Micro- and Nanocapsules Based on Artificial Peptides
Abstract
:1. Introduction
2. Current Methods for Peptide Micro/Nanocapsules Production
2.1. Capsule Formation by Self-Assembly
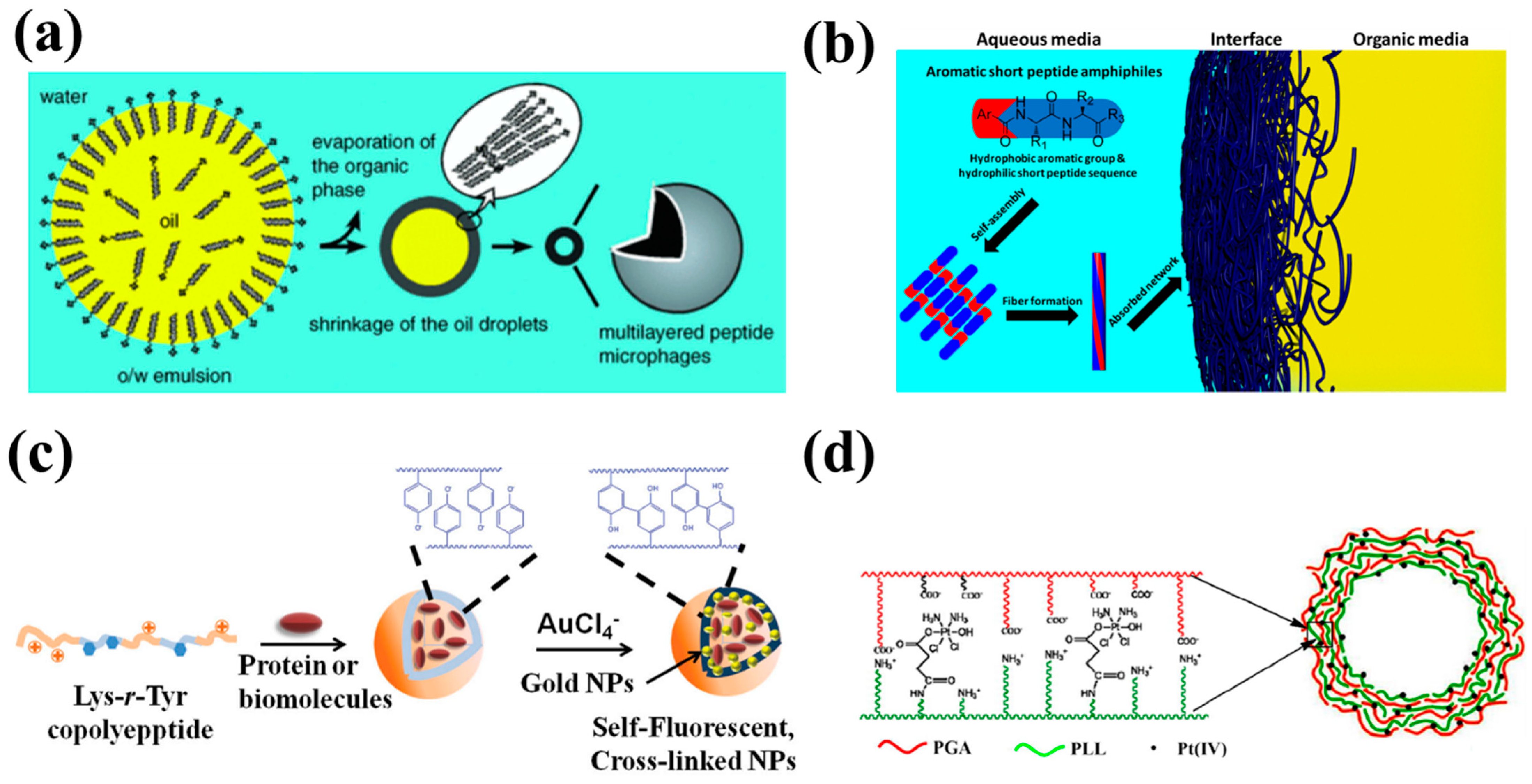
2.2. Capsule Formation by Polymerization and Crosslinking
2.3. Capsule Formation by Layer-by-Layer (LbL) Technology
3. Properties of Peptide Capsules
4. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Rasines Mazo, A.; Allison-Logan, S.; Karimi, F.; Chan, N.J.; Qiu, W.; Duan, W.; O’Brien-Simpson, N.M.; Qiao, G.G. Ring opening polymerization of alpha-amino acids: Advances in synthesis, architecture and applications of polypeptides and their hybrids. Chem. Soc. Rev. 2020, 49, 4737–4834. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Sudo, A. Well-defined construction of functional macromolecular architectures based on polymerization of amino acid urethanes. Biomedicines 2020, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Bai, T.; Tao, X.; Ling, J. An inspection into multifarious ways to synthesize poly(amino acid)s. Macromol. Rapid Commun. 2021, 42, e2100453. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Peptide nanotubes. Angew. Chem. Int. Ed. 2014, 53, 6866–6881. [Google Scholar] [CrossRef]
- Arslan, E.; Garip, I.C.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Bioactive supramolecular peptide nanofibers for regenerative medicine. Adv. Healthc. Mater. 2014, 3, 1357–1376. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xing, R.; Bai, S.; Yan, X. Recent advances of self-assembling peptide-based hydrogels for biomedical applications. Soft Matter 2019, 15, 1704–1715. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Lamprou, D.A.; Urquhart, A.J.; Yannopoulos, S.N.; Vizirianakis, I.S.; Zhang, S.; Koutsopoulos, S. Lipid-like self-assembling peptide nanovesicles for drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 8184–8189. [Google Scholar] [CrossRef]
- Matsuura, K.; Watanabe, K.; Matsushita, Y.; Kimizuka, N. Guest-binding behavior of peptide nanocapsules self-assembled from viral peptide fragments. Polym. J. 2013, 45, 529–534. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zhuang, X.; Tang, Z.; Tian, H.; Chen, X. Stimuli-sensitive synthetic polypeptide-based materials for drug and gene delivery. Adv. Healthc. Mater. 2012, 1, 48–78. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Chen, C.; Shen, G.; Yan, L.; Zou, Q.; Ma, G.; Mohwald, H.; Yan, X. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. Int. Ed. Engl. 2015, 54, 500–505. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Zhang, X.; Yu, D.; Jiang, X.; Wang, Z.; Cao, M.; Xia, Y.; Liu, H. Short peptide-regulated aggregation of porphyrins for photoelectric conversion. Sustain. Energy Fuels 2019, 3, 529–538. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Martinez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodriguez, S.A.; Roman, R.A.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C. Nanocapsules as drug delivery systems. Int. J. Artif. Organs 2005, 28, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Fleming, S.; Ulijn, R.V. Design of nanostructures based on aromatic peptide amphiphiles. Chem. Soc. Rev. 2014, 43, 8150–8177. [Google Scholar] [CrossRef]
