Abstract
This study evaluated the pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization approaches for processing of Prunus mume regarding browning factors and microorganisms, compared with non-sterilization (control check, CK) treatment. The microorganisms (total bacterial count and fungi and yeast count) in the juice were identified after different sterilization techniques, while the quality parameter changes (degree of browning, color measurements, total phenolic content, reducing sugar, ascorbic acid, 5-hydroxymethyl furaldehyde (5-HMF), amino acid nitrogen, total soluble solids (TSS), pH value) were investigated. The results indicate that P and HHP treatment reduced non-enzymatic browning while substantially impacting the color measurements, TSS, and pH, while the sterilization effect was remarkable, with a rate exceeding 90%. Furthermore, the Prunus mume juices treated with P and HHP sterilization were used as the objects, and the CK group was used as the control group. They were placed at 4 °C, 25 °C and 37 °C, respectively, and stored in dark for 15 d. Sampling and determination were carried out on 0, 3, 6, 9, 12, and 15 d, respectively. M-&-Y (molds and yeasts) were not detected in the late storage period, and no obvious microbial growth was observed during storage, indicating that P and HHP treatments could ensure the microbial safety of Prunus mume juice. P- and HHP- treated Prunus mume juice has better quality and low temperature storage is beneficial for maintaining the quality of Prunus mume juice. Therefore, P treatment or HHP treatment combined with low temperature storage could achieve a more ideal storage effect. Overall, this study conclusively established that P and HHP methods were suitable for sterilizing Prunus mume juice. These techniques minimally affected overall product quality while better maintaining the quality parameters than the untreated juice samples and those exposed to O3 and US treatment.
1. Introduction
Prunus mume Sieb. et Zucc., popularly known as Chinese plum, is one of the most important seasonal fruits in southern China. Prunus mume is a uniquely flavored, highly acidic yellow or green fruit [1] with a sugar-to-acid ratio of 0.2 [2]. Known as Fructus mume in its unripe state, it is used in traditional medicinal food. Since Prunus mume is rich in organic acids, amino acids, vitamins, flavonoids, and polyphenols and presents antibacterial [3], antiallergic [4], and antioxidant activity [5], it is an excellent natural health food. The water content in fresh Prunus mume generally exceeds 88%, which is suitable for juice, however, its acidity renders it unacceptable to consumers. Therefore, Prunus mume juice is mixed with other materials to produce compound beverages after juice extraction, which forms the markets demand for Prunus mume juice.
Processing and storage lead to substantial Prunus mume juice browning [6], which can be classified into enzymatic and non-enzymatic browning. Enzyme-driven browning mostly occurs during the crushing, juicing, and filtration stages, while the oxidation that occurs during sterilization and the subsequent storage process is primarily ascribed to non-enzymatic browning. Thermal sterilization, traditionally used for fruit and vegetable juice, is not suitable for nutrient preservation, quickly leading to browning and a decline in product quality [3]. Contrarily, non-thermal sterilization is highly successful in retaining the original nutritional composition and quality of juice and mainly includes ozone (O3) [7,8], ultrasonic (US) [9], high-hydrostatic-pressure (HHP) [10], and high-voltage pulsed electric field sterilization [11]. Such processes minimize the negative thermal effects on food nutritional and quality parameters [12], while ensuring safety from a microbiological point of view. The application efficiency of ozone in fruit juices has been studied, mainly in apple and orange juices [13] focusing on quality and safety characteristics. You [14] showed that compared with the traditional thermal sterilization treatment, HHP sterilization treatment can better reduce the damage to anthocyanin and improve the quality of mulberry juice. However, no studies are available regarding its application to Prunus mume juice. Therefore, this work compared the effect of pasteurization (P), as well as O3, US, and HHP sterilization, on the physicochemical properties (degree of browning, color measurements, total phenolic content, reducing sugar, ascorbic acid, 5-hydroxymethylfurfural, amino acid nitrogen, total soluble solids (TSS), pH value) and microorganisms total number of the bacterial colony and aspergillus and yeast (APC and M&Y) of Prunus mume juice. Furthermore, the changes in physicochemical indexes and microorganism of Prunus mume juice treated by P and HHP treatments during storage were further observed to identify the most suitable technique for Prunus mume juice application.
2. Results and Discussion
2.1. The Effect of Different Sterilization Methods on the Browning Degree and Color Measurements of Prunus mume Juice
The degree of browning denotes a reference index to measure the browning of juice during the sterilization treatment process. Figure 1 shows that the browning degree of P-, O3-, US-, and HHP-treated Prunus mume juice decreased significantly compared with non-sterilization treatment (p < 0.05). Although oxidation changes were more distinct during P and HHP treatment, no significant differences were evident between the two groups (p > 0.05), indicating that both low-temperature P and HHP sterilization had a less substantial impact on the browning degree of Prunus mume juice.
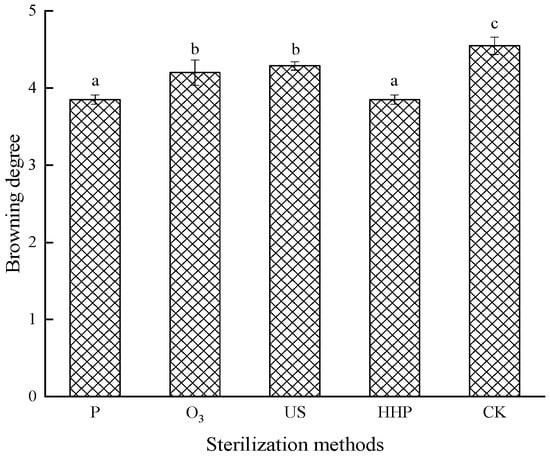
Figure 1.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the browning degree of Prunus mume juice. Values with the different letters in the column are significantly different by Duncan’s multiple range test (p < 0.05).
