Artificial Solar Light-Driven APTES/TiO2 Photocatalysts for Methylene Blue Removal from Water
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the APTES-Modified TiO2
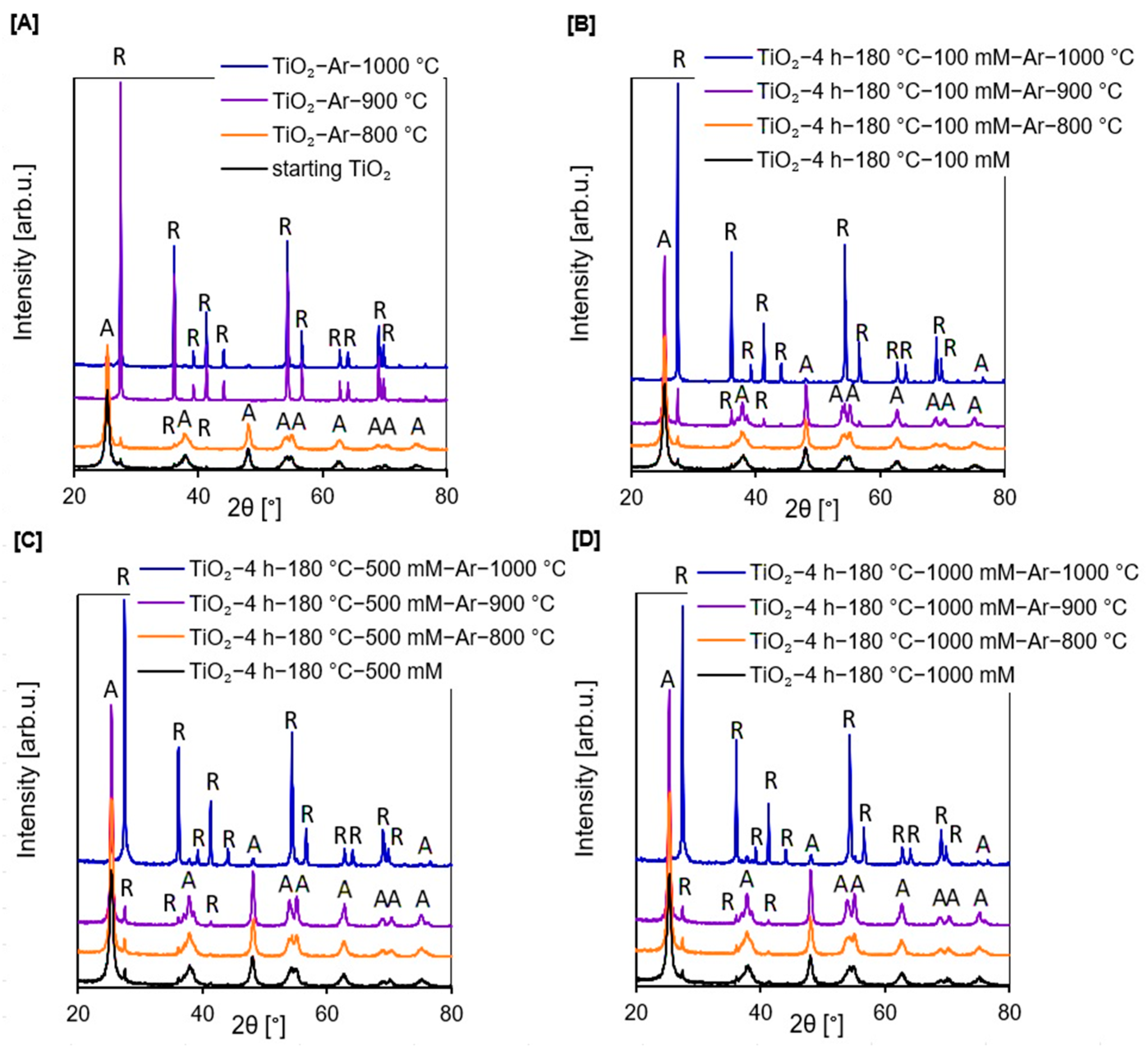
2.1.1. XRD Analysis
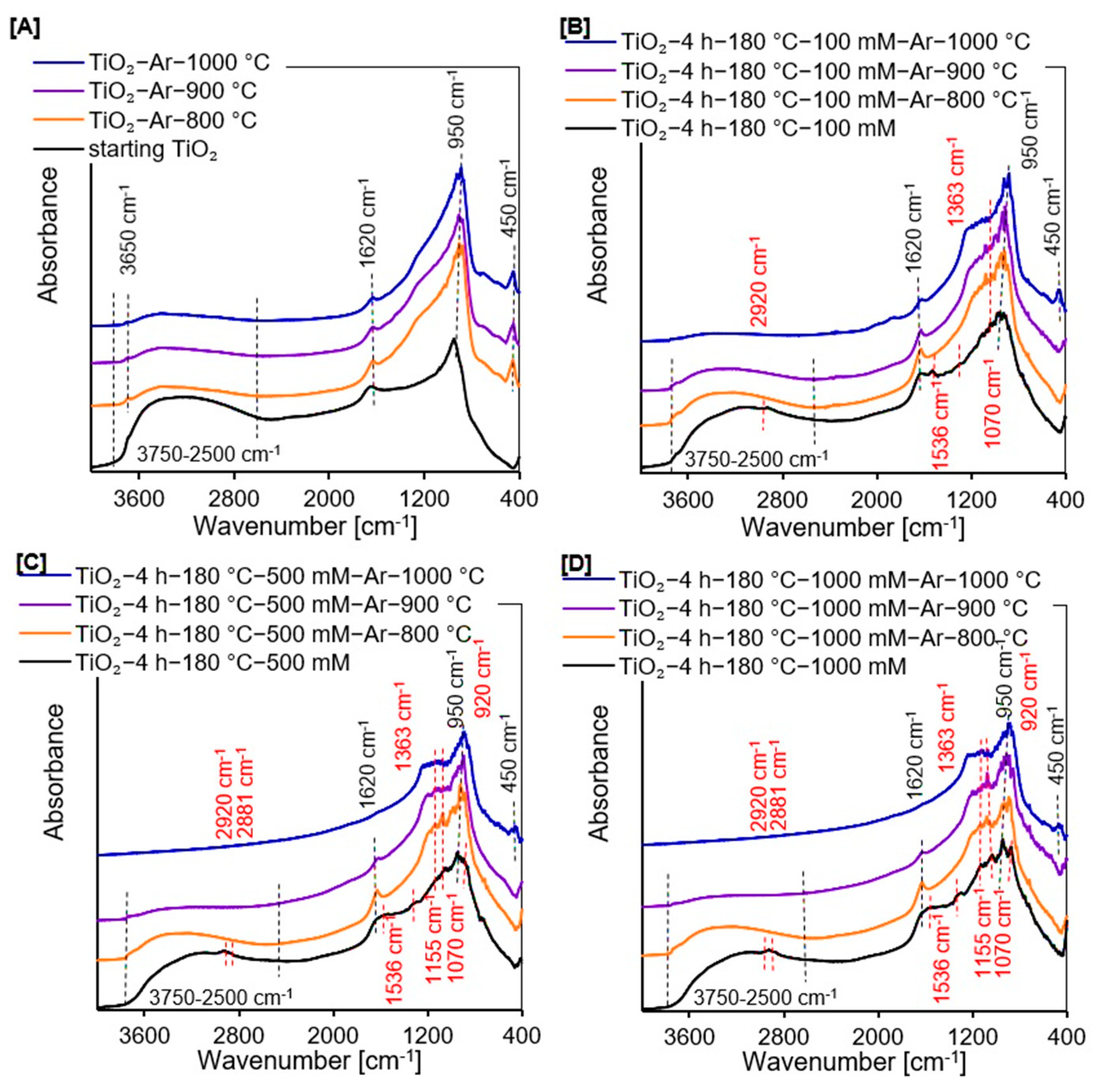
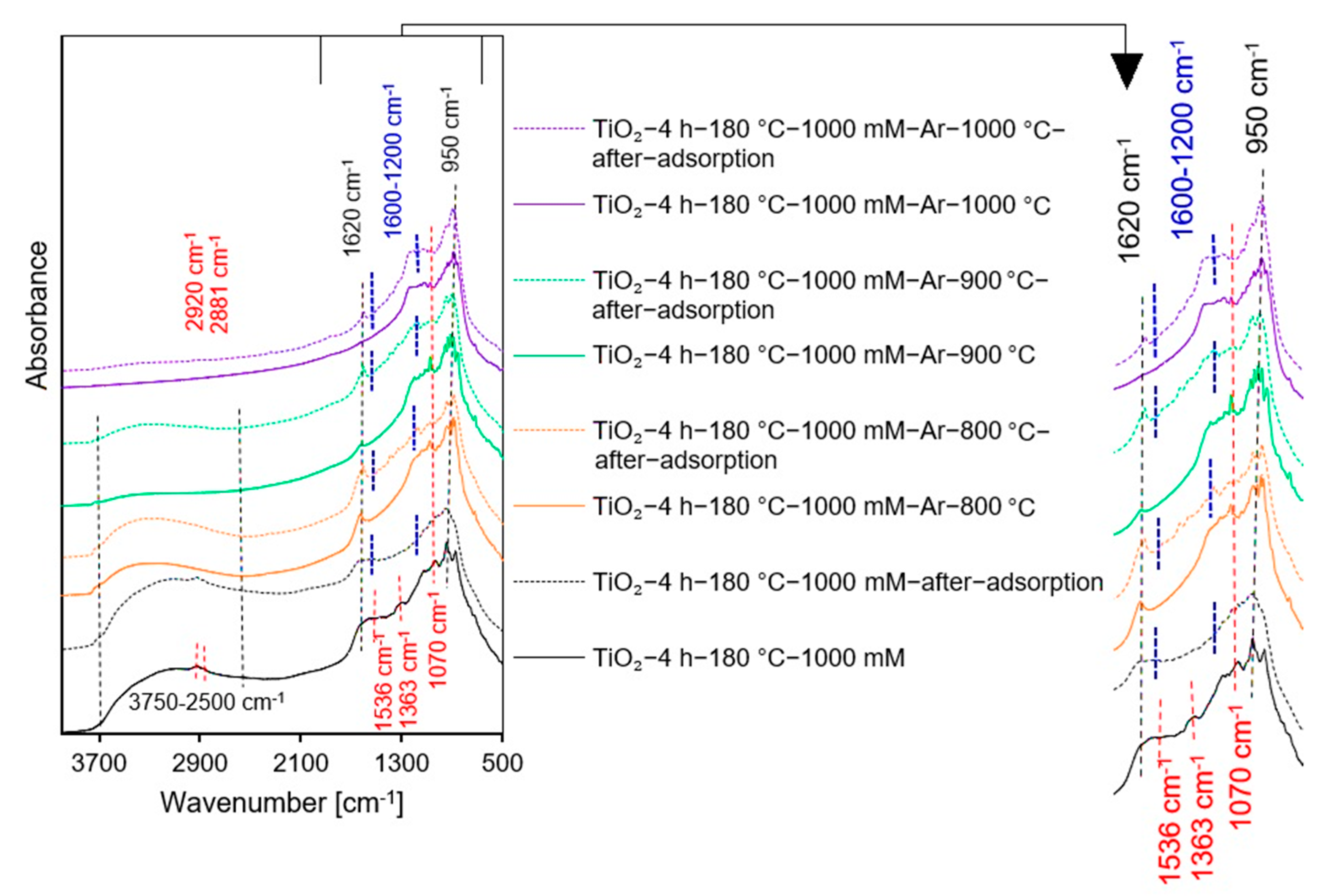
2.1.2. DRIFTS Measurements
2.1.3. BET Measurements
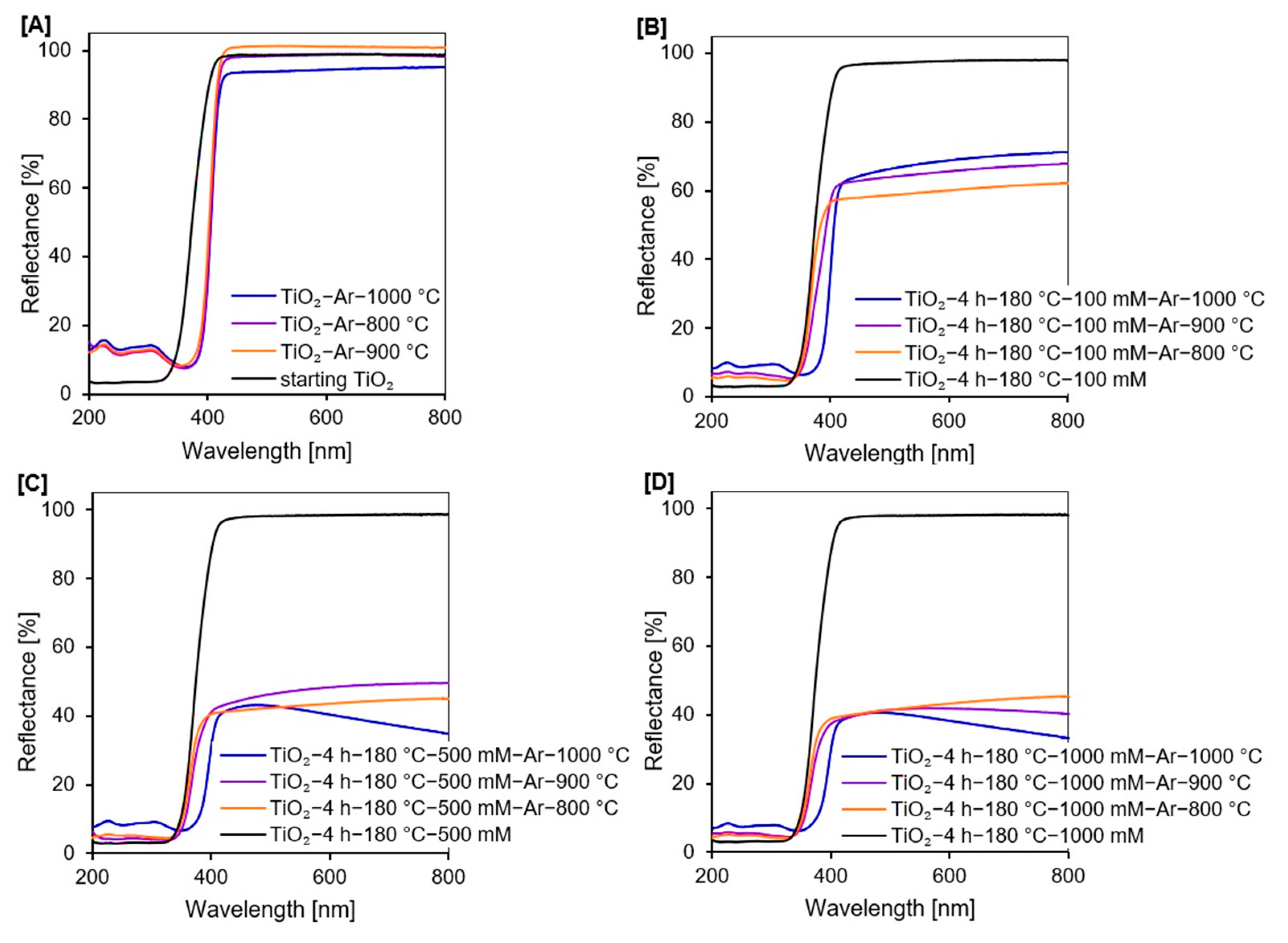
2.1.4. UV-Vis Diffuse Absorbance Spectroscopy
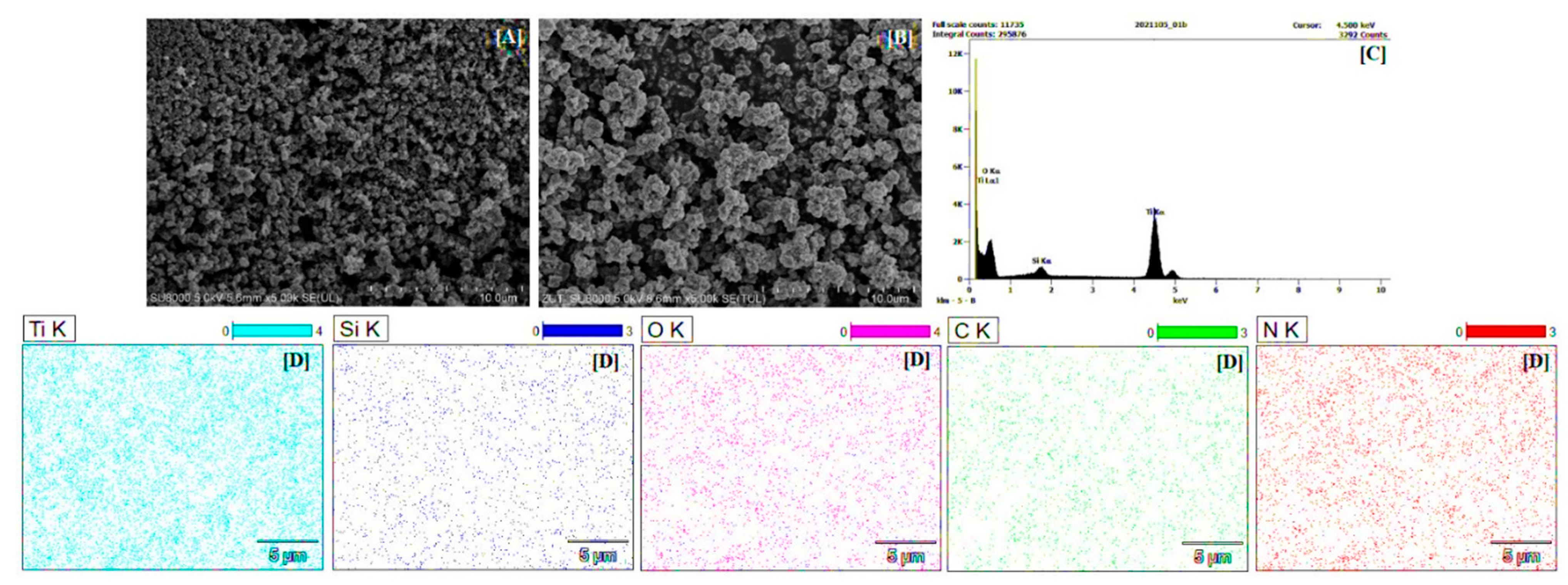
2.1.5. SEM and EDX Mapping Analysis
2.1.6. Carbon and Nitrogen Content Analysis
2.1.7. Zeta Potential Measurements
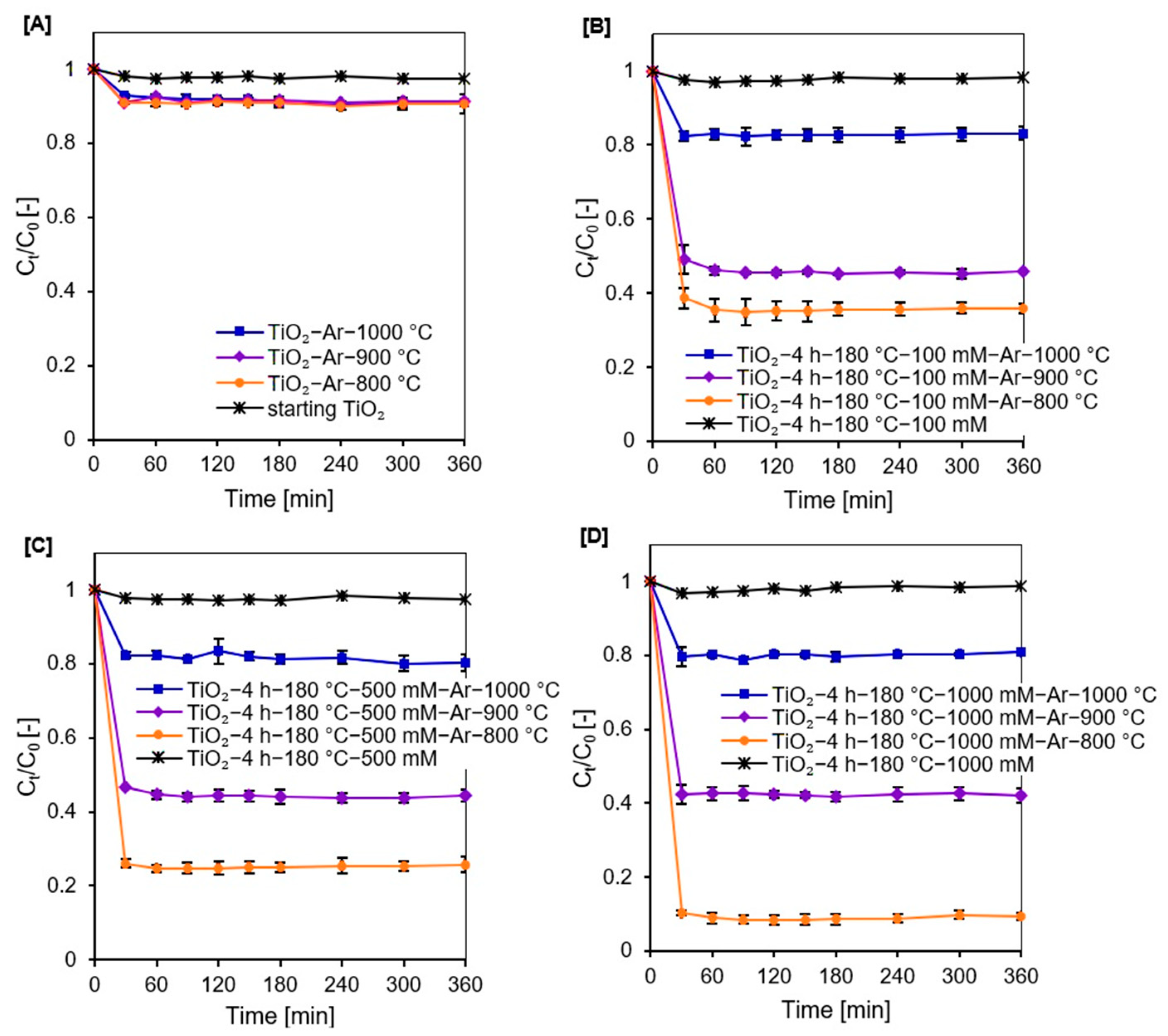
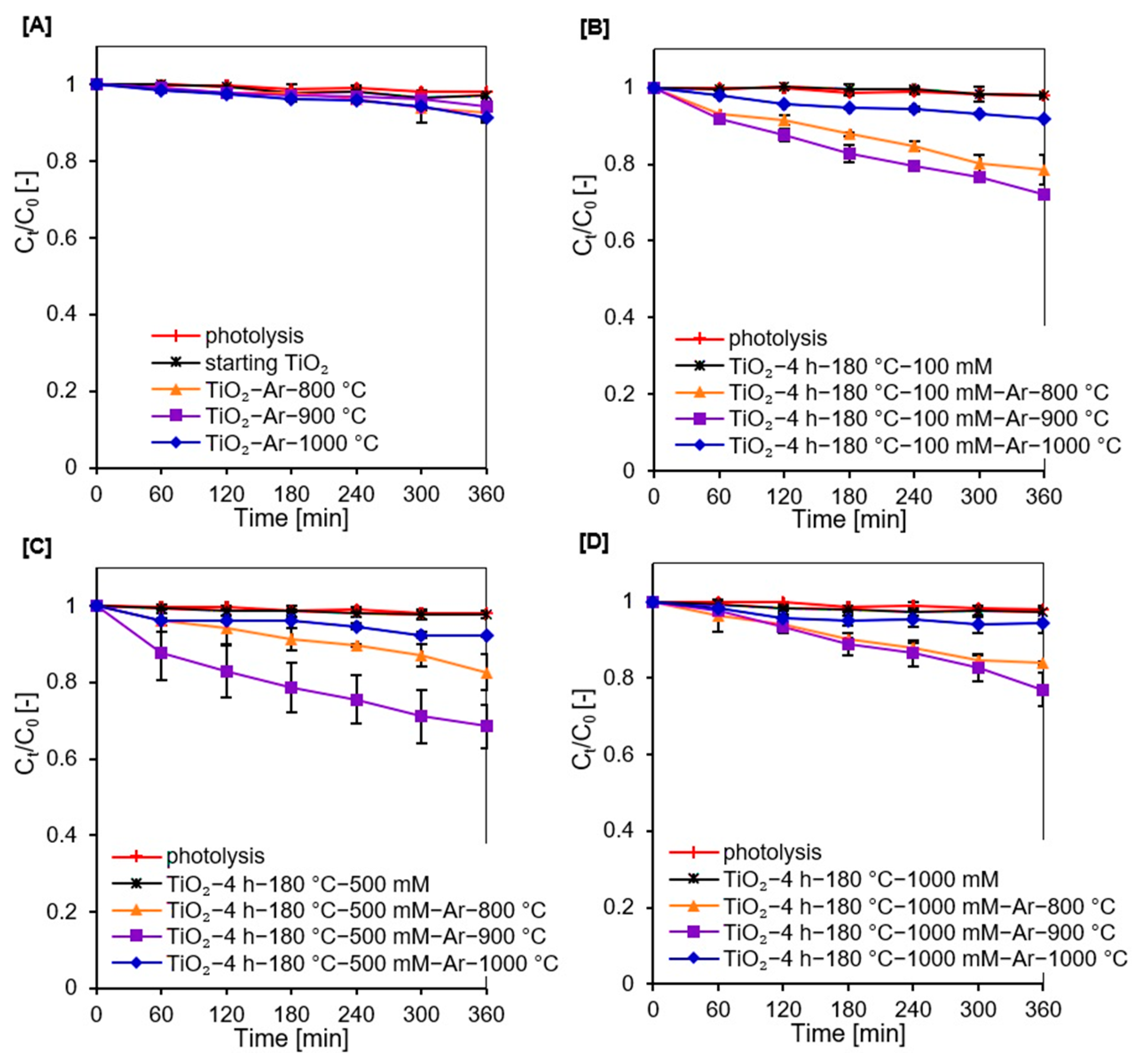
2.2. Adsorption and Photocatalytic Studies
3. Experimental
3.1. Materials and Reagents
3.2. Preparation Procedure of APTES/TiO2 Nanomaterials
3.3. Characterization of Photocatalysts
3.4. Photocatalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piątkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-,N- and S-doped TiO2 photocatalysts: A review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Li, X.; Yi, X.; Chu, H.; Chen, X.; Wang, C.-C. Facile fabrication of BUC-21/Bi24O31Br10 composites for enhanced photocatalytic Cr(VI) reduction under white light. Chem. Eng. J. 2020, 389, 123431. [Google Scholar] [CrossRef]
- Reghunath, S.; Pinheiro, D.; Devi, K.R.S. A review of hierarchical nanostructures of TiO2: Advances and applications. Appl. Surf. Sci. Adv. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2020, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Liu, X.; Ren, C.; Miao, X.; Li, X. Engineering of Gd/Er/Lu-triple-doped Bi2MoO6 to synergistically boost the photocatalytic performance in three different aspects: Oxidizability, light absorption and charge separation. Appl. Surf. Sci. 2019, 463, 556–565. [Google Scholar] [CrossRef]
- Nasirian, M.; Lin, Y.P.; Bustillo-Lecompte, C.F.; Mehrvar, M. Enhancement of photocatalytic activity of titanium dioxide using non-metal doping methods under visible light: A review. Int. J. Environ. Sci. Technol. 2017, 15, 2009–2032. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal modified TiO2: A step towards visible light photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Ramandi, S.; Entezari, M.H.; Ghows, N. Sono-synthesis of solar light responsive S-N-C-tri doped TiO2 photo-catalyst under optimized conditions for degradation and mineralization of diclofenac. Ultrason. Sonochem. 2017, 38, 234–245. [Google Scholar] [CrossRef]
