Relationship between Apolipoprotein E Genotype and Lipoprotein Profile in Patients with Coronary Heart Disease
Abstract
1. Introduction
2. Results
2.1. Baseline Clinical Characteristics
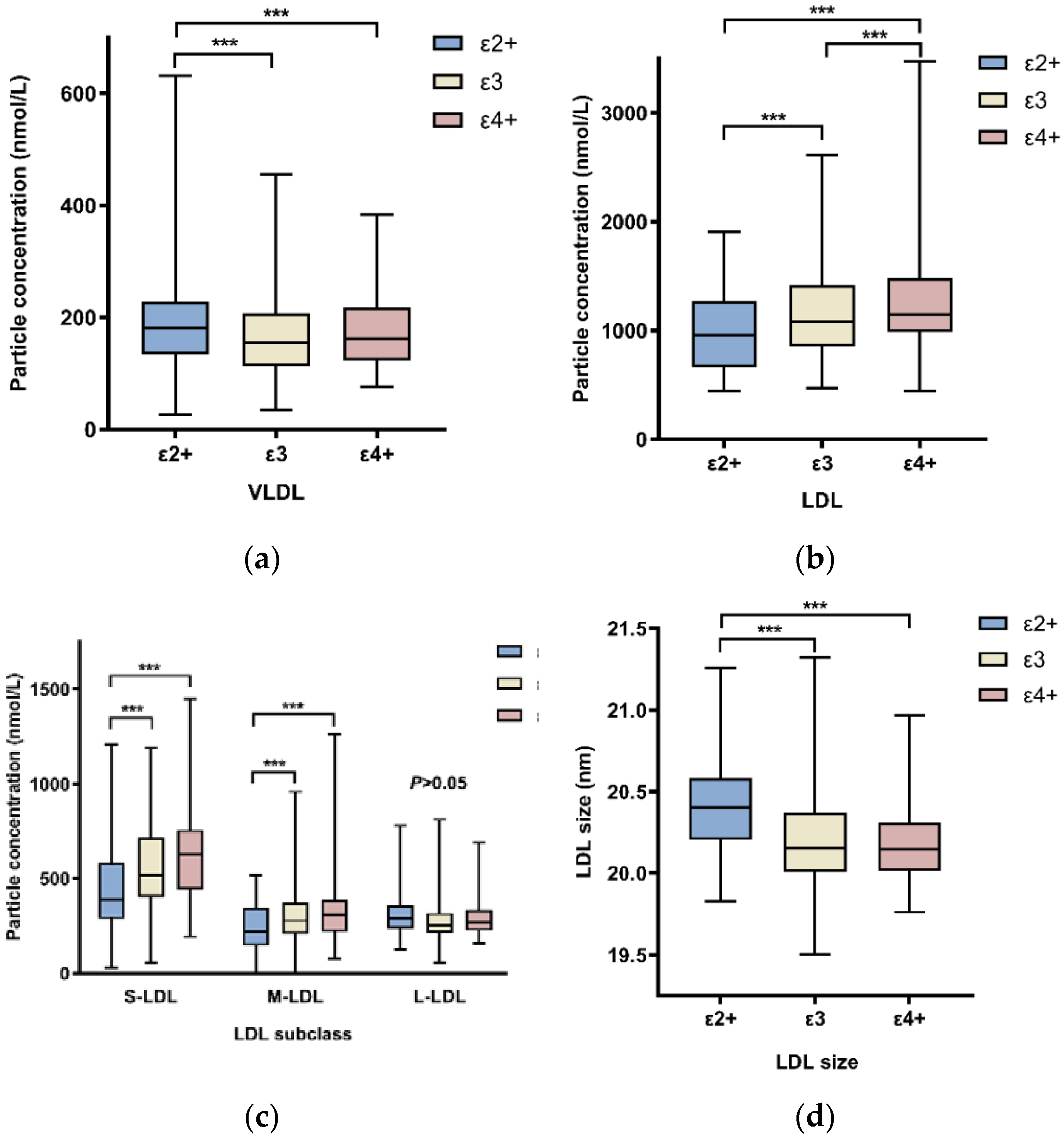
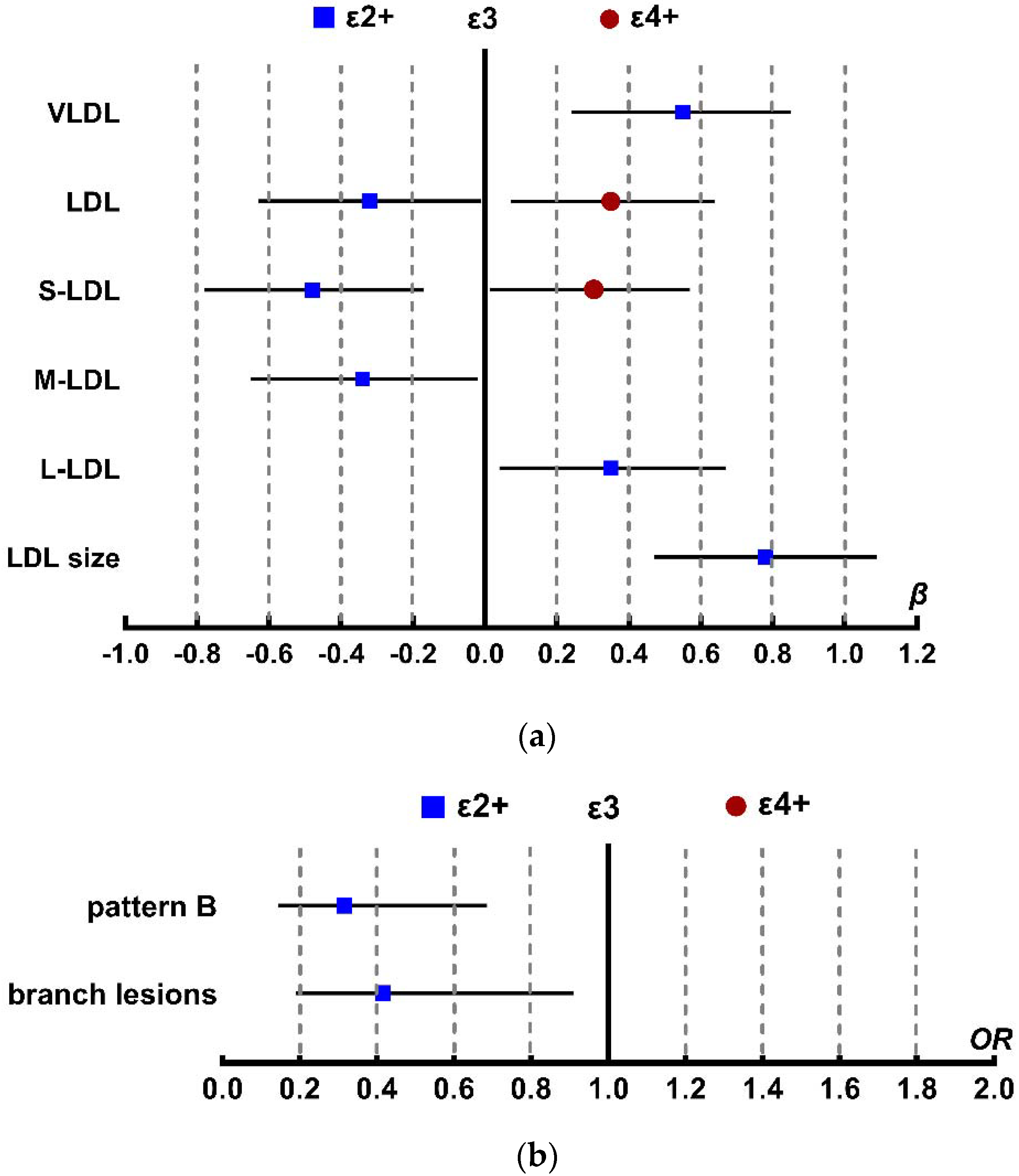
2.2. APOE Genotypes Influence on Lipoprotein Particle Concentrations
2.3. Association between APOE Genotype and LDL Density Patterns
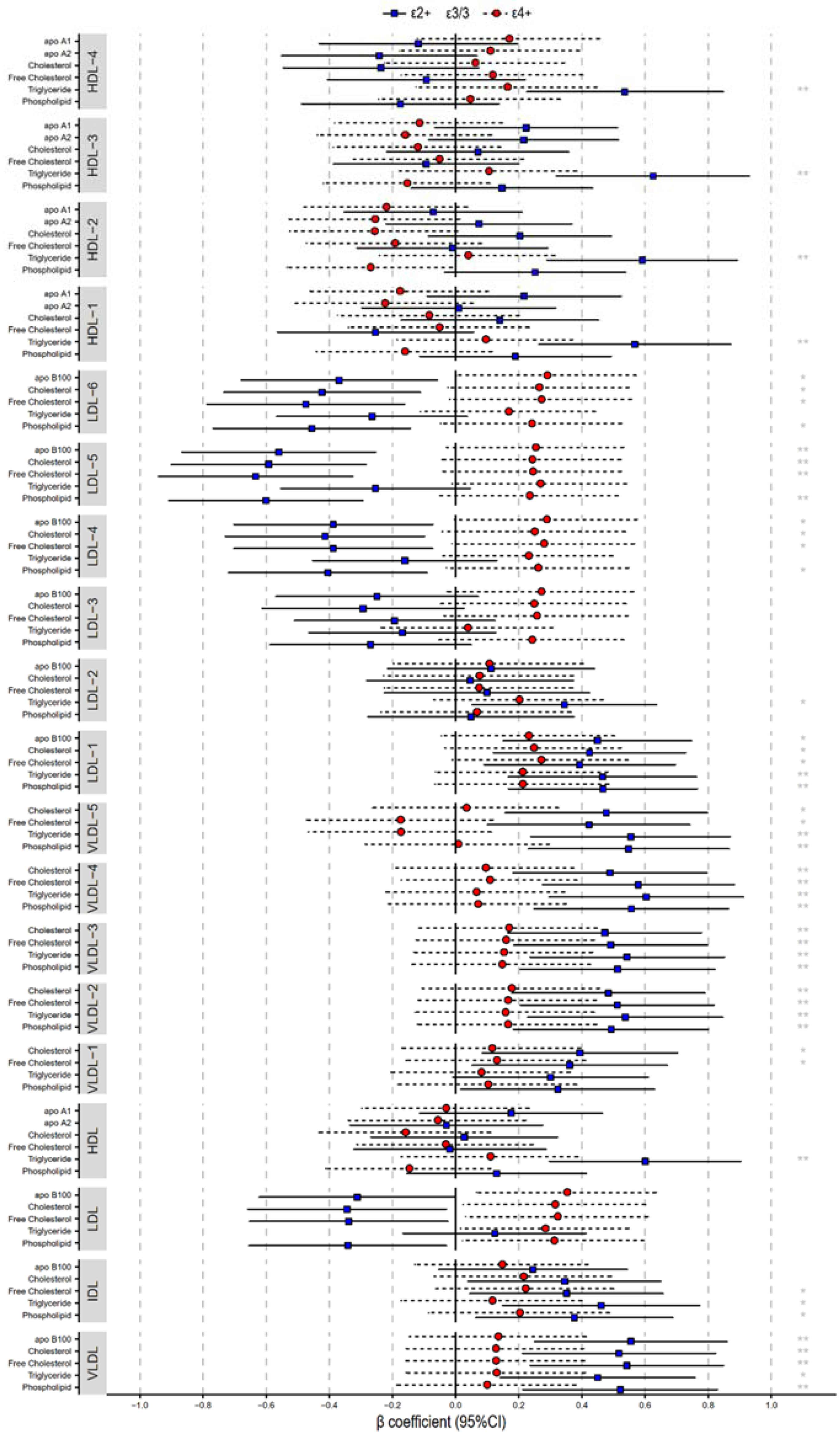
2.4. Lipoprotein Compositions
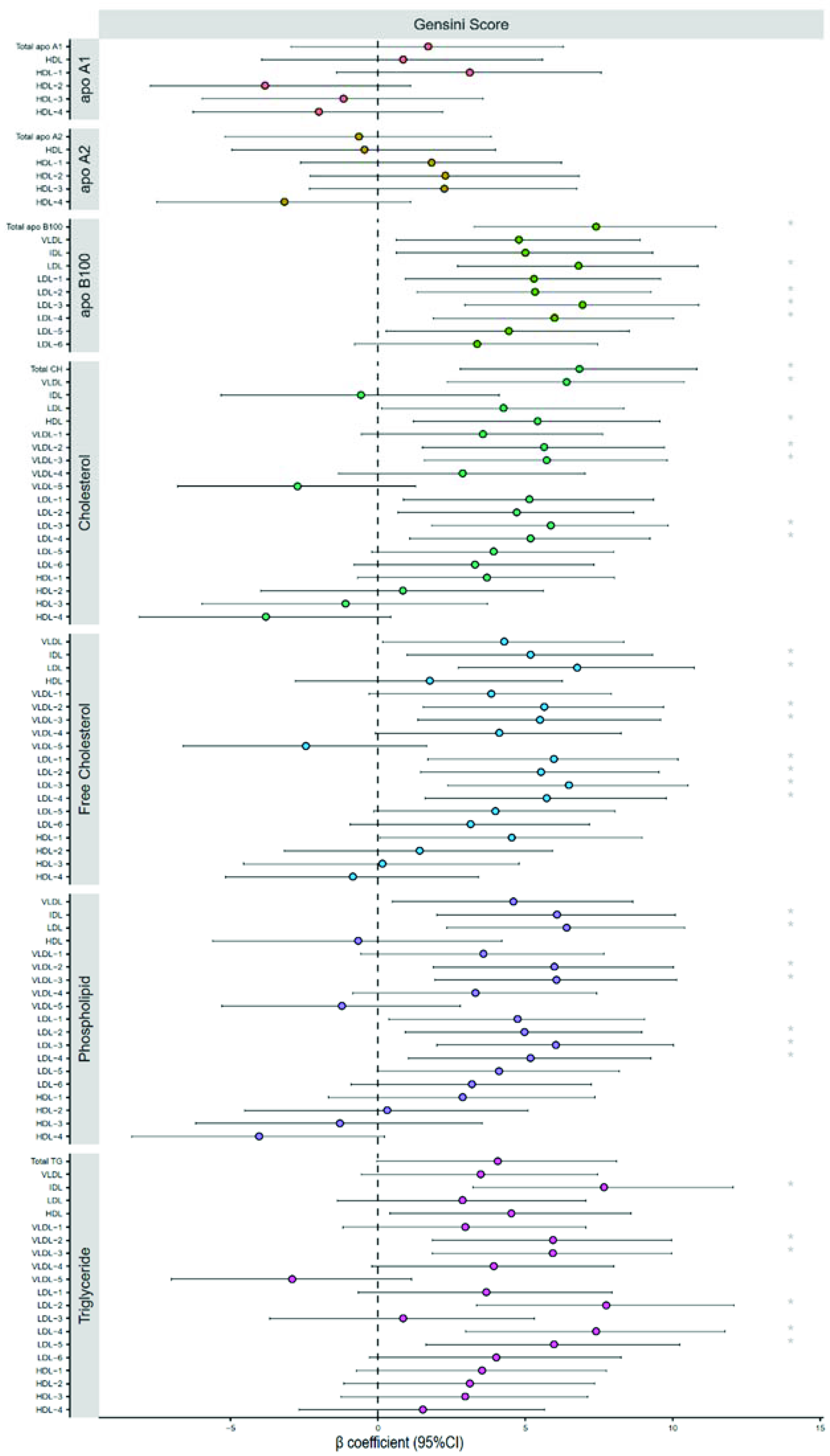
2.5. Severity of Coronary Heart Disease
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical Data Collection and Laboratory Measurements
4.3. APOE Genotyping
4.4. Lipoprotein Profile Measured by NMR Spectroscopy
4.5. Severity of Coronary Heart Disease
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mora, S.; Wenger, N.; Demicco, D.; Breazna, A.; Boekholdt, S.; Arsenault, B.; Deedwania, P.; Kastelein, J.; Waters, D. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: The Treating to New Targets (TNT) study. Circulation 2012, 125, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Delatour, V.; Clouet-Foraison, N.; Gaie-Levrel, F.; Marcovina, S.; Hoofnagle, A.; Kuklenyik, Z.; Caulfield, M.; Otvos, J.; Krauss, R.; Kulkarni, K.; et al. Comparability of Lipoprotein Particle Number Concentrations Across ES-DMA, NMR, LC-MS/MS, Immunonephelometry, and VAP: In Search of a Candidate Reference Measurement Procedure for apoB and non-HDL-P Standardization. Clin. Chem. 2018, 64, 1485–1495. [Google Scholar] [CrossRef]
