Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Plant Materials
2.1.2. Chemicals Assay
2.1.3. NA Enzymes Preparation
2.2. Methods
2.2.1. Column Chromatography
2.2.2. General Experiments and Spectroscopy
2.2.3. Isolation of Compounds from GAF
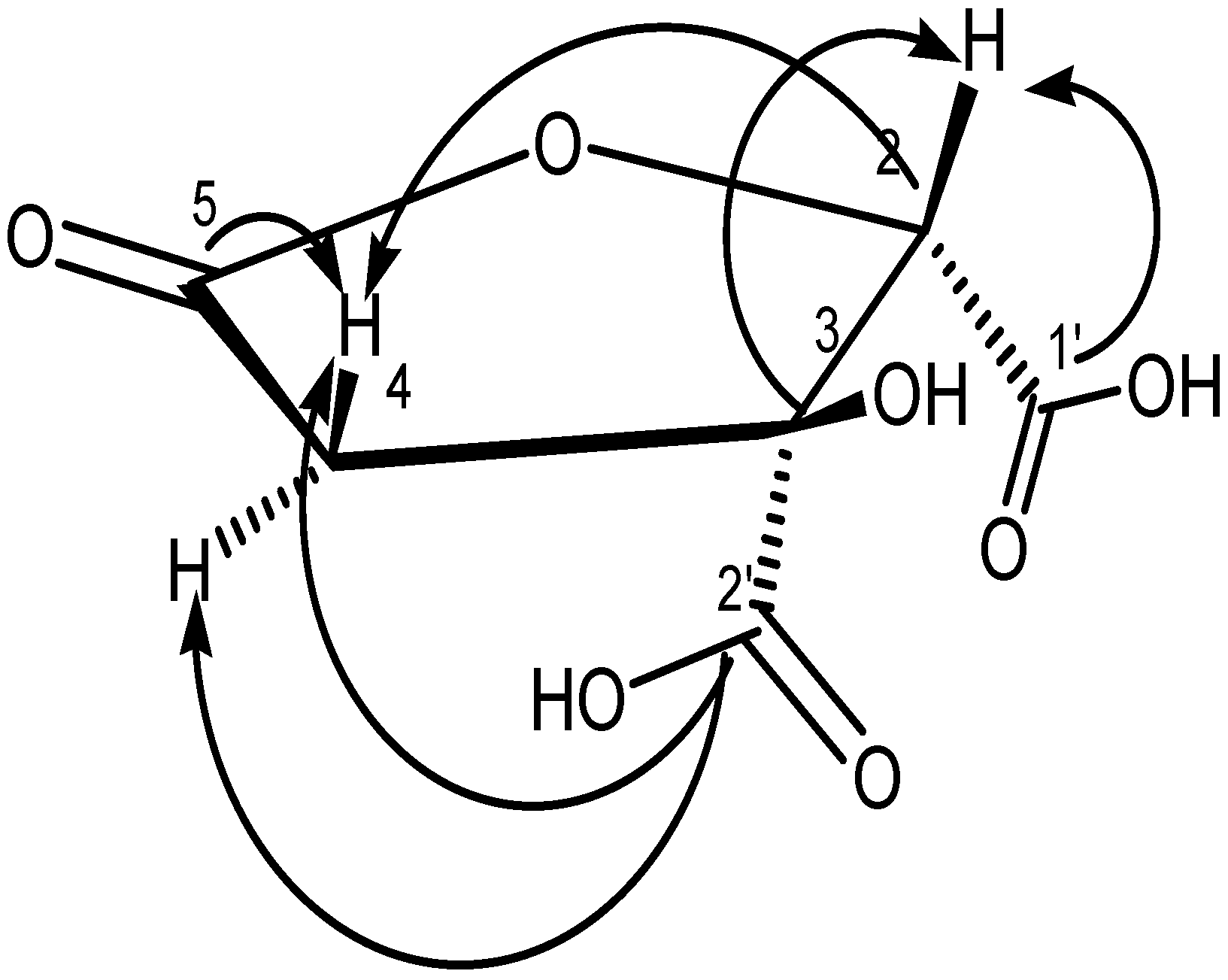
2.2.4. Spectral Data of Garcinia Acid (GM6 or D2)
2.2.5. Extraction and Isolation of Compounds from GAL
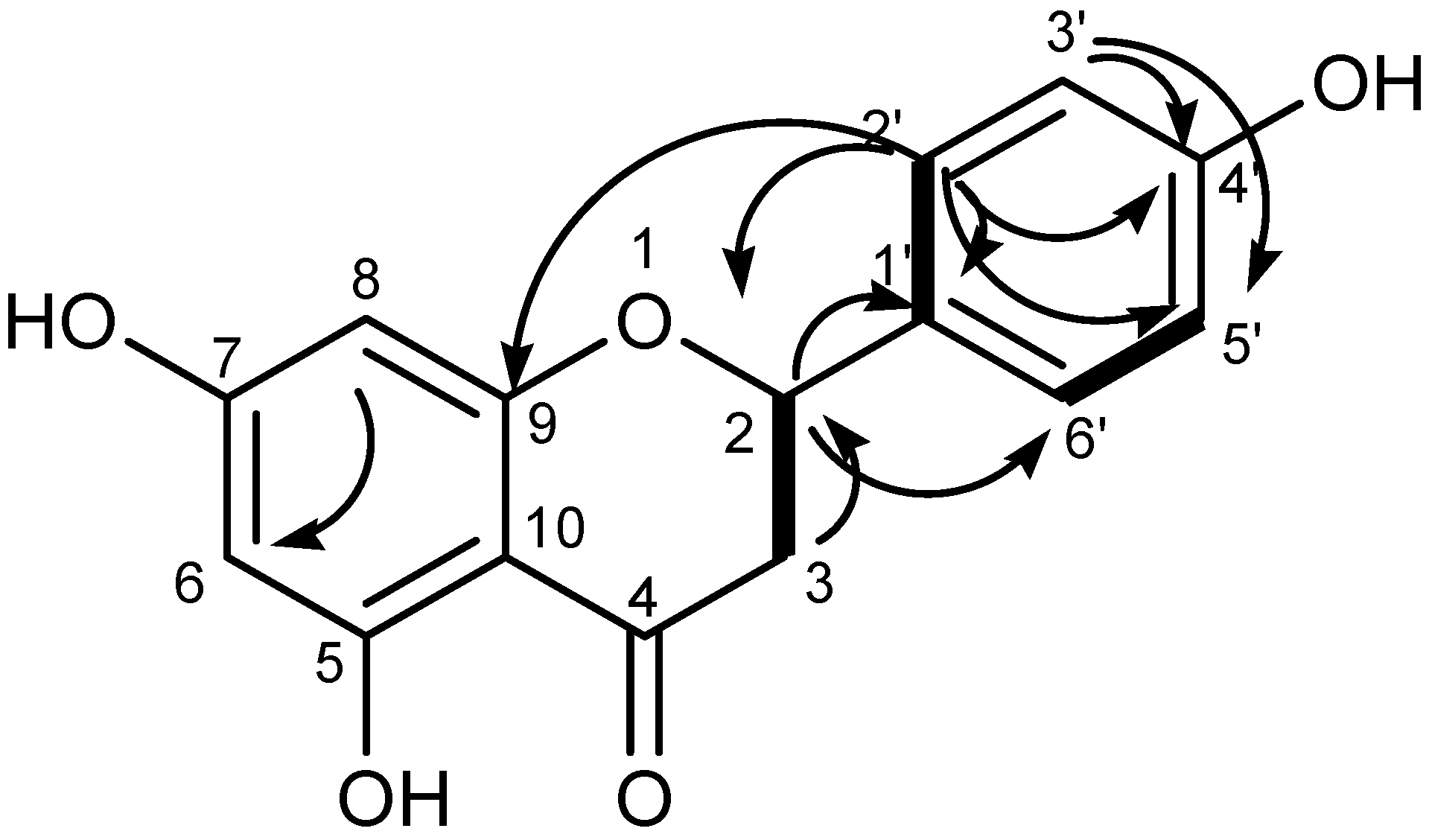
2.2.6. Spectral Data of Naringenin (GAL1)
2.2.7. MUNANA Assays
2.2.8. Data Analysis of Assay
2.2.9. Molecular Docking
3. Results
3.1. Bioassay-Guided Isolation of Active Compounds from GA
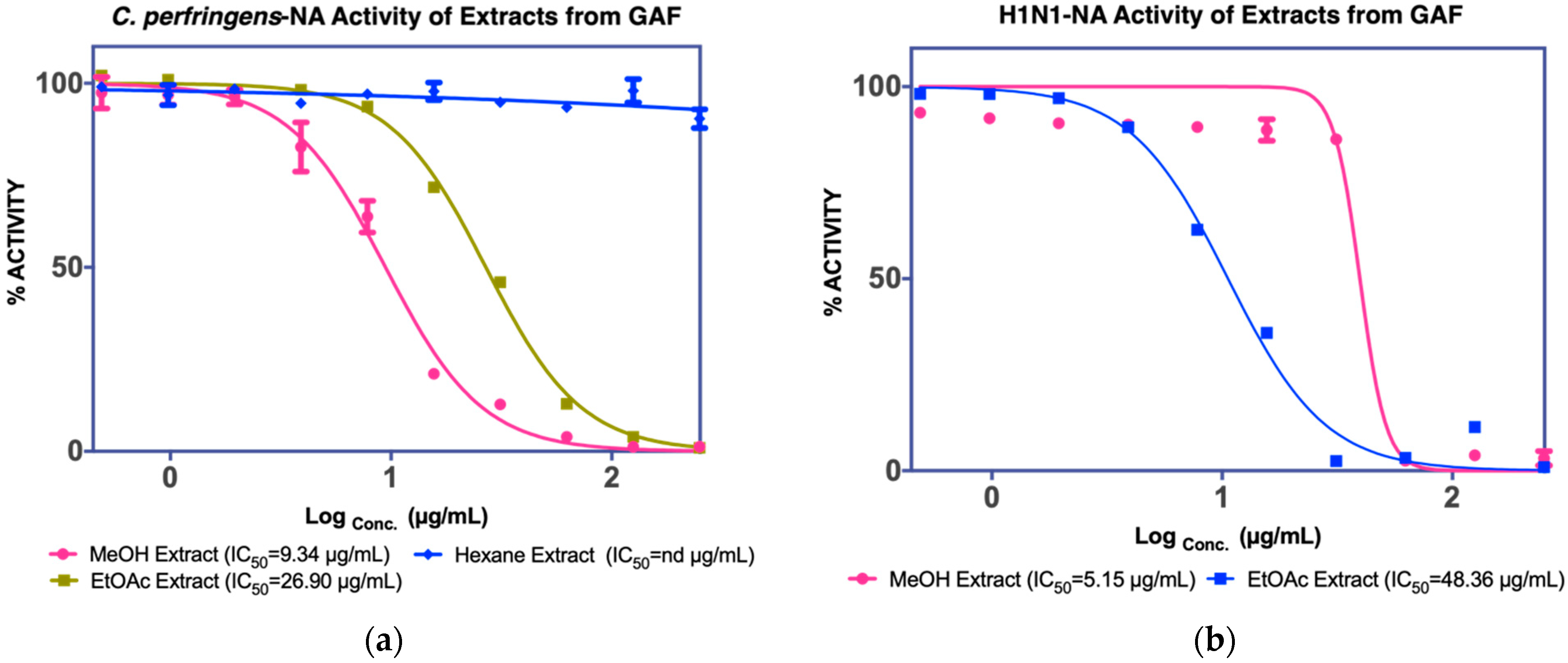
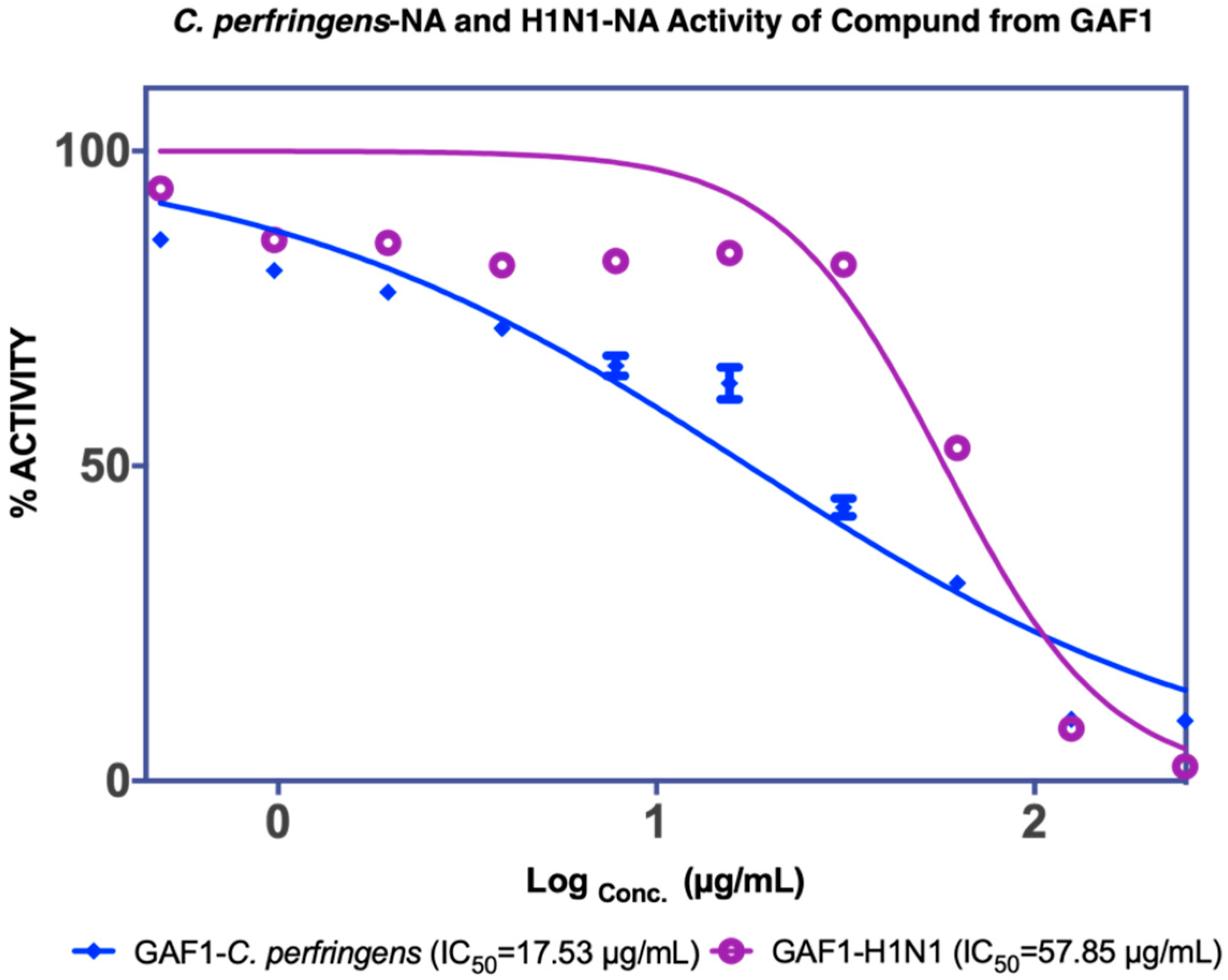
3.1.1. Isolation of Compounds from GAF (G. atroviridis Fruits)
3.1.2. Isolation of Compounds from GAL (G. atroviridis Leaves)
3.2. Structure Characterisation
3.2.1. Structure of GAF1 Compound
