The Alleviating Effect of Lagerstroemia indica Flower Extract on Stretch Marks through Regulation of Mast Cells
Abstract
1. Introduction
2. Results
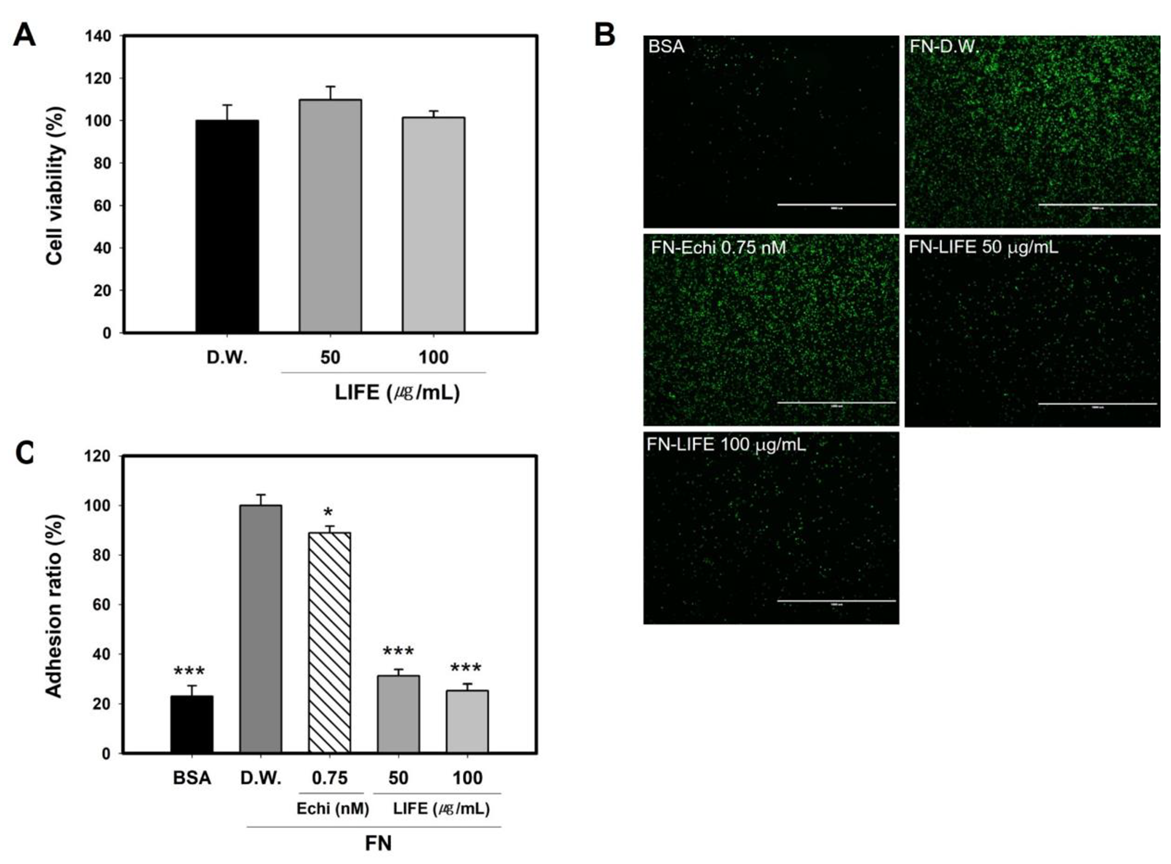
2.1. LIFE Inhibits Mast Cell Binding to FN
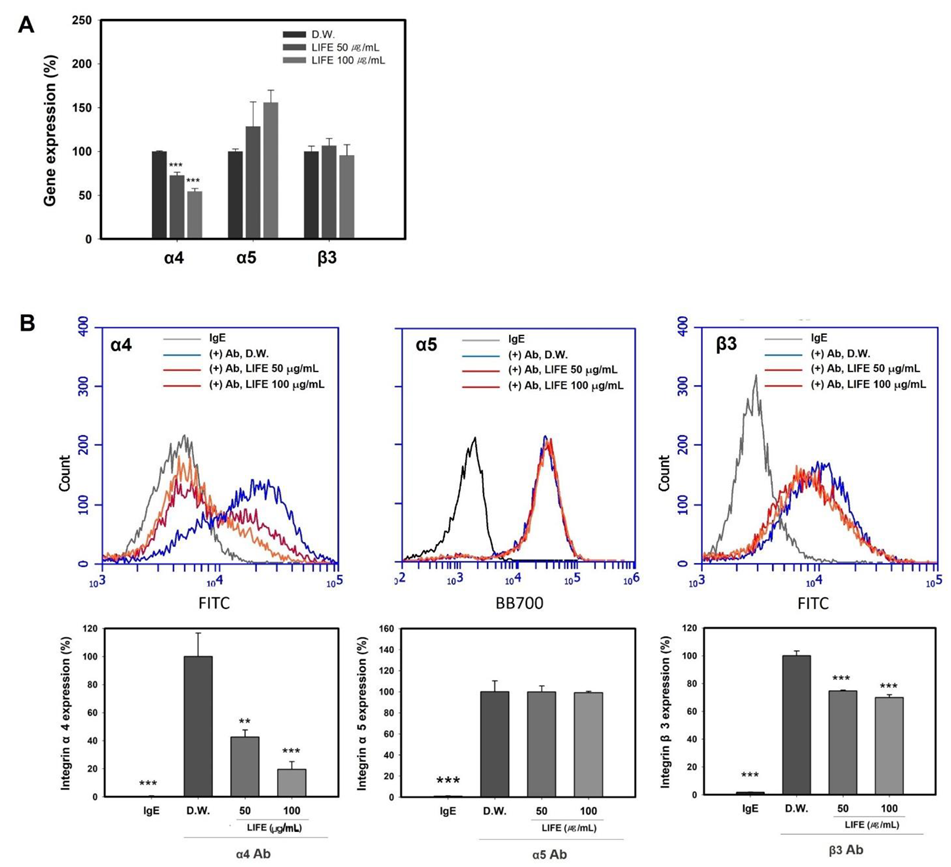
2.2. LIFE Decreases the Expression of Integrin α4 and β3
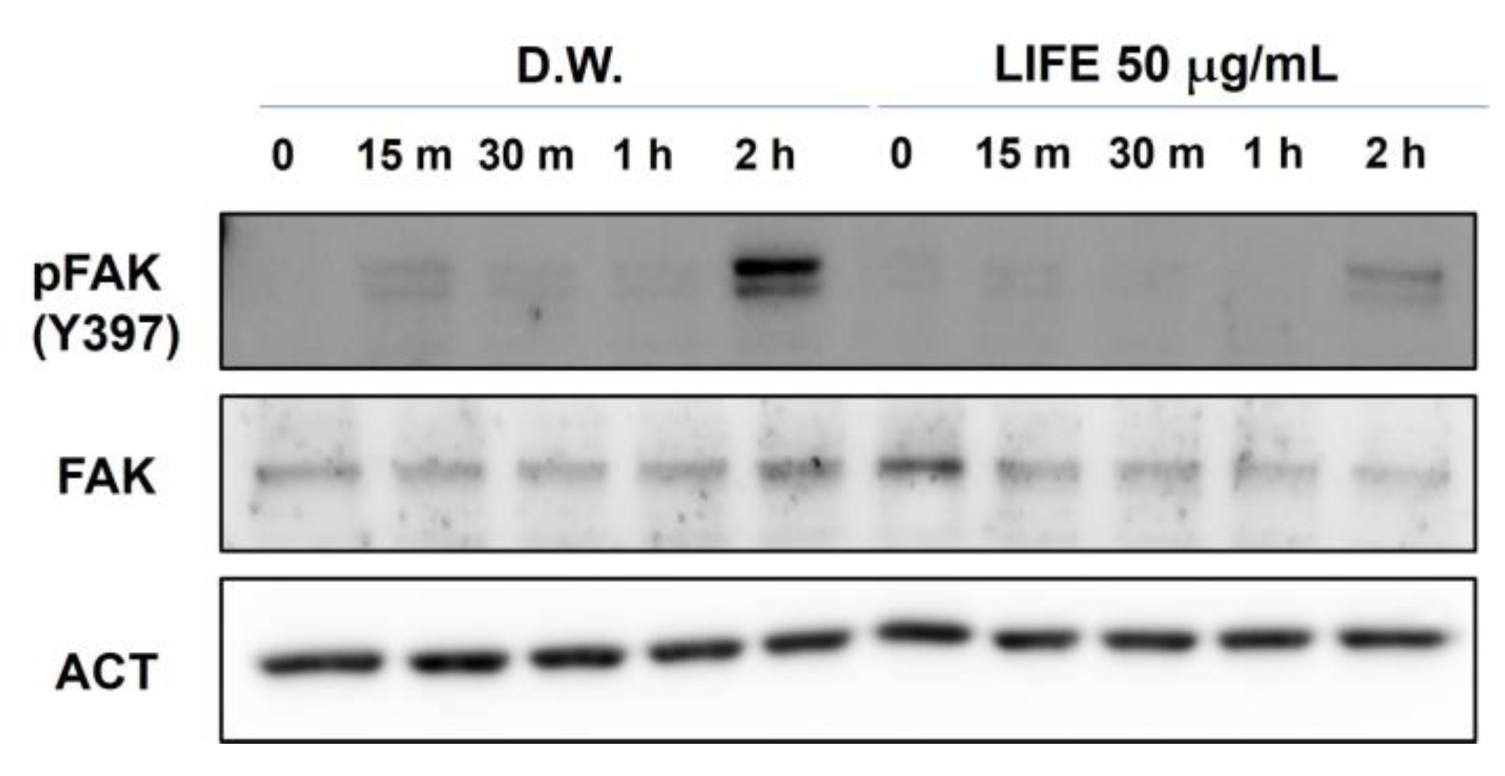
2.3. LIFE Inhibits FAK Phosphorylation but Not Intact FAK Protein
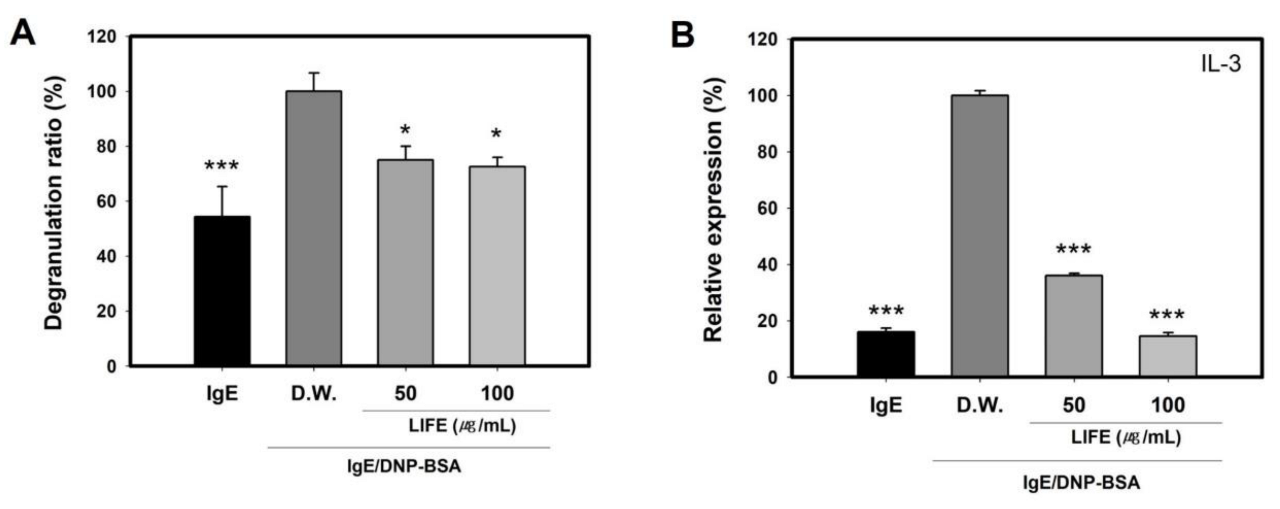
2.4. LIFE Decrease the IgE-Induced Relases of Graunles and Cytokine IL-3 on FN-Coated Plate
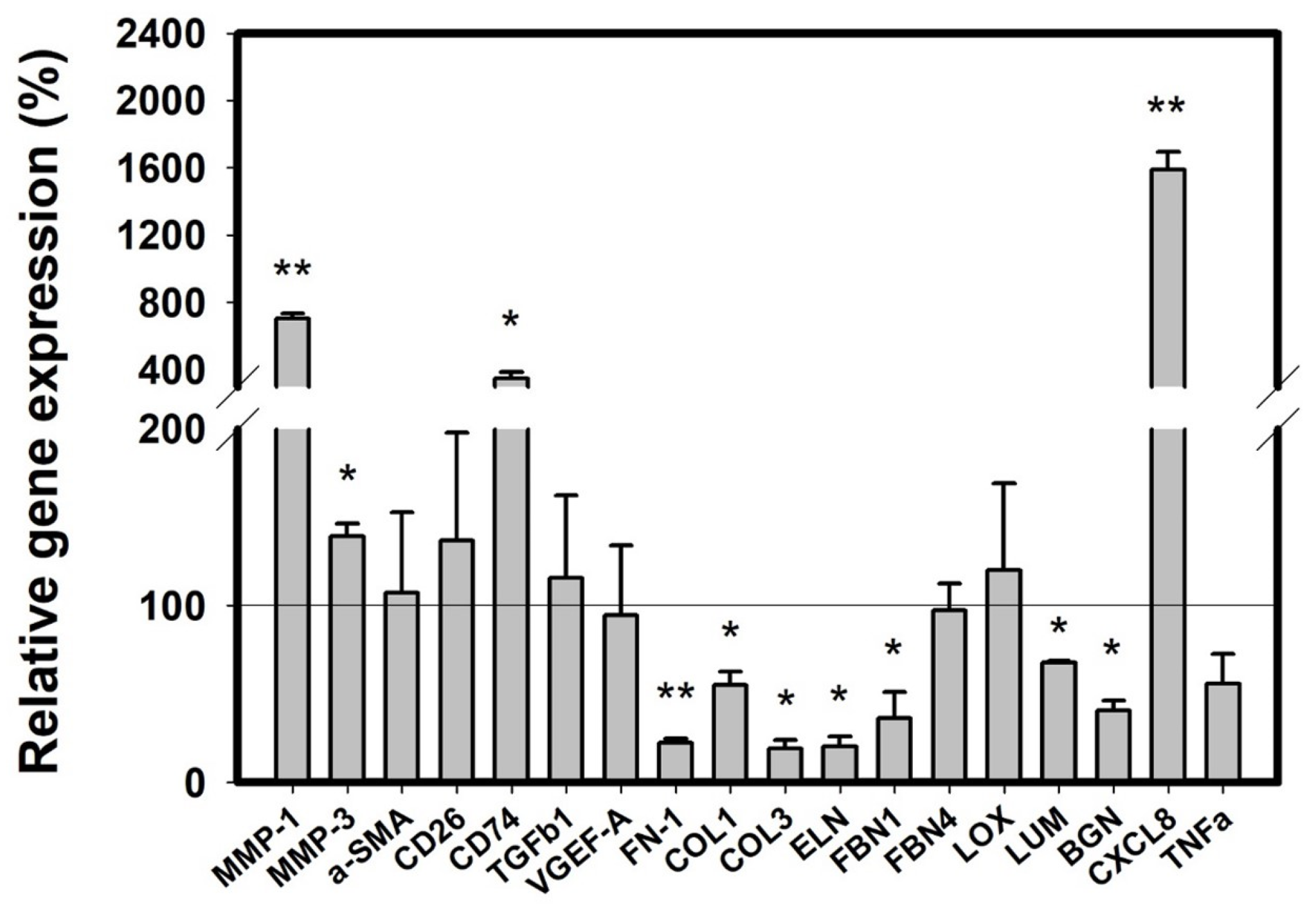
2.5. Conditioned Medium from Activated RBL-2H3 Changes the Expression of ECM Components and Inflammatory Mediators in Fibroblast
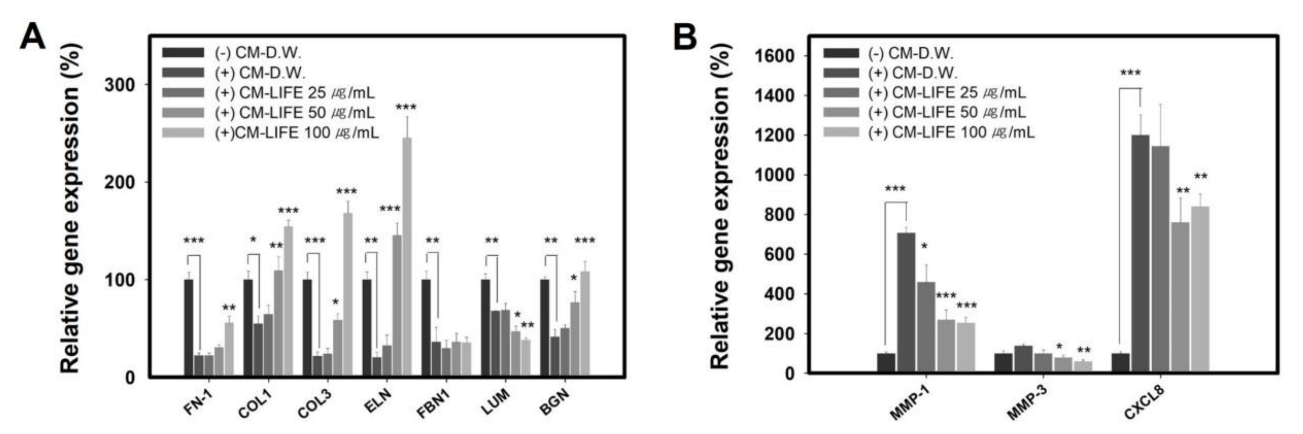
2.6. LIFE Restores Abnormal Genes Expression in Fibroblast in Response to the Conditioned Medium from the Activated Mast Cells
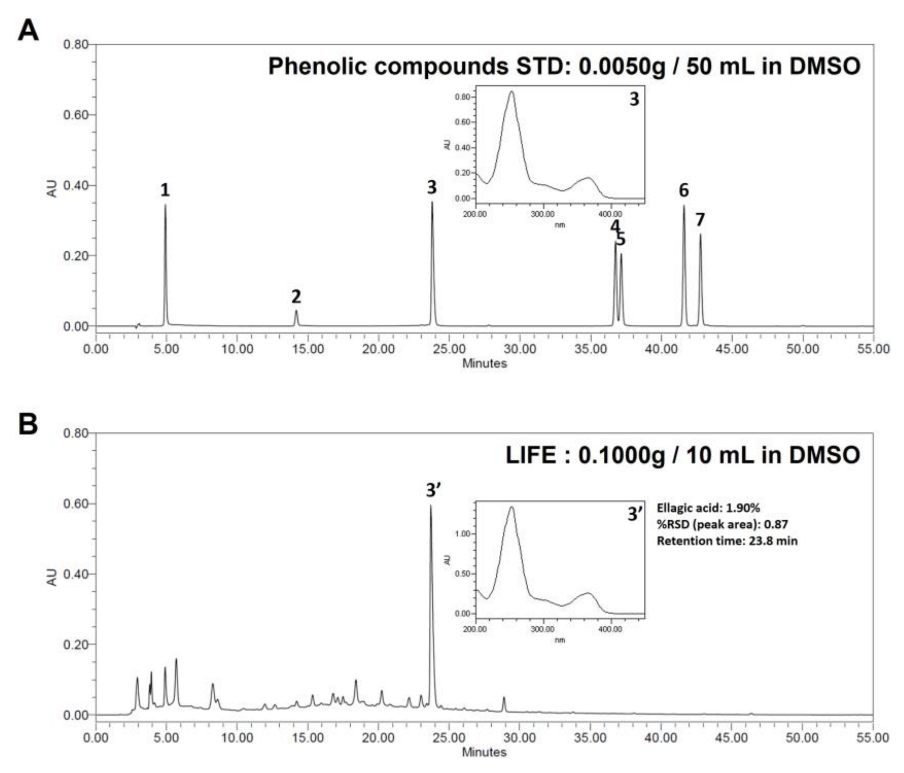
2.7. Phenolic Compounds Analysis of LIFE
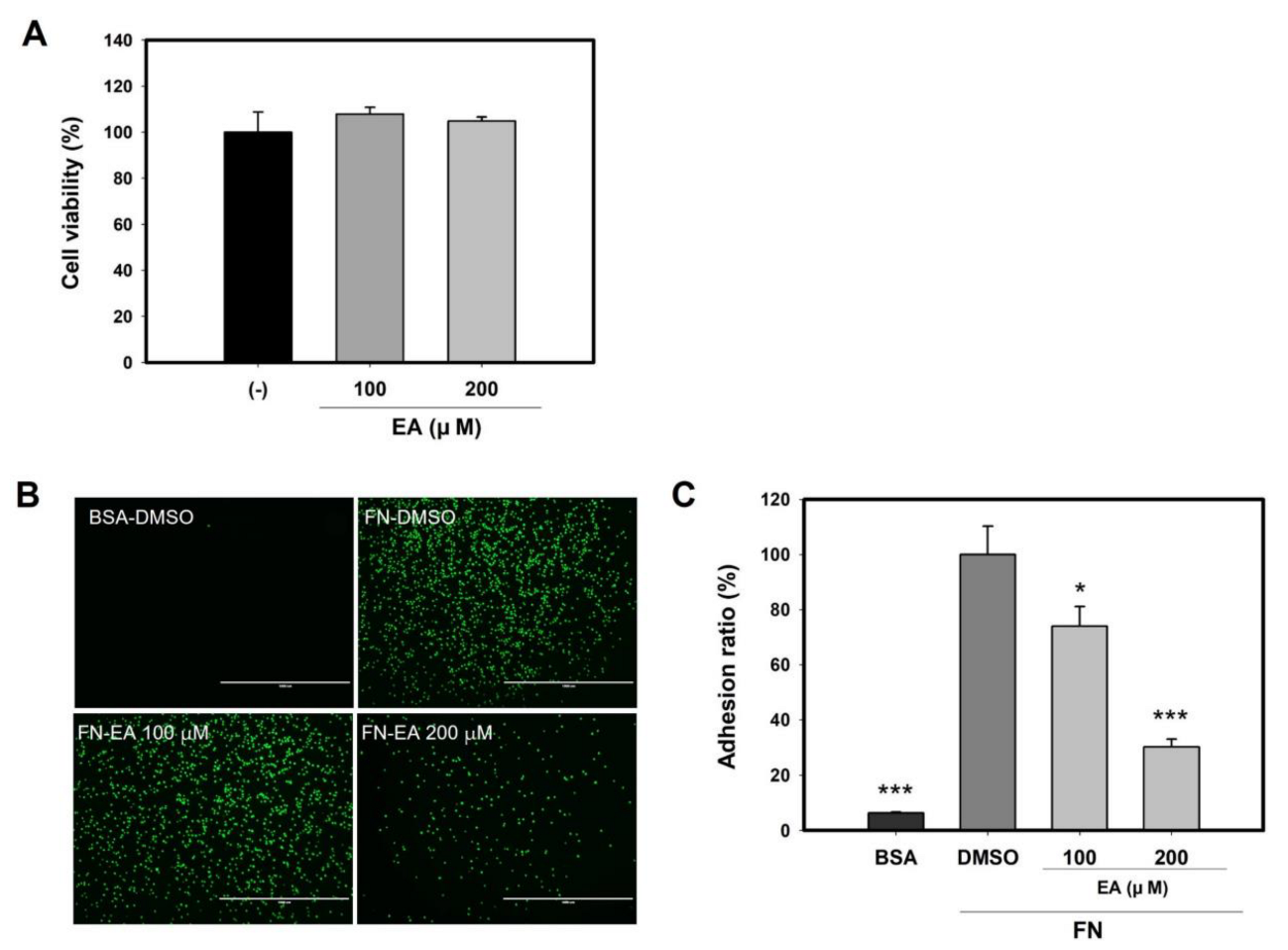
2.8. Ellagic Acid Inhibits Mast Cell Binding to FN
3. Discussion
4. Materials and Method
4.1. Preparation of LIF Extracts
4.2. Cell Culture and Cell Treatment
4.3. Adhesion Assay
4.4. β-Hexosaminidase Release Assay
4.5. Enzyme-Linked Immunosorbent Assay
4.6. RNA Isolation and Quantitative Real-Time RT–PCR
4.7. Flow Cytometry Analysis
4.8. Western Blotting
4.9. Preparation of Activated Mast Cell-CM and Cell Treatment
4.10. High-Performance Liquid Chromatography (HPLC)
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
List of Abbreviations
| Abbreviations | Full Name |
| DNP-BSA | 2,4-dinitrophenylated BSA |
| ACT | actin |
| α-SMA | alpha-smooth muscle actin |
| RGD | arginine-glycine-aspartic acid |
| BGN | biglycan |
| BSA | bovine serum albumin |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| CD26 | cluster of differentiation 26 |
| CD74 | cluster of differentiation 74 |
| COL1 | collagen type I |
| COL3 | collagens type III |
| CM | conditioned medium |
| CS | connecting segment |
| DMEM | Dulbecco’s modified Eagle’s medium |
| EMEM | eagle’s minimum essential medium |
| Echi | echistatin |
| ELN | elastin |
| ELISA | enzyme-linked immunosorbent assay |
| ECM | extracellular matrix |
| FceR1 | Fc epsilonR1 alpha (FceR1) |
| FBN | fibrillin |
| FN | fibronectin |
| FAK | focal adhesion kinase |
| HBSS | hanks balanced salt solution |
| IgE | immunoglobulin E |
| IL-3 | interleukin-3 |
| LI | Lagerstroemia indica |
| LIF | Lagerstroemia indica flower |
| LIFE | Lagerstroemia indica flower extract |
| LUM | lumican |
| LOX | lysyl oxidase |
| MMP1 | matrix metallopeptidase 1 |
| MMP3 | matrix metallopeptidase 3 |
| NHDF | normal human dermal fibroblast |
| PBS | phosphaste-buffered saline |
| RBL | rat basophilic leukemia |
| SA | striae albae |
| SD | striae distensae |
| SR | striae rubrae |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor-α |
| VEGF-A | vascular endothelial growth factor A |
References
- Al-Himdani, S.; Ud-Din, S.; Gilmore, S.; Bayat, A. Striae Distensae: A Comprehensive Review and Evidence-Based Evaluation of Prophylaxis and Treatment. Br. J. Dermatol. 2014, 170, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A. Striae Distensae: What’s New at the Horizon? Br. J. Med Pract. 2016, 9, 7. [Google Scholar]
- Borrelli, M.R.; Griffin, M.; Ngaage, L.M.; Longaker, M.T.; Lorenz, H.P. Striae Distensae: Scars without Wounds. Plast. Reconstr. Surg. 2021, 148, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S. The Cause of Striae Distensae. Acta Derm. Venereol. Suppl. (Stockh.) 1979, 59, 161–169. [Google Scholar]
- McDaniel, D.H. Laser Therapy of Stretch Marks. Dermatol. Clin. 2002, 20, 67–76. [Google Scholar] [CrossRef]
- Ud-Din, S.; McGeorge, D.; Bayat, A. Topical Management of Striae Distensae (Stretch Marks): Prevention and Therapy of Striae Rubrae and Albae. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 211–222. [Google Scholar] [CrossRef]
- Lee, K.S.; Rho, Y.J.; Jang, S.I.; Suh, M.H.; Song, J.Y. Decreased Expression of Collagen and Fibronectin Genes in Striae Distensae Tissue. Clin. Exp. Dermatol. 1994, 19, 285–288. [Google Scholar] [CrossRef]
- Perez-Aso, M.; Roca, A.; Bosch, J.; Martínez-Teipel, B. Striae Reconstructed, a Full Thickness Skin Model That Recapitulates the Pathology behind Stretch Marks. Int. J. Cosmet. Sci. 2019, 41, 311–319. [Google Scholar] [CrossRef]
- Sheu, H.-M.; Yu, H.-S.; Chang, C.-H. Mast Cell Degranulation and Elastolysis in the Early Stage of Striae Distensae. J. Cutan. Pathol. 1991, 18, 410–416. [Google Scholar] [CrossRef]
- Kanata, M.; Oka, M.; Nagai, H.; Kunisada, M.; Nishigori, C. Urticariform Striae Distensae with Severe Pruritus and Pain in an Obese Woman. Eur. J. Dermatol. EJD 2011, 21, 799–800. [Google Scholar] [CrossRef]
- Krüger-Krasagakes, S.; Czarnetzki, B.M. Cytokine Secretion by Human Mast Cells. Exp. Dermatol. 1995, 4, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Henz, B.M.; Grützkau, A.; Lippert, U.; Aragane, Y.; Schwarz, T.; Krüger-Krasagakes, S. Comparative Cytokine Gene Expression: Regulation and Release by Human Mast Cells. Immunology 1998, 93, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Dabbous, M.K.; Walker, R.; Haney, L.; Carter, L.M.; Nicolson, G.L.; Woolley, D.E. Mast Cells and Matrix Degradation at Sites of Tumour Invasion in Rat Mammary Adenocarcinoma. Br. J. Cancer 1986, 54, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Wang, L.; Sikora, J.; Hu, L.; Shen, X.; Grygorczyk, R.; Schwarz, W. ATP Release from Mast Cells by Physical Stimulation: A Putative Early Step in Activation of Acupuncture Points. Evid. Based Complement. Alternat. Med. 2013, 2013, e350949. [Google Scholar] [CrossRef]
- Shimbori, C.; Upagupta, C.; Bellaye, P.-S.; Ayaub, E.A.; Sato, S.; Yanagihara, T.; Zhou, Q.; Ognjanovic, A.; Ask, K.; Gauldie, J.; et al. Mechanical Stress-Induced Mast Cell Degranulation Activates TGF-Β1 Signalling Pathway in Pulmonary Fibrosis. Thorax 2019, 74, 455–465. [Google Scholar] [CrossRef]
- Zhang, D.; Spielmann, A.; Wang, L.; Ding, G.; Huang, F.; Gu, Q.; Schwarz, W. Mast-Cell Degranulation Induced by Physical Stimuli Involves the Activation of Transient-Receptor-Potential Channel TRPV2. Physiol. Res. 2012, 113–124. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The Extracellular Matrix In Development and Morphogenesis: A Dynamic View. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Buck, C.A.; Horwitz, A.F. Cell Surface Receptors for Extracellular Matrix Molecules. Annu. Rev. Cell Biol. 1987, 3, 179–205. [Google Scholar] [CrossRef]
- Krüger-Krasagakes, S.; Grützkau, A.; Krasagakis, K.; Hoffmann, S.; Henz, B.M. Adhesion of Human Mast Cells to Extracellular Matrix Provides a Co-Stimulatory Signal for Cytokine Production. Immunology 1999, 98, 253–257. [Google Scholar] [CrossRef]
- Ra, C.; Yasuda, M.; Yagita, H.; Okumura, K. Fibronectin Receptor Integrins Are Involved in Mast Cell Activation. J. Allergy Clin. Immunol. 1994, 94, 625–628. [Google Scholar] [CrossRef]
- Yasuda, M.; Hasunuma, Y.; Adachi, H.; Sekine, C.; Sakanishi, T.; Hashimoto, H.; Ra, C.; Yagita, H.; Okumura, K. Expression and Function of Fibronectin Binding Integrins on Rat Mast Cells. Int. Immunol. 1995, 7, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, V.; Wilson, C.G.; Carver, W.; Goldsmith, E.C. Mechanical Loading Promotes Mast Cell Degranulation via RGD-Integrin Dependent Pathways. J. Biomech. 2013, 46, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, H.; Miyake, K.; Asai, K.; Mizuno, K.; Shimada, T. Cyclical Mechanical Stretch Enhances Degranulation and IL-4 Secretion in RBL-2H3 Mast Cells. Cell Biochem. Funct. 2014, 32, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Butler, J.; Ingber, D. Mechanotransduction across the Cell Surface and through the Cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Gilmore, S.J.; Vaughan, B.L.; Madzvamuse, A.; Maini, P.K. A Mechanochemical Model of Striae Distensae. Math. Biosci. 2012, 240, 141–147. [Google Scholar] [CrossRef]
- Viennet, C.; Bride, J.; Armbruster, V.; Aubin, F.; Gabiot, A.-C.; Gharbi, T.; Humbert, P. Contractile Forces Generated by Striae Distensae Fibroblasts Embedded in Collagen Lattices. Arch. Dermatol. Res. 2005, 297, 10–17. [Google Scholar] [CrossRef]
- Das, K. Study of Medicinal Plants in Nuhashpalli, Bangladesh. Bachelor’s Thesis, Daffodil International University, Dhaka, Bangladesh, 2015. [Google Scholar]
- Al-Snafi, A.E. A Review on Lagerstroemia Indica: A Potential Medicinal Plant. IOSR J. Pharm. 2019, 9, 36–42. [Google Scholar]
- Yang, E.J.; Lee, J.S.; Song, B.B.; Yun, C.Y.; Kim, D.H.; Kim, I.S. Anti-inflammatory effects of ethanolic extract from Lagerstroemia indica on airway inflammation in mice. J. Ethnopharmacol. 2011, 136, 422–427. [Google Scholar] [CrossRef]
- Ayoub, N.; Singab, A.B.; Labib, R.M. Chemical Constituents and Pharmacological Studies of Lagerstroemia Indica. Phytopharmacology 2013, 4, 373–389. [Google Scholar]
- Woo, K.W.; Sim, M.O.; Park, E.J.; Kim, M.S.; Suh, W.S.; Cho, H.W.; Kwon, H.C.; Park, J.C.; Lee, K.R. Chemical Constituents from the Stems of Lagerstroemia indica and Their Anti-oxidant Effect. Korean J. Pharmacogn. 2016, 47, 204–210. [Google Scholar]
- Beaven, M.A.; Metzger, H. Signal Transduction by Fc Receptors: The Fc Epsilon RI Case. Immunol. Today 1993, 14, 222–226. [Google Scholar] [CrossRef]
- Metzger, H. The Receptor with High Affinity for IgE. Immunol. Rev. 1992, 125, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Apgar, J.R. Increased Degranulation and Phospholipase A2, C, and D Activity in RBL Cells Stimulated through FcεR1 Is Due to Spreading and Not Simply Adhesion. J. Cell Sci. 1997, 110, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Patynowski, I.; Niewiarowski, S.; Marcinkiewicz, C.; Calvete, J.J.; Marcinkiewicz, M.M.; McLane, M.A. Structural Requirements of Echistatin for the Recognition of Avβ3 and A5β1Integrins*. J. Biol. Chem. 1999, 274, 37809–37814. [Google Scholar] [CrossRef]
- Brockbank, E.C.; Bridges, J.; Marshall, C.J.; Sahai, E. Integrin Β1 Is Required for the Invasive Behaviour but Not Proliferation of Squamous Cell Carcinoma Cells in Vivo. Br. J. Cancer 2005, 92, 102–112. [Google Scholar] [CrossRef][Green Version]
- Richardson, A.; Parsons, T. A Mechanism for Regulation of the Adhesion-Associated Proteintyrosine Kinase Pp125FAK. Nature 1996, 380, 538–540. [Google Scholar] [CrossRef]
- Paszek, M.J.; Boettiger, D.; Weaver, V.M.; Hammer, D.A. Integrin Clustering Is Driven by Mechanical Resistance from the Glycocalyx and the Substrate. PLOS Comput. Biol. 2009, 5, e1000604. [Google Scholar] [CrossRef]
- Borrelli, M.R.; Henn, D.; Mascharak, S.; Ngaage, L.M.; Griffin, M.; Patel, A.; Nazerali, R.; Lee, G.; Januszyk, M.; Wan, D.C.; et al. Abstract 79: Striae Distensae Are Rich In Mechanoresponsive And Cd26-Positive Human Dermal Fibroblasts And Exhibit Increased Profibrotic Signaling. Plast. Reconstr. Surg. Glob. Open 2020, 8, 51–52. [Google Scholar] [CrossRef]
- Razzak, M.A.; Hossain, M.S.; Radzi, Z.B.; Yahya, N.A.B.; Czernuszka, J.; Rahman, M.T. Cellular and Molecular Responses to Mechanical Expansion of Tissue. Front. Physiol. 2016, 7, 540. [Google Scholar] [CrossRef]
- Schwartz, M.A.; DeSimone, D.W. Cell Adhesion Receptors in Mechanotransduction. Curr. Opin. Cell Biol. 2008, 20, 551–556. [Google Scholar] [CrossRef]
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction Gone Awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Bradding, P.; Pejler, G. The Controversial Role of Mast Cells in Fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef]
- Hua, Y. The Role of the Mast Cell in Skin Aging. J. Dermatol. Res. Ther. 2016, 2, 035. [Google Scholar] [CrossRef]
- Behzad, H.; Sharma, A.; Mousavizadeh, R.; Lu, A.; Scott, A. Mast Cells Exert Pro-Inflammatory Effects of Relevance to the Pathophyisology of Tendinopathy. Arthritis Res. Ther. 2013, 15, R184. [Google Scholar] [CrossRef]
- Ceci, C.; Lacal, P.M.; Tentori, L.; De Martino, M.G.; Miano, R.; Graziani, G. Experimental Evidence of the Antitumor, Antimetastatic and Antiangiogenic Activity of Ellagic Acid. Nutrition 2018, 10, 1756. [Google Scholar] [CrossRef]
- Ríos, J.-L.; Giner, R.; Marín, M.; Recio, M. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yan, G.H. Ellagic Acid Attenuates Immunoglobulin E-Mediated Allergic Response in Mast Cells. Biol. Pharm. Bull. 2009, 32, 1118–1121. [Google Scholar] [CrossRef]
- Lam, V.; Kalesnikoff, J.; Lee, C.W.K.; Hernandez-Hansen, V.; Wilson, B.S.; Oliver, J.M.; Krystal, G. IgE Alone Stimulates Mast Cell Adhesion to Fibronectin via Pathways Similar to Those Used by IgE + Antigen but Distinct from Those Used by Steel Factor. Blood 2003, 102, 1405–1413. [Google Scholar] [CrossRef]
| Gene Name | Accession Number | Primer Sequence |
|---|---|---|
| Integrin α4 | NM_001107737.1 | F: ctccccacaggcctttattt |
| R: tctctgtcacgtcgcagttt | ||
| Integrin α5 | NM_001108118.1 | F: agctgcatttccgagtctg |
| R: ctcacactgaaggctgaacg | ||
| Integrin β3 | NM_153720.1 | F: cacctgcatgtccaccaa |
| R: cagctgccacactcacagtt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeom, M.; Ji, H.; Shin, J.; Cho, E.; Ryu, D.-H.; Park, D.; Jung, E. The Alleviating Effect of Lagerstroemia indica Flower Extract on Stretch Marks through Regulation of Mast Cells. Molecules 2022, 27, 1274. https://doi.org/10.3390/molecules27041274
Yeom M, Ji H, Shin J, Cho E, Ryu D-H, Park D, Jung E. The Alleviating Effect of Lagerstroemia indica Flower Extract on Stretch Marks through Regulation of Mast Cells. Molecules. 2022; 27(4):1274. https://doi.org/10.3390/molecules27041274
Chicago/Turabian StyleYeom, Miji, Hyanggi Ji, Jongheon Shin, Eunae Cho, De-Hun Ryu, Deokhoon Park, and Eunsun Jung. 2022. "The Alleviating Effect of Lagerstroemia indica Flower Extract on Stretch Marks through Regulation of Mast Cells" Molecules 27, no. 4: 1274. https://doi.org/10.3390/molecules27041274
APA StyleYeom, M., Ji, H., Shin, J., Cho, E., Ryu, D.-H., Park, D., & Jung, E. (2022). The Alleviating Effect of Lagerstroemia indica Flower Extract on Stretch Marks through Regulation of Mast Cells. Molecules, 27(4), 1274. https://doi.org/10.3390/molecules27041274