- Morikawa, M.A.; Yoshihara, M.; Endo, T.; Kimizuka, N. alpha-Helical polypeptide microcapsules formed by emulsion-templated self-assembly. Chemistry 2005, 11, 1574–1578. [Google Scholar] [CrossRef]
- Feng, H.; Linders, J.; Myszkowska, S.; Mayer, C. Capsules from synthetic diblock-peptides as potential artificial oxygen carriers. J. Microencapsul. 2021, 38, 276–284. [Google Scholar] [CrossRef]
- Hanson, J.A.; Chang, C.B.; Graves, S.M.; Li, Z.; Mason, T.G.; Deming, T.J. Nanoscale double emulsions stabilized by single-component block copolypeptides. Nature 2008, 455, 85–88. [Google Scholar] [CrossRef]
- Hanson, J.A.; Deming, T.J. Functionalized nanoscale through microscale polypeptide stabilized emulsions for display of biomolecules. Polym. Chem. 2011, 2, 1473–1475. [Google Scholar] [CrossRef]
- Bai, S.; Pappas, C.; Debnath, S.; Frederix, P.J.M.; Leckie, J.; Fleming, S.; Ulijn, R.V. Stable emulsions formed by self-assembly of interfacial networks of dipeptide derivatives. ACS Nano 2014, 8, 7005–7013. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.G.; McKnight, P.J.; Tuttle, T.; Ulijn, R.V. Tripeptide emulsifiers. Adv. Mater. 2016, 28, 1381–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, Y.; Qi, W.; Su, R.; He, Z. Bioorganometallic ferrocene-tripeptide nanoemulsions. Nanoscale 2017, 9, 15323–15331. [Google Scholar] [CrossRef] [PubMed]
- Holowka, E.P.; Pochan., D.J.; Deming, A.T.J. Charged polypeptide vesicles with controllable diam. JACS 2005, 127, 12423–12428. [Google Scholar] [CrossRef]
- Hamley, I.W. Self-assembly of amphiphilic peptides. Soft Matter 2011, 7, 4122–4138. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, E.G.; Wyrsta, M.D.; Pakstis, L.; Pochan, D.J.; Deming, T.J. Stimuli-responsive polypeptide vesicles by conformation-specific assembly. Nat. Mater. 2004, 3, 244–248. [Google Scholar] [CrossRef]
- Hell, A.J.v.; Costa, C.I.C.A.; Flesch, F.M.; Sutter, M.; Jiskoot, W.; Crommelin, D.J.A.; Hennink, W.E.; Mastrobatti, E. Self-Assembly of recombinant amphiphilic oligopeptides into vesicles. Biomacromolecules 2007, 8, 2753–2761. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lu, S.C.; Huang, Y.C.; Jan, J.S. Cross-linked, self-fluorescent gold nanoparticle/polypeptide nanocapsules comprising dityrosine for protein encapsulation and label-free imaging. Small 2014, 10, 1939–1944. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, H.; Meng, F.; Zhou, S.; Guo, J.; Li, X.; Jing, X.; Huang, Y. Layer-by-layer assembled polypeptide capsules for platinum-based pro-drug delivery. Bioconjug. Chem. 2012, 23, 2335–2343. [Google Scholar] [CrossRef]