- Sushma, C.; Kumar, S.G. C–N–S tridoping into TiO2 matrix for photocatalytic applications: Observations, speculations and contradictions in the codoping process. Inorg. Chem. Front. 2017, 4, 1250–1267. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, W.; Wu, Z. Characterization and photocatalytic activities of C, N and S co-doped TiO2 with 1D nanostructure prepared by the nano-confinement effect. Nanotechnology 2008, 19, 365607. [Google Scholar] [CrossRef]
- Lei, X.F.; Xue, X.X.; Yang, H.; Chen, C.; Li, X.; Niu, M.C.; Gao, X.Y.; Yang, Y.T. Effect of calcination temperature on the structure and visible-light photocatalytic activities of (N, S and C) co-doped TiO2 nano-materials. Appl. Surf. Sci. 2015, 332, 172–180. [Google Scholar] [CrossRef]
- Klaysri, R.; Tubchareon, T.; Praserthdam, P. One-step synthesis of amine-functionalized TiO2 surface for photocatalytic decolorization under visible light irradiation. J. Ind. Eng. Chem. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Xu, G.Q.; Zheng, Z.X.; Wu, Y.C.; Feng, N. Effect of silica on the microstructure and photocatalytic properties of titania. Ceram. Int. 2009, 35, 1–5. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Tucci, A.; Skapin, A.S.; Esposito, L. Effects of SiO2 addition on TiO2 crystal structure and photocatalytic activity. J. Eur. Ceram. Soc. 2010, 30, 2481–2490. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Al-Thani, N.J.; Tawbi, K.; Al-Kandari, H. Carbon/nitrogen-doped TiO2: New synthesis route, characterization and application for phenol degradation. Arab. J. Chem. 2016, 9, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Yang, L.; Xu, S.; Luo, S.; Cai, Q. Photocatalytic activities of C–N-doped TiO2 nanotube array/carbon nanorod composite. Electrochem. Commun. 2009, 11, 1748–1751. [Google Scholar] [CrossRef]
