Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy
Abstract
:1. Introduction
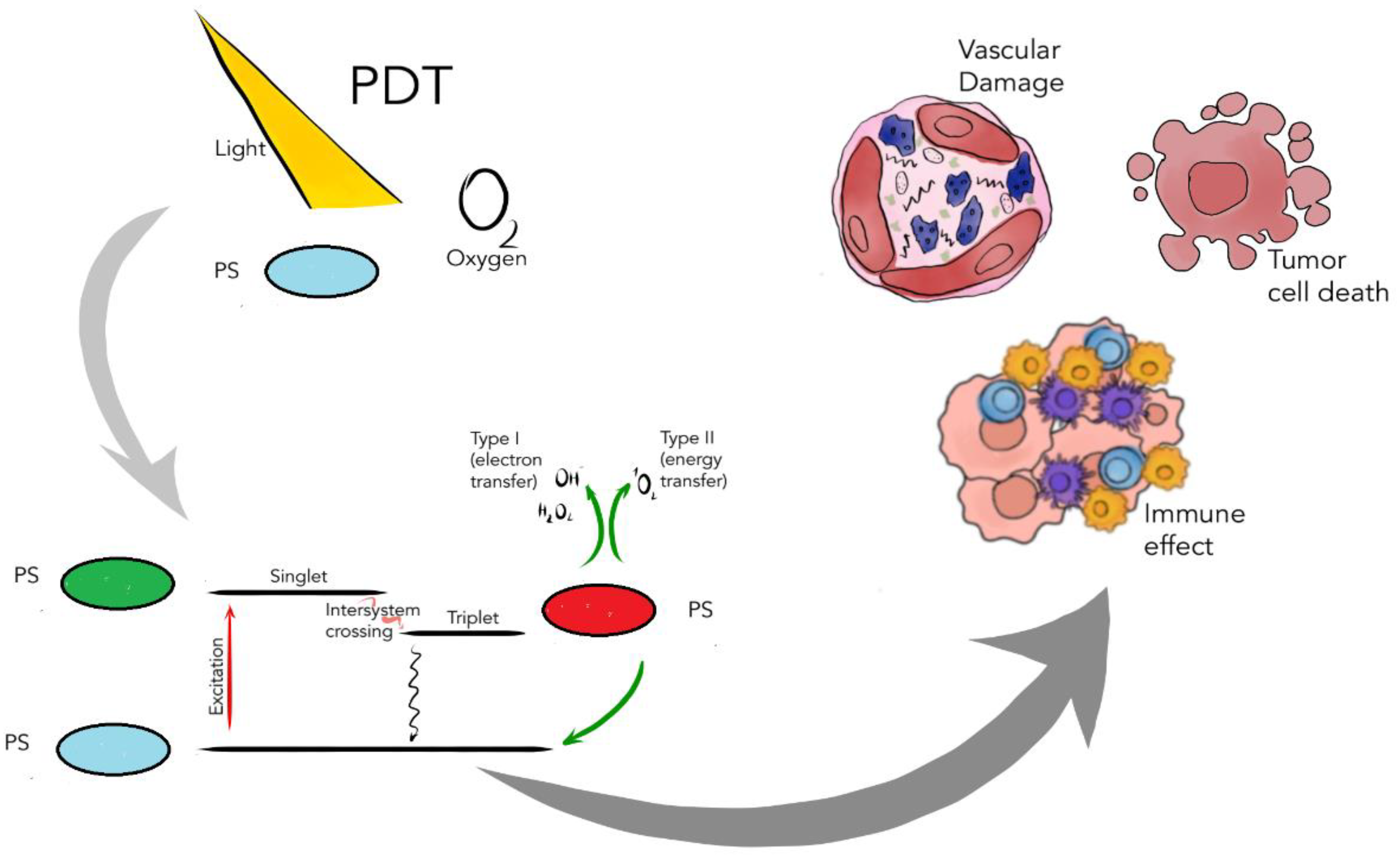
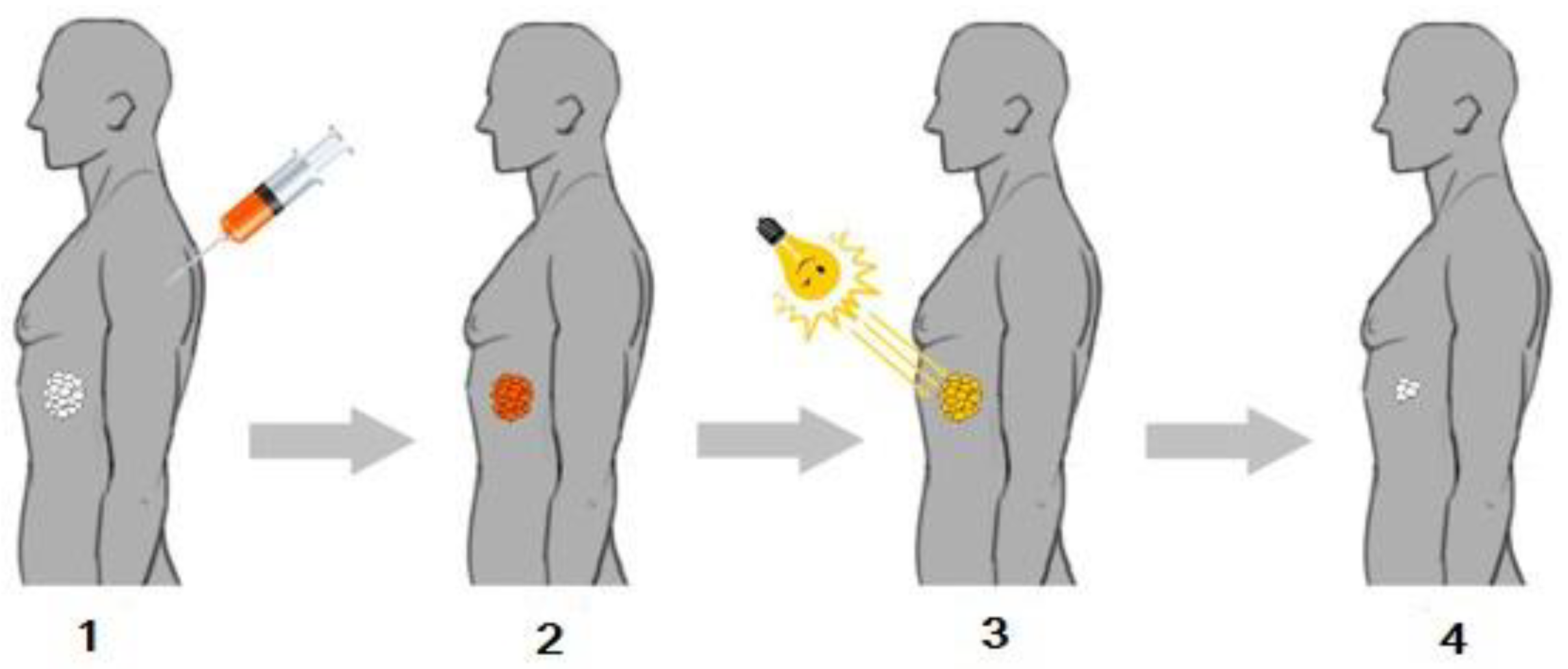
2. Photochemical Reactions in Photodynamic Therapy
3. The Role of Photosensitizers in PDT
- The PS should selectively accumulate in neoplastic tissue;
- The PS should preferably be readily available, in the form of a pure compound, and its chemical properties must be carefully established beforehand;
- The PS should not have phototoxic effects in healthy tissue;
- The PS should be characterized by a high coefficient of absorption in the spectral range of 600–800 nm, with maximum light penetration through the tissue;
- The absorption bands of the photosensitizer must not overlap the absorption bands of endogenous dyes such as melanin or hemoglobin and the water absorption bands in the near infrared region;
- The PS should react efficiently with light to generate singlet oxygen or radicals;
- The PS and these photoproducts should be characterized by optimal pharmacokinetic properties;