- Wong, M.S.; Cha, J.N.; Choi, K.-S.; Deming, T.J.; Stucky, G.D. Assembly of nanoparticles into hollow spheres using blcok copolypeptides. Nano Lett. 2002, 2, 583–587. [Google Scholar] [CrossRef]
- Lee, J.; Ju, M.; Cho, O.H.; Kim, Y.; Nam, K.T. Tyrosine-rich peptides as a platform for assembly and material synthesis. Adv. Sci. 2019, 6, 1801255. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.; Pavlovic, D.; Prydderch, H.; Moradi, M.A.; Ibarboure, E.; Heuts, J.P.A.; Lecommandoux, S.; Heise, A. Polypeptide nanoparticles obtained from emulsion polymerization of amino acid N-carboxyanhydrides. J. Am. Chem. Soc. 2019, 141, 12522–12526. [Google Scholar] [CrossRef] [PubMed]
- Min, K.I.; Yun, G.; Jang, Y.; Kim, K.R.; Ko, Y.H.; Jang, H.S.; Lee, Y.S.; Kim, K.; Kim, D.P. Covalent self-assembly and one-step photocrosslinking of tyrosine-rich oligopeptides to form diverse nanostructures. Angew. Chem. Int. Ed. Engl. 2016, 55, 6925–6928. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Qi, W.; Zhang, J.; Zhang, L.; Huang, R.; Su, R.; He, Z. Photo-induced polymerization and reconfigurable assembly of multifunctional ferrocene-tyrosine. Small 2018, 14, e1800772. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.H.; Wong, E.H.; Sulistio, A.; Guntari, S.N.; Blencowe, A.; Caruso, F.; Qiao, G.G. Assembly of free-standing polypeptide films via the synergistic combination of hyperbranched macroinitiators, the grafting-from approach, and cross-chain termination. Adv. Mater. 2013, 25, 4619–4624. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Beretta, G.L.; Cui, J.; Braunger, J.A.; Yan, Y.; Richardson, J.J.; Tinelli, S.; Folini, M.; Zaffaroni, N.; Caruso, F. Redox-sensitive PEG-polypeptide nanoporous particles for survivin Ssilencing in prostate cancer cells. Biomacromolecules 2015, 16, 2168–2178. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, C.; Chi, P. One-step preparation of glycopeptide microspheres based on alpha-amino acid-N-carboxyanhydride polymerization using interfacial protocols. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 45–54. [Google Scholar] [CrossRef]
- Xiao, F.X.; Pagliaro, M.; Xu, Y.J.; Liu, B. Layer-by-layer assembly of versatile nanoarchitectures with diverse dimensionality: A new perspective for rational construction of multilayer assemblies. Chem. Soc. Rev. 2016, 45, 3088–3121. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, T.; Shi, P.; Yang, D.; Luo, S.; Voit, B.; Appelhans, D.; Zan, X.; Chen, H. The construction and effect of physical properties on intracellular drug delivery of poly(amino acid) capsules. Colloids Surf. B Biointerfaces 2019, 177, 178–187. [Google Scholar] [CrossRef]
