Elemental Chemometrics as Tools to Depict Stalked Barnacle (Pollicipes pollicipes) Harvest Locations and Food Safety
Abstract
1. Introduction
2. Results
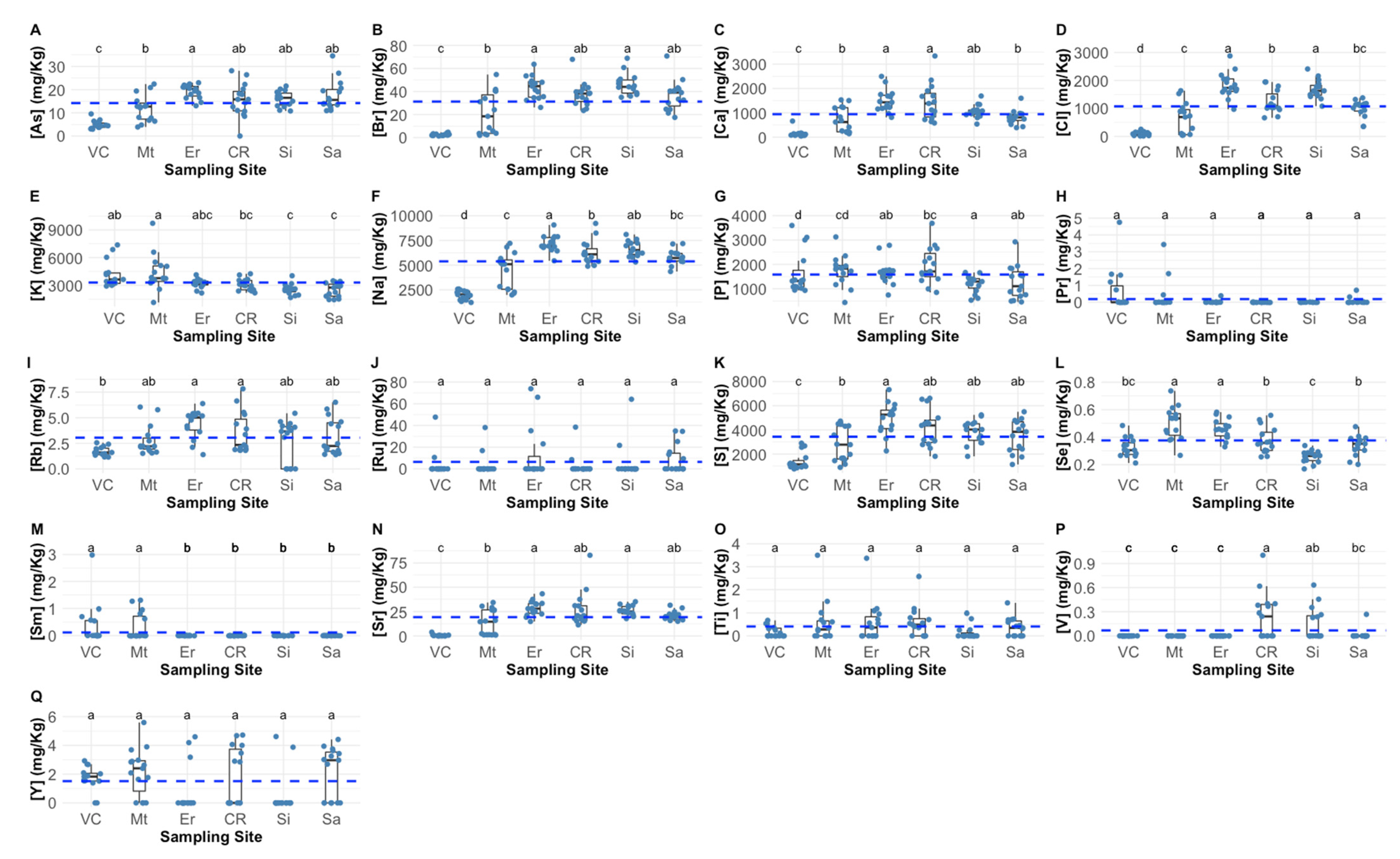
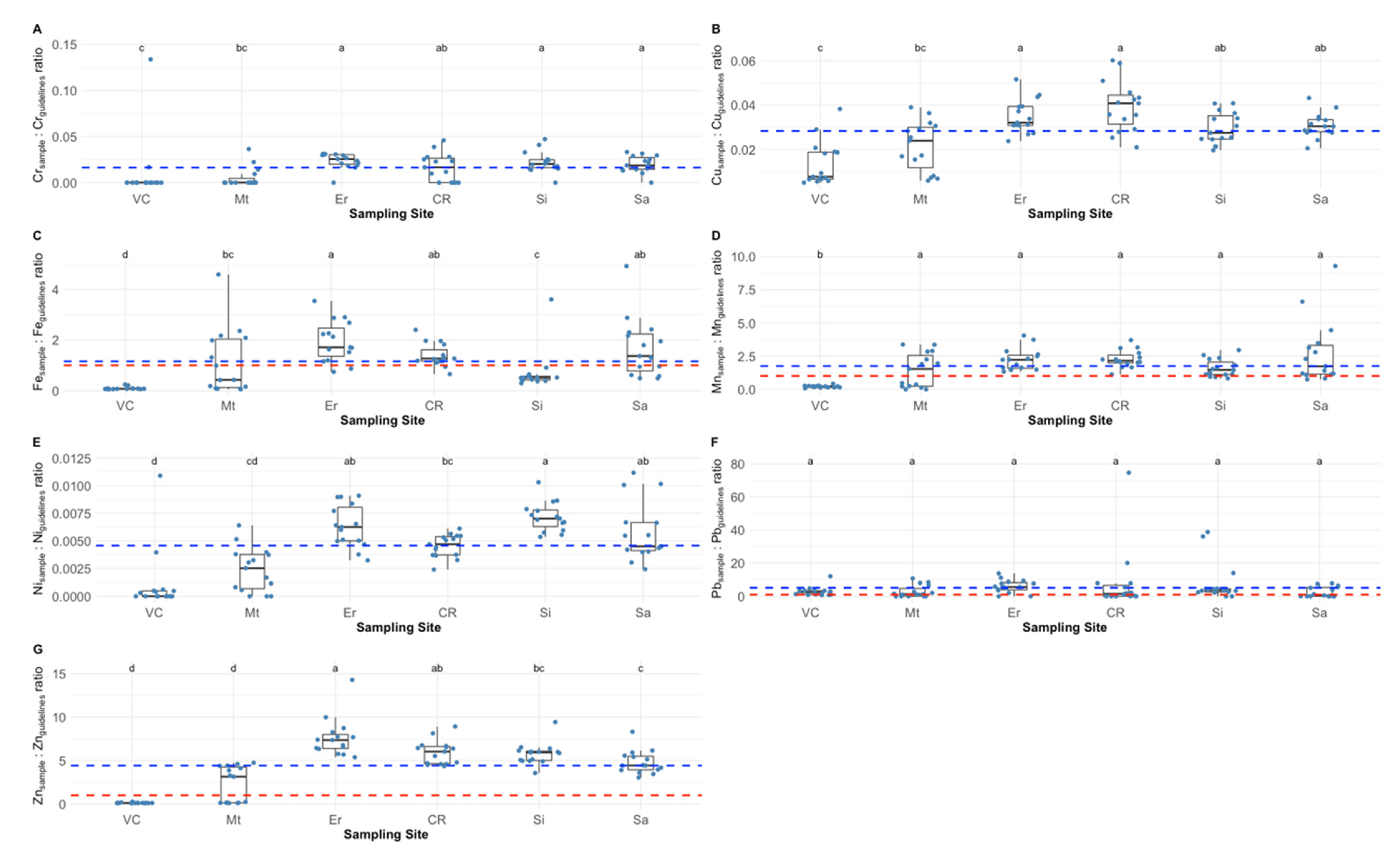
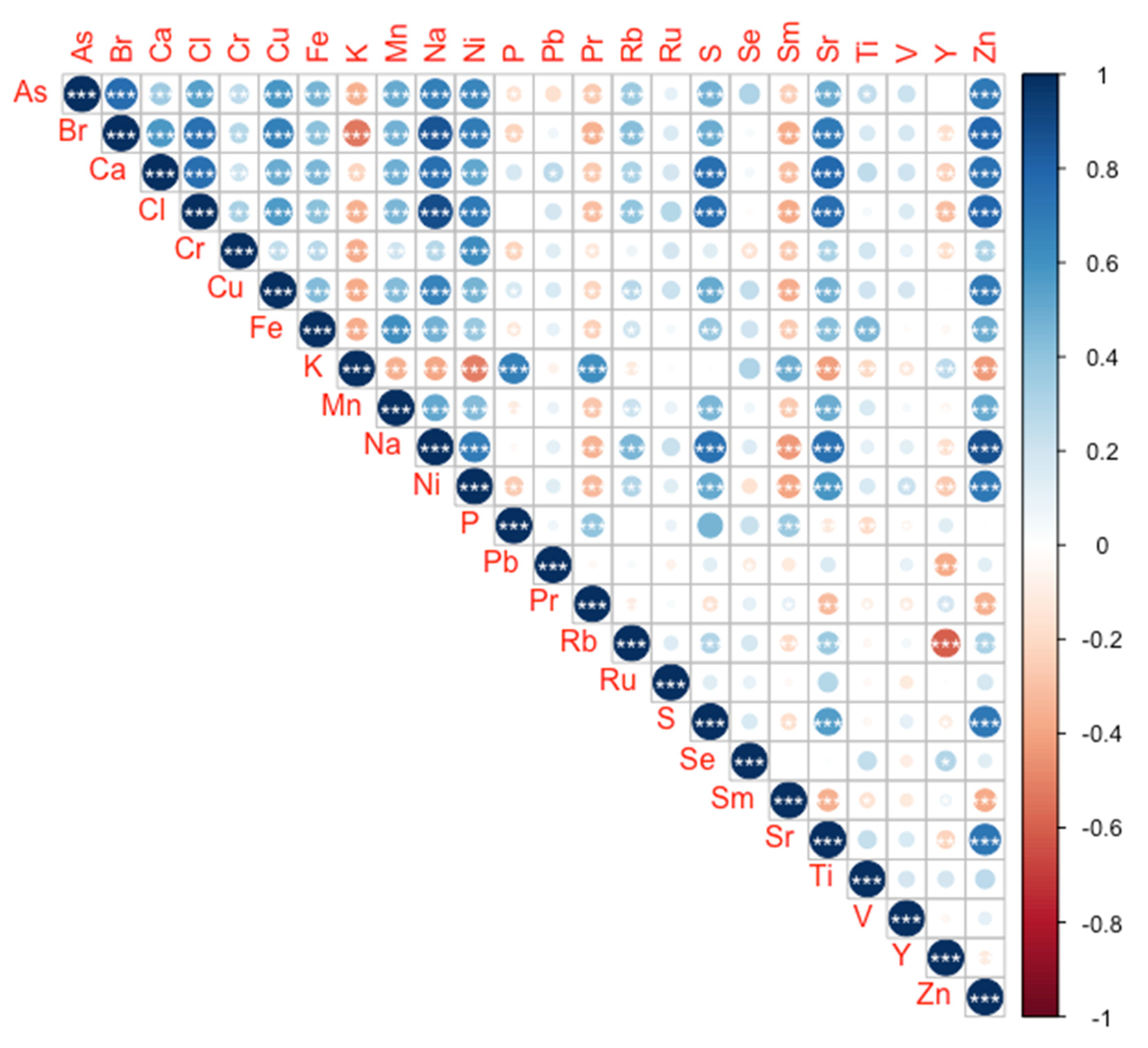
2.1. Barnacle Peduncle Elemental Concentrations and Safety Values
2.2. Chemometric Provenance Classification
3. Discussion
4. Materials and Methods
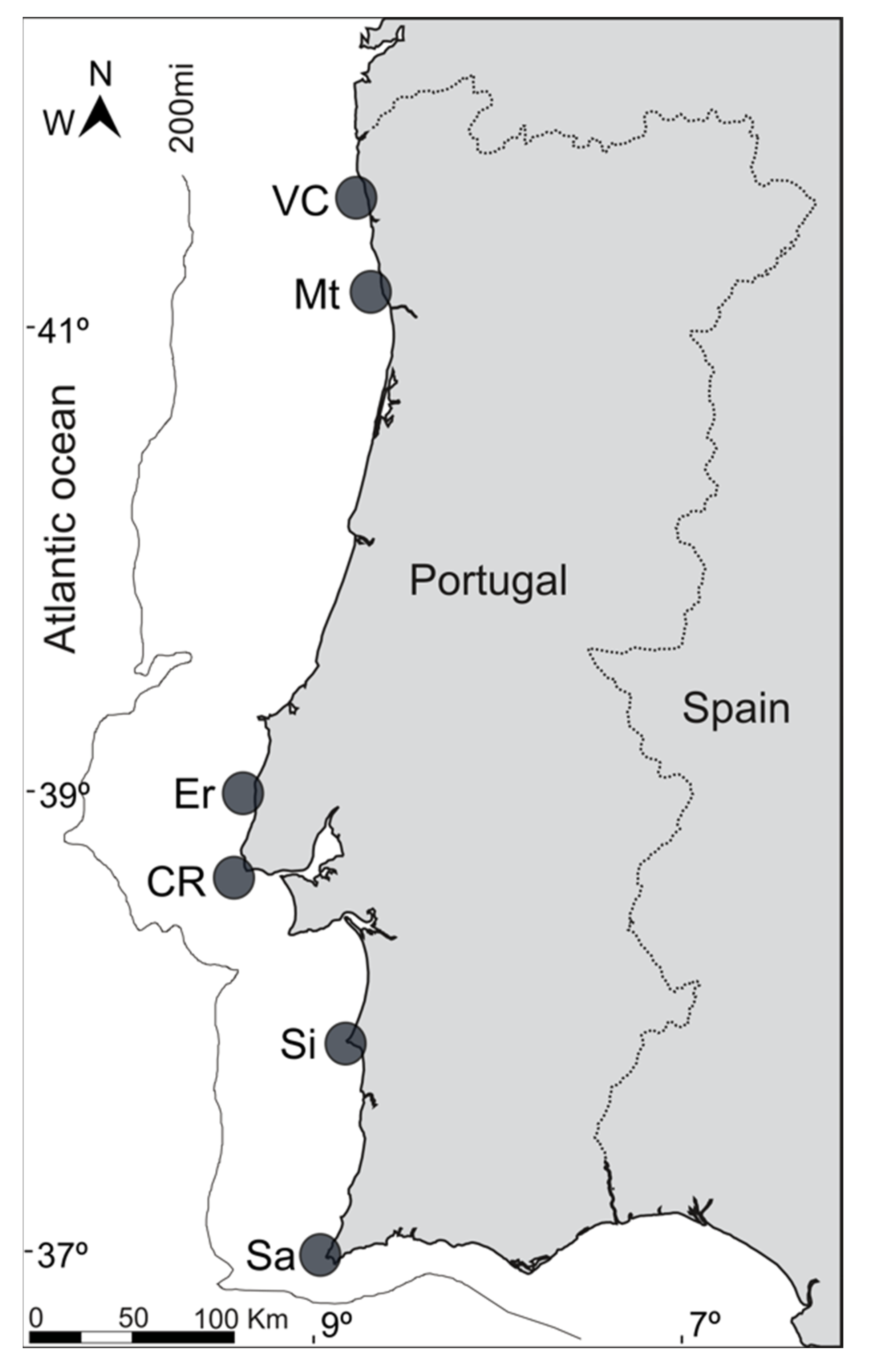
4.1. Sample Collection
4.2. Total Elemental Fingerprinting
4.3. Statistical and Chemometric Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Reilly, A. Overview of Food Fraud in the Fisheries Sector; Fisheries and Aquaculture Circular; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2018; p. 32. [Google Scholar]
- He, J. From Country-of-Origin Labelling (COOL) to Seafood Import Monitoring Program (SIMP): How Far Can Seafood Traceability Rules Go? Mar. Policy 2018, 96, 163–174. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) No 1379/2013 of the European Parliament and of the Council of 11 December 2013 on the Common Organization of the Markets in Fishery and Aquaculture Products, Amending Council Regulations (EC) No 1184/2006 and (EC) No 1224/2009 and Repealing Council Regulation (EC) No 104/2000, European Parliament and Council of the European Union: Brussels, Belgium, 11 December 2013; Volume 354.