- Martinez-Oviedo, A.; Kshetri, Y.K.; Joshi, B.; Wohn Lee, S. Surface modification of blue TiO2 with silane coupling agent for NOx abatement. Prog. Nat. Sci. Mater. 2021, 31, 230–238. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Nguyen, T.D.; Vu, D.T.; Dinh, D.P.; Nguyen, A.H.; Ly, T.N.L.; Dao, P.H.; Nguyen, T.L.; Bach, L.G.; Thai, H. Modification of titanium dioxide nanoparticles with 3-(trimethoxysilyl)propyl methacrylate silane coupling agent. J. Chem. 2020, 1381407. [Google Scholar] [CrossRef]
- Meroni, D.; Lo Presti, L.; Di Liberto, G.; Ceotto, M.; Acres, R.G.; Prince, K.C.; Bellani, R.; Soliveri, G.; Ardizzone, S. A close look at the structure of the TiO2 APTES interface in hybrid nanomaterials and degradation pathway: An experimental and theoretical study. J. Phys. Chem. C 2017, 121, 430–440. [Google Scholar] [CrossRef]
- Shakeri, A.; Yip, D.; Badv, M.; Imani, S.M.; Sanjari, M.; Didar, T.F. Self-cleaning ceramic tiles produced via stable coating of TiO2 nanoparticles. Materials 2018, 11, 1003. [Google Scholar] [CrossRef] [Green Version]
- Nadzirah, S.; Zainudin, N.; Hashim, U.; Derman, M.N.B.; Chong, K.F.; Rahim Ruslinda, A. Rapid and sensitive E. coli DNA detection by titanium dioxide nanoparticles. In Proceedings of the 2014 IEEE Conference on Biomedical Engineering and Sciences, Miri, Malaysia, 8–10 December 2014. [Google Scholar]
- Andrzejewska, A.; Krysztafkiewicz, A.; Jesionowski, T. Adsorption of organic dyes on the aminosilane modified TiO2 surface. Dyes Pigments 2004, 62, 121–130. [Google Scholar] [CrossRef]
- Lee, K.N.; Kim, Y.; Lee, C.W.; Lee, J.S. Simultaneous amination of TiO2 nanoparticles in the gas phase synthesis for bio-medical applications. IOP Conf. Ser. Mater. Sci. Eng. 2011, 18, 082021. [Google Scholar] [CrossRef]
- Bao, N.; Wu, G.; Niu, J.; Zhang, Q.; He, S.; Wang, J. Wide spectral response and enhanced photocatalytic activity of TiO2 continuous fibers modified with aminosilane coupling agents. J. Alloys Compd. 2014, 599, 40–48. [Google Scholar] [CrossRef]