- Sachdeva, A.; Cannon, C.; Deedwania, P.; Labresh, K.; Smith, S.; Dai, D.; Hernandez, A.; Fonarow, G. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get with The Guidelines. Am. Heart J. 2009, 157, 111–117.e2. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [PubMed]
- Pallarés-Carratalá, V.; Quesada, J.; Orozco-Beltrán, D.; Amigó-Grau, N.; Lopez-Pineda, A.; Fernández Giménez, A.; Gil-Guillén, V.; Correig, X.; Masana, L.; Monleón, D.; et al. Analysis of LDL and HDL size and number by nuclear magnetic resonance in a healthy working population: The LipoLab Study. Int. J. Clin. Pract. 2021, 75, e13610. [Google Scholar] [CrossRef]
- Everett, B.; Ridker, P. Biomarkers for Cardiovascular Screening: Progress or Passé? Clin. Chem. 2017, 63, 248–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arsenault, B.; Lemieux, I.; Després, J.; Wareham, N.; Stroes, E.; Kastelein, J.; Khaw, K.; Boekholdt, S. Comparison between gradient gel electrophoresis and nuclear magnetic resonance spectroscopy in estimating coronary heart disease risk associated with LDL and HDL particle size. Clin. Chem. 2010, 56, 789–798. [Google Scholar] [CrossRef]
- Jiménez, B.; Holmes, E.; Heude, C.; Tolson, R.; Harvey, N.; Lodge, S.; Chetwynd, A.; Cannet, C.; Fang, F.; Pearce, J.; et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by H NMR Spectroscopy in a Multilaboratory Trial. Anal. Chem. 2018, 90, 11962–11971. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, J.; Mononen, N.; Hutri-Kähönen, N.; Lehtimäki, M.; Juonala, M.; Ala-Korpela, M.; Kähönen, M.; Raitakari, O.; Lehtimäki, T. The effect of apolipoprotein E polymorphism on serum metabolome—A population-based 10-year follow-up study. Sci. Rep. 2019, 9, 458. [Google Scholar] [CrossRef]
- Gonzàlez, M.; Ribalta, J.; Vives, G.; Iftimie, S.; Ferré, R.; Plana, N.; Guardiola, M.; Dallinga-Thie, G.; Masana, L.; Castro, A. Nuclear magnetic resonance lipoprotein subclasses and the APOE genotype influence carotid atherosclerosis in patients with systemic lupus erythematosus. J. Rheumatol. 2010, 37, 2259–2267. [Google Scholar] [CrossRef]
- Vekic, J.; Joppa, P.; Habalova, V.; Tisko, R.; Zeljkovic, A.; Pobeha, P.; Gojkovic, T.; Spasojevic-Kalimanovska, V.; Strbova, Z.; Kuklisova, Z.; et al. Relationship Between the Apolipoprotein E Genotype and LDL Particle Size in Patients With Obstructive Sleep Apnea. Angiology 2016, 67, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease—Executive Summary Complete Appendix to Guidelines. Endocr. Pract. 2017, 23, 479–497. Available online: http://journals.aace.com (accessed on 23 November 2020). [CrossRef] [PubMed]
- Austin, M.; King, M.; Vranizan, K.; Krauss, R. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation 1990, 82, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.; Breslow, J.; Hennekens, C.; Buring, J.; Willett, W.; Krauss, R. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, Y.; Mu, Y.; Yu, B.; Ye, P.; Yan, X.; Li, Z.; Wei, Y.; Ambegaonakr, B.; Hu, D. Prevalence of dyslipidaemia in patients treated with lipid-lowering agents in China: Results of the DYSlipidemia International Study (DYSIS). Atherosclerosis 2014, 235, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Otvos, J.; Mora, S.; Shalaurova, I.; Greenland, P.; Mackey, R.; Goff, D. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J. Clin. Lipidol. 2011, 5, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, W.; Otvos, J.; Keyes, M.; Pencina, M.; Sullivan, L.; Vasan, R.; Wilson, P.; D’Agostino, R. LDL Particle Number and Risk of Future Cardiovascular Disease in the Framingham Offspring Study—Implications for LDL Management. J. Clin. Lipidol. 2007, 1, 583–592. [Google Scholar] [CrossRef]
- Diffenderfer, M.; Schaefer, E. The composition and metabolism of large and small LDL. Curr. Opin. Lipidol. 2014, 25, 221–226. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef]
- Garber, A.; Abrahamson, M.; Barzilay, J.; Blonde, L.; Bloomgarden, Z.; Bush, M.; Dagogo-Jack, S.; DeFronzo, R.; Einhorn, D.; Fonseca, V.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2016 Executive Summary. Endocr. Pract. 2016, 22, 84–113. [Google Scholar] [CrossRef]
- Ma, S.; Xia, M.; Gao, X. Biomarker Discovery in Atherosclerotic Diseases Using Quantitative Nuclear Magnetic Resonance Metabolomics. Front. Cardiovasc. Med. 2021, 8, 681444. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.C. Apolipoprotein E isoforms and lipoprotein metabolism. IUBMB Life 2014, 66, 616–623. [Google Scholar] [CrossRef]