Colorimetric analysis of the color characteristics after the different sterilization treatments indicated distinct changes. The impact of P, O3, US, and HHP processing on color is shown in Table 1. The L* value of the P-, O3-, and HHP-treated juice exhibited a significant decrease (p < 0.05) compared with freshly squeezed juice, while that of the US-treated juice remained unchanged (p < 0.05). The a* value of the samples treated with P, US, and HHP increased significantly (p < 0.05), while that of the O3-treated juice exhibited a substantial decline (p < 0.05) compared with freshly squeezed juice. A considerable increase in the b* value was evident in all the treated juice samples compared with untreated juice. The ΔE value indicated significant color differences (p < 0.05) between the untreated and treated samples, as shown in Table 1. The ΔE values between the sterilization treatment groups and the non-sterilization treatment group were P > O3 > US > HHP > Non-sterilization (p < 0.05) in descending order. This showed that P treatment had the most significant impact on the Prunus mume juice color, which could be attributed to the fact that the thermal effect during heat treatment accelerated the browning of Prunus mume juice [14,15]. Jabber [16] found that thermal treatment reduces the activity of enzymes, which in turn inhibits browning caused by enzymes and hence improves the color of the product. The effect of O3 treatment on color may be ascribed to the attack of coupling double bonds by O3 molecules and hydroxyl radicals, resulting in the partial oxidation of organic acids, aldehydes, and ketones [17]. HHP treatment had an effect on the Prunus mume juice color, but it was better at maintaining the original color compared to other sterilization treatments. Justyna [18] indicated that HHP-treated carrot juices were less red and yellow compared with fresh carrot juice. Elizabeth [19] indicated that there was a significant (p < 0.05) effect of pressure on color characteristics of pomegranate juice with increased pressure and that these decreases in a* and L* values can be attributed to the degradation or polymerization of anthocyanins and indicate a fading of the typical red color of pomegranate juice. Therefore, HHP can better maintain the color of Prunus mume juice in terms of the changes in browning degree and color.

Table 1.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the ΔE of the Prunus mume juice.
2.2. The Effect of Different Sterilization Methods on the Total Phenols and Reducing Sugar in the Prunus mume Juice
The impact of different sterilization treatments on the total phenols is shown in Figure 2a. The initial phenolic content in the control sample (untreated) was 0.0647 mg GAE/mL while decreasing significantly (p < 0.05) in the P-, O3-, and HHP-treated juice. No significant differences were evident between the US and HHP-treated juice. The total phenolic content in the Prunus mume juice decreased after P treatment, which could be attributed to the thermal instability of phenolic compounds. High temperatures accelerated the degradation of some phenolic compounds [19]. The total phenolic content loss was the highest in the O3-treated juice at 0.0551 mg GAE/mL, which could be ascribed to various chemical reactions, including direct chemical reactions between the O3 and the target compounds or their intermediates or free radical reactions between the hydroxyl radicals [20], which was consistent with the findings of Tiwari [21,22]. The decreased total phenolic content in Prunus mume juice after HHP treatment may be due to an increase in the extractability of phenolic compounds or non-enzymatic oxidative degradation reaction in samples after high-pressure treatment [23]. The effect of US treatment on the total phenolic content in Prunus mume juice was relatively mild. Saeeduddin [24] reported similar research results, indicating that US sterilization could better maintain polyphenols in fruits and vegetables. Moreover, Bhat [25] found that the total phenolic content increased significantly after US treatment.
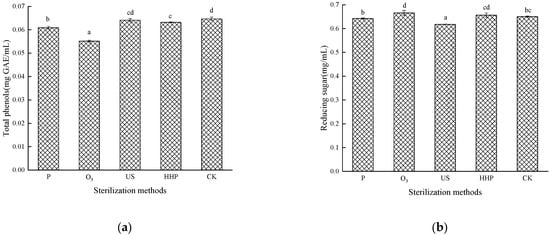
Figure 2.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on total phenolic content (a) and (b) the reducing sugar content in the Prunus mume juice. Values with the different letters in the column are significantly different by Duncan’s multiple range test (p < 0.05).
The impact of the different sterilization treatments on reducing sugar is shown in Figure 2b. The reducing sugar content in the US-treated Prunus mume juice exhibited a significant decrease (p < 0.05) at 0.618 mg/mL, which could be attributed to the thermal, cavitation, and mechanical impact [26]. The reducing sugar content increased substantially in the Prunus mume juice after O3 treatment (p < 0.05) at 0.666 mg/mL, which could be ascribed to the reaction between O3 and polysaccharides, resulting in the glycosidic bond cleavage and hydrolysis to produce reducing monosaccharides [27]. The decrease in reducing sugars by ultrasound and heat treatment may be due to the formation of 5-HMF via sugar dehydration and amino acid interaction at elevated temperatures [28]. The results indicate that HHP treatment could maintain the reducing sugar content in the Prunus mume juice since no significant differences (p > 0.05) were evident, as was the case with P (p > 0.05).
2.3. The Effect of Different Sterilization Methods on the 5-Hydroxymethyl Furaldehyde Levels and the Amino Acid Nitrogen Content in the Prunus mume Juice
The impact of different sterilization treatments on 5-HMF is shown in Figure 3a. The 5-HMF content in the Prunus mume juice treated with P, O3, and HHP did not exhibit a significant change (p > 0.05) but increased considerably (p < 0.05) in the US treatment group. The 5-HMF is mainly produced by the Maillard reaction and the dehydration of carbohydrates under acidic conditions. Therefore, on the one hand, this may involve the Maillard reaction between β-lactoglobulin and glucose, galactose, lactose, fructose, ribose, and arabinose in a water system, induced by high-intensity US treatment. On the other hand, this may denote the hydrolysis of reducing sugar under acidic conditions. Zhang [29] found that acidic and high-temperature experimental conditions favor the fructofuranosyl cation pathway, while a higher pH and lower temperature are conducive to HMF formation.
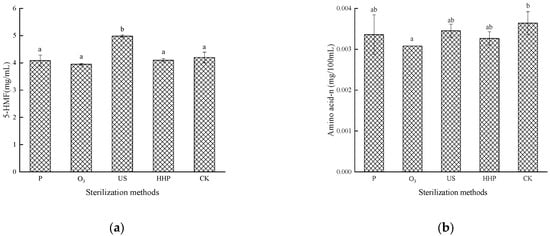
Figure 3.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the 5-hydroxymethyl furaldehyde levels (a) and the amino acid- nitrogen levels (b) in the Prunus mume juice. Values with the different letters in the column are significantly different by Duncan’s multiple range test (p < 0.05).
Amino acid nitrogen indicates the amino acid content in food while indirectly reflecting the Maillard reaction [19]. As shown in Figure 3b, no significant differences were evident in the amino acid nitrogen levels in the Prunus mume juice after P, US, and HHP treatment (p > 0.05), while a substantial decrease (p < 0.05) was apparent in the samples treated with O3, at a level of 0.0031 mg/mL. The reason for this may be that O3 can oxidize amino acid components (cysteine, tryptophan, methionine, and histidine) and glycolipids in cell membrane proteins, decreasing the amino acid content in Prunus mume juice. In addition, O3 may aggravate the Maillard reaction in the juice, leading to amino acid consumption.
2.4. The Effect of Different Sterilization Methods on the Total Soluble Solid and pH of the Prunus mume Juice
The TSS content in the Prunus mume juice treated with P, O3, US, and HHP was significantly higher than in the non-sterilization treatment, as shown in Table 2 (p < 0.05), suggesting that these treatments were beneficial to TSS dissolution. The reason for the highest TSS content under US treatment may be that ultrasonic treatment accelerated the hydrolysis of reducing sugar under acidic conditions. Furthermore, the juice displayed substantially higher sucrose, fructose, and glucose levels [19].

Table 2.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the total soluble solid and pH of the Prunus mume juice.
The pH values of the P-treated samples were considerably higher than in the non-sterilization treatment (p < 0.05), indicating that the treatment strategies could cause strong physical or chemical reactions in the juice, leading to acid substance formation. However, the pH value of the US- and O3- and HHP-treated samples changed slightly. These results are in line with the findings of Abid [30,31,32]. This may be attributed to a higher citric acid level in the form of free acid, as well as the formation of buffer substances with inorganic salts and other substances, preventing significant pH changes. Additionally, Jaramillo-Sánchez [33] found that no significant difference was observed for pear juice treated with ozone. Lower ozone concentration has no effect on pH and titratable acidity of fruit juices [34].
2.5. The Effect of Different Sterilization Methods on the Microbial Counts in the Prunus mume Juice
The APC and M&Y content in the Prunus mume juice treated using different sterilization methods are listed in Table 3, showing distinct variations in their impact on the juice samples. Compared with the CK group, the P, O3, US, and HHP sterilization methods had bactericidal effect, and no M&Y content was detected. P exhibited the best sterilization effect, while APC and M&Y were not detected after treatment. Furthermore, the bactericidal effect of different sterilization methods on APC content was P, HHP, US, O3. Therefore, P, O3, US, and HHP sterilization decreased the microbial indexes of the Prunus mume juice. Shah [17] demonstrated that ozone treatment reduces the number of microorganisms in pummelo fruit juice. Khandpur [35] evaluated ultrasound-based sterilization approaches showed an obvious inhibitory effect on microorganisms.

Table 3.
The effect of pasteurization (P), ozone (O3), ultrasonic (US), and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the microbial counts in the Prunus mume juice.
2.6. Changes in Physicochemical Properties and Microorganisms during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
2.6.1. Changes in Browning Degree and Color Measurement of Prunus mume Juice during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
The Prunus mume juices of P, HHP, and CK sterilization groups were stored at different temperatures (4 °C, 25 °C, 37 °C), and the browning changes during storage are shown in Figure 4a. After 15 days storage, compared with untreated juice, the browning degree of HHP- and P-treated juice changed little, and the browning degree of HHP was more stable. At 4 °C storage temperature, the browning degree of Prunus mume juice treated with P and HHP fluctuated slightly, but there was no significant difference (p > 0.05). The browning degree of Prunus mume juice in CK group increased from 4.55 ± 0.11 to 5.16 ± 0.03. At 25 °C storage temperature, the browning degree of Prunus mume juice treated with P increased from 3.85 ± 0.06 to 5.14 ± 0.06, the browning degree of juice treated with HHP increased from 3.85 ± 0.06 to 4.49 ± 0.10, and the browning degree of control CK group increased from 4.55 ± 0.11 to 6.28 ± 0.14. At 37 °C storage temperature, the browning degree of Prunus mume juice treated with P increased from 3.85 ± 0.06 to 5.93 ± 0.07, the browning degree of juice treated with HHP increased from 3.85 ± 0.06 to 5.30 ± 0.11, and the browning degree of control CK group increased from 4.55 ± 0.11 to 6.42 ± 0.12. It can be seen that with the extension of storage time, different sterilization methods can increase the browning degree of Prunus mume juice to varying degrees. The lower the storage temperature, the lower the browning degree of Prunus mume juice, and the better the stability. Compared with the control group and pasteurization treatment, the color of Prunus mume juice after HHP sterilization was better and the browning degree was smaller.
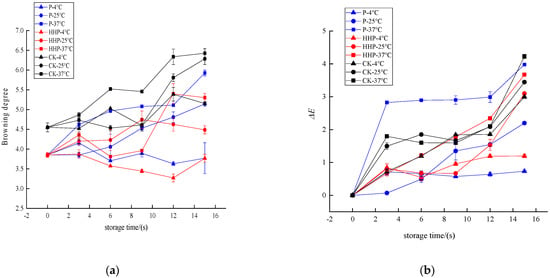
Figure 4.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on browning degree (a) and color measurement (b) in the Prunus mume juice.
Color change is an important indicator for measuring the change of sensory quality of fruit and vegetable juice during storage. The juices of P, HHP, and CK groups were stored at different temperatures, and the changes in ΔE value during storage are shown in Figure 4b. After 15 days of storage, the storage temperature had a great influence on the color change of P and HHP treated Prunus mume juice. Under the storage condition of 4 °C, the ΔE value of Prunus mume juice did not change significantly (p < 0.05), but with the increase in storage temperature, the ΔE value of Prunus mume juice obtained by the two sterilization treatments changed significantly, reaching 3.98 ± 0.01 and 3.67 ± 0.05 at 37 °C, respectively. The same law can be found in the control group, and the color change was mainly caused by browning reaction, which was consistent with the change trend of browning degree of Prunus mume juice during storage. It can be seen that at 4 °C, 25 °C and 37 °C, HHP treatment had the best effect on maintaining the color of Prunus mume juice, followed by P treatment, and the color of Prunus mume juice in the CK group was the most unstable. This is because compared with heat treatment, HHP treatment does not destroy the covalent bond of molecules. At this time, small molecular substances have strong stability and can better maintain the sensory characteristics such as nutrients and pigments in food [36,37]. Wang [38] and Liu [39] also reported similar research results.
2.6.2. Changes in Total Phenol Content and Reducing Sugar of Prunus mume Juice during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
The Prunus mume juices of P, HHP, and CK groups were stored at different temperatures, and the changes in total phenol content during storage are shown in Figure 5a. After 15 days of storage, at 4 °C storage temperature, the total phenol content of Prunus mume juice treated with P and HHP had no significant difference (p < 0.05). The total phenol content of control CK group decreased from 0.0647 mg GAE/mL to 0.0591 mg GAE/mL. At 25 °C storage temperature, the total phenol content of Prunus mume juice treated with P decreased from 0.0608 mg GAE/mL to 0.0559 mg GAE/mL, the total phenol content of Prunus mume juice treated with HHP decreased from 0.0632 mg GAE/mL to 0.0568 mg GAE/mL, and the total phenol content of control CK group decreased from 0.0647 mg GAE/mL to 0.0562 mg GAE/mL. At 37 °C storage temperature, the total phenol content of Prunus mume juice treated with P decreased from 0.0608 mg GAE/mL to 0.0514 mg GAE/mL, the total phenol content of Prunus mume juice treated with HHP decreased from 0.0632 mg GAE/mL to 0.0558 mg GAE/mL, and the total phenol content of control CK group decreased from 0.0647 mg GAE/mL to 0.0518 mg GAE/mL.
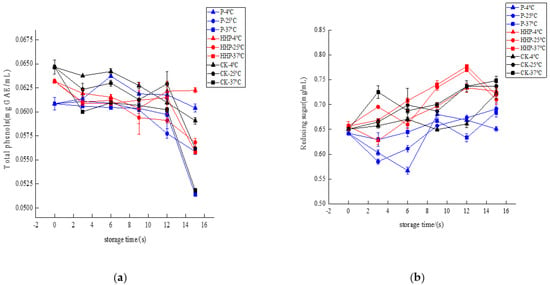
Figure 5.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on total phenol content (a) and reducing sugar (b) in the Prunus mume juice.
Therefore, with the extension of storage time, different sterilization methods can reduce the total phenol content in Prunus mume juice to varying degrees. The higher the storage temperature, the more unstable the total phenol in Prunus mume juice. At the same storage temperature, compared with the control group and pasteurization treatment, the total phenols of Prunus mume juice after HHP treatment remained better, which may be that the low temperature of HHP treatment delayed the oxidative degradation of phenolic substances by oxygen free radicals formed by dissolved oxygen decomposition in the sample [40,41].
The Prunus mume juices of P, HHP and CK groups were stored at different temperatures, and the change in reducing sugar content of Prunus mume juice during storage is shown in Figure 5b. After 15 days of storage, at 4 °C, 25 °C and 37 °C, compared with 0 d, the reducing sugar content of Prunus mume juice in the three groups increased to varying degrees and finally gradually tended to be flat or decreased. This is contrary to the browning degree and ΔE value of Prunus mume juice, which may be because the non-enzymatic browning of juice occurs slowly during storage and the reducing sugar is continuously consumed. However, during the browning process, non-reducing sugars such as sucrose in Prunus mume juice will be hydrolyzed under acidic conditions to produce reducing sugars such as glucose and fructose, and the increase in storage temperature will accelerate the hydrolysis reaction to ensure the continuous non-enzymatic browning [42]. Therefore, when the hydrolysis rate was far greater than the consumption rate of reducing sugar in browning reaction, the reducing sugar in Prunus mume juice increased. Then, with the non-enzymatic browning reaction, the content of reducing sugar began to stabilize or even decrease.
2.6.3. Changes in the 5-Hydroxymethyl Furaldehyde Levels and the Amino Acid Nitrogen Content of Prunus mume Juice during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
5-HMF is an intermediate product of Maillard reaction, which not only leads to the deterioration of color and flavor of fruit and vegetable juice, but also affects its edible safety [42]. The Prunus mume juices of P, HHP, and CK groups were stored at different temperatures, and the changes in 5-HMF content during storage are shown in Figure 6a. In the early stage of storage, the content of 5-HMF in the three groups of Prunus mume juice was low, and with the extension of storage time, it showed an indigenous upward trend. The 5-HMF of CK group was the fastest, and the increment was also the largest. It can be seen that P and HHP treatments can effectively inhibit the formation of 5-HMF, thereby inhibiting the browning reaction of Prunus mume juice. The increase in 5-HMF content is relatively slow at 4 °C storage temperature. When the storage temperature is 25 °C and 37 °C, the increase rate of 5-HMF content is larger. Therefore, the increase in storage temperature will accelerate the formation of 5-HMF in Prunus mume juice. During the storage process, the 5-HMF content fluctuated irregularly, which was due to the fact that when the accumulation of 5-HMF reached a certain level, it would participate in the Maillard reaction and polymerize to produce dark substances, resulting in the deepening of the color of Prunus mume juice. This process would consume part of 5-HMF. When the consumption rate was less than the generation rate, the content of 5-HMF decreased.
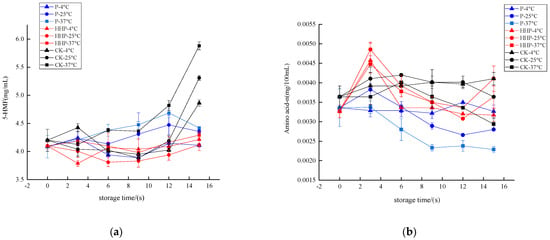
Figure 6.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the 5-HMF levels (a) and the amino acid nitrogen content (b) in the Prunus mume juice.
Maillard reaction is a complex reaction with amino acids and reducing sugars as substrates, and the content of amino acid nitrogen can directly reflect the content of amino acids, so the content of amino acid nitrogen can indirectly reflect the Maillard reaction. The Prunus mume juices of P, HHP, and CK groups were stored at different temperatures, and the changes in amino acid nitrogen content during storage are shown in Figure 6b. After 15 days of storage, there was no significant difference in amino acid nitrogen content between P- and HHP-treated Prunus mume juice at 4 °C storage temperature (p > 0.05). The amino acid nitrogen content of Prunus mume juice in the control CK group increased slightly, from 0.0036 mg GAE/mL to 0.0041 mg GAE/mL. At 25 °C and 37 °C, the loss of amino acid nitrogen may be due to Maillard reaction with reducing sugar during storage. The content of amino acid nitrogen in Prunus mume juice treated with HHP increased first and then decreased. Ultrahigh pressure would destroy the hydrogen bond of protein molecules, and some polar groups were dissociated, so that the molecules on the surface of protein molecules had the same charge, which promoted the separation of the binding substance and protein, thereby increasing the solubility of protein, dissociating to produce small molecular substances such as amino acids, and increasing its content [43]. Then, with the Maillard reaction, amino acids were consumed, and the hydrolysis rate of protein was less than the consumed rate, resulting in a gradual decrease in the content. Because temperature can also affect the hydrolysis of protein, and thus the P group and CK group of Prunus mume juice stored at 25 °C and 37 °C, there is the same trend of amino acid nitrogen content being increased at first and then decreased.
2.6.4. Changes in Total Soluble Solid of Prunus mume Juice during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
TSS refers to the general term for all compounds dissolved in water in liquid or liquid foods, including sugar, acid, vitamins, minerals and so on [44]. The Prunus mume juices of P, HHP, and CK groups were stored at different temperatures, and the changes of TSS content during storage are shown in Figure 7. Although the TSS content in the three groups showed a downward trend with the extension of storage time, the retention rate was above 90.00%, and there was no significant difference between groups (p > 0.05). Therefore, in general, TSS was relatively stable during the storage of Prunus mume juice.
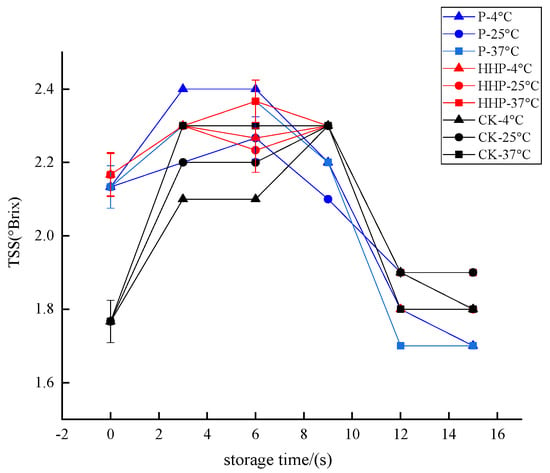
Figure 7.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on the TSS in the Prunus mume juice.
The pH value of juice can reflect the degree of deterioration of Prunus mume juice quality [45]. The Prunus mume juices of P, HHP, and CK groups were stored at different temperatures. The pH changes in Prunus mume juice during storage are shown in Table 4. There was no significant difference in pH among the three groups of Prunus mume juice stored at 4 °C, 25 °C and 37 °C (p > 0.05). This may be because the juice contains more citric acid in the form of free acid, and it contains inorganic salts and other substances to form a buffer, so the pH value did not change significantly.

Table 4.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on pH of Prunus mume juice during storage.
2.6.5. Changes in Microbial counts of Prunus mume Juice during Storage with Pasteurization and High-Hydrostatic-Pressure Sterilization
The Prunus mume juices of P, HHP and CK groups were stored at different temperatures. The changes in APC and M & Y during storage are shown in Table 5. The table shows that different sterilization methods have different sterilization effects on Prunus mume juice, that the pasteurization treatment had the best sterilization effect on Prunus mume juice, and that APC and M-&-Y were not detected within 15 days after storage. Although HHP treatment failed to completely kill APC, it did not increase during the later storage period. It may be that HHP caused damage to the cells of microorganisms, making them unable to be repaired during storage, thereby being inactivated [46].

Table 5.
The effect of pasteurization (P) and high-hydrostatic-pressure (HHP) sterilization methods and non-sterilization (control check, CK) on microbial counts of Prunus mume juice during storage.
3. Materials and Methods
3.1. Juice Preparation
The Prunus mume was obtained from the Tianqing Prunus mume Planting Cooperative (Dazhou, Sichuan), from which fruits of uniform size, color, and maturity were selected and washed. The Prunus mume kernels were removed. One volume of the pulp was mixed with three volumes of water and squeezed using a juice extractor (JYL-C91T, Joyoung Co., Ltd., Zhejiang, China). Next, 0.06% of a pectinase (Yuan Ye Biotechnology Co., Ltd., Shanghai, China) was added for enzymatic hydrolysis at 34.6 °C for 1.4 h. A mixture of 0.26% (m/v) of citric acid, 0.26% (m/v) of ascorbic acid, and 0.20% (m/v) of calcium chloride (Cologne Chemicals Co., Ltd, Chengdu, Sichuan, China) was prepared and added to the clarified juice to prevent juice browning. Then, the clarified juice was filtered using a muslin cloth prior to treatment.
3.2. Pasteurization Sterilization
For the thermal P treatment, the parameter according to methodology previously reported by Marsellés-Fontanet [47] was used with modifications. The juice samples were packed in low density polyethylene (LDPE) packets (30 mL), sealed using a film sealing machine (SF-150, Afanlao Machinery Co., Ltd., Shanghai, China), and pasteurized in a bain-marie (HH-S4, Jintan Medical Instrument Factory, Jiangsu, China) at 80 °C for 10 min.
3.3. Ozone Sterilization
The O3 treatment was performed using a method described by Fundo [34] with some modifications. Oxygen was passed through a corona discharge generator (YS-MJCB-S17, Hangzhou Yishi Technology Co., Ltd., Zhejiang, China) to produce O3 at 5 g/h. Then, 120 mL of Prunus mume juice was subjected to constant stirring (magnetic agitation) to ensure equal O3 distribution throughout the sample. The iodometric titration method [48] was used to determine the O3 concentration of 7.0 ± 2 mg/m3. The sample was prepared after O3 treatment for 45 min. The treatments were performed in triplicate.
3.4. Ultrasonic Sterilization
The US treatment was performed using a method described by Elżbieta [49] with some modifications. Directly after the fresh juice extraction, sonication was performed at 40 W and 200 W for 15 min using a cleaning bath (Scientz-IID, Ningbo Xinzhi Biotechnology Co., Ltd., Zhejiang, China). The process consisted of a 2 s turn-on and 2 s turn-off cycle. All experiments were conducted in triplicate.
3.5. High-Hydrostatic-Pressure Sterilization
The pressure experiments were performed in a laboratory-scale high-pressure processor (HHP-600, Baotou Kefa High-Pressure Technology Co., Ltd., Inner Mongolia, China). The HHP sterilization treatment referenced Elizabeth [19] with modification. The samples were placed in 100 mL bottles and enclosed in the pressure vessel already equilibrated at 4 °C and 500 MPa. The vessel was then pressurized and maintained for 10 min, followed by decompression.
3.6. Physicochemical Analysis
3.6.1. Determining the Degree of Browning
The browning degree was measured using the spectrophotometric method described by Zhou [50]. Distilled water was used as a control. A volume of juice (1.5 mL) was mixed with an equal quantity of distilled water at 25 °C and left to stand for 5 min, after which the absorbance was measured at 410 nm using a UV-Vis Spectrophotometer (UV2400, Sunny Hengping Scientific Instrument Co., Ltd., Shanghai, China). The results are expressed as a ten-fold absorbance value.
3.6.2. Color Measurements
The color measurements were performed using a precision colorimeter (WF32, Weifu Optoelectronic Technology Co., Ltd., Shenzhen, China). The color values were expressed as brightness (L*), redness (a*), and yellowness (b*) values. The L* value changed from black (0) to white (100). Furthermore, a* and b* displayed color value changes from greenness (−a*) to redness (+a*) and blueness (−b*) to yellowness (+b*). In addition, the total color difference (∆E) was calculated from the following equation according to Jung [51].
where L0, a0, and b0 represented the control values, while L, a, b denoted the values of the treated juice.
3.6.3. Total Phenolic Content
The total phenolic content was determined according to a method delineated by Singleton [52] with some modifications. A calibration curve was prepared using standard gallic acid solutions (0.01~0.08 mg/mL); the total phenolic content in the samples (reported as µg gallic acid equivalent per mL of juice) was calculated from interpolating the respective absorbance values in the calibration curve. The result was expressed as the gallic acid equivalent (mg GAE/mL), and the measurement was repeated three times. Here, 2 mL of Prunus mume juice was mixed with 16 mL of a pre-chilled hydrochloric acid (HCl)/methanol (1%, v/v, 1 mol/L HCl/methanol:distilled water = 1:80:19) mixture and centrifuged at 10,000 rpm for 20 min at 4 °C using a refrigerated centrifuge (5810 R, Eppendorf Company, Hamburg, Germany). Next, 1 mL of clarified juice was diluted to 2 mL with distilled water and mixed with 8 mL of a 7.5% sodium carbonate solution and 4 mL Folin–Ciocalteau reagent diluted six times. The absorbance of the mixture was measured at 765 nm after standing for 1.5 h at room temperature in the dark. The measurement was repeated three times. The total phenol content in Prunus mume juice was calculated using a standard linear regression curve (Y = 1.3202X − 0.0344, R2 = 0.9993).
3.6.4. Reducing Sugar
The reducing sugar levels were determined via 3,5-dinitrosalicylic acid colorimetry [53]. A calibration curve was prepared using a standard glucose solution (1.0 mg/mL). Respective concentrations of 1.0 mL, 2.0 mL, 3.0 mL, 4.0 mL, 5.0 mL, 6.0 mL, 7.0 mL, and 8.0 mL were placed in 10 mL volumetric flasks with distilled water. After accurately weighing 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, and 8.0 mL (1.0 mL/mL) of the prepared solution separately to different 10 mL colorimetric tube, 1.5 mL 3,5-Dinitrosalicylic acid solution was added to each colorimetric tube and held on in boiling water for 5 min, after which rapid cooling and distilled water was fixed to scale. After the absorption value was measured at 540 nm, the standard curve was drawn (Y = 1.1436X − 0.0701, R2 = 0.9990).
Sample tests: Prunus mume juice was diluted five times with distilled water, 1 ml was measured to determine its absorbance, and the same method used for the standard curve was employed to measure the wavelength. The reducing sugar content in the juice was calculated according to the standard curve without the dilution factor.
3.6.5. 5-Hydroxymethyl Furaldehyde
The 5-hydroxymethylfurfural was determined using a method described by Aguilo [54] with some modifications. Here, 30 mL, 40 mL, 50 mL, 60 mL, 70 mL, 80 mL, and 90 mL of the 5-hydroxymethylfurfural standard solution were respectively placed in 10 mL volumetric flasks with distilled water. The absorbance was measured at 433 nm to obtain a standard curve (Y = 0.1262X − 0.1911, R2 = 0.9991).
A 6 mL sample was added to the test tube, followed by a 4 mL trichloroacetic acid solution (g/L) and 4 mL 2-thiobarbituric acid (0.025 mol/L) at 40 °C water for 50 min. The mixtures were cooled rapidly, and the absorbance was measured at 443 nm, after which the results were calculated according to the standard curve.
3.6.6. Amino Acid Nitrogen
An appropriate amount of the sample solution (1 mg- to 5 mg of amino nitrogen) was added to a beaker with five drops of 30% hydrogen peroxide. The organic acids in the sample were gradually neutralized with a 0.1 mol/L sodium hydroxide standard solution using an electromagnetic stirrer. When the pH reached 7.5, the 0.05 mol/L sodium hydroxide standard titration solution was adjusted to pH 8.1 and remained unchanged for 1 min, after which a 10 mL~15 mL neutral formaldehyde solution was gradually added. After 1 min, the 0.05 mol/L sodium hydroxide standard solution was titrated to pH 8.1, and the ml consumption was recorded.
3.6.7. Determination of the pH and Total Soluble Solid
A total of 25 mL Prunus mume juice was placed in a beaker, and the pH value was recorded after the reading was stable. Each sample was measured three times, and the results were averaged.
An Abbe refractometer (A610, Jinan Haieng Instrument Co., Ltd., Shandong, China) was zeroed with distilled water, after which 200 uL Prunus mume juice was absorbed on the surface of the glass mirror hole, yielding a reading of 25 ± 2 °C at room temperature. The results were calculated using °Brix.
3.7. Microbiological Examination
The microbiological counts were determined using a method described by Chen [55] with some modifications. Here, 25 mL of the sample was mixed with a 225 mL 0.85% sodium chloride solution, homogenized, and used to prepare 10−1 dilutions. The aerobic bacterial count was determined via the pour plate method, using plate count agar (Beijing Aobo Star Biotechnology Co., Ltd., Beijing, China) as a medium. The plates were incubated at 37 °C for 48 h ± 2 h. Rose- Bengal medium was used to determine the total mold-yeast counts. The plates were incubated at 28 °C for 72 h.
3.8. Storage Treatment of Prunus mume Juice
The Prunus mume juice samples were treated with P and HHP sterilization, and then stored at 4 °C, 25 °C, and 35 °C (temperature fluctuation range ± 1.0 °C, relative humidity 75%) for 15 days in dark. The CK group was used as the control. The samples were sampled and determined at 0, 3, 6, 9, 12, and 15 days, respectively. The 15th day was the test end point of the storage test.
3.9. Statistical Analysis
Each group of experiments was repeated three times, and the experimental results are expressed as mean ± SD, while the data were analyzed using SPSS 23.0. The PCA and LDA graphs of the E-nose were obtained via WinMuster software, while those presenting the other experimental results were drawn using Origin 8.5.
4. Conclusions
This study compared the impact of different sterilization methods, such as P, O3, US, and HHP, on the physicochemical properties, microbial indexes, and changes in physicochemical properties and microorganisms of Prunus mume juice during storage to determine the most suitable processing technique. The results indicate P treatment reduces the non-enzymatic browning degree while significantly affecting (p < 0.05) the total phenols, TSS, and pH; however, reducing sugar, 5-HMF, and amino acid nitrogen content did not exhibit a significant change (p > 0.05); the sterilization effect of P treatment was significant; sterilization rate was 100%. O3 treatment substantially affected the browning degree, ΔE, total phenols, reducing sugar, amino acid nitrogen, TSS and pH value in the Prunus mume juice (p < 0.05), the sterilization effect of O3 treatment was general, and the sterilization rate was 70%. US treatment had a considerable impact on the degree of browning, ΔE, reducing sugar, 5-HMF, TSS, and pH value (p < 0.05); the sterilization effect on the juice was general; and the sterilization rate was 80%. HHP treatment decreased the degree of non-enzymatic browning of the Prunus mume juice and significantly affected ΔE, TSS, and pH (p < 0.05); while the sterilization rate was as high as 90%. During storage at 4 °C, 25 °C and 37 °C, the browning degree and ΔE value of Prunus mume juice increased significantly, and the contents of Maillard reaction substrates such as total phenols, reducing sugar and amino acid nitrogen decreased significantly. The content of 5-HMF fluctuated and increased in the dynamic reaction system of Prunus mume juice. The TSS and pH values of Prunus mume juice were relatively stable and did not change significantly. Under the same sterilization condition, the transverse comparison of storage temperature showed that P and HHP treatments had better quality at 4 °C, and that low temperature storage was beneficial for maintaining the quality of Prunus mume juice. Therefore, P treatment or HHP treatment combined with low temperature storage could achieve more ideal storage effect. Therefore, P and HHP technology are a more suitable for Prunus mume juice sterilization, minimally affecting its overall quality and better preserving its color, and physicochemical components.
Author Contributions
The manuscript was written through the contributions of all authors. Conceptualization, Y.C. and Y.X.; methodology, Y.X.; software, Y.C. and Y.X.; validation, Y.M., A.M.; formal analysis, Y.X. and Y.C.; investigation, Y.M.; resources, Y.M.; data curation, Y.X. and Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, P.L., K.Y., A.Y.; visualization, A.M.; supervision, Y.M.; project administration, Y.M.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by Innovation Fund of Postgraduate, Xihua University (grant number YCJJ2021098).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Ma, Y.; Luo, M.; Xu, Y.; Liu, Y.; Liu, X.; Bi, X.; Yuan, Y.; Su, F.; Yin, X. Purification and characterization of a thaumatin-like protein-1 with polyphenol oxidase activity found in Prunus mume. RSC Adv. 2020, 10, 28746–28754. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilo-Aguayo, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martin-Belloso, O. Changes in quality attributes throughout storage of strawberry juice processed by high-intensity pulsed electric fields or heat treatments. LWT Food Sci. Technol. 2008, 42, 813–818. [Google Scholar] [CrossRef]
- Kono, R.; Nakamura, M.; Nomura, S.; Kitano, N.; Kagiya, T.; Okuno, Y.; Inada, K.-I.; Tokuda, A.; Utsunomiya, H.; Ueno, M. Biological and epidemiological evidence of anti-allergic effects of traditional Japanese food ume (Prunus mume). Sci. Rep. 2018, 8, 11638. [Google Scholar] [CrossRef]
- Shi, J.; Gong, J.; Liu, J.; Wu, X.; Zhang, Y. Antioxidant capacity of extract from edible flowers of Prunus mume in China and its active components. LWT 2009, 42, 477–482. [Google Scholar] [CrossRef]
- Igual, M.; García-Martínez, E.; Camacho, M.D.M.; Martínez-Navarrete, N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010, 118, 291–299. [Google Scholar] [CrossRef]
- Dash, K.; Chakraborty, S. Food Processing: Advances in Thermal and Non-Thermal Technologies, Two Volume Set; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Patil, S.; Valdramidis, V.P.; Tiwari, B.K.; Cullen, P.; Bourke, P. Quantitative assessment of the shelf life of ozonated apple juice. Eur. Food Res. Technol. 2011, 232, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Kentish, S.; Feng, H. Applications of Power Ultrasound in Food Processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Jayachandran, L.E.; Chakraborty, S.; Rao, P.S. Effect of high pressure processing on physicochemical properties and bioactive compounds in litchi based mixed fruit beverage. Innov. Food Sci. Emerg. Technol. 2015, 28, 1–9. [Google Scholar] [CrossRef]
- Bi, X.; Liu, F.; Rao, L.; Li, J.; Liu, B.; Liao, X.; Wu, J. Effects of electric field strength and pulse rise time on physicochemical and sensory properties of apple juice by pulsed electric field. Innov. Food Sci. Emerg. Technol. 2013, 17, 85–92. [Google Scholar] [CrossRef]
- Mertens, B.; Knorr, D. Developments of nonthermal processes for food preservation. Food Technol. 1992, 46, 124–133. [Google Scholar]
- Fátima, A.M.; Cristina, L.M.S.; Teresa, R.S.B. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar]
- You, Y.; Li, N.; Han, X.; Guo, J.; Zhao, Y.; Liu, G.; Huang, W.; Zhan, J. Influence of different sterilization treatments on the color and anthocyanin contents of mulberry juice during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 1–10. [Google Scholar] [CrossRef]
- Kanwate, B.W.; Ballari, R.V.; Kudre, T.G. Influence of spray-drying, freeze-drying and vacuum-drying on physicochemical and functional properties of gelatin from Labeo rohita swim bladder. Int. J. Biol. Macromol. 2019, 121, 135–141. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Saeeduddin, M.; Lei, S.; Wu, T.; Zeng, X. Influence of sonication and high hydrostatic pressure on the quality of carrot juice. Int. J. Food Sci. Technol. 2014, 49, 2449–2457. [Google Scholar] [CrossRef]
- Shah, N.N.A.K.; Supian, N.A.M.; Hussein, N.A. Disinfectant of pummelo (Citrus grandis L. Osbeck) fruit juice using gaseous ozone. J. Food Sci. Technol. 2019, 56, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Elizabeth, V.; Araceli, O.; Gipsy, T.; Juan, E.R.; Mario, P.; Vilbett, B.; Juliana, M. Effect of high hydrostatic pressure (HHP) processing on physicochemical properties, bioactive compounds and shelf-life of pomegranate juice. Innov. Food Sci. Emerg. Technol. 2012, 13, 13–22. [Google Scholar]
- Nowicka, A.; Kucharska, A.; Sokol-Letowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef]
- Cullen, P.; Tiwari, B.K.; O’Donnell, C.; Muthukumarappan, K. Modelling approaches to ozone processing of liquid foods. Trends Food Sci. Technol. 2009, 20, 125–136. [Google Scholar] [CrossRef]
- Torres, B.; Tiwari, B.K.; Patras, A.; Wijngaard, H.H.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ozone processing on the colour, rheological properties and phenolic content of apple juice. Food Chem. 2011, 124, 721–726. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef] [PubMed]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Awad, F.; Hu, B.; Lei, S.; Zeng, X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT 2015, 64, 452–458. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.; Tze, L. Sonication ameliorates Kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef]
- Hu, A.; Li, Y.; Zheng, J. Dual-frequency ultrasonic effect on the structure and properties of starch with different size. LWT 2019, 106, 254–262. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Kondratiuk-Janyska, A.; Piątkowski, M.; Śmigielski, K. Modelling the Ozone-Based Treatments for Inactivation of Microorganisms. Int. J. Environ. Res. Public Health 2017, 14, 1196. [Google Scholar] [CrossRef] [Green Version]
- Kavousi, P.; Mirhosseini, H.; Ghazali, H.; Ariffin, A.A. Formation and reduction of 5-hydroxymethylfurfural at frying temperature in model system as a function of amino acid and sugar composition. Food Chem. 2015, 182, 164–170. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, Y.; Wu, T.; Huang, C.; Pei, K.; Zhang, G.; Lin, X.; Bai, W.; Ou, S. Chlorogenic acid increased 5-hydroxymethylfurfural formation when heating fructose alone or with aspartic acid at two pH levels. Food Chem. 2016, 190, 832–835. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.; Wu, T.; Lei, S.; Zhang, X.; Zeng, X. Effect ofultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Lei, S.; Khan, M.A.; Zeng, X. Thermosonication as a potential quality enhancement technique of apple juice. Ultrason. Sonochem. 2014, 21, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Sánchez, G.M.; Loredo, A.B.G.; Gómez, P.L.; Alzamora, S.M. Ozone Processing of Peach Juice: Impact on Physicochemical Parameters, Color, and Viscosity. Ozone Sci. Eng. 2017, 40, 305–312. [Google Scholar] [CrossRef]
- Fundo, J.; Miller, F.; Tremarin, A.; Garcia, E.; Brandao, T.; Silva, C. Quality assessment of Cantaloupe melon juice under ozone processing. Innov. Food Sci. Emerg. Technol. 2018, 47, 461–466. [Google Scholar] [CrossRef]
- Khandpur, P.; Gogate, R. Evaluation of ultrasound based sterilization approaches in terms of shelf life and quality parameters of fruit and vegetable juices. Ultrason. Sonochem. 2016, 29, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Cheftel, J.C. High-pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 1995, 1, 75–90. [Google Scholar] [CrossRef]
- Oey, I.; Van der Plancken, I.; Van Loey, A. Does high pressure processing influence nutritional aspects of plant based food systems. Trends Food Sci. Technol. 2008, 19, 300–308. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Cao, X. Comparison of high hydrostatic pressure and high temperature short time processing on quality of purple sweet potato nectar. Innov. Food Sci. Emerg. Technol. 2012, 16, 326–334. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Bi, X. Comparison of Microbial Inactivation and Rheological Characteristics of Mango Pulp after High Hydrostatic Pressure Treatment and High Temperature Short Time Treatment. Food Bioprocess Technol. 2013, 6, 2675–2684. [Google Scholar] [CrossRef]
- Bree, I.V.; Meulenaer, B.D.; Samapundo, S. Predicting the headspace oxygen level due to oxygen permeation across multilayer polymer packaging materials: A practical software simulation tool. Innov. Food Sci. Emerg. Technol. 2010, 11, 511–519. [Google Scholar] [CrossRef]
- Chen, D.; Pan, S.; Chen, J. Comparing the Effects of High Hydrostatic Pressure and Ultrahigh Temperature on Quality and Shelf Life of Cloudy Ginger Juice. Food Bioprocess Technol. 2016, 9, 1779–1793. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Martinon, M.E.; Moreira, R.G.; Castell-Perez, M.E. Development of a multilayered antimicrobial edible coating for shelf-life extension of fresh-cut cantaloupe (Cucumis melo L.) stored at 4 °C. LWT-Food Sci. Technol. 2014, 56, 341–350. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S. Nutritional, microbial and physicochemical changes in pear juice under ultrasound and commercial pasteurization during storage. J. Food Processing Preserv. 2017, 41, e13237. [Google Scholar] [CrossRef]
- Serment-Moreno, V.; Barbosa-Cánovas, G.; Torres, J.A. High-pressure Processing: Kinetic Models for Microbial and Enzyme Inactivation]. Food Eng. Rev. 2014, 6, 56–88. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, Á.R.; Anna, P.; Paola, O.; Santiago, M.; Olga, M. A Comparison of the Effects of Pulsed Electric Field and Thermal Treatments on Grape Juice. Food Bioprocess Technol. 2013, 6, 978–987. [Google Scholar] [CrossRef]
- I.O.A. Iodometric Method for the Determination of Ozone in a Progress Gas-Revised Standardized Procedure 001/96. 1996. [Google Scholar]
- Elżbieta, R.; Artur, S.; Henryk, R.; Kinga, N. Effect of ultrasound, heating and enzymatic pre-treatment on bioactive compounds in juice from Berberis amurensis Rupr. Ultrason. Sonochem. 2020, 63, 104971. [Google Scholar]
- Zhou, L.; Wang, Y.; Hu, X.; Wu, J.; Liao, X. Effect of high pressure carbon dioxide on the quality of carrot juice. Innov. Food Sci. Emerg. Technol. 2009, 10, 321–327. [Google Scholar] [CrossRef]
- Leong, L.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Toma, R.; Leung, H. Determination of reducing sugars in French fried potatoes by 3,5-dinitrosalicylic acid. Food Chem. 1987, 23, 29–33. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
- Chen, L.; Bi, X.; Cao, X.; Liu, L.; Che, Z. Effects of high-power ultrasound on microflora, enzymes and some quality attributes of a strawberry drink. J. Sci. Food Agric. 2018, 98, 5378–5385. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).