- López-Zamora, L.; Martínez-Martínez, H.N.; González-Calderón, J.A. Improvement of the colloidal stability of titanium dioxide particles in water through silicon based coupling agent. Mater. Chem. Phys. 2018, 217, 285–290. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2020, 357, 630–637. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Wanag, A.; Kusiak-Nejman, E.; Ekiert, E.; Rokicka-Konieczna, P.; Morawski, A.W. Effect of calcination on the photocatalytic activity and stability of TiO2 photocatalysts modified with APTES. J. Environ. Chem. Eng. 2021, 9, 104794. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Taha, S.Y. Synthesis and characterization of TiO2 nanoparticles via sol-gel method by pulse laser ablation. Eng. Technol. J. 2015, 33, 761–771. [Google Scholar]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A.; MacKenzie, K.J.D. Effect of silica additive on the anatase-to-rutile phase transition. Ceram. Soc. 2001, 84, 1591–1596. [Google Scholar] [CrossRef]
- Morawski, A.W.; Janus, M.; Tryba, B.; Toyoda, M.; Tsumura, T.; Inagaki, M. Carbon modified TiO2 photocatalysts for water purification. Pol. J. Chem. Technol. 2009, 11, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Fu, G.; Zhou, P.; Zhao, M.; Zhu, W.; Yan, S.; Yu, T.; Zou, T. Carbon coating stabilized Ti3+-doped TiO2 for photocatalytic hydrogen generation under visible light irradiation. Dalton Trans. 2015, 44, 12812–12817. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Zheng, M.; Jin, Y.; Huang, Q.; Gu, M. Preparation and characterization of silica-doped titania photocatalyst through sol–gel method. Mater. Lett. 2003, 57, 2989–2994. [Google Scholar] [CrossRef]
- Wu, N.L.; Wang, S.Y.; Rusakova, A. Inhibition of crystallite growth in the sol-gel synthesis of nanocrystalline metal oxides. Science 1999, 285, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Morawski, A.W. Preparation and characterisation of TiO2 thermally modified with cyclohexane vapours. Int. J. Mater. Prod. Technol. 2016, 52, 286–297. [Google Scholar] [CrossRef]
- Zanchetta, E.; Della Giustina, G.; Brusatin, G. One-step patterning of double tone high contrast and high refractive index inorganic spin-on resist. J. Appl. Phys. 2014, 116, 103504. [Google Scholar] [CrossRef]
- Pramanik, A.; Bhattacharjee, K.; Mitra, M.K.; Das, G.C.; Duari, B. A mechanistic study of the initial stage of the sintering of sol–gel derived silica nanoparticles. Int. J. Mod. Eng. Res. 2013, 3, 1066–1070. [Google Scholar]