- Otvos, J.; Shalaurova, I.; Freedman, D.; Rosenson, R. Effects of pravastatin treatment on lipoprotein subclass profiles and particle size in the PLAC-I trial. Atherosclerosis 2002, 160, 41–48. [Google Scholar] [CrossRef]
- Rosenson, R.; Otvos, J.; Hsia, J. Effects of rosuvastatin and atorvastatin on LDL and HDL particle concentrations in patients with metabolic syndrome: A randomized, double-blind, controlled study. Diabetes Care 2009, 32, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Scharnagl, H.; Abletshauser, C.; Hoffmann, M.; Berg, A.; Keul, J.; Wieland, H.; Baumstark, M. Fluvastatin lowers atherogenic dense low-density lipoproteins in postmenopausal women with the atherogenic lipoprotein phenotype. Circulation 2001, 103, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Mackey, R.; Greenland, P.; Goff, D.; Lloyd-Jones, D.; Sibley, C.; Mora, S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J. Am. Coll. Cardiol. 2012, 60, 508–516. [Google Scholar] [CrossRef]
- Dona, A.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.; Pearce, J.; Holmes, E.; Lindon, J.; Nicholson, J. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef]
- Gensini, G. A more meaningful scoring system for determining the severity of coronary heart disease. Am. J. Cardiol. 1983, 51, 606. [Google Scholar] [CrossRef]