3.2.2. Structure of GAL1 Compounds
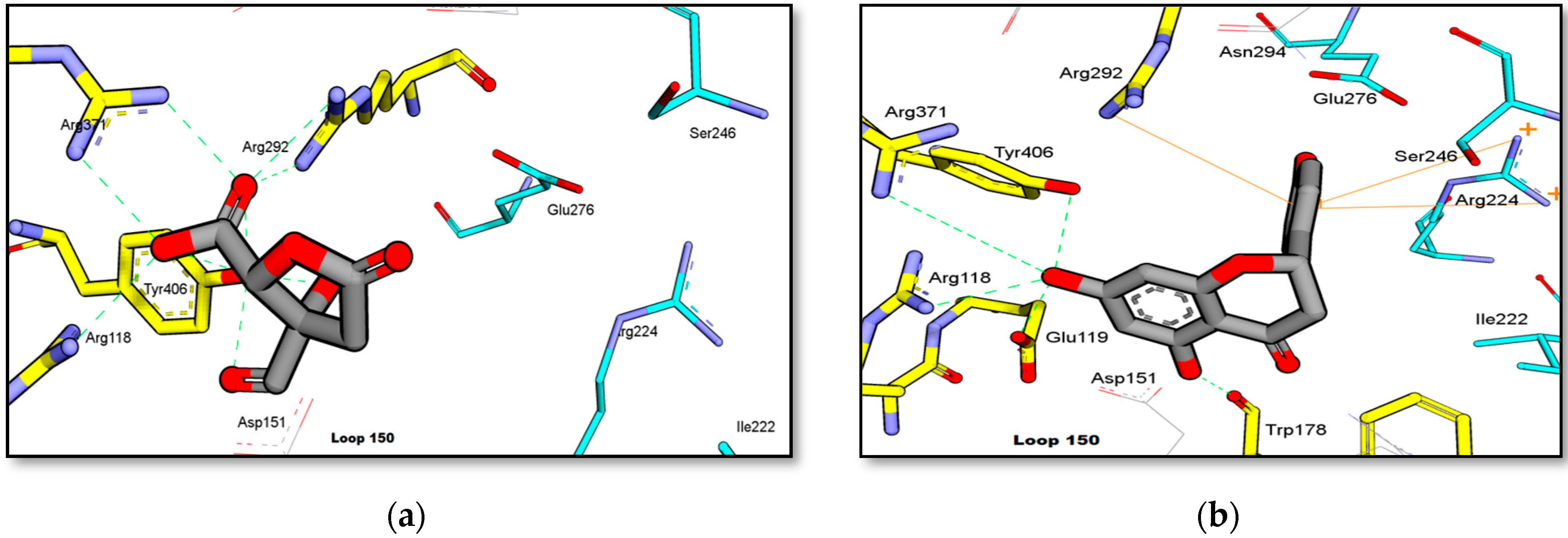
3.3. Binding Interaction of Isolated Compound from G. atroviridis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Lee, C.K. Influenza A/H1N1 Pandemic: The Scare of 2009. Malays. J. Med. Sci. 2009, 16, 1–4. [Google Scholar]
- Wignjadiputro, I.; Widaningrum, C.; Setiawaty, V.; Widuri Wulandari, E.; Sihombing, S.; Prasetyo, W.A.; Azhar, M.; iL Rim, K.; Pang Junxiong, V.; Waworuntu, W.; et al. Whole–of–society approach for influenza pandemic epicenter Containment exercise in Indonesia. J. Infect. Public Health 2020, 13, 994–997. [Google Scholar] [CrossRef]
- Gaymard, A.; Le Briand, N.; Frobert, E.; Lina, B.; Escuret, V. Functional balance between neuraminidase and haemagglutinin in influenza viruses. Clin. Microbiol. Infect. 2016, 22, 975–983. [Google Scholar] [CrossRef]
- Schwerdtfeger, S.M.; Melzig, M.F. Sialidases in biological systems. Pharmazie 2010, 65, 551–561. [Google Scholar]
- Sander-Wewer, M.; Schauer, R.; Corfield, A.P. Substrate specificity of viral, bacterial and mammalian sialidases with regard to different N,O-acetylated sialic acids and GM1. Adv. Exp. Med. Biol. 1982, 152, 215–222. [Google Scholar] [PubMed]
- Garman, E.; Laver, G. Controlling influenza by inhibiting the virus′s neuraminidase. Curr. Drug Targets 2004, 5, 119–136. [Google Scholar] [CrossRef]
- Barnard, K.N.; Alford-Lawrence, B.K.; Buchholz, D.W.; Wasik, B.R.; LaClair, J.R.; Yu, H.; Honce, R.; Ruhl, S.; Pajic, P.; Daugherity, E.K.; et al. Modified Sialic Acids on Mucus and Erythrocytes Inhibit Influenza A Virus Hemagglutinin and Neuraminidase Functions. J. Virol. 2020, 94, e01567-19. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef] [PubMed]
- von Itzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.E.; Gabay, M.P.; Steinke, L.M.; Vanosdol, S.J. Peramivir: An intravenous neuraminidase inhibitor for the treatment of 2009 H1N1 influenza. Ann. Pharm. 2010, 44, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A. Method of Isolating Shikimic Acid from a Plant. U.S. Patent 8,203,020, 19 June 2012. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981-2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef] [PubMed]
- Mackeen, M.M.; Mooi, L.Y.; Amran, M.; Mat, N.; Lajis, N.H.; Ali, A.M. Noncytotoxic and Antitumour-Promoting Activities of Garcinia Acid Esters from Garcinia atroviridis Griff. ex T. Anders (Guttiferae). Evid. Based Complementary Altern. Med. 2012, 2012, 829814. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, P.W.; Gothard, P.K.; McWilliam, N.C.; Supriono, A.; Gray, D.O. Medicinal plants from Riau province, Sumatra, Indonesia. Part 1: Uses. J. Ethnopharmacol. 1995, 45, 75–95. [Google Scholar] [CrossRef]
- Burkill, I.H.; William, B.; Foxworthy, F.W.; Scrivenor, J.B.; Watson, J.G. Dictionary of the Economic Products of the Malay Peninsula, 2nd ed.; Crown Agent: Kuala Lumpur, Malaysia, 1966; p. 255. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Ikram, N. Discovery of New Neuraminidase Inhibitors from Plant Natural Compounds: Virtual Screening and Bioassays Studies; USM: Penang, Malaysia, 2012. [Google Scholar]
- Lewis, Y.S.; Neelakantan, S. (−)-Hydroxycitric acid—The principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 1965, 4, 619–625. [Google Scholar] [CrossRef]
- Hida, H.; Yamada, T.; Yamada, Y. Production of hydroxycitric acid by microorganisms. Biosci. Biotechnol. Biochem. 2005, 69, 1555–1561. [Google Scholar] [CrossRef][Green Version]
- Andrade, C.A.d.; Carvalho, J.L.d.S.; Cunico, M.M.; Lordello, A.L.L.; Higaskino, C.E.K.; Almeida, S.C.d.C.; Dias, J.d.F.G.; Kerber, V.A.; Miguel, M.D.; Miguel, O.G. Antioxidant and antibacterial activity of extracts, fractions and isolated substances from the flowers of Acacia podalyriifolia A. Cunn. ex G. Don. Braz. J. Pharm. Sci. 2010, 46, 715–722. [Google Scholar] [CrossRef]
- Ibrahim, A.R. Sulfation of naringenin by Cunninghamella elegans. Phytochemistry 2000, 53, 209–212. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Preparation of Three Flavonoids from the Bark of Salix alba by High-Speed Countercurrent Chromatographic Separation. J. Liq. Chromatogr. Relat. Technol. 2005, 27, 3257–3264. [Google Scholar] [CrossRef]
- Hurt, A. Fluorometric Neuraminidase Inhibition Assay. Available online: http://www.nisn.org/documents/A.Hurt_Protocol_for_NA_fluorescence.pdf (accessed on 3 March 2015).
- Muchtaridi, M.; Sugijanto, M.; Mohd Gazzali, A.; Wahab, H.A. Anti-Neuraminidase Bioactives from Manggis Hutan (Garcinia celebica L.) Leaves: Partial Purification and Molecular Characterization. Molecules 2020, 25, 821. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, X.; Dwek, R.A.; Stevens, J.; Wilson, I.A. Structural characterization of the 1918 influenza virus H1N1 neuraminidase. J. Virol. 2008, 82, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, W.; Morris, G.M.; Weber, L.; Huey, R. Using AutoDock for Virtual Screening. Available online: http://autodock.scripps.edu/faqs-help/tutorial/using-autodock-for-virtual-screening/UsingAutoDockforVirtualScreening_v7.pdf (accessed on 14 February 2003).
- Ibnusaud, I.; Thomas, P.T.; Rani, R.N.; Sasi, P.V.; Beena, T.; Hisham, A. Chiral γ-butyrolactones related to optically active 2-hydroxycitric acids. Tetrahedron 2002, 58, 4887–4892. [Google Scholar] [CrossRef]
- Polavarapu, P.L.; Scalmani, G.; Hawkins, E.K.; Rizzo, C.; Jeirath, N.; Ibnusaud, I.; Habel, D.; Nair, D.S.; Haleema, S. Importance of solvation in understanding the chiroptical spectra of natural products in solution phase: Garcinia acid dimethyl ester. J. Nat. Prod. 2011, 74, 321–328. [Google Scholar] [CrossRef]
- Mabry, T.; Markham, K.; Thomas, M. The Systematic Identification of Flavonoids; Springer: Berlin-Heidelberg, Germany; New York, NY, USA, 1970; Volume 1, pp. 84–234. [Google Scholar]
- Mackeen, M.M.; Ali, A.M.; Lajis, N.H.; Kawazu, K.; Kikuzaki, H.; Nakatani, N. Antifungal garcinia acid esters from the fruits of Garcinia atroviridis. Z. Für Nat. C J. Biosci. 2002, 57, 291–295. [Google Scholar] [CrossRef]
- Amran, A.A.; Zaiton, Z.; Faizah, O.; Morat, P. Effects of Garcinia atroviridis on serum profiles and atherosclerotic lesions in the aorta of guinea pigs fed a high cholesterol diet. Singap. Med. J. 2009, 50, 295–299. [Google Scholar]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The Use of Garcinia Extract (Hydroxycitric Acid) as a Weight loss Supplement: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Allison, D.B.; Vasselli, J.R.; Pietrobelli, A.; Greenfield, D.; Nunez, C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: A randomized controlled trial. J. Am. Med Assoc. 1998, 280, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Kosin, J.; Ruangrungsi, N.; Ito, C.; Furukawa, H. A xanthone from Garcinia atroviridis. Phytochemistry 1998, 47, 1167–1168. [Google Scholar] [CrossRef]
- Venkateswara Rao, G.; Karunakara, A.C.; Santhosh Babu, R.R.; Ranjit, D.; Chandrasekara Reddy, G. Hydroxycitric acid lactone and its salts: Preparation and appetite suppression studies. Food Chem. 2010, 120, 235–239. [Google Scholar] [CrossRef]
- Collins, D.; Kopic, S.; Geibel, J.P.; Hogan, A.M.; Medani, M.; Baird, A.W.; Winter, D.C. The flavonone naringenin inhibits chloride secretion in isolated colonic epithelia. Eur. J. Pharmacol. 2011, 668, 271–277. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muniz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.D.; Epifano, F.; Grenier, D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal Res. 2008, 43, 400–407. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Zierau, O.; Morrissey, C.; Watson, R.W.; Schwab, P.; Kolba, S.; Metz, P.; Vollmer, G. Antiandrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl)naringenin and 8-prenylnaringenin. Planta Med. 2003, 69, 856–858. [Google Scholar] [CrossRef]