- Martra, G. Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: Relationships between surface morphology and chemical behaviour. Appl. Catal. A Gen. 2000, 200, 275–285. [Google Scholar] [CrossRef]
- Kuroda, Y.; Mori, T.; Yagi, K.; Makihata, N.; Kawahara, Y.; Nagao, M.; Kittaka, S. Preparation of visible-light-responsive TiO2-xNx photocatalyst by a sol−gel method: analysis of the active center on TiO2 that reacts with NH3. Langmuir 2005, 21, 8026–8034. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating photodegradation properties of anatase and rutile TiO2 nanoparticles for organic compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- San Andrés, E.; Toledano-Luque, M.; Del Prado, A.; Navacerrada, M.; Mártil, I.; González-Díaz, G. Physical properties of high pressure reactively sputtered TiO2. J. Vac. Sci. Technol. A 2005, 23, 1523–1530. [Google Scholar] [CrossRef]
- Gracia, F.; Holgado, J.P.; Contreras, L.; Girardeau, T.; González-Elipe, A.R. Optical and crystallisation behaviour of TiO2 and V/TiO2 thin films prepared by plasma and ion beam assisted methods. Thin Solid Films 2003, 429, 84–90. [Google Scholar] [CrossRef]
- Pambudi, A.B.; Kurniawati, R.; Iryani, A.; Hartanto, D. Effect of calcination temperature in the synthesis of carbon doped TiO2 without external carbon source. In Proceedings of the 3rd International Seminar on Chemistry, AIP Conference Proceedings, Surabaya, Indonesia, 18–19 July 2018; Volume 2049, p. 020074. [Google Scholar]
- Razmjou, A.; Mansouri, J.; Chena, V. The effects of mechanical and chemical modification of TiO2 nanoparticles on the surface chemistry, structure and fouling performance of PES ultrafiltration membranes. J. Membr. Sci. 2011, 378, 73–84. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Wang, W. Construction of a crossed-layer-structure MoS2/g-C3N4 heterojunction with enhanced photocatalytic performance. RSC Adv. 2017, 7, 6131–6139. [Google Scholar] [CrossRef] [Green Version]
- Grill, A.; Neumayer, D.A. Structure of low dielectric constant to extreme low dielectric constant SiCOH films: Fourier transform infrared spectroscopy characterization. J. Appl. Phys. 2003, 94, 6697–6707. [Google Scholar] [CrossRef]