- Leal, M.C.; Pimentel, T.; Ricardo, F.; Rosa, R.; Calado, R. Seafood Traceability: Current Needs, Available Tools, and Biotechnological Challenges for Origin Certification. Trends Biotechnol. 2015, 33, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Labra, M.; Sandionigi, A.; Bruno, A.; Mezzasalma, V.; De Mattia, F. DNA Barcoding for Minor Crops and Food Traceability. Adv. Agric. 2014, 2014, 831875. [Google Scholar] [CrossRef]
- Ogden, R. Fisheries Forensics: The Use of DNA Tools for Improving Compliance, Traceability and Enforcement in the Fishing Industry. Fish Fish. 2008, 9, 462–472. [Google Scholar] [CrossRef]
- Portarena, S.; Farinelli, D.; Lauteri, M.; Famiani, F.; Esti, M.; Brugnoli, E. Stable Isotope and Fatty Acid Compositions of Monovarietal Olive Oils: Implications of Ripening Stage and Climate Effects as Determinants in Traceability Studies. Food Control 2015, 57, 129–135. [Google Scholar] [CrossRef]
- Ricardo, F.; Gonçalves, D.; Pimentel, T.; Mamede, R.; Rosário, M.; Domingues, M.; Lillebø, A.I.; Calado, R. Prevalence of Phylogenetic over Environmental Drivers on the Fatty Acid Profiles of the Adductor Muscle of Marine Bivalves and Its Relevance for Traceability. Ecol. Indic. 2021, 129, 108017. [Google Scholar] [CrossRef]
- Montet, D.; Nguyen, D.D.L.; Sheikha, A.F.E.; Condur, A.; Métayer, I.; Loiseau, G. Application of PCR-DGGE in Determining Food Origin. Asp. Appl. Biol. 2008, 87, 12. [Google Scholar]
- Albuquerque, R.; Queiroga, H.; Swearer, S.E.; Calado, R.; Leandro, S.M. Harvest Locations of Goose Barnacles Can Be Successfully Discriminated Using Trace Elemental Signatures. Sci. Rep. 2016, 6, 27787. [Google Scholar] [CrossRef]
- Bennion, M.; Morrison, L.; Shelley, R.; Graham, C. Trace Elemental Fingerprinting of Shells and Soft Tissues Can Identify the Time of Blue Mussel (Mytilus Edulis) Harvesting. Food Control 2021, 121, 107515. [Google Scholar] [CrossRef]
- Davis, R.P.; Boyd, C.E.; Davis, D.A. The Utility of Discriminant Analysis to Determine the Geographic Origin of Commercially Important Seafood and Aquaculture Species: A Meta-Analysis. Rev. Fish. Sci. Aquac. 2021, 29, 791–799. [Google Scholar] [CrossRef]
- Duarte, B.; Duarte, I.A.; Caçador, I.; Reis-Santos, P.; Vasconcelos, R.P.; Gameiro, C.; Tanner, S.E.; Fonseca, V.F. Elemental Fingerprinting of Thornback Ray (Raja Clavata) Muscle Tissue as a Tracer for Provenance and Food Safety Assessment. Food Control 2022, 133, 108592. [Google Scholar] [CrossRef]
- Mamede, R.; Ricardo, F.; Gonçalves, D.; Ferreira da Silva, E.; Patinha, C.; Calado, R. Assessing the Use of Surrogate Species for a More Cost-Effective Traceability of Geographic Origin Using Elemental Fingerprints of Bivalve Shells. Ecol. Indic. 2021, 130, 108065. [Google Scholar] [CrossRef]
- Vieira, S.; Barrulas, P.; Chainho, P.; Dias, C.B.; Sroczyńska, K.; Adão, H. Spatial and Temporal Distribution of the Multi-Element Signatures of the Estuarine Non-Indigenous Bivalve Ruditapes Philippinarum. Biol. Trace Elem. Res. 2022, 200, 385–401. [Google Scholar] [CrossRef]
- Kelly, S.; Heaton, K.; Hoogewerff, J. Tracing the Geographical Origin of Food: The Application of Multi-Element and Multi-Isotope Analysis. Trends Food Sci. Technol. 2005, 16, 555–567. [Google Scholar] [CrossRef]
- Lim, C.M.; Carey, M.; Williams, P.N.; Koidis, A. Rapid Classification of Commercial Teas According to Their Origin and Type Using Elemental Content with X-Ray Fluorescence (XRF) Spectroscopy. Curr. Res. Food Sci. 2021, 4, 45–52. [Google Scholar] [CrossRef]
- Rajapaksha, D.; Waduge, V.; Padilla-Alvarez, R.; Kalpage, M.; Rathnayake, R.M.N.P.; Migliori, A.; Frew, R.; Abeysinghe, S.; Abrahim, A.; Amarakoon, T. XRF to Support Food Traceability Studies: Classification of Sri Lankan Tea Based on Their Region of Origin. X-ray Spectrom. 2017, 46, 220–224. [Google Scholar] [CrossRef]
- López, D.A.; López, B.A.; Pham, C.K.; Isidro, E.J.; De Girolamo, M. Barnacle Culture: Background, Potential and Challenges. Aquac. Res. 2010, 41, e367–e375. [Google Scholar] [CrossRef]
- Aguión, A.; Ojea, E.; García-Flórez, L.; Cruz, T.; Garmendia, J.M.; Davoult, D.; Queiroga, H.; Rivera, A.; Acuña-Fernández, J.L.; Macho, G. Establishing a Governance Threshold in Small-Scale Fisheries to Achieve Sustainability. Ambio 2021, 51, 652–665. [Google Scholar] [CrossRef]
- Jacinto, D.; Cruz, T.; Silva, T.; Castro, J.J. Stalked Barnacle (Pollicipes Pollicipes) Harvesting in the Berlengas Nature Reserve, Portugal: Temporal Variation and Validation of Logbook Data. ICES J. Mar. Sci. 2010, 67, 19–25. [Google Scholar] [CrossRef]
- Jacinto, D.; Cruz, T.; Silva, T.; Castro, J.J. Management of the Stalked Barnacle (Pollicipes Pollicipes) Fishery in the Berlengas Nature Reserve (Portugal): Evaluation of Bag and Size Limit Regulation Measures. Sci. Mar. 2011, 75, 439–445. [Google Scholar] [CrossRef]
- Sousa, A.; Jacinto, D.; Penteado, N.; Martins, P.; Fernandes, J.; Silva, T.; Castro, J.J.; Cruz, T. Patterns of Distribution and Abundance of the Stalked Barnacle (Pollicipes Pollicipes) in the Central and Southwest Coast of Continental Portugal. J. Sea Res. 2013, 83, 187–194. [Google Scholar] [CrossRef]
- Caçador, I.; Costa, J.L.; Duarte, B.; Silva, G.; Medeiros, J.P.; Azeda, C.; Castro, N.; Freitas, J.; Pedro, S.; Almeida, P.R.; et al. Macroinvertebrates and Fishes as Biomonitors of Heavy Metal Concentration in the Seixal Bay (Tagus Estuary): Which Species Perform Better? Ecol. Indic. 2012, 19, 184–190. [Google Scholar] [CrossRef]
- Fonseca, V.F.; França, S.; Duarte, B.; Caçador, I.; Cabral, H.N.; Mieiro, C.L.; Coelho, J.P.; Pereira, E.; Reis-Santos, P. Spatial Variation in Mercury Bioaccumulation and Magnification in a Temperate Estuarine Food Web. Front. Mar. Sci. 2019, 6, 117. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Tanner, S.E.; França, S.; Vasconcelos, R.P.; Gillanders, B.M.; Cabral, H.N. Connectivity within Estuaries: An Otolith Chemistry and Muscle Stable Isotope Approach. Ocean. Coast. Manag. 2015, 118, 51–59. [Google Scholar] [CrossRef]
- Cruz, T.; Jacinto, D.; Sousa, A.; Penteado, N.; Pereira, D.; Fernandes, J.N.; Silva, T.; Castro, J.J. The State of the Fishery, Conservation and Management of the Stalked Barnacle Pollicipes Pollicipes in Portugal. Mar. Environ. Res. 2015, 112, 73–80. [Google Scholar] [CrossRef]
- Varrà, M.O.; Husáková, L.; Patočka, J.; Ghidini, S.; Zanardi, E. Multi-Element Signature of Cuttlefish and Its Potential for the Discrimination of Different Geographical Provenances and Traceability. Food Chem. 2021, 356, 129687. [Google Scholar] [CrossRef]
- Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics; Princeton University Press: Princeton Woodstock, NJ, USA, 2006. [Google Scholar]
- Barnes, M. Pedunculate Cirripedes of the Genus Pollicipes. Oceanogr. Mar. Biol. 1996, 4, 303–394. [Google Scholar]
- Rodríguez-Navarro, A.; Rubio, F. High-Affinity Potassium and Sodium Transport Systems in Plants. J. Exp. Bot. 2006, 57, 1149–1160. [Google Scholar] [CrossRef]
- Smith, G. Step Away from Stepwise. J. Big Data 2018, 5, 32. [Google Scholar] [CrossRef]
- Rashid, N.A.; Hussain, W.S.E.C.; Ahmad, A.R.; Abdullah, F.N. Performance of Classification Analysis: A Comparative Study between PLS-DA and Integrating PCA+LDA. Math. Stat. 2019, 7, 24–28. [Google Scholar] [CrossRef]
- Brereton, R.G.; Lloyd, G.R. Partial Least Squares Discriminant Analysis: Taking the Magic Away. J. Chemom. 2014, 28, 213–225. [Google Scholar] [CrossRef]
- Ricardo, F.; Génio, L.; Costa Leal, M.; Albuquerque, R.; Queiroga, H.; Rosa, R.; Calado, R. Trace Element Fingerprinting of Cockle (Cerastoderma Edule) Shells Can Reveal Harvesting Location in Adjacent Areas. Sci. Rep. 2015, 5, 11932. [Google Scholar] [CrossRef]
- Cruz, T.; Hawkins, S.J. Reproductive Cycle of Pollicipes Pollicipes at Cabo De Sines, South-West Coast of Portugal. J. Mar. Biol. Assoc. United Kingd. 1998, 78, 483–496. [Google Scholar] [CrossRef]
- Costas-Rodríguez, M.; Lavilla, I.; Bendicho, C. Classification of Cultivated Mussels from Galicia (Northwest Spain) with European Protected Designation of Origin Using Trace Element Fingerprint and Chemometric Analysis. Anal. Chim. Acta 2010, 664, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, R.P.; Reis-Santos, P.; Fonseca, V.; Maia, A.; Ruano, M.; França, S.; Vinagre, C.; Costa, M.J.; Cabral, H. Assessing Anthropogenic Pressures on Estuarine Fish Nurseries along the Portuguese Coast: A Multi-Metric Index and Conceptual Approach. Sci. Total Environ. 2007, 374, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.A.; Salgado, M.A.; Vasconcelos, V. Goose Barnacle Pollicipes Pollicipes as Biomonitor of Metal Contamination in the Northwest Coast of Portugal. Environ. Monit. Assess. 2012, 184, 6987–7000. [Google Scholar] [CrossRef]
- Reis, P.A.; Salgado, M.A.; Vasconcelos, V. Seasonal Variation of Metal Contamination in the Barnacles Pollicipes Pollicipes in Northwest Coast of Portugal Show Clear Correlation with Levels in the Surrounding Water. Mar. Pollut. Bull. 2013, 70, 155–161. [Google Scholar] [CrossRef]
- Towett, E.K.; Shepherd, K.D.; Cadisch, G. Quantification of Total Element Concentrations in Soils Using Total X-Ray Fluorescence Spectroscopy (TXRF). Sci. Total Environ. 2013, 463–464, 374–388. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Test Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; Environmental Protection Agency (EPA): Washington, DC, USA, 1996; p. 20. [Google Scholar]
- European Commission Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs, European Commission: Tokyo, Japan, 2006.
- FAO/WHO Evaluation of Certain Food Additives and the Contaminants Mercury, Lead and Cadmium; WHO Technical Report; WHO: Geneva, Switzerland, 1989.
- FAO/WHO List of Maximum Levels Recommended for Contaminants by the Joint FAO/WHO Codex Alimentarius Commission; Second Series CAC/FAL; WHO: Rome, Italy, 1984; p. 8.
- De Mendiburu, F.; Simon, R. Agricolae-Ten Years of an Open Source Statistical Tool for Experiments in Breeding, Agriculture and Biology. PeerJ Prepr. 2015, 3, e1404v1. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Use R! Springer: New York, NY, USA, 2009; ISBN 978-0-387-98141-3. [Google Scholar]
- Taiyun. Taiyun/Corrplot; Taiyun: Guangzhou, China, 2021. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006; Volume 192. [Google Scholar] [CrossRef]
- Duarte, B.; Carreiras, J.; Feijão, E.; Reis-Santos, P.; Caçador, I.; Matos, A.R.; Fonseca, V.F. Fatty Acid Profiles of Estuarine Macroalgae Are Biomarkers of Anthropogenic Pressures: Development and Application of a Multivariate Pressure Index. Sci. Total Environ. 2021, 788, 147817. [Google Scholar] [CrossRef]
- Sanchez, G. Package ‘DiscriMiner’. 2013. Available online: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.408.5145&rep=rep1&type=pdf (accessed on 26 January 2022).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: Berlin, Germany, 2002. [Google Scholar]
- Röver, C.; Raabe, N.; Luebke, K.; Ligges, U. KlaR: A Package Including Various Classification Tools; University Dortmund: Dortmund, Germany, 2004. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2–3, 18–22. [Google Scholar]
- Fawcett, T. An Introduction to ROC Analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
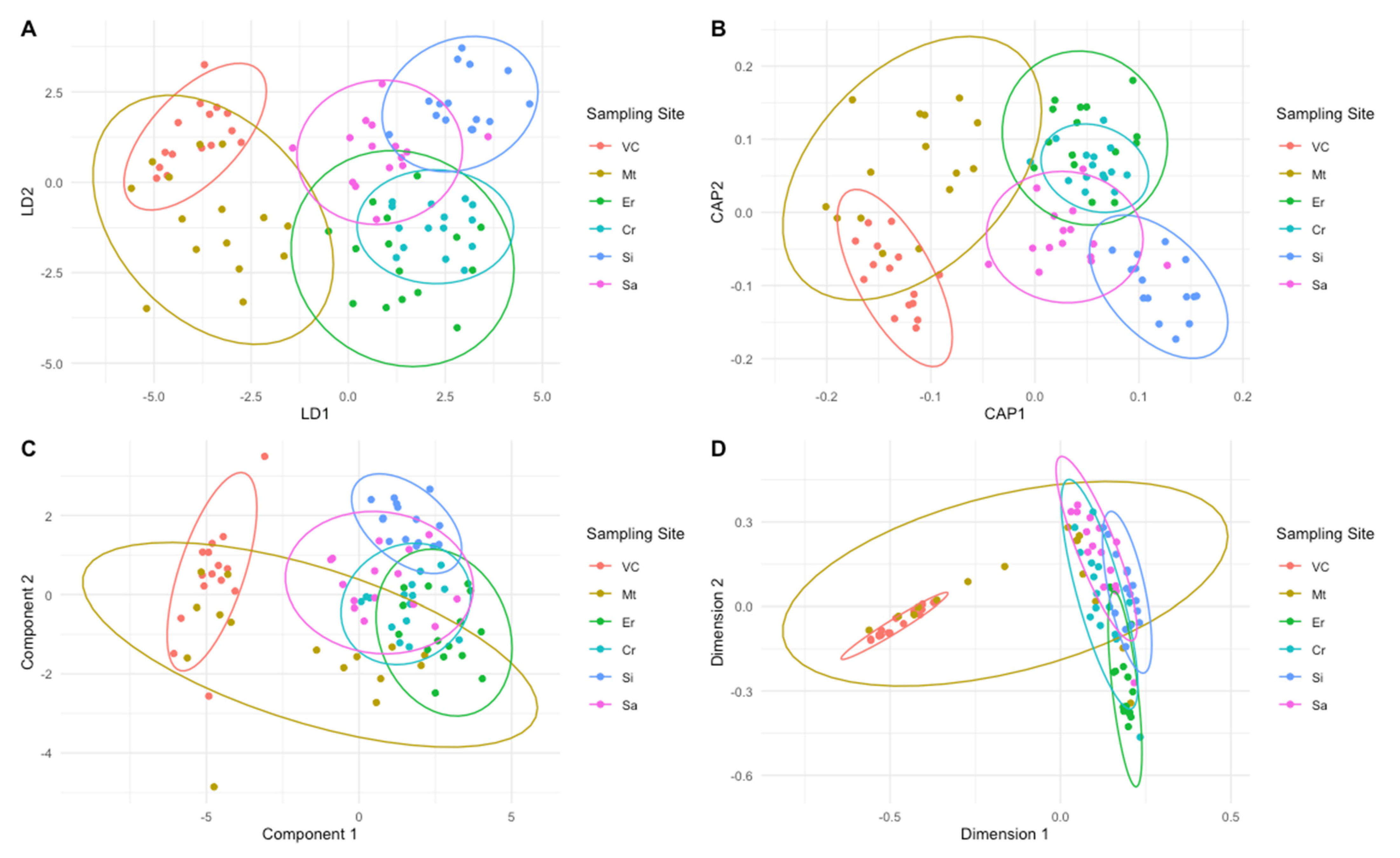

| Predicted | Overall Accuracy (%) | Accuracy (%) | Precision (%) | Sensitivity (%) | Specificity (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | Origin | VC | Mt | Er | CR | Si | Sa | |||||
| PLS-DA | VC | 14 | 1 | 0 | 0 | 0 | 0 | 77.8 | 90.9 | 70.0 | 93.3 | 90.3 |
| Mt | 6 | 6 | 2 | 0 | 0 | 1 | 85.4 | 66.7 | 40.0 | 95.5 | ||
| Er | 0 | 0 | 14 | 0 | 0 | 1 | 90.9 | 70.0 | 93.3 | 90.3 | ||
| CR | 0 | 2 | 1 | 11 | 1 | 1 | 93.3 | 91.7 | 73.3 | 98.3 | ||
| Si | 0 | 0 | 0 | 0 | 15 | 0 | 98.6 | 93.8 | 100.0 | 98.2 | ||
| Sa | 0 | 2 | 1 | 1 | 1 | 10 | 89.7 | 76.9 | 66.7 | 95.2 | ||
| VIP-PLS-DA | VC | 14 | 1 | 0 | 0 | 0 | 0 | 77.8 | 90.9 | 70.0 | 93.3 | 90.3 |
| Mt | 5 | 10 | 0 | 0 | 0 | 0 | 88.6 | 71.4 | 66.7 | 93.8 | ||
| Er | 0 | 0 | 13 | 0 | 1 | 1 | 94.6 | 86.7 | 86.7 | 96.6 | ||
| CR | 0 | 3 | 1 | 8 | 1 | 2 | 89.7 | 88.9 | 53.3 | 98.4 | ||
| Si | 0 | 0 | 1 | 0 | 14 | 0 | 93.33 | 77.78 | 93.33 | 93.33 | ||
| Sa | 1 | 0 | 0 | 1 | 2 | 11 | 90.9 | 78.6 | 73.3 | 95.2 | ||
| LDA (validation set) | VC | 3 | 0 | 0 | 0 | 0 | 0 | 72.7 | 91.4 | 50.0 | 100.0 | 90.6 |
| Mt | 1 | 4 | 0 | 0 | 0 | 0 | 97.0 | 100.0 | 80.0 | 100.0 | ||
| Er | 0 | 0 | 6 | 2 | 0 | 2 | 84.2 | 75.0 | 60.0 | 92.9 | ||
| CR | 2 | 0 | 1 | 7 | 0 | 1 | 84.2 | 77.8 | 63.6 | 92.6 | ||
| Si | 0 | 0 | 1 | 0 | 7 | 1 | 91.4 | 87.5 | 77.8 | 96.2 | ||
| Sa | 0 | 0 | 0 | 0 | 1 | 5 | 86.5 | 55.6 | 83.3 | 87.1 | ||
| S-LDA | VC | 14 | 1 | 0 | 0 | 0 | 0 | 84.4 | 93.8 | 77.8 | 93.3 | 93.9 |
| Mt | 4 | 11 | 0 | 0 | 0 | 0 | 93.8 | 91.6 | 73.3 | 98.5 | ||
| Er | 0 | 0 | 14 | 0 | 1 | 0 | 93.8 | 77.8 | 93.3 | 93.9 | ||
| CR | 0 | 0 | 3 | 11 | 0 | 1 | 93.8 | 91.6 | 73.3 | 98.5 | ||
| Si | 0 | 0 | 0 | 0 | 14 | 1 | 96.2 | 87.4 | 93.3 | 96.9 | ||
| Sa | 0 | 0 | 1 | 1 | 1 | 12 | 93.8 | 85.7 | 79.9 | 97.0 | ||
| RF | VC | 12 | 3 | 0 | 0 | 0 | 0 | 70.0 | 92.6 | 85.7 | 80.0 | 96.2 |
| Mt | 2 | 10 | 2 | 0 | 0 | 1 | 87.5 | 71.4 | 66.7 | 93.0 | ||
| Er | 0 | 0 | 12 | 0 | 1 | 2 | 88.7 | 70.6 | 80.0 | 91.1 | ||
| CR | 0 | 1 | 2 | 8 | 2 | 2 | 86.3 | 72.7 | 53.3 | 94.8 | ||
| Si | 0 | 0 | 0 | 0 | 13 | 2 | 88.7 | 68.4 | 86.7 | 89.3 | ||
| Sa | 0 | 0 | 1 | 3 | 3 | 8 | 81.8 | 53.3 | 53.3 | 88.7 | ||
| CAP | VC | 11 | 3 | 0 | 0 | 0 | 1 | 73.3 | 88.0 | 68.8 | 73.3 | 91.7 |
| Mt | 4 | 10 | 1 | 0 | 0 | 0 | 88.0 | 71.4 | 66.7 | 93.3 | ||
| Er | 0 | 0 | 12 | 1 | 1 | 1 | 89.2 | 70.6 | 80.0 | 91.5 | ||
| CR | 0 | 1 | 2 | 11 | 0 | 1 | 89.2 | 73.3 | 73.3 | 93.2 | ||
| Si | 0 | 0 | 0 | 2 | 12 | 1 | 93.0 | 85.7 | 80.0 | 96.4 | ||
| Sa | 1 | 0 | 2 | 1 | 1 | 10 | 88.0 | 71.4 | 66.7 | 93.3 | ||
| Element | Certified Value | Uncertainty | Measured Value | Extraction Efficiency (%) |
|---|---|---|---|---|
| Cr | 0.73 | 0.22 | 0.67 ± 0.10 | 91.3 ± 5.3 |
| Mn | 4.88 | 0.24 | 3.35 ± 0.10 | 68.6 ± 1.9 |
| Fe | 161.0 | 8.0 | 198.03 ± 0.67 | 123.0 ± 0.4 |
| Ni | 0.69 | 0.15 | 0.78 ± 0.05 | 113.1 ± 5.8 |
| Cu | 5.98 | 0.27 | 7.10 ± 0.07 | 118,8 ± 1.0 |
| Zn | 71.0 | 4.0 | 73.29 ± 0.28 | 103.2 ± 0.4 |
| As | 6.7 | 0.4 | 7.25 ± 0.07 | 108.2 ± 0.9 |
| Se | 1.62 | 0.12 | 1.51 ± 0.03 | 93.3 ± 1.9 |
| Rb | 2.46 | 0.16 | 2.45 ± 0.05 | 99.6 ± 1.9 |
| Sr | 19.0 | 0.0 | 18.55 ± 0.34 | 97.6 ± 1.8 |
| Cd | 0.336 | 0.025 | 0.32 ± 0.02 | 96.6 ± 4.5 |
| Pb | 2.18 | 0.18 | 2.47 ± 0.05 | 113.3 ± 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, B.; Mamede, R.; Duarte, I.A.; Caçador, I.; Tanner, S.E.; Silva, M.; Jacinto, D.; Cruz, T.; Fonseca, V.F. Elemental Chemometrics as Tools to Depict Stalked Barnacle (Pollicipes pollicipes) Harvest Locations and Food Safety. Molecules 2022, 27, 1298. https://doi.org/10.3390/molecules27041298
Duarte B, Mamede R, Duarte IA, Caçador I, Tanner SE, Silva M, Jacinto D, Cruz T, Fonseca VF. Elemental Chemometrics as Tools to Depict Stalked Barnacle (Pollicipes pollicipes) Harvest Locations and Food Safety. Molecules. 2022; 27(4):1298. https://doi.org/10.3390/molecules27041298
Chicago/Turabian StyleDuarte, Bernardo, Renato Mamede, Irina A. Duarte, Isabel Caçador, Susanne E. Tanner, Marisa Silva, David Jacinto, Teresa Cruz, and Vanessa F. Fonseca. 2022. "Elemental Chemometrics as Tools to Depict Stalked Barnacle (Pollicipes pollicipes) Harvest Locations and Food Safety" Molecules 27, no. 4: 1298. https://doi.org/10.3390/molecules27041298
APA StyleDuarte, B., Mamede, R., Duarte, I. A., Caçador, I., Tanner, S. E., Silva, M., Jacinto, D., Cruz, T., & Fonseca, V. F. (2022). Elemental Chemometrics as Tools to Depict Stalked Barnacle (Pollicipes pollicipes) Harvest Locations and Food Safety. Molecules, 27(4), 1298. https://doi.org/10.3390/molecules27041298