- Zhi, Z.L.; Haynie, D.T. Straightforward and effective protein encapsulation in polypeptide-based artificial cells. Artif. Cells Blood Substit. Biotechnol. 2006, 34, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Shutava., T.G.; Balkundi., S.S.; Vangala., P.; Steffan., J.J.; Bigelow., R.L.; Cardelli., J.A.; O’Neal., D.P.; Yuri, M.L. Layer-by-layer-coated gelatin nanoparticles as a vehicle for delivery of natural polyphenols. ACS Nano 2009, 3, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Shutava, T.G.; Pattekari, P.P.; Arapov, K.A.; Torchilin, V.P.; Lvov, Y.M. Architectural layer-by-layer assembly of drug nanocapsules with PEGylated polyelectrolytes. Soft Matter 2012, 8, 9418–9427. [Google Scholar] [CrossRef] [PubMed]
- Ruttala, H.B.; Ramasamy, T.; Shin, B.S.; Choi, H.G.; Yong, C.S.; Kim, J.O. Layer-by-layer assembly of hierarchical nanoarchitectures to enhance the systemic performance of nanoparticle albumin-bound paclitaxel. Int. J. Pharm. 2017, 519, 11–21. [Google Scholar] [CrossRef]
- Ye, C.; Combs, Z.A.; Calabrese, R.; Dai, H.; Kaplan, D.L.; Tsukruk, V.V. Robust microcapsules with controlled permeability from silk fibroin reinforced with graphene oxide. Small 2014, 10, 5087–5097. [Google Scholar] [CrossRef]
- Muriel Mundo, J.L.; Zhou, H.; Tan, Y.; Liu, J.; McClements, D.J. Stabilization of soybean oil-in-water emulsions using polypeptide multilayers: Cationic polylysine and anionic polyglutamic acid. Food Res. Int. 2020, 137, 109304. [Google Scholar] [CrossRef]
- Mundo, J.L.M.; Zhou, H.; Tan, Y.; Liu, J.; McClements, D.J. Enhancing emulsion functionality using multilayer technology: Coating lipid droplets with saponin-polypeptide-polysaccharide layers by electrostatic deposition. Food Res. Int. 2021, 140, 109864. [Google Scholar] [CrossRef]
- Ye, C.; Drachuk, I.; Calabrese, R.; Dai, H.; Kaplan, D.L.; Tsukruk, V.V. Permeability and micromechanical properties of silk ionomer microcapsules. Langmuir 2012, 28, 12235–12244. [Google Scholar] [CrossRef]
- Ochs, C.J.; Such, G.K.; Städler, B.; Caruso, F. Low-fouling, biofunctionalized, and biodegradable click capsules. Biomacromolecules 2008, 9, 3389–3396. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid. Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef] [Green Version]
- Shimanovich, U.; Bernardes, G.J.; Knowles, T.P.; Cavaco-Paulo, A. Protein micro- and nano-capsules for biomedical applications. Chem. Soc. Rev. 2014, 43, 1361–1371. [Google Scholar] [CrossRef] [Green Version]
- Wibowo, S.H.; Sulistio, A.; Wong, E.H.; Blencowe, A.; Qiao, G.G. Polypeptide films via N-carboxyanhydride ring-opening polymerization (NCA-ROP): Past, present and future. Chem. Commun. 2014, 50, 4971–4988. [Google Scholar] [CrossRef]