- Schramm, C. High temperature ATR-FTIR characterization of the interaction of polycarboxylic acids and organotrialkoxysilanes with cellulosic material. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 243, 118815. [Google Scholar] [CrossRef] [PubMed]
- Ukaji, E.; Furusawa, T.; Sato, M.; Suzuki, M. The effect of surface modification with silane coupling agent on suppressing the photo-catalytic activity of fine TiO2 particles as inorganic UV filter. Appl. Surf. Sci. 2007, 254, 563–569. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Kutarov, V.V.; Tarasevich, Y.I.; Aksenenko, E.V.; Ivanova, Z.G. Adsorption hysteresis for a slit-like pore model. Russ. J. Phys. Chem. 2011, 85, 1222–1227. [Google Scholar] [CrossRef]
- Cheng, F.; Sajedin, S.M.; Kelly, S.M.; Lee, A.F.; Kornherr, A. UV-stable paper coated with APTES-modified P25 TiO2 nanoparticles. Carbohydr. Polym. 2014, 114, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Zhang, Y.; He, L.; An, R.; Li, B.; Ying, H.; Wu, J.; Chen, Y.; Zhou, J.; Lu, X. Facile synthesis of amino-functionalized mesoporous TiO2 microparticles for adenosine deaminase immobilization. Microporous Mesoporous Mater. 2017, 239, 158–166. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Luu, B.; Sengpiel, R.G.; Ye, Y.; Wessling, M.; Chen, V. Hybrid membrane with TiO2 based bio-catalytic nanoparticle suspension system for the degradation of bisphenol-A. Bioresour. Technol. 2014, 169, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Luís, A.M.; Neves, M.C.; Mendonça, M.H.; Monteiro, O.C. Influence of calcination parameters on the TiO2 photocatalytic properties. Mater. Chem. Phys. 2011, 125, 20–25. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of photocatalytic performance with the use of noble-metal-decorated TiO2 nanocrystals as highly active catalysts for aerobic oxidation under visible-light irradiation. Appl. Catal. B Environ. 2017, 210, 352–367. [Google Scholar] [CrossRef]
- Wawrzyniak, B.; Morawski, A.W. Solar-light-induced photocatalytic decomposition of two azo dyes on new TiO2 photocatalyst containing nitrogen. Appl. Catal. B Environ. 2006, 62, 150–158. [Google Scholar] [CrossRef]
- Beranek, R.; Kisch, H. Tuning the optical and photoelectrochemical properties of surface-modified TiO2. Photochem. Photobiol. Sci. 2008, 7, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Chong, F.K.; Dutta, B.L.; Man, Z.B.; Saqib Khan, M.; Nurlaela, E. Photodegradation of Orange II under visible light using Cu–Ni/TiO2: Effect of calcination temperature. Chem. Eng. J. 2012, 185–186, 108–119. [Google Scholar] [CrossRef]
- Ponaryadov, A.; Kotova, O. Leucoxene photocatalysts for water purification. IOP Conf. Ser. Mater. Sci. Eng. 2013, 47, 012039. [Google Scholar] [CrossRef] [Green Version]
- Nurlaela, E.; Chong, F.K.; Dutta, B.K.; Riaz, N. Bimetallic Cu-Ni/TiO2 as photocatalyst for hydrogen production from water. In Proceedings of the International Conference on Fundamental and Applied Sciences (ICFAS2010), Kuala Lumpur, Malaysia, 15–17 June 2010. [Google Scholar]
- Mohammad Jafri, N.N.; Jaafar, J.; Alias, N.H.; Samitsu, S.; Aziz, F.; Wan Salleh, W.N.; Mohd Yusop, M.Z.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.F.; et al. Synthesis and characterization of titanium dioxide hollow nanofiber for photocatalytic degradation of methylene blue dye. Membranes 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Choudhury, A. Investigation of the optical property and photocatalytic activity of mixed phase nanocrystalline titania. Appl. Nanosci. 2014, 4, 839–847. [Google Scholar] [CrossRef] [Green Version]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef] [Green Version]
- Saranya, K.S.; Vellora Thekkae Padil, V.; Senan, C.; Pilankatta, R.; Saranya, K.; George, B.; Wacławek, S.; Černík, M. Green synthesis of high temperature stable anatase titanium dioxide nanoparticles using gum kondagogu: Characterization and solar driven photocatalytic degradation of organic dye. Nanomaterials 2018, 8, 1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwińska-Stefańska, K.; Ciesielczyk, F.; Nowacka, M.; Jesionowski, T. Influence of selected alkoxysilanes on dispersive properties and surface chemistry of titanium dioxide and TiO2-SiO2 composite material. J. Nanomater. 2012, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Boscaro, P.; Cacciaguerra, T.; Cot, D.; Fajula, F.; Hulea, V.; Galarneau, A. C,N-doped TiO2 monoliths with hierarchical macro-/mesoporosity for water treatment under visible light. Microporous Mesoporous Mater. 2019, 280, 37–45. [Google Scholar] [CrossRef]