- Zierau, O.; Gester, S.; Schwab, P.; Metz, P.; Kolba, S.; Wulf, M.; Vollmer, G. Estrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl)naringenin and 8-prenylnaringenin. Planta Med. 2002, 68, 449–451. [Google Scholar] [CrossRef]
- Sabarinathan, D.; Mahalakshmi, P.; Vanisree, A.J. Naringenin promote apoptosis in cerebrally implanted C6 glioma cells. Mol. Cell. Biochem. 2010, 345, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Wang, H.D.; Lee, S.M.; Wang, Y.T.; Du, G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza Virus Neuraminidase Structure and Functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Lew, W.; Williams, M.A.; Wu, H.; Zhang, L.; Chen, X.; Escarpe, P.A.; Mendel, D.B.; Laver, W.G.; Stevens, R.C. Structure-activity relationship studies of novel carbocyclic influenza neuraminidase inhibitors. J. Med. Chem. 1998, 41, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Chen, X.; Mendel, D.B. Neuraminidase inhibitors as anti-influenza virus agents. Antivir. Chem. Chemother. 1999, 10, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-L.; Hoffmann, E.; Taylor, G.; Scholtissek, C.; Monto, A.S.; Webster, R.G.; Govorkova, E.A. Importance of neuraminidase active-site residues to the neuraminidase inhibitor resistance of influenza viruses. J. Virol. 2006, 80, 8787–8795. [Google Scholar] [CrossRef]





| Fractions-1 | Fractions-2 | Compounds | ||
|---|---|---|---|---|
| Fruits | MeOH extract IC50 C. perfringens-NA 9.4 µg/mL IC50 NA-H1N1 5.15 µg/mL | Hexane fraction | ||
| EtOAC fraction IC50 C. perfringens-NA 26.9 µg/mL IC50 NA-H1N1 48.36 µg/mL | F1 Not active | - | ||
| F2 2.82 µg/mL a nd c | - | |||
| F3 2.82 µg/mL a 1.73 µg/mL b | Garcinia acid (GAF 1) 17.53 µg/mL a 57.85 µg/mL b | |||
| F4 2.52 µg/mL a 12.4 µg/mL b | Garcinia acid (GAF 1) | |||
| Leaves | MeOH extract IC50 C. perfringens-NA 36.17 µg/mL IC50 NA-H1N1 36.34 µg/mL | Hexane fraction | - | - |
| EtOAC fraction IC50 C. perfringens-NA 38.39 µg/mL IC50 NA-H1N1 48.36 µg/mL | F1 nd c | - | ||
| F2 12.80 µg/mL a 15.03 µg/mL b | Naringenin (GAL 1) 107 µg/mL a 123 µg/mL b | |||
| F3 6.78 µg/mL a 15.03 µg/mL b | Garcinia acid (GAL 2) 17.34 µg/mL a 56.71 µg/mL b | |||
| F4 145.20 µg/mL a nd c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muchtaridi, M.; Nuwarda, R.F.; Ikram, E.H.K.; Abdul Rahim, A.S.; Gazzali, A.M.; Wahab, H.A. Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization. Molecules 2022, 27, 949. https://doi.org/10.3390/molecules27030949
Muchtaridi M, Nuwarda RF, Ikram EHK, Abdul Rahim AS, Gazzali AM, Wahab HA. Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization. Molecules. 2022; 27(3):949. https://doi.org/10.3390/molecules27030949
Chicago/Turabian StyleMuchtaridi, Muchtaridi, Rina Fajri Nuwarda, Emmy Hainida Khairul Ikram, Aisyah Saad Abdul Rahim, Amirah Mohd Gazzali, and Habibah A. Wahab. 2022. "Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization" Molecules 27, no. 3: 949. https://doi.org/10.3390/molecules27030949
APA StyleMuchtaridi, M., Nuwarda, R. F., Ikram, E. H. K., Abdul Rahim, A. S., Gazzali, A. M., & Wahab, H. A. (2022). Neuraminidase Inhibitor of Garcinia atroviridis L. Fruits and Leaves Using Partial Purification and Molecular Characterization. Molecules, 27(3), 949. https://doi.org/10.3390/molecules27030949