- Lv, S.; Tang, Z.; Li, M.; Lin, J.; Song, W.; Liu, H.; Huang, Y.; Zhang, Y.; Chen, X. Co-delivery of doxorubicin and paclitaxel by PEG-polypeptide nanovehicle for the treatment of non-small cell lung cancer. Biomaterials 2014, 35, 6118–6129. [Google Scholar] [CrossRef]
- Holowka, E.P.; Sun, V.Z.; Kamei, D.T.; Deming, T.J. Polyarginine segments in block copolypeptides drive both vesicular assembly and intracellular delivery. Nat. Mater. 2007, 6, 52–57. [Google Scholar] [CrossRef]

| Formation Process | Main Interaction | Peptide | Mechanical Stability | Diameter | Ref. |
|---|---|---|---|---|---|
| Self-assembly | Amphiphilic interaction and hydrogen bonding | Polypeptide | Average | 1–5 μm | [17] |
| Self-assembly | Amphiphilic interaction | Polypeptide | Average | 100 nm−1 μm | [18] |
| Self-assembly | Amphiphilic interaction | Polypeptide | Average | 10–100 nm | [19] |
| Self-assembly | Aromatic π-π stacking and hydrogen bonding | Dipeptide | High | 5–50 μm | [21] |
| Self-assembly | Aromatic π-π stacking and hydrogen bonding | Tripeptide | High | 1–10 μm | [22] |
| Self-assembly | Amphiphilic interaction and hydrogen bonding | Polypeptide | Average | 1–10 μm | [24] |
| Self-assembly | Amphiphilic interaction and hydrogen bonding | Oligopeptides | Average | 100–200 nm | [27] |
| Crosslinking | Covalent bonding | Polypeptide | High | 100–500 nm | [32] |
| Crosslinking | Covalent bonding | Polypeptide | High | 20–200 nm | [28] |
| Crosslinking | Covalent bonding | Oligopeptides | High | 100–200 nm | [33] |
| Crosslinking | Covalent bonding | Polypeptide | High | 5 μm | [35] |
| Crosslinking | Covalent bonding | Polypeptide | High | 1–2 μm | [36] |
| LbL technology | Electrostatic interaction | Polypeptide | Average | 500 nm−2 μm | [29] |
| LbL technology | Electrostatic interaction | Polypeptide | Average | 1–5 μm | [40] |
| LbL technology | Electrostatic interaction | Polypeptide | Average | 4–5 μm | [41] |
| LbL technology | Electrostatic interaction | Polypeptide | Average | 100–200 nm | [44] |
| LbL technology | Electrostatic interaction | Polypeptide | Average | 500 nm–2 μm | [46] |
| LbL technology | Electrostatic interaction and covalent bonding | Polypeptide | High | 3–6 μm | [48] |
| LbL technology | Electrostatic interaction and covalent bonding | Polypeptide | High | 5–10 μm | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H. Micro- and Nanocapsules Based on Artificial Peptides. Molecules 2022, 27, 1373. https://doi.org/10.3390/molecules27041373
Feng H. Micro- and Nanocapsules Based on Artificial Peptides. Molecules. 2022; 27(4):1373. https://doi.org/10.3390/molecules27041373
Chicago/Turabian StyleFeng, Huayang. 2022. "Micro- and Nanocapsules Based on Artificial Peptides" Molecules 27, no. 4: 1373. https://doi.org/10.3390/molecules27041373
APA StyleFeng, H. (2022). Micro- and Nanocapsules Based on Artificial Peptides. Molecules, 27(4), 1373. https://doi.org/10.3390/molecules27041373





