | Positive | 0.17 | 0.01 |
| 11 | NADP | Positive | 0.65 | 0.02 |
| 12 | 5′-Adenylyl sulfate | Positive | 4.24 | 0.00 |
| 13 | 6-Phospho-D-gluconate | Positive | 0.18 | 0.00 |
| 14 | Cyclic-AMP | Negative | 2.05 | 0.03 |
| 15 | Inositol 1-phosphate | Negative | 2.25 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yelamanchi, S.D.; Arun Kumar, S.T.; Mishra, A.; Keshava Prasad, T.S.; Surolia, A. Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis. Molecules 2022, 27, 1520. https://doi.org/10.3390/molecules27051520
Yelamanchi SD, Arun Kumar ST, Mishra A, Keshava Prasad TS, Surolia A. Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis. Molecules. 2022; 27(5):1520. https://doi.org/10.3390/molecules27051520
Chicago/Turabian StyleYelamanchi, Soujanya D., Sumaithangi Thattai Arun Kumar, Archita Mishra, Thottethodi Subrahmanya Keshava Prasad, and Avadhesha Surolia. 2022. "Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis" Molecules 27, no. 5: 1520. https://doi.org/10.3390/molecules27051520
APA StyleYelamanchi, S. D., Arun Kumar, S. T., Mishra, A., Keshava Prasad, T. S., & Surolia, A. (2022). Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis. Molecules, 27(5), 1520. https://doi.org/10.3390/molecules27051520