- Janus, M.; Bubacz, K.; Zatorska, J.; Kusiak-Nejman, E.; Czyżewski, A.; Morawski, A.W. Preliminary studies of photocatalytic activity of gypsum plasters containing TiO2 co-modified with nitrogen and carbon. Pol. J. Chem. Technol. 2015, 17, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Al-Anbari, R.; Kadhim, G.; Jameel, Z. Synthesis and photocatalytic activity for TiO2 nanoparticles as air purification. In Proceedings of the 3rd International Conference on Buildings, Construction and Environmental Engineering (BCEE3-2017), 23–25 October 2017, Sharm el-Shiekh, Egypt. MATEC Web Conf. 2018, 162, 05006. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro Pinton, A.; De, S.; Bulhões, L.O. Synthesis, characterization, and photostability of manganese-doped titanium dioxide nanoparticles and the effect of manganese content. Mater. Res. Express 2019, 6, 125015. [Google Scholar] [CrossRef]
- Chellappah, K.; Tarleton, E.S.; Wakeman, R.J. Filtration and sedimentation behaviour of fibre/particle binary suspensions. Filtration 2009, 9, 286–294. [Google Scholar]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.F.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of different amounts of APTES on physicochemical and structural properties of amino-functionalized MCM-41-MSNs. J. Sol-Gel Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I. APTES-functionalized mesoporous silica as a vehicle for antipyrine-adsorption and release studies. Colloids Surf. A 2017, 533, 187–196. [Google Scholar] [CrossRef]
- Li, Q.Y.; Chen, Y.F.; Zeng, D.D.; Gao, W.M.; Wu, Z.J. Photocatalytic characterization of silica coated titania nanoparticles with tunable coatings. J. Nanopart. Res. 2005, 7, 295–299. [Google Scholar] [CrossRef]
- Ferreira-Neto, E.P.; Ullah, S.; Simões, M.B.; Perissinotto, A.P.; Vicente, F.S.; Noeske, P.L.M.; Ribeiro, S.J.L.; Rodrigues-Filho, U.P. Solvent-controlled deposition of titania on silica spheres for the preparation of SiO2@TiO2 core@shell nanoparticles with enhanced photocatalytