Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells
Abstract
1. Introduction
2. Results
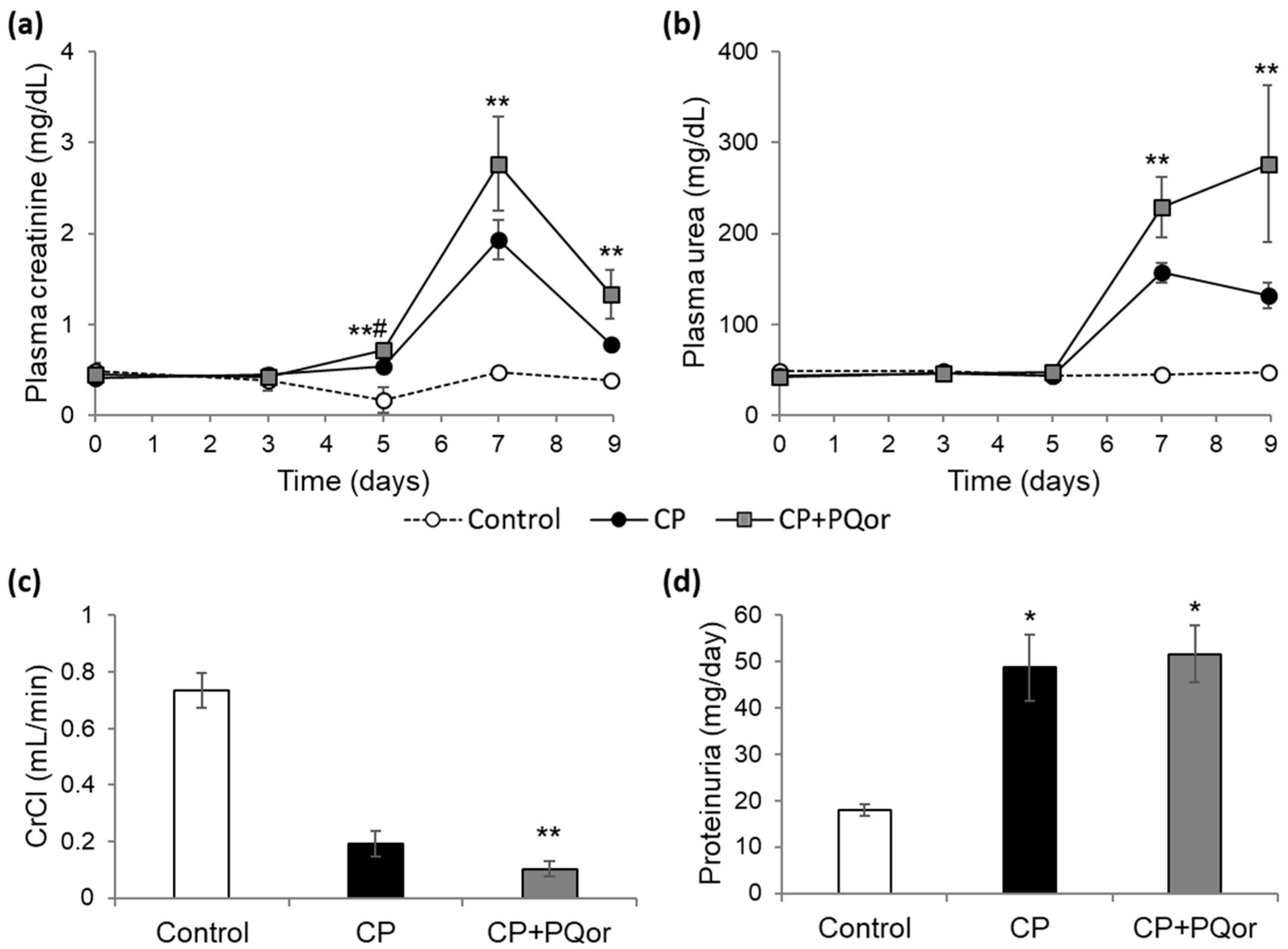
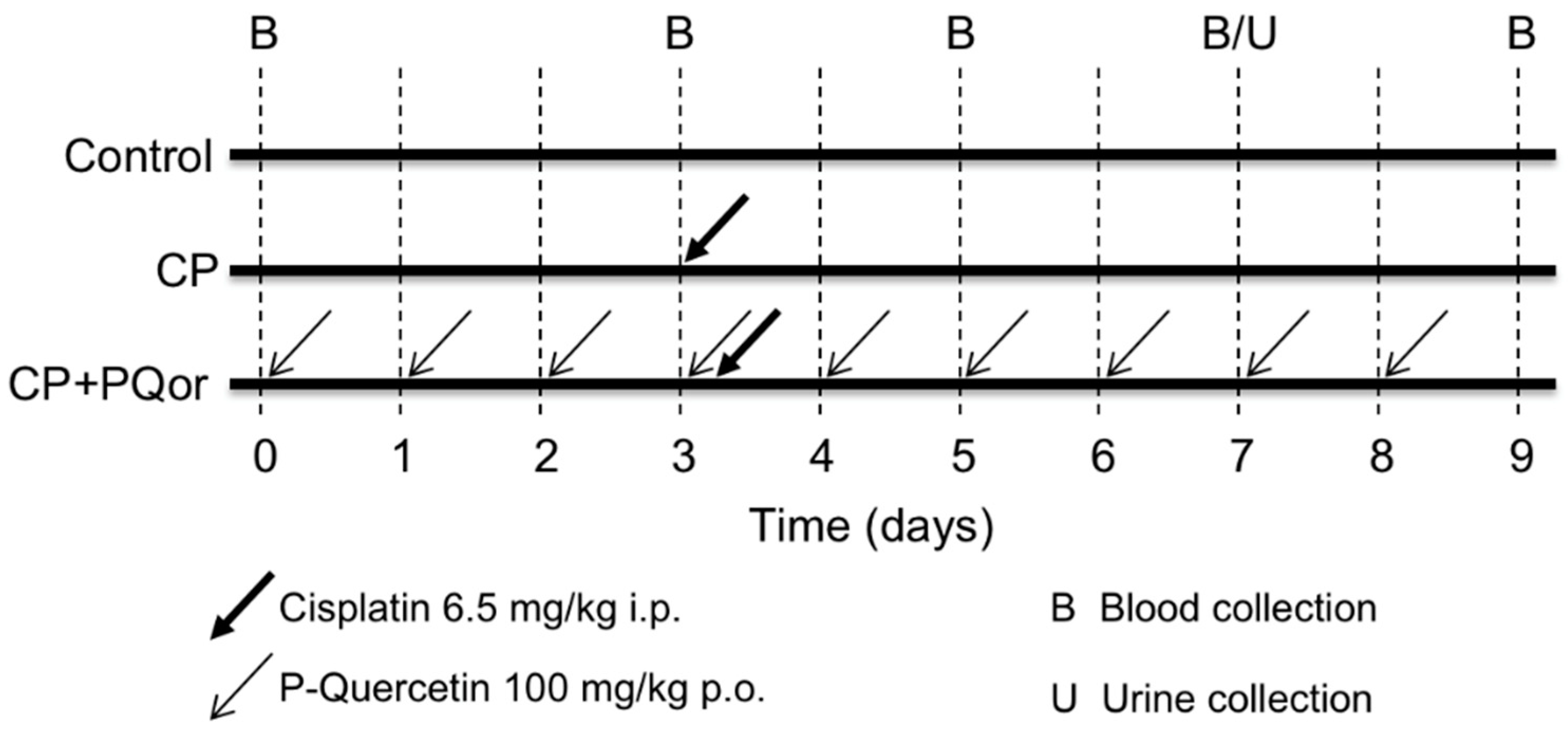
2.1. Nephroprotection Study with Oral P-quercetin
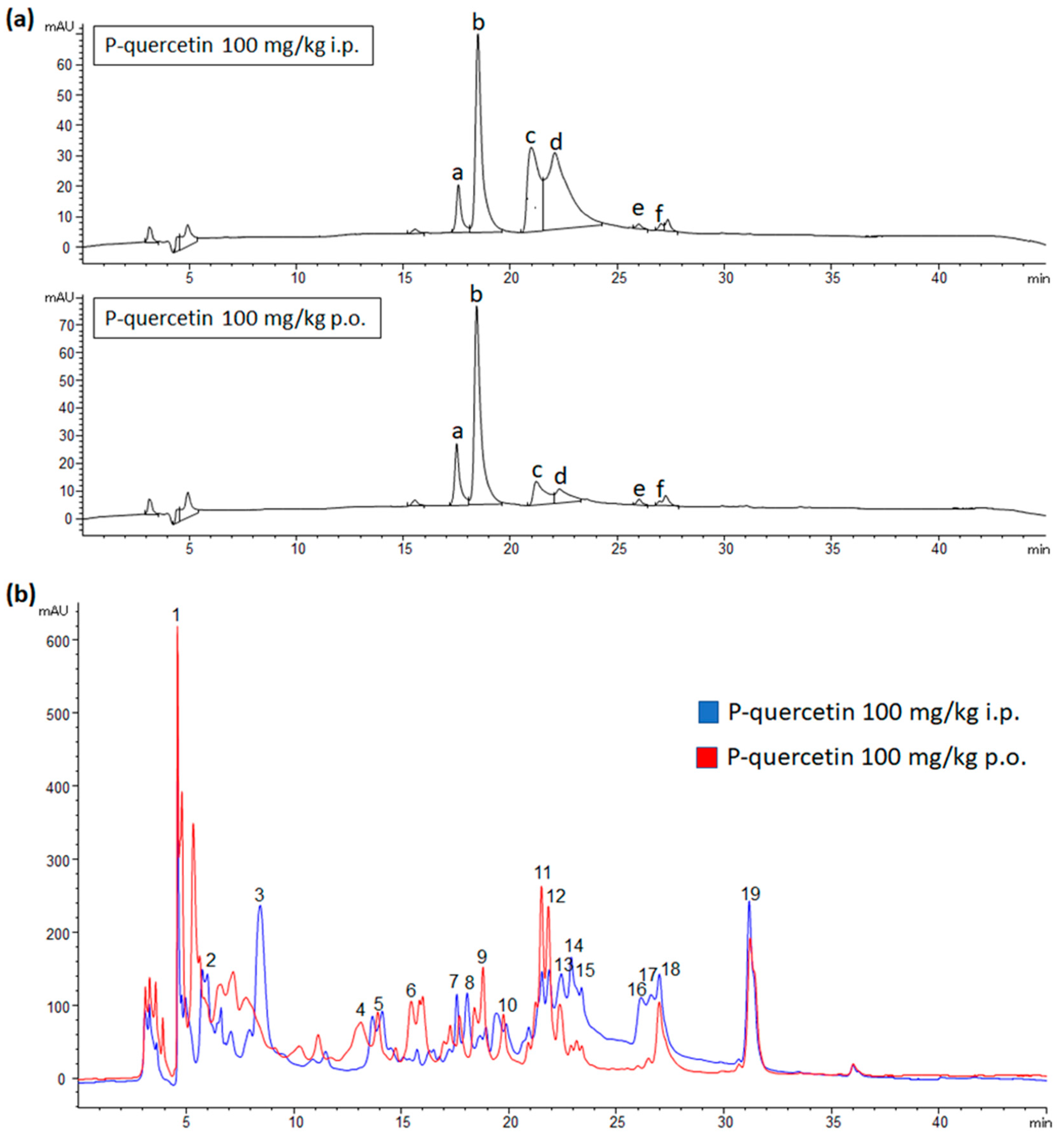
2.2. Metabolites Distribution in Plasma and Urine after P-quercetin Administration
2.3. Evaluation of the Protective Capacity of Quercetin and Its Metabolites against Cisplatin Cytotoxicity in HK-2 Cells
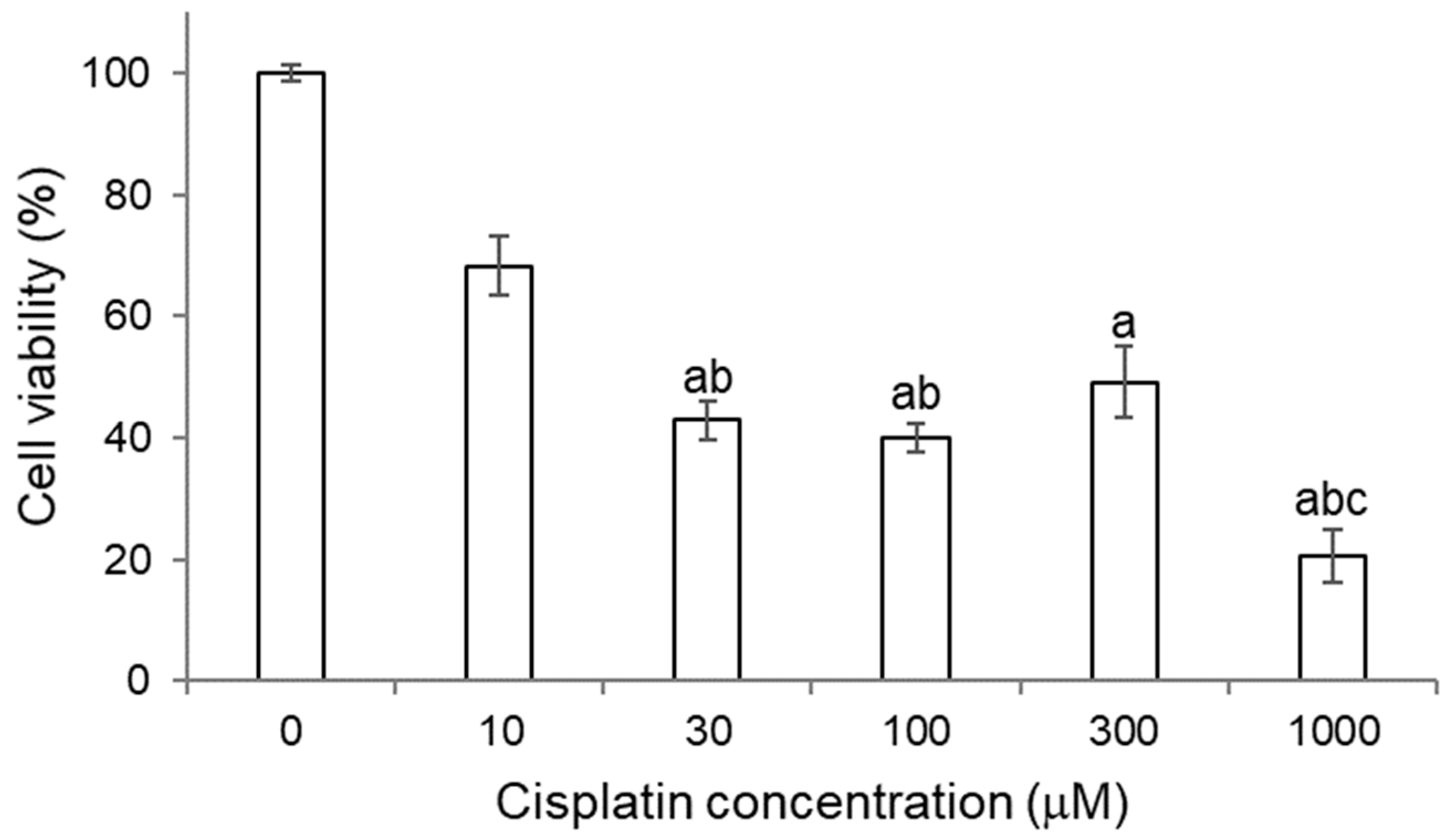
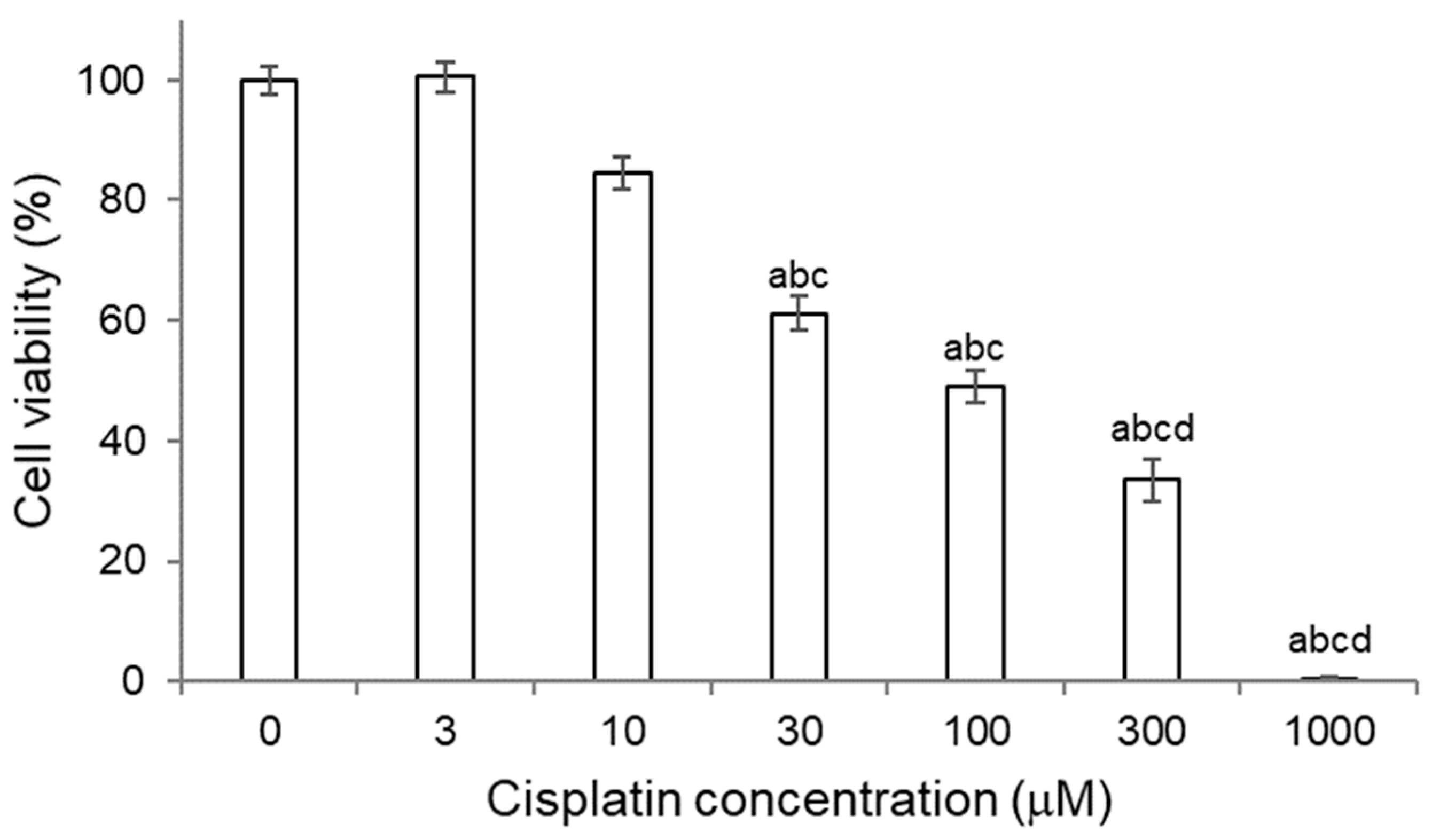
2.3.1. Titration of Cisplatin in HK-2 Cells
2.3.2. Titration of Quercetin and Its Metabolites in HK-2 Cells
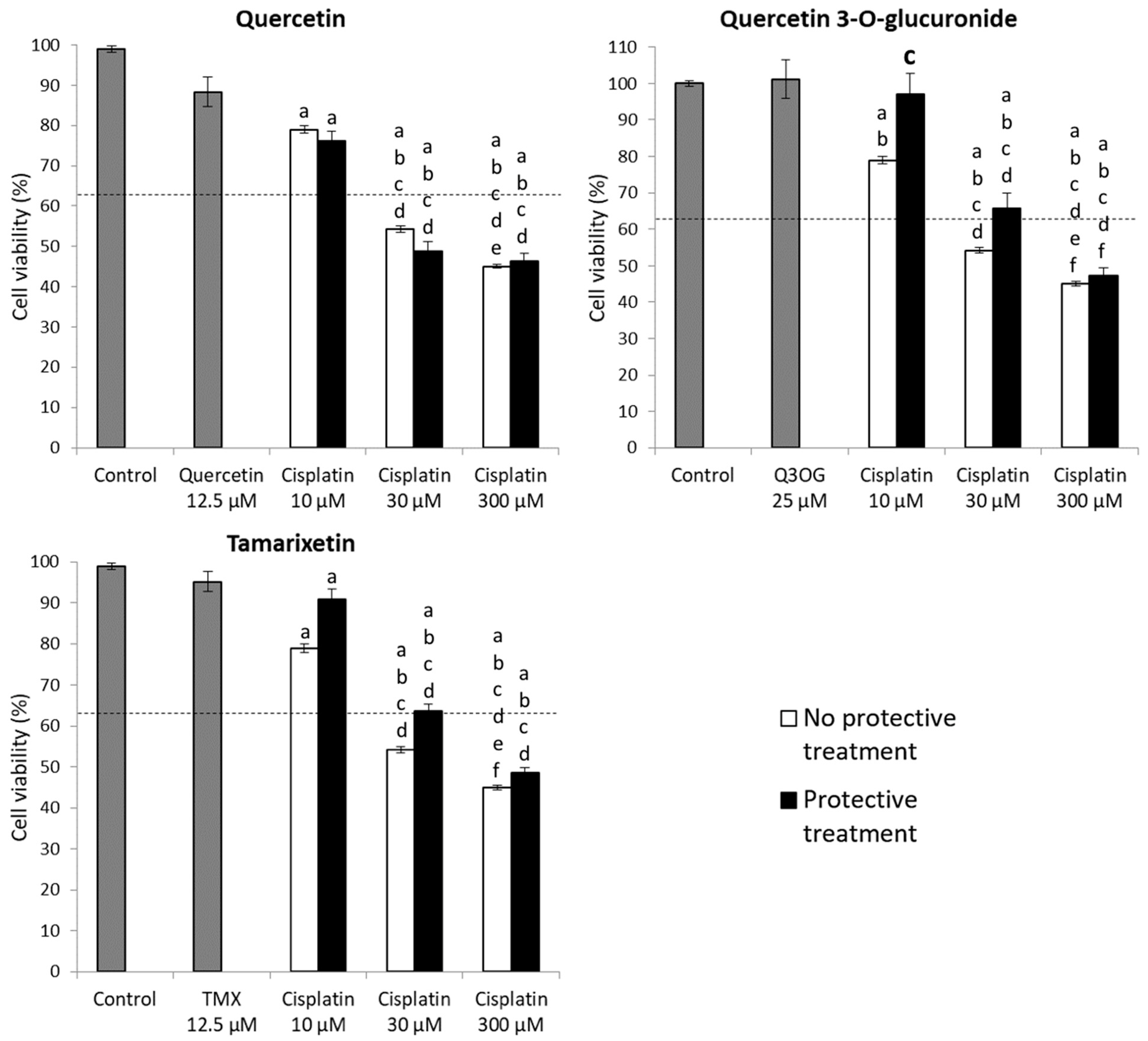
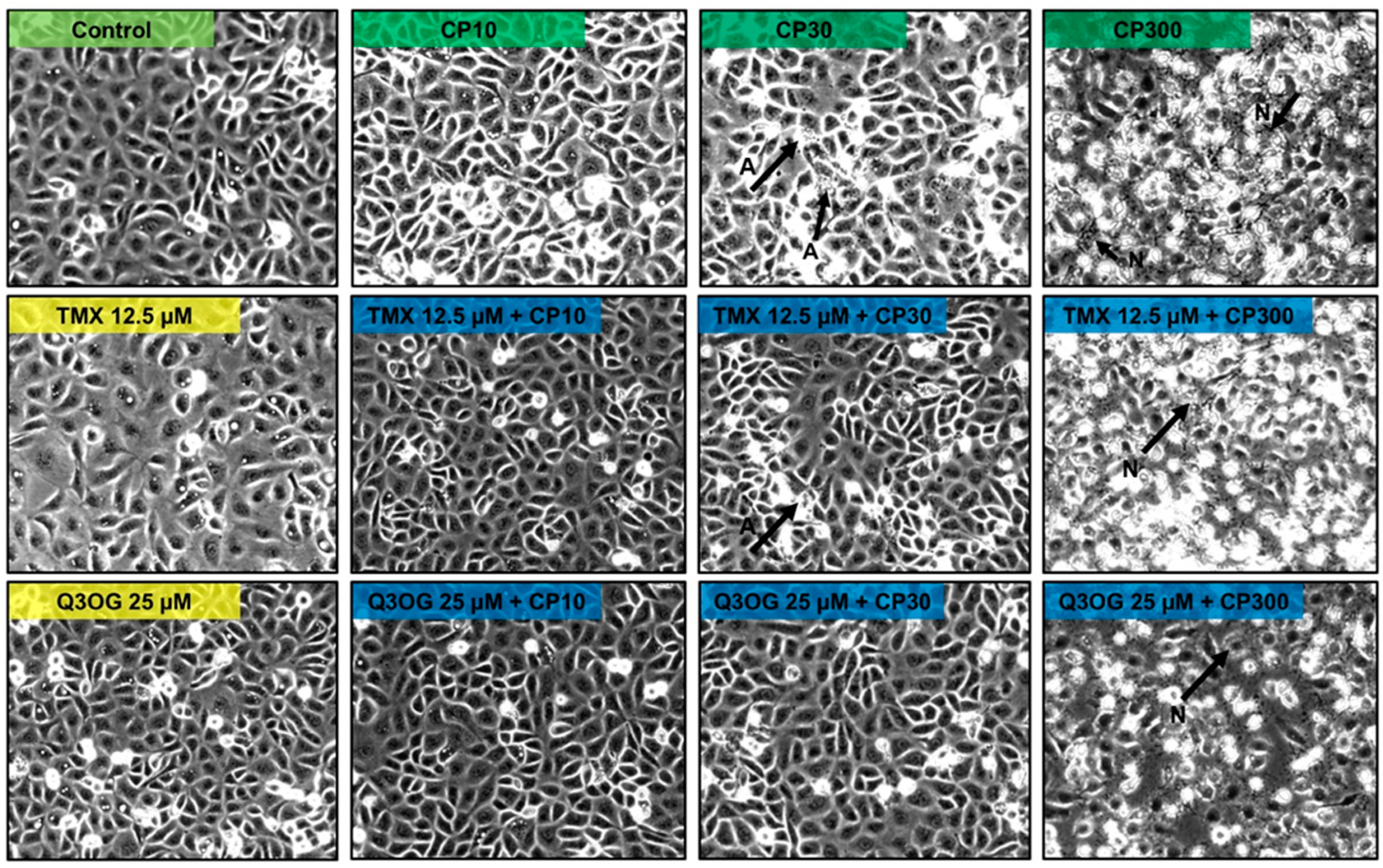
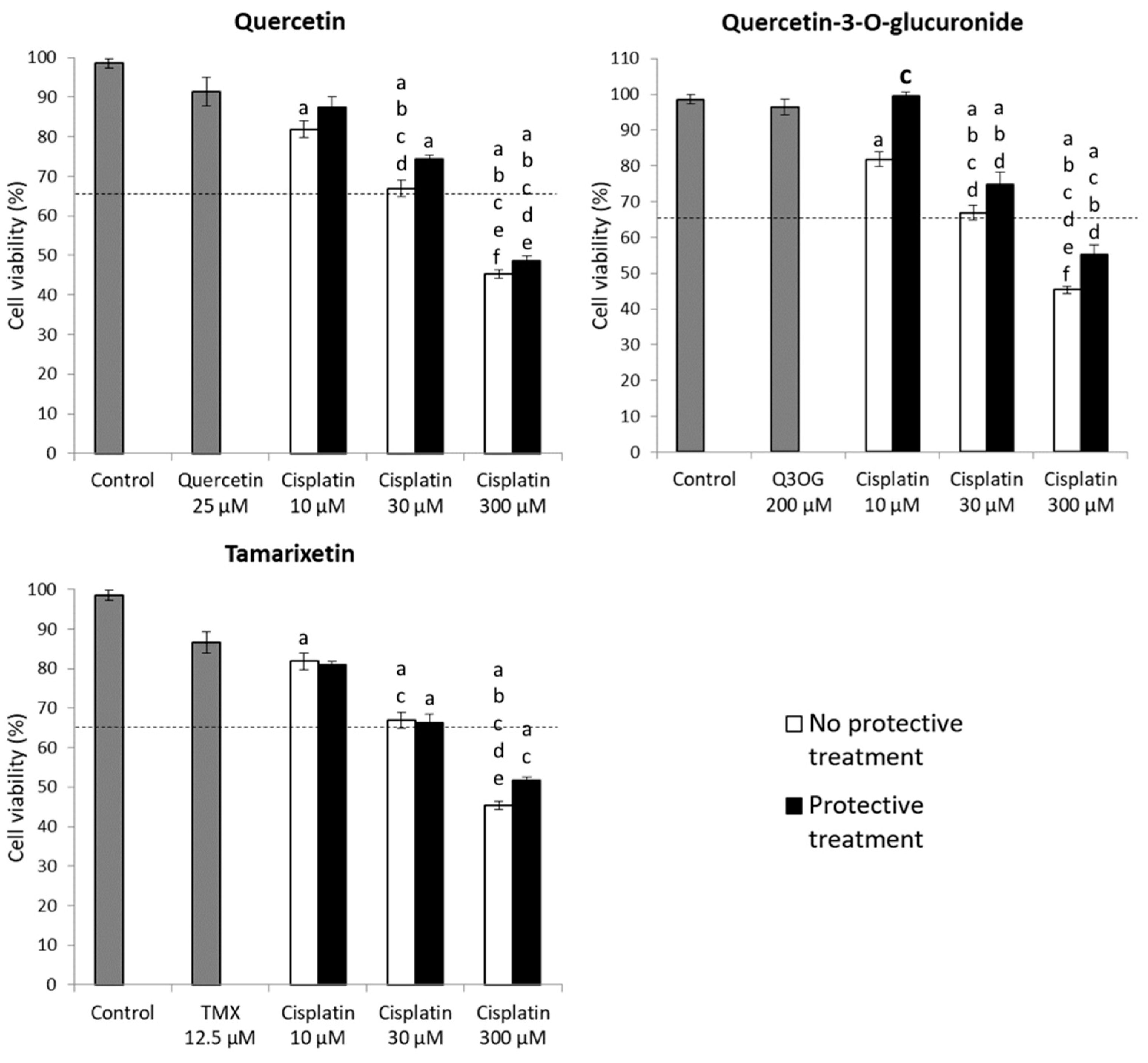
2.3.3. Protection Assays of Quercetin and Its Metabolites against Cisplatin Cytotoxicity in HK-2 Cells
2.4. Evaluation of the Protective Capacity of Quercetin and Its Metabolites against Cisplatin Cytotoxicity in NRK-52E Cells
2.4.1. Titration of Cisplatin in NRK-52E Cells
2.4.2. Titration of Quercetin and Its Metabolites in NRK-52E
2.4.3. Protection Assays of Quercetin and Its Metabolites against Cisplatin Cytotoxicity in NRK-52E Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Bioethics
4.2. Nephroprotection Study with Oral P-quercetin
4.3. Analysis of Quercetin and Its Metabolites in Blood and Urine Samples
4.4. Renal Cells
4.5. Quercetin Metabolites
4.6. Design of Experiments In Vitro
4.6.1. Titration of Cisplatin
4.6.2. Titration of Quercetin and Its Metabolites
4.6.3. Efficacy Experiments of Quercetin and Its Metabolites against Cisplatin Cytotoxicity
4.7. MTT Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin Nephrotoxicity: A Review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Leung, N. Cisplatin Nephrotoxicity: A Review of the Literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, P.D.; Lopez-Hernandez, F.J.; Perez-Barriocanal, F.; Morales, A.I.; Lopez-Novoa, J.M. Quercetin Reduces Cisplatin Nephrotoxicity in Rats without Compromising Its Anti-Tumour Activity. Nephrol. Dial. Transplant. 2011, 26, 3484–3495. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, M.; Burkhardt, G.; Kohler, H. Insights into Potential Cellular Mechanisms of Cisplatin Nephrotoxicity and Their Clinical Application. Nephrol. Dial. Transplant. 1997, 12, 2478–2480. [Google Scholar] [CrossRef]
- Sancho-Martínez, S.M.; Piedrafita, F.J.; Cannata-Andía, J.B.; López-Novoa, J.M.; López-Hernández, F.J. Necrotic Concentrations of Cisplatin Activate the Apoptotic Machinery but Inhibit Effector Caspases and Interfere with the Execution of Apoptosis. Toxicol. Sci. 2011, 122, 73–85. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin Nephrotoxicity: Mechanisms and Renoprotective Strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- DeHaan, R.D.; Yazlovitskaya, E.M.; Persons, D.L. Regulation of P53 Target Gene Expression by Cisplatin-Induced Extracellular Signal-Regulated Kinase. Cancer Chemother. Pharm. 2001, 48, 383–388. [Google Scholar] [CrossRef]
- Gluba, A.; Banach, M.; Hannam, S.; Mikhailidis, D.P.; Sakowicz, A.; Rysz, J. The Role of Toll-like Receptors in Renal Diseases. Nat. Rev. Nephrol. 2010, 6, 224–235. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Spagnuolo, C. The Pleiotropic Flavonoid Quercetin: From Its Metabolism to the Inhibition of Protein Kinases in Chronic Lymphocytic Leukemia. Food Funct. 2014, 5, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Reyes, D.; Morales, A.I.; Prieto, M. Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- González-Paramás, A.M.; Ayuda-Durán, B.; Martínez, S.; González-Manzano, S.; Santos-Buelga, C. The Mechanisms Behind the Biological Activity of Flavonoids. Curr. Med. Chem. 2019, 26, 6976–6990. [Google Scholar] [CrossRef]
- Yang, H.; Song, Y.; Liang, Y.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2018, 24, 4760–4766. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, P.D.; López-Hernández, F.J.; Dueñas, M.; Prieto, M.; Sánchez-López, E.; Thomale, J.; Ruiz-Ortega, M.; López-Novoa, J.M.; Morales, A.I. Differential Effect of Quercetin on Cisplatin-Induced Toxicity in Kidney and Tumor Tissues. Food Chem. Toxicol. 2017, 107, 226–236. [Google Scholar] [CrossRef]
- Casanova, A.G.; Prieto, M.; Colino, C.I.; Gutiérrez-Millán, C.; Ruszkowska-Ciastek, B.; de Paz, E.; Martín, Á.; Morales, A.I.; López-Hernández, F.J. A Micellar Formulation of Quercetin Prevents Cisplatin Nephrotoxicity. Int. J. Mol. Sci. 2021, 22, 729. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute Renal Failure—Definition, Outcome Measures, Animal Models, Fluid Therapy and Information Technology Needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004, 8, R204. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an Initiative to Improve Outcomes in Acute Kidney Injury. Crit Care 2007, 11, R31. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- De Corte, W.; Vanholder, R.; Dhondt, A.W.; De Waele, J.J.; Decruyenaere, J.; Danneels, C.; Claus, S.; Hoste, E.A.J. Serum Urea Concentration Is Probably Not Related to Outcome in ICU Patients with AKI and Renal Replacement Therapy. Nephrol. Dial. Transplant. 2011, 26, 3211–3218. [Google Scholar] [CrossRef][Green Version]
- Diskin, C.J. Creatinine and Glomerular Filtration Rate: Evolution of an Accommodation. Ann. Clin. Biochem. 2007, 44, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, P.D.; López-Hernández, F.J.; López-Novoa, J.M.; Morales, A.I. An Integrative View of the Pathophysiological Events Leading to Cisplatin Nephrotoxicity. Crit. Rev. Toxicol. 2011, 41, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Stafford, R.; Petrasovits, B.; Boone, M.; Valentovic, M. Establishment of HK-2 Cells as a Relevant Model to Study Tenofovir-Induced Cytotoxicity. Int. J. Mol. Sci. 2017, 18, 531. [Google Scholar] [CrossRef] [PubMed]
- Lash, L.H.; Putt, D.A.; Matherly, L.H. Protection of NRK-52E Cells, a Rat Renal Proximal Tubular Cell Line, from Chemical-Induced Apoptosis by Overexpression of a Mitochondrial Glutathione Transporter. J. Pharm. Exp. 2002, 303, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; González-Manzano, S.; Zarzuelo, M.J.; Gómez-Guzmán, M.; Quintela, A.M.; González-Paramás, A.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; Duarte, J.; Jiménez, R. Different Cardiovascular Protective Effects of Quercetin Administered Orally or Intraperitoneally in Spontaneously Hypertensive Rats. Food Funct. 2012, 3, 643. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation: Variability in Quercetin Bioavailability. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Nemeth, K.; Piskula, M.K. Food Content, Processing, Absorption and Metabolism of Onion Flavonoids. Crit. Rev. Food Sci. Nutr. 2007, 47, 397–409. [Google Scholar] [CrossRef]
- Semenova, S.; Rozov, S.; Panula, P. Distribution, Properties, and Inhibitor Sensitivity of Zebrafish Catechol-O-Methyl Transferases (COMT). Biochem. Pharmacol. 2017, 145, 147–157. [Google Scholar] [CrossRef]
- Wong, C.C.; Botting, N.P.; Orfila, C.; Al-Maharik, N.; Williamson, G. Flavonoid Conjugates Interact with Organic Anion Transporters (OATs) and Attenuate Cytotoxicity of Adefovir Mediated by Organic Anion Transporter 1 (OAT1/SLC22A6). Biochem. Pharmacol. 2011, 81, 942–949. [Google Scholar] [CrossRef]
- Baral, S.; Pariyar, R.; Kim, J.; Lee, H.-S.; Seo, J. Quercetin-3-O-Glucuronide Promotes the Proliferation and Migration of Neural Stem Cells. Neurobiol. Aging 2017, 52, 39–52. [Google Scholar] [CrossRef]
- Chen, X.; Wu, B.; Wang, P. Glucuronides in Anti-Cancer Therapy. Curr. Med. Chem.-Anti-Cancer Agents 2003, 3, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Incani, A.; Rosa, A.; Atzeri, A.; Loru, D.; Cabboi, B.; Paola Melis, M.; Lucas, R.; Morales, J.C.; Assunta Dessì, M. Hydroxytyrosol Glucuronides Protect Renal Tubular Epithelial Cells against H2O2 Induced Oxidative Damage. Chem.-Biol. Interact. 2011, 193, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Hirata, J.; Watanabe-Akanuma, M. Indoxyl Glucuronide, a Protein-Bound Uremic Toxin, Inhibits Hypoxia-Inducible Factor—dependent Erythropoietin Expression through Activation of Aryl Hydrocarbon Receptor. Biochem. Biophys. Res. Commun. 2018, 504, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, A.; Watanabe, K.; Akai, S.; Nishinosono, T.; Tsuneyama, K.; Oda, S.; Kume, T.; Yokoi, T. Zomepirac Acyl Glucuronide Is Responsible for Zomepirac-Induced Acute Kidney Injury in Mice. Drug Metab. Dispos. 2016, 44, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Steffen, P.; Keller, F. Analgesic Drug Therapy in Kidney Patients. Dtsch. Med. Wochenschr. 2021, 146, 1009–1015. [Google Scholar] [CrossRef]
- Yang, L.-L.; Xiao, N.; Li, X.-W.; Fan, Y.; Alolga, R.N.; Sun, X.-Y.; Wang, S.-L.; Li, P.; Qi, L.-W. Pharmacokinetic Comparison between Quercetin and Quercetin 3-O-β-Glucuronide in Rats by UHPLC-MS/MS. Sci. Rep. 2016, 6, 35460. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Santos-Buelga, C. The Flavonoid Paradox: Conjugation and Deconjugation as Key Steps for the Biological Activity of Flavonoids: The Flavonoid Paradox. J. Sci. Food Agric. 2012, 92, 1822–1825. [Google Scholar] [CrossRef]
- Menendez, C.; Dueñas, M.; Galindo, P.; González-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodríguez-Gómez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular Deconjugation of Quercetin Glucuronide: The Flavonoid Paradox Revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The Flavonoid Quercetin Induces Acute Vasodilator Effects in Healthy Volunteers: Correlation with Beta-Glucuronidase Activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Galindo, P.; Rodriguez-Gómez, I.; González-Manzano, S.; Dueñas, M.; Jiménez, R.; Menéndez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Pérez-Vizcaíno, F.; et al. Glucuronidated Quercetin Lowers Blood Pressure in Spontaneously Hypertensive Rats via Deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Angeles Ocete, M.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive Effects of the Flavonoid Quercetin in Spontaneously Hypertensive Rats: Quercetin and Hypertension. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Duarte, J.; Andriantsitohaina, R. Endothelial Function and Cardiovascular Disease: Effects of Quercetin and Wine Polyphenols. Free Radic. Res. 2006, 40, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Fraile, M.; Buratto, R.; Gómez, B.; Martín, Á.; Cocero, M.J. Enhanced Delivery of Quercetin by Encapsulation in Poloxamers by Supercritical Antisolvent Process. Ind. Eng. Chem. Res. 2014, 53, 4318–4327. [Google Scholar] [CrossRef]
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, Excretion and Metabolite Profiling of Methyl-, Glucuronyl-, Glucosyl- and Sulpho-Conjugates of Quercetin in Human Plasma and Urine after Ingestion of Onions. Br. J. Nutr. 2006, 96, 107. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; Santos-Buelga, C.; Cueva, C.; Martín-Cabrejas, M.A.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Chemical Characterization and in Vitro Colonic Fermentation of Grape Pomace Extracts. J. Sci. Food Agric. 2017, 97, 3433–3444. [Google Scholar] [CrossRef]


| Maximum Non-Toxic Concentration (μM) in HK-2 Cells | |
|---|---|
| Isorhamnetin | 1.56 |
| Quercetin | 12.5 |
| Quercetin 3-O-glucoside | 0.78 |
| Quercetin 3-O-glucuronide | 25 |
| Rutin | 25 |
| Tamarixetin | 12.5 |
| Maximum Non-Toxic Concentration in NRK-52E Cells (μM) | |
|---|---|
| Quercetin | 25 |
| Quercetin 3-O-glucuronide | 200 |
| Tamarixetin | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Reyes, D.; Casanova, A.G.; González-Paramás, A.M.; Martín, Á.; Santos-Buelga, C.; Morales, A.I.; López-Hernández, F.J.; Prieto, M. Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells. Molecules 2022, 27, 1319. https://doi.org/10.3390/molecules27041319
Muñoz-Reyes D, Casanova AG, González-Paramás AM, Martín Á, Santos-Buelga C, Morales AI, López-Hernández FJ, Prieto M. Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells. Molecules. 2022; 27(4):1319. https://doi.org/10.3390/molecules27041319
Chicago/Turabian StyleMuñoz-Reyes, Daniel, Alfredo G. Casanova, Ana María González-Paramás, Ángel Martín, Celestino Santos-Buelga, Ana I. Morales, Francisco J. López-Hernández, and Marta Prieto. 2022. "Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells" Molecules 27, no. 4: 1319. https://doi.org/10.3390/molecules27041319
APA StyleMuñoz-Reyes, D., Casanova, A. G., González-Paramás, A. M., Martín, Á., Santos-Buelga, C., Morales, A. I., López-Hernández, F. J., & Prieto, M. (2022). Protective Effect of Quercetin 3-O-Glucuronide against Cisplatin Cytotoxicity in Renal Tubular Cells. Molecules, 27(4), 1319. https://doi.org/10.3390/molecules27041319