| ε2+ (n = 47) | ε3 (n = 252) | ε4+ (n = 61) | p Value | |
|---|---|---|---|---|
| Male (%) | 25.00 (53.19) | 138.00 (54.76) | 38.00 (62.30) | 0.446 |
| DM (%) | 12.00 (25.53) | 86.00 (34.13) | 23.00 (37.70) | 0.394 |
| Age (years) | 59.17 ± 8.69 | 60.88 ± 9.34 | 61.57 ± 8.60 | 0.381 |
| BMI (kg/m²) | 26.10 (23.10, 28.35) | 25.70 (23.70, 27.70) | 26.00 (23.80, 27.70) | 0.977 |
| HbA1c (%) | 6.00 (5.60, 6.90) | 6.10 (5.70, 6.80) | 6.00 (5.70, 6.80) | 0.963 |
| hs-CRP (mg/L) | 1.34 (0.48, 3.50) | 1.56 (0.51, 3.28) | 0.99 (0.39, 2.63) | 0.276 |
| ALT (IU/L) | 20.00 (15.00, 29.00) | 21.00 (15.00, 31.00) | 18.00 (12.50, 27.50) | 0.206 |
| Cr (μmol/L) | 79.13 ± 19.41 | 77.71 ± 17.15 | 81.19 ± 16.44 | 0.360 |
| TC (mmol/L) | 4.25 ± 1.23 | 4.21 ± 1.05 | 4.34 ± 1.20 | 0.704 |
| TG (mmol/L) | 1.62 (1.18, 2.24) | 1.45 (1.03, 1.90) | 1.47 (1.18, 2.17) | 0.074 |
| HDL-C (mmol/L) | 1.23 (1.01, 1.35) | 1.18 (0.98, 1.36) | 1.11 (0.96, 1.25) | 0.064 |
| LDL-C (mmol/L) | 2.29 (1.46, 2.98) | 2.27 (1.80, 3.00) | 2.48 (2.04, 3.07) | 0.254 |
| apoA1 (g/L) * | 1.49 ± 0.24 | 1.39 ± 0.33 | 1.31 ± 0.26 | 0.016 |
| apoB (g/L) * | 0.74 ± 0.23 | 0.78 ± 0.23 | 0.86 ± 0.28 | 0.034 |
| Lp(a) (mg/L) | 149.00(46.00,345.24) | 212.00 (82.00, 443.07) | 204.00 (100.64, 741.00) | 0.055 |
| Pattern A | Pattern B | Total | |
|---|---|---|---|
| ε2+ | 15 | 32 | 47 |
| ε3 | 35 | 217 | 252 |
| ε4+ | 7 | 54 | 61 |
| Total | 57 | 303 | 360 |
| B | 95%CI | p | |
|---|---|---|---|
| cholesterol | −6.589 | −30.084~16.907 | 0.581 |
| triglyceride | 5.517 | −3.029~14.063 | 0.205 |
| HDL-C | 1.117 | −26.503~28.737 | 0.937 |
| LDL-C | 2.099 | −23.4~27.598 | 0.871 |
| Lp(a) * | 0.012 | 0~0.023 | 0.041 |
| Small LDL * | 0.058 | 0.008~0.108 | 0.024 |
| Medium LDL | 0.03 | −0.011~0.071 | 0.154 |
| Large LDL | −0.031 | −0.109~0.047 | 0.438 |
| LDL size * | 42.254 | 2.193~82.315 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Yang, Q.; Liu, Z.; Su, B.; Xu, F.; Li, Y.; Kang, J.; Zhou, Z. Relationship between Apolipoprotein E Genotype and Lipoprotein Profile in Patients with Coronary Heart Disease. Molecules 2022, 27, 1377. https://doi.org/10.3390/molecules27041377
Lin Y, Yang Q, Liu Z, Su B, Xu F, Li Y, Kang J, Zhou Z. Relationship between Apolipoprotein E Genotype and Lipoprotein Profile in Patients with Coronary Heart Disease. Molecules. 2022; 27(4):1377. https://doi.org/10.3390/molecules27041377
Chicago/Turabian StyleLin, Yahui, Qiong Yang, Zhaohui Liu, Baoman Su, Fen Xu, Yang Li, Jinsuo Kang, and Zhou Zhou. 2022. "Relationship between Apolipoprotein E Genotype and Lipoprotein Profile in Patients with Coronary Heart Disease" Molecules 27, no. 4: 1377. https://doi.org/10.3390/molecules27041377
APA StyleLin, Y., Yang, Q., Liu, Z., Su, B., Xu, F., Li, Y., Kang, J., & Zhou, Z. (2022). Relationship between Apolipoprotein E Genotype and Lipoprotein Profile in Patients with Coronary Heart Disease. Molecules, 27(4), 1377. https://doi.org/10.3390/molecules27041377