- The PS should have few side effects;
- The PS should be of low toxicity and easily excreted from the body to avoid phototoxicity after treatment.
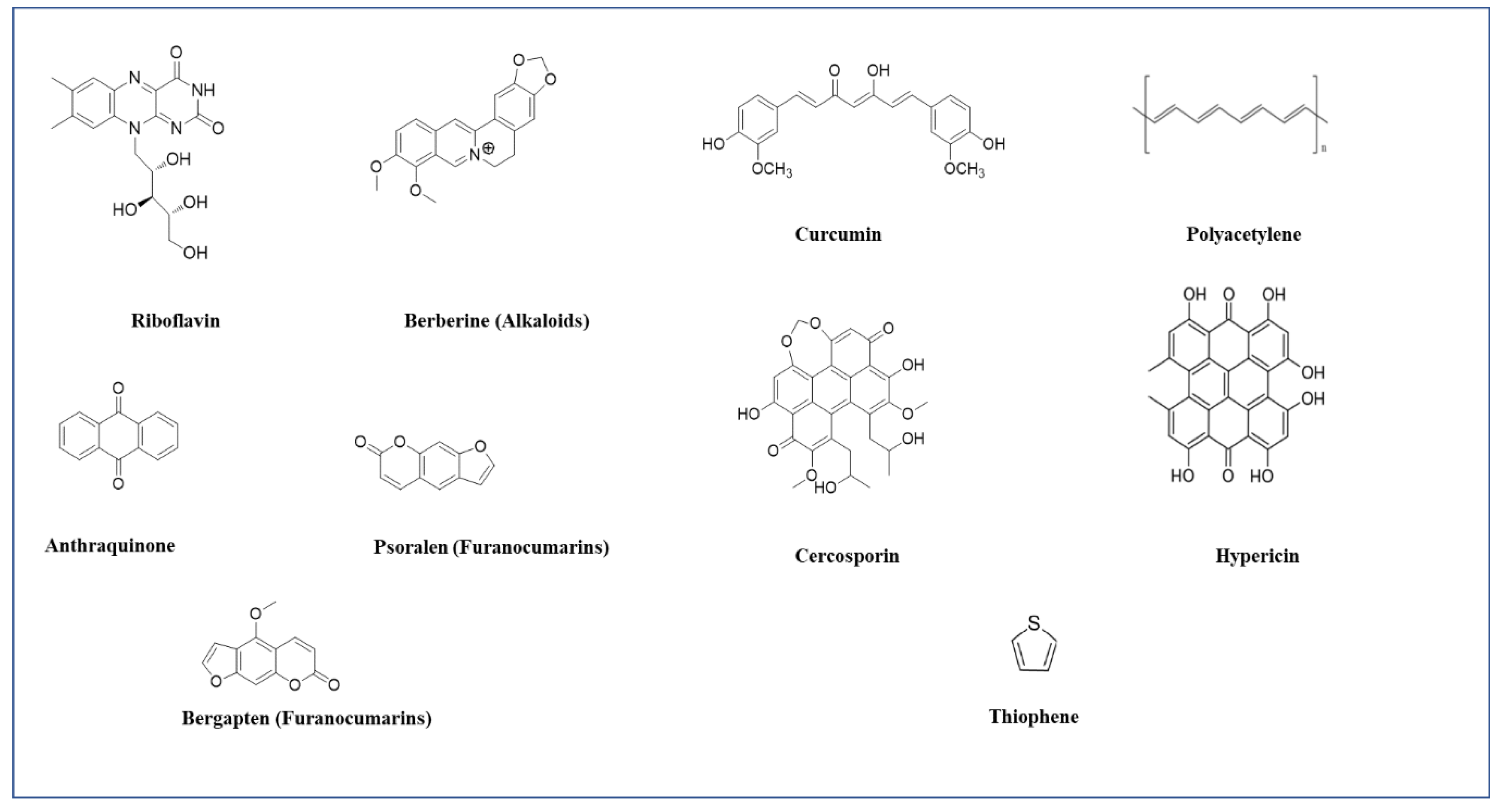
3.1. Naturally Occurring Photosensitizers in PDT
3.1.1. Pheophorbide A
| Absorption Maxima | Origin |
| 670 nm | Pheophorbide A |
3.1.2. Curcumins
| Absorption Maxima | Origin |
| 420–480 nm | Dicinnamoylmethane, curcumin, curcuminoids, demethoxycurcumin, bisdemethoxycurcumin |
3.1.3. Anthraquinones
| Absorption Maxima | Origin |
| 437 nm | Polygonum cuspidatum, Heterophyllaea pustulata, H. lycioides Aloe vera, Rheum palmatum, Rumex crispus Polyathia suberosa, Dactylopius coccus, Xanthoria parietina, Drechslera avenae, Ramularia collo-cygni. H. perforatum, Fagopyrum esculentum. |
3.1.4. Polyacetylene and Thiophenes
| Absorption Maxima | Origin |
| 314–350 nm | Asteraceae spp., Heliopsisa, Rudbeckia spp., Arnica, Centaurea scabiosa, Tagetes erecta, Porophyllum obscurum, Echinops, Bidens, Ambrosia chamissonis, T. minuta, E. latifolius, E. sgrijissi, Rhaponticum uniflorum. |
3.1.5. Tolyporphin
3.1.6. Chlorophyllin
3.1.7. Hypericin
3.1.8. Hypocrellin
3.1.9. Cercosporin
3.1.10. Riboflavin
3.1.11. Alkaloids
3.1.12. Furanocoumarins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 1 December 2021).
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yavari, N.; Andersson-Engels, S.; Segersten, U.; Malmstrom, P.U. An overview on preclinical and clinical experiences with photodynamic therapy for bladder cancer. Can. J. Urol. 2011, 18, 5778–5786. [Google Scholar] [PubMed]
- Railkar, R.; Agarwal, P.K. Photodynamic Therapy in the Treatment of Bladder Cancer: Past Challenges and Current Innovations. Eur. Urol. Focus 2018, 4, 509–511. [Google Scholar] [CrossRef]
- Champeau, M.; Vignoud, S.; Mortier, L.; Mordon, S. Photodynamic therapy for skin cancer: How to enhance drug penetration? J. Photochem. Photobiol. B 2019, 197, 111544. [Google Scholar] [CrossRef]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Ikeda, N. Photodynamic Therapy for Lung Cancer. Kyobu Geka 2016, 69, 694–699. [Google Scholar]
- Wang, K.; Yu, B.; Pathak, J.L. An update in clinical utilization of photodynamic therapy for lung cancer. J. Cancer 2021, 12, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Shishkova, N.; Kuznetsova, O.; Berezov, T. Photodynamic Therapy in Gastroenterology. J. Gastrointest. Canc. 2013, 44, 251–259. [Google Scholar] [CrossRef]
- Wu, H.; Minamide, T.; Yano, T. Role of photodynamic therapy in the treatment of esophageal cancer. Dig. Endosc. 2019, 31, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef]
- Sundaram, P.; Abrahamse, H. Effective Photodynamic Therapy for Colon Cancer Cells Using Chlorin e6 Coated Hyaluronic Acid-Based Carbon Nanotubes. Int. J. Mol. Sci. 2020, 21, 4745. [Google Scholar] [CrossRef]
- Bicalho, L.S.; Figueiró Longo, J.P.; Villamizar, I.; Meneses de Almeida Santos, M.; Bentes Azevedo, R. Photodynamic Therapy, a new approach in the treatment of oral cancer. Rev. Univ. Ind. Santander. Salud 2010, 42, 167–174. [Google Scholar]
- Prażmo, E.J.; Kwaśny, M.; Łapiński, M.; Mielczarek, A. Photodynamic Therapy as a Promising Method Used in the Treatment of Oral Diseases. Adv. Clin. Exp. Med. 2016, 25, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniogo, E.C.; Plackal Adimuriyil George, B.; Abrahamse, H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostańska, E.; Aebisher, D.; Bartusik-Aebisher, D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed. Pharmacother. 2021, 137, 111302. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural photosensitizers in photodynamic therapy: In vitro activity against monolayers and spheroids of human colorectal adenocarcinoma SW480 cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Petrakou, K.; Iatrou, G.; Lamari, F.N. Ethnopharmacological survey of medicinal plants traded in herbal markets in the Peloponnisos Greece. J. Herb. Med. 2020, 19, 100305. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Ben Mihoub, A.; Larue, L.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of Cyclodextrins in Anticancer Photodynamic Therapy Treatment. Molecules 2018, 23, 1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [Green Version]
- Greer, A. Christopher Foote’s discovery of the role of singlet oxygen 1O2 (1∆g) in photosensitized oxidation reactions. Acc. Chem. Res. 2006, 39, 797–804. [Google Scholar] [CrossRef]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar]
- Zhang, Q.; Li, L. Photodynamic combinational therapy in cancer treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagn. Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef] [Green Version]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)-Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Klaber, R. Phyto-photo-dermatitis. Br. J. Dermatol. 1942, 54, 193–211. [Google Scholar] [CrossRef]
- Schild, A.L.; Motta, A.C.; Riet-Correa, F.; Karam, F.C.; Grecco, F.B. Photosensitization in cattle in Southern Brazil. In Poisonous Plants and Related Toxins; CABI International: London, UK, 2009; pp. 162–166. [Google Scholar]
- Le Quesne, W.; Do, M.N.; Ikram, M.; Israrkkhan, M.M.; Mir, I. Furocoumarins from the fruit of ammi visnaga. J. Nat. Prod. 1985, 48, 496. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Colegate, S.M.; Knoppel, E.L.; Rood, K.A.; Collett, M.G. Wild parsnip (Pastinaca sativa)-induced photosensitization. Toxicon 2019, 167, 60–66. [Google Scholar] [CrossRef]
- Egyed, M.N.; Williams, M.C. Photosensitizing effects of cymopterus watsonii and cymopterus longipes in chickens and turkey poults. Avian Dis. 1977, 21, 566–575. [Google Scholar] [CrossRef]
- Del Río, J.A.; Ortuño, A.; Pérez, I.; Bennett, R.G.; Real, D.; Correal, E. Furanocoumarin content in Bituminaria bituminosa varieties and Cullen species Options Méditerranéennes. Sér. A Mediterr. Semin. 2010, 92, 67–70. [Google Scholar]
- Schempp, C.M.; Schöpf, E.; Simon, J.C. Dermatitis bullosa striata pratensis durch Ruta graveolens L. (Gartenraute). Hautarzt 1999, 50, 432–434. [Google Scholar] [CrossRef]
- Moan, J. Properties for optimal PDT sensitizers. J. Photochem. Photobiol. B 1990, 5, 521–524. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.H.; Childs, C.J.; Sibata, C.H. Photosensitizers in clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Lange, C.; Bednarski, P.J. Photosensitizers for Photodynamic Therapy: Photochemistry in the Service of Oncology. Curr. Pharm. Des. 2016, 22, 6956–6974. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-I.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Muehlmann, L.A.; Longo, J.P.F.; Silva, R.C.; Graebner, I.B.; Degterev, I.A.; Lucci, C.M.; Azevedo, R.B.; Garci, M.P. Photodynamic Therapy Based on Arrabidaea chica (Crajiru) Extract Nanoemulsion: In vitro Activity against Monolayers and Spheroids of Human Mammary Adenocarcinoma MCF-7 Cells. J. Nanomed. Nanotechnol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Huang, M.Y.; Zhang, L.L.; Ding, J.; Lu, J.J. Anticancer drug discovery from Chinese medicinal herbs. Chin. Med. 2018, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- Cordell, G.A.; Shin, Y.G. Finding the needle in the haystack. The dereplication of natural product extracts. Pure Appl. Chem. 1999, 71, 1089–1094. [Google Scholar] [CrossRef] [Green Version]
- Alali, F.Q.; Tawaha, K. Dereplication of bioactive constituents of the genus hypericum using LC-(+,-)-ESI-MS and LC-PDA techniques: Hypericum triquterifolium as a case study. Saudi Pharm. J. 2009, 17, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Tan, P.J.; Appleton, D.R.; Mustafa, M.R.; Lee, H.B. Rapid identification of cyclic tetrapyrrolic photosensitisers for photodynamic therapy using on-line hyphenated LC-PDA-MS coupled with photo-cytotoxicity assay. Phytochem. Anal. 2012, 23, 52–59. [Google Scholar] [CrossRef]
- Jong, W.W.; Tan, P.J.; Kamarulzaman, F.A.; Mejin, M.; Lim, D.; Ang, I.; Naming, M.; Yeo, T.C.; Ho, A.S.; Teo, S.H.; et al. Photodynamic activity of plant extracts from Sarawak, Borneo. Chem. Biodivers. 2013, 10, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Samat, N.; Tan, P.J.; Shaari, K.; Abas, F.; Lee, H.B. Prioritization of natural extracts by LC-MS-PCA for the identification of new photosensitizers for photodynamic therapy. Anal. Chem. 2014, 86, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Villacorta, R.B.; Roque, K.F.J.; Tapang, G.A.; Jacinto, S.D. Plant extracts as natural photosensitizers in photodynamic therapy: In vitro activity against human mammary adenocarcinoma MCF-7 cells. Asian Pac. J. Trop. Biomed. 2017, 7, 358–366. [Google Scholar] [CrossRef]
- Sokolov, V.V.; Chissov, V.I.; Filonenko, E.V.; Sukhin, G.M.; Yakubovskaya, R.I.; Belous, T.A.; Zharkova, N.N.; Kozlov, D.N.; Smirnov, V.V. Photodynamic therapy of cancer with the photosensitizer PHOTOGEM. In Photodynamic Therapy of Cancer II; SPIE: Bellingham, WA, USA, 1995; Volume 2325. [Google Scholar]
- Romanko, Y.S.; Tsyb, A.F.; Kaplan, M.A.; Popuchiev, V.V. Effect of photodynamic therapy with photodithazine on morphofunctional parameters of M-1 sarcoma. Bull. Exp. Biol. Med. 2004, 138, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shengen, Y.; Liangwu, L.; He, H.; Tao, P.; Zhongtao, L.; Zhipeng, L.; Dongni, P.; Xiongying, M.; Yu, W.; et al. Anticancer Effect of Photodynamic Therapy with Photosan-Loaded Titanium Dioxide Nanoparticles on Panc-1 Pancreatic Cancer Cells. In Vitro. Sci. Adv. Mat. 2016, 8, 1145–1153. [Google Scholar]
- Sperandio, F.F.; Huang, Y.Y.; Hamblin, M.R. Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardivo, J.P.; Adami, F.; Correa, J.A.; Pinhal, M.A.; Baptista, M.S. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagn. Photodyn. Ther. 2014, 11, 342–350. [Google Scholar] [CrossRef]
- Petelin, M.; Perkič, K.; Seme, K.; Gašpirc, B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015, 30, 1647–1656. [Google Scholar] [CrossRef]
- Machorowska-Pieniążek, A.; Morawiec, T.; Olek, M.; Mertas, A.; Aebisher, D.; Bartusik-Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. Advantages of using toothpaste containing propolis and plant oils for gingivitis prevention and oral cavity hygiene in cleft lip/palate patients. Biomed. Pharmacother. 2021, 142, 111992. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, W.Y.; Hahn, B.S.; Han, M.J.; Yang, W.I.; Kim, B.S. Chlorophyll derivatives: A new photosensitizer for photodynamic therapy of cancer in mice. Yonsei Med. J. 1989, 30, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.; Tang, P.M.; Hon, P.M.; Au, S.W.; Tsui, S.K.; Waye, M.M.; Kong, S.K.; Mak, T.C.; Fung, K.P. Pheophorbide a, a major antitumor component purified from Scutellaria barbata, induces apoptosis in human hepatocellular carcinoma cells. Planta Med. 2006, 72, 28–33. [Google Scholar] [CrossRef]
- Chou, S.T.; Chan, H.H.; Peng, H.Y.; Liou, M.J.; Wu, T.S. Isolation of substances with antiproliferative and apoptosis-inducing activities against leukemia cells from the leaves of Zanthoxylum ailanthoides Sieb. & Zucc. Phytomedicine 2011, 18, 344–348. [Google Scholar]
- Tang, P.M.; Chan, J.Y.; Au, S.W.; Kong, S.K.; Tsui, S.K.; Waye, M.M.; Mak, T.C.; Fong, W.P.; Fung, K.P. Pheophorbide a an active compound isolated from Scutellaria barbata, possesses photodynamic activities by inducing apoptosis in human hepatocellular carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, P.M.; Liu, X.Z.; Zhang, D.M.; Fong, W.P.; Fung, K.P. Pheophorbide a based photodynamic therapy induces apoptosis via mitochondrial-mediated pathway in human uterine carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.M.; Kim, S.N.; Amna, T.; Lee, S.; Shin, W.S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. J. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, L.C.; Vucenik, I.; Hagen, I.K.; Chernomorsky, S.A.; Poretz, R.D. In-vitro photocytotoxicity of lysosomotropic immunoliposomes containing pheophorbide a with human bladder carcinoma cells. J. Photochem. Photobiol. B 1994, 24, 17–23. [Google Scholar] [CrossRef]
- Chung, P.S.; He, P.; Shin, J.I.; Hwang, H.J.; Lee, S.J.; Ahn, J.C. Photodynamic therapy with 9-hydroxypheophorbide alpha on AMC-HN-3 human head and neck cancer cells: Induction of apoptosis via photoactivation of mitochondria and endoplasmic reticulum. Cancer Biol. Ther. 2009, 8, 1343–1351. [Google Scholar] [CrossRef]
- Qumseya, B.J.; David, W.; Wolfsen, H.C. Photodynamic therapy for barrett’s esophagus and esophageal carcinoma. Clin. Endosc. 2013, 46, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.Y.; Lim, D.S.; Ko, S.H.; Park, Y.J.; Ryu, K.S.; Ahn, M.Y.; Kim, Y.R.; Lee, D.W.; Cho, C.W. Photoactivation of pheophorbide a induces a mitochondrial-mediated apoptosis in Jurkat leukaemia cells. J. Photochem. Photobiol. B 2004, 75, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.D.; Lam, H.M.; Hoeven, R.; Xu, C.B.; Leung, A.W.; Cho, W.C. Photodynamic therapy induced cell death of hormone insensitive prostate cancer PC-3 cells with autophagic characteristics. Photodiagn. Photodyn. Ther. 2013, 10, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Della Pietra, E.; Simonella, F.; Bonavida, B.; Xodo, L.E.; Rapozzi, V. Repeated sub-optimal photodynamic treatments with pheophorbide a induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide 2015, 45, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gheewala, T.; Skwor, T.; Munirathinam, G. Photodynamic therapy using pheophorbide and 670 nm LEDs exhibits anti-cancer effects in-vitro in androgen dependent prostate cancer. Photodiagn. Photodyn. Ther. 2018, 21, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liu, Z.; Fu, Y.; Zhang, Y.; Tang, N.; Wang, Q.; Tao, L. Efficacy of 2-(1-hexyloxyethyl)-2-devinyl pyropheophorbide-a in photodynamic therapy of human esophageal squamous cancer cells. Oncol. Lett. 2013, 6, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Lin, J.-N.; Ma, J.-W.; Yang, N.-S.; Ho, C.-T.; Kuo, S.-C.; Way, T.-D. Demethoxycurcumin induces autophagic and apoptotic responses on breast cancer cells in photodynamic therapy. J. Funct. Foods 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Comini, L.; Fernandez, I.; Vittar, N.R.; Montoya, S.N.; Cabrera, J.L.; Rivarola, V.A. Photodynamic activity of anthraquinones isolated from Heterophyllaea pustulata Hook f. (Rubiaceae) on MCF-7c3 breast cancer cells. Phytomedicine 2011, 18, 1093–1095. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Koon, H.; Leung, A.W.; Yue, K.K.; Mak, N.K. Photodynamic effect of curcumin on NPC/CNE2 cells. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 205–215. [Google Scholar] [CrossRef]
- Dujic, J.; Kippenberger, S.; Ramirez-Bosca, A.; Diaz-Alperi, J.; Bereiter-Hahn, J.; Kaufmann, R.; Bernd, A.; Hofmann, M. Curcumin in combination with visible light inhibits tumor growth in a xenograft tumor model. Int. J. Cancer 2009, 124, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic action of LED-activated curcumin against Staphylococcus aureus involving intracellular ROS increase and membrane damage. Int. J. Photoener. 2014, 2014, 637601. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Hou, W.; Cao, B.; Zuo, T.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q. Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bioaccumulated in oysters. Photodiagn. Photodyn. 2015, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mou, H.; Xue, C.; Leung, A.W.; Xu, C.; Tang, Q. Photodynamic effect of curcumin on Vibrio parahaemolyticus. Photodiagn. Photodyn. 2016, 15, 34–39. [Google Scholar] [CrossRef]
- Zeng, X.B.; Leung, A.W.N.; Xia, X.S.; Yu, H.P.; Bai, D.Q.; Xiang, J.Y.; Jiang, Y.; Xu, C.S. Effect of Blue Light Radiation on Curcumin-Induced Cell Death of Breast Cancer Cells. Laser Phys. 2010, 20, 1500–1503. [Google Scholar] [CrossRef]
- Randazzo, W.; Aznar, R.; Sánchez, G. Curcumin-Mediated Photodynamic Inactivation of Norovirus Surrogates. Food Environ. Virol. 2016, 8, 244–250. [Google Scholar] [CrossRef]
- Felouat, A.; D’Aléo, A.; Fages, F. Synthesis and photophysical properties of difluoroboron complexes of curcuminoid derivatives bearing different terminal aromatic units and a mesoaryl ring. J. Org. Chem. 2013, 78, 4446–4455. [Google Scholar] [CrossRef]
- Fang, X.; Fang, L.; Gou, S.; Cheng, L. Design and synthesis of dimethylaminomethyl-substituted curcumin derivatives/analogues: Potent antitumor and antioxidant activity, improved stability and aqueous solubility compared with curcumin. Bioorg. Med. Chem. Lett. 2013, 23, 1297–1301. [Google Scholar] [CrossRef]
- Montoya, S.C.; Comini, L.R.; Sarmiento, M.; Becerra, C.; Albesa, I.; Argüello, G.A.; Cabrera, J.L. Natural anthraquinones probed as Type I and Type II photosensitizers: Singlet oxygen and superoxide anion production. J. Photochem. Photobiol. B 2005, 78, 77–83. [Google Scholar] [CrossRef]
- Rumie Vittar, N.B.; Comini, L.; Fernadez, I.M.; Agostini, E.; Nuñez-Montoya, S.; Cabrera, J.L.; Rivarola, V.A. Photochemotherapy using natural anthraquinones: Rubiadin and Soranjidiol sensitize human cancer cell to die by apoptosis. Photodiagn. Photodyn. Ther. 2014, 11, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Marles, R.J.; Hudson, J.B.; Graham, E.A.; Soucy-Breau, C.; Morand, P.; Compadre, R.L.; Compadre, C.M.; Towers, G.H.; Arnason, J.T. Structure-activity studies of photoactivated antiviral and cytotoxic tricyclic thiophenes. Photochem. Photobiol. 1992, 56, 479–487. [Google Scholar] [CrossRef]
- Marles, R.J.; Compadre, R.L.; Compadre, C.M.; Soucy-Breau, C.; Redmond, R.W.; Duval, F.; Mehta, B.; Morand, P.; Scaiano, J.C.; Arnason, J.T. Thiophenes as mosquito larvicides: Structure-toxicity relationship analysis. Pestic. Biochem. Physiol. 1991, 41, 89–100. [Google Scholar] [CrossRef]
- Zhang, P.; Jin, W.-R.; Shi, Q.; He, H.; Ma, Z.; Qu, H.-B. Two novel thiophenes from Echinops grijissi Hance. J. Asian Nat. Prod. Res. 2008, 10, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Morlière, P.; Mazière, J.C.; Santus, R.; Smith, C.D.; Prinsep, M.R.; Stobbe, C.C.; Fenning, M.C.; Golberg, J.L.; Chapman, J.D. Tolyporphin: A natural product from cyanobacteria with potent photosensitizing activity against tumor cells in vitro and in vivo. Cancer Res. 1998, 58, 3571–3578. [Google Scholar] [PubMed]
- Singh, R.K.; Tiwari, S.P.; Rai, A.K.; Mohapatra, T.M. Cyanobacteria: An emerging source for drug discovery. J. Antibiot. 2011, 64, 401–412. [Google Scholar] [CrossRef] [Green Version]
- Naithani, R.; Mehta, R.G.; Shukla, D.; Chandersekera, S.N.; Moriarty, R.M. Antiviral Activity of Phytochemi-cals: A Current Perspective. Diet. Compon. Immune Funct. 2010, 421–468. [Google Scholar]
- Barnhart-Dailey, M.; Zhang, Y.; Zhang, R.; Anthony, S.M.; Aaron, J.S.; Miller, E.S.; Lindsey, J.S.; Timlin, J.A. Cellular localization of tolyporphins, unusual tetrapyrroles, in a microbial photosynthetic community determined using hyperspectral confocal fluorescence microscopy. Photosynth. Res. 2019, 141, 259–271. [Google Scholar] [CrossRef]
- Gomaa, I.; Ali, S.E.; El-Tayeb, T.A.; Abdel-Kader, M.H. Chlorophyll derivative mediated PDT versus methotrexate: An in vitro study using MCF-7 cells. Photodiagn. Photodyn. Ther. 2012, 9, 362–368. [Google Scholar] [CrossRef]
- Ichimaru, Y.; Kanaeda, N.; Tominaga, S.; Suzui, M.; Maeda, T.; Fujii, H.; Nakao, M.; Yoshioka, H. Sasa veitchii extract induces anticancer effects via inhibition of cyclin D1 expression in MCF-7 cells. Nagoya J. Med. Sci. 2020, 82, 509–518. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jiang, C.; Li, Y.; Liu, W.; Yao, N.; Gao, M.; Ji, Y.; Huang, D.; Yin, Z.; Sun, Z.; et al. Evaluation of hypericin: Effect of aggregation on targeting biodistribution. J. Pharm. Sci. 2015, 104, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Theodossiou, T.A.; Hothersall, J.S.; De Witte, P.A.; Pantos, A.; Agostinis, P. The multifaceted photocytotoxic profile of hypericin. Mol. Pharm. 2009, 6, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Paba, V.; Quarto, M.; Varriale, L.; Crescenzi, E.; Palumbo, G. Photo-activation of hypericin with low doses of light promotes apparent photo-resistance in human histiocytic lymphoma U937 cells. J. Photochem. Photobiol. B 2001, 60, 87–96. [Google Scholar] [CrossRef]
- Krammer, B.; Verwanger, T. Molecular response to hypericin-induced photodamage. Curr. Med. Chem. 2012, 19, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Davids, L.M.; Kleemann, B.; Cooper, S.; Kidson, S.H. Melanomas display increased cytoprotection to hypericin-mediated cytotoxicity through the induction of autophagy. Cell Biol. Int. 2009, 33, 1065–1072. [Google Scholar] [CrossRef]
- Karioti, A.; Bilia, A.R. Hypericins as Potential Leads for New Therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schempp, C.M.; Winghofer, B.; Langheinrich, M.; Schöpf, E.; Simon, J.C. Hypericin levels in human serum and interstitial skin blister fluid after oral single-dose and steady-state administration of Hypericum perforatum extract (St. John’s wort). Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 299–304. [Google Scholar] [CrossRef]
- Vantieghem, A.; Xu, Y.; Declercq, W.; Vandenabeele, P.; Denecker, G.; Vandenheede, J.R.; Merlevede, W.; de Witte, P.A.; Agostinis, P. Different pathways mediate cytochrome c release after photodynamic therapy with hypericin. Photochem. Photobiol. 2001, 74, 133–142. [Google Scholar] [CrossRef]
- Vantieghem, A.; Assefa, Z.; Vandenabeele, P.; Declercq, W.; Courtois, S.; Vandenheede, J.R.; Merlevede, W.; de Witte, P.; Agostinis, P. Hypericin-induced photosensitization of HeLa cells leads to apoptosis or necrosis. Involvement of cytochrome c and procaspase-3 activation in the mechanism of apoptosis. FEBS Lett. 1998, 440, 19–24. [Google Scholar] [CrossRef]
- Weller, M.; Trepel, M.; Grimmel, C.; Schabet, M.; Bremen, D.; Krajewski, S.; Reed, J.C. Hypericin-induced apoptosis of human malignant glioma cells is light-dependent, independent of bcl-2 expression, and does not require wild-type p53. Neurol Res. 1997, 19, 459–470. [Google Scholar] [CrossRef]
- Zupkó, I.; Kamuhabwa, A.R.; D’Hallewin, M.A.; Baert, L.; De Witte, P.A. In vivo photodynamic activity of hypericin in transitional cell carcinoma bladder tumors. Int. J. Oncol. 2001, 18, 1099–1105. [Google Scholar] [CrossRef]
- Xu, C.; Leung, A. Light-activated hypericin induces cellular destruction of nasopharyngeal carcinoma cells. Laser. Phys. Lett. 2010, 7, 68. [Google Scholar] [CrossRef]
- Liu, C.D.; Kwan, D.; Saxton, R.E.; McFadden, D.W. Hypericin and photodynamic therapy decreases human pancreatic cancer in vitro and in vivo. J. Surg. Res. 2000, 93, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; de Witte, P.A. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell. Biol. 2002, 34, 221–241. [Google Scholar] [CrossRef]
- Ali, S.M.; Olivo, M. Efficacy of hypocrellin pharmacokinetics in phototherapy. Int. J. Oncol. 2002, 21, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Besic Gyenge, E.; Forny, P.; Lüscher, D.; Laass, A.; Walt, H.; Maake, C. Effects of hypericin and a chlorin based photosensitizer alone or in combination in squamous cell carcinoma cells in the dark. Photodiagn. Photodyn. Ther. 2012, 9, 321–331. [Google Scholar] [CrossRef]
- Gyenge, E.B.; Lüscher, D.; Forny, P.; Antoniol, M.; Geisberger, G.; Walt, H.; Patzke, G.; Maake, C. Photodynamic mechanisms induced by a combination of hypericin and a chlorin based-photosensitizer in head and neck squamous cell carcinoma cells. Photochem. Photobiol. 2013, 89, 150–162. [Google Scholar] [CrossRef]
- Jin, S.; Zhou, L.; Gu, Z.; Tian, G.; Yan, L.; Ren, W.; Yin, W.; Liu, X.; Zhang, X.; Hu, Z.; et al. A new near infrared photosensitizing nanoplatform containing blue-emitting up-conversion nanoparticles and hypocrellin A for photodynamic therapy of cancer cells. Nanoscale 2013, 5, 11910–11918. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014, 90, 575–579. [Google Scholar] [CrossRef]
- Jiang, Y.; Leung, W.; Tang, Q.; Zhang, H.; Xu, C. Effect of light-activated hypocrellin B on the growth and membrane permeability of gram-negative Escherichia coli cells. Int. J. Photoenergy 2014, 2014, 521209. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Leung, A.W.; Wang, X.; Zhang, H.; Xu, C. Inactivation of Staphylococcus aureus by photodynamic action of hypocrellin B. Photodiagn. Photodyn. 2013, 10, 600–606. [Google Scholar] [CrossRef]
- Miller, G.G.; Brown, K.; Ballangrud, A.M.; Barajas, O.; Xiao, Z.; Tulip, J.; Lown, J.W.; Leithoff, J.M.; Allalunis-Turner, M.J.; Mehta, R.D.; et al. Preclinical assessment of hypocrellin B and hypocrellin B derivatives as sensitizers for photodynamic therapy of cancer: Progress update. Photochem. Photobiol. 1997, 65, 714–722. [Google Scholar] [CrossRef]
- Jiang, Y.; Xia, X.; Leung, A.W.; Xiang, J.; Xu, C. Apoptosis of breast cancer cells induced by hypocrellin B under light-emitting diode irradiation. Photodiagn. Photodyn. 2012, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelopoulou, M.; Grigalavicius, M.; Berg, K.; Ménard, M.; Theodossiou, T.A. Cytotoxic and Photocytotoxic Effects of Cercosporin on Human Tumor Cell Lines. Photochem. Photobiol. 2019, 95, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Bouillaguet, S.; Wataha, J.C.; Zapata, O.; Campo, M.; Lange, N.; Schrenzel, J. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed. Laser Surg. 2010, 28, 519–525. [Google Scholar] [CrossRef]
- Corbin, F., 3rd. Pathogen inactivation of blood components: Current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 2002, 76, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Galloway, J.R.; McCormick, D.B. Pharmacokinetics of orally and intravenously administered riboflavin in healthy humans. Am. J. Clin. Nutr. 1996, 63, 54–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Administration USFaD. Listing of Specific Substances Affirmed as GRAS. Available online: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1695 (accessed on 6 December 2021).
- Hassan, I.; Chibber, S.; Khan, A.A.; Naseem, I. Riboflavin ameliorates cisplatin induced toxicities under photoillumination. PLoS ONE 2012, 7, e36273. [Google Scholar] [CrossRef]
- Hassan, I.; Chibber, S.; Naseem, I. Vitamin B2: A promising adjuvant in cisplatin based chemoradiotherapy by cellular redox management. Food Chem. Toxi. 2013, 59, 715–723. [Google Scholar] [CrossRef]
- Juarez, A.V.; Sosa Ldel, V.; De Paul, A.L.; Costa, A.P.; Farina, M.; Leal, R.B.; Torres, A.I.; Pons, P. Riboflavin acetate induces apoptosis in squamous carcinoma cells after photodynamic therapy. J. Photochem. Photobiol. B 2015, 153, 445–454. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chang, C.J.; Chen, L.Y. Blue light induced reactive oxygen species from flavin mononucleotide and flavin adenine dinucleotide on lethality of HeLa cells. J. Photochem. Photobiol. B 2017, 173, 325–332. [Google Scholar] [CrossRef]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 4, 9679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benyhe, S. Morphine: New aspects in the study of an ancient compound. Life Sci. 1994, 55, 969–979. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine-A Summary Update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Kukielczak, B.M.; Bilski, P.; Sandvik, S.L.; Chignell, C.F. Photochemistry and Photocytotoxicity of Alkaloids from Goldenseal (Hydrastis canadensis L.) 1. Berberine. Chem. Res. Toxicol. 2001, 14, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, N.L.; Vevert-Bizet, C.; Bourg-Heckly, G.; Sureau, F.; Salvador, M.J.; Bonneau, S. Berberine as a Photosensitizing Agent for Antitumoral Photodynamic Therapy: Insights into its Association to Low Density Lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Lopes, T.Z.; de Moraes, F.R.; Tedesco, A.C.; Arni, R.K.; Rahal, P.; Calmon, M.F. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed. Pharmacother. 2020, 123, 109794. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Lopes, T.Z.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of berberine associated with photodynamic therapy in cell lines. Photodiagn. Photodyn. Ther. 2020, 32, 102045. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-L.; Wang, M.; Zhu, H.; Li, K.; Zhu, R.-R.; Sun, X.-Y.; Yao, S.-D.; Wu, Q.-S.; Wang, S.-L. Characterization of the transient species generated by the photoionization of Berberine: A laser flash photolysis study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 955–959. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Gupta, P.; Bandyopadhyay, S.K.; Patro, B.S.; Chattopadhyay, S. Coralyne, a protoberberine alkaloid, causes robust photosenstization of cancer cells through ATR-p38 MAPK-BAX and JAK2-STAT1-BAX pathways. Chem. Interact. 2018, 285, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.P.; Labrador, V.; Freire, P.F.; Molero, M.L.; Hazen, M. Ultrastructural changes induced in HeLa cells after phototoxic treatment with harmine. J. Appl. Toxicol. 2004, 24, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Jantova, S.; Letašiová, S.; Brezová, V.; Cipak, L.; Lábaj, J. Photochemical and phototoxic activity of berberine on murine fibroblast NIH-3T3 and Ehrlich ascites carcinoma cells. J. Photochem. Photobiol. B 2006, 85, 163–176. [Google Scholar] [CrossRef]
- Kubrak, T.; Podgórski, R.; Stompor-Gorący, M. Natural and Synthetic Coumarins and their Pharmacological Activity. Eur. J. Clin. Exp. Med. 2017, 15, 169–175. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Palma, M.G.; Bartella, V.; Rago, V.; Maggiolini, M.; Sisci, D.; Lanzino, M.; De Amicis, F.; Ando, S. Evidence that bergapten, independently of its photoactivation, enhances p53 gene expression and induces apoptosis in human breast cancer cells. Curr. Cancer Drug Targets 2009, 9, 469–481. [Google Scholar] [CrossRef]
- Panno, M.L.; Giordano, F.; Rizza, P.; Pellegrino, M.; Zito, D.; Giordano, C.; Mauro, L.; Catalano, S.; Aquila, S.; Sisci, D.; et al. Bergapten induces ER depletion in breast cancer cells through SMAD4-mediated ubiquitination. Breast Cancer Res. Treat. 2012, 136, 443–455. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.-S.; Shim, B.S.; Ahn, K.S.; Kim, S.-H. Bergamottin, a natural furanocoumarin obtained from grapefruit juice induces chemosensitization and apoptosis through the inhibition of STAT3 signaling pathway in tumor cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, E.-J.; Lee, J.H.; Yang, W.M.; Nam, D.; Lee, J.H.; Lee, S.-G.; Um, J.-Y.; Shim, B.S.; Ahn, K.S. Simvastatin in combination with bergamottin potentiates TNF-induced apoptosis through modulation of NF-kB signalling pathway in human chronic myelogenous leukaemia. Pharm. Biol. 2016, 54, 2050–2060. [Google Scholar] [CrossRef] [Green Version]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Provenzano, E.; de Cindio, B.; Menichini, F. In vitro photo-induced cytotoxic activity of Citrus bergamia and C. medica L. cv. Diamante peel essential oils and identified active coumarins. Pharm Biol. 2010, 48, 1059–1065. [Google Scholar] [CrossRef]
- Nagatani, T.; Matsuzaki, T.; Kim, S.; Baba, N.; Ichiyama, S.; Miyamoto, H.; Nakajima, H. Treatment of cutaneous T-cell lymphoma (CTCL) with extracorporeal photochemotherapy. J. Dermatol. Sci. 1990, 1, 226. [Google Scholar] [CrossRef]
- Bethea, D.; Fullmer, B.; Syed, S.; Seltzer, G.; Tiano, J.; Rischko, C.; Gillespie, L.; Brown, D.; Gasparro, F.P. Psoralen photobiology and photochemotherapy: 50 years of science and medicine. J. Dermatol. Sci. 1999, 19, 78–88. [Google Scholar] [CrossRef]
- Sumiyoshi, M.; Sakanaka, M.; Taniguchi, M.; Baba, K.; Kimura, Y. Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation. J. Nat. Med. 2014, 68, 83–94. [Google Scholar] [CrossRef] [PubMed]


| Common Name | Plant Species | Phototoxin(s) | References |
|---|---|---|---|
| Ammi majus L. | Bishop’s weed | e.g., Xanthotoxin, bergapten, oxypeucedanin | [35] |
| Ammi visnaga (L.) | Apiaceae | e.g., Xanthotoxin; 8-hydroxybergapten; imperatorin | [36] |
| Pastinaca sativa | Wild parsnip | e.g., Xanthotoxin; bergapten; imperatorin | [37] |
| Cymopterus watsonii | Apiaceae | e.g., Xanthotoxin; bergapten | [38] |
| Cullen cinereum | Hoary Scurf-pea | e.g., Psoralen | [39] |
| Ruta graveolens L. | Rua | e.g., Psoralen; bergapten; isorutarin | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubrak, T.P.; Kołodziej, P.; Sawicki, J.; Mazur, A.; Koziorowska, K.; Aebisher, D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules 2022, 27, 1192. https://doi.org/10.3390/molecules27041192
Kubrak TP, Kołodziej P, Sawicki J, Mazur A, Koziorowska K, Aebisher D. Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules. 2022; 27(4):1192. https://doi.org/10.3390/molecules27041192
Chicago/Turabian StyleKubrak, Tomasz Piotr, Przemysław Kołodziej, Jan Sawicki, Anna Mazur, Katarzyna Koziorowska, and David Aebisher. 2022. "Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy" Molecules 27, no. 4: 1192. https://doi.org/10.3390/molecules27041192
APA StyleKubrak, T. P., Kołodziej, P., Sawicki, J., Mazur, A., Koziorowska, K., & Aebisher, D. (2022). Some Natural Photosensitizers and Their Medicinal Properties for Use in Photodynamic Therapy. Molecules, 27(4), 1192. https://doi.org/10.3390/molecules27041192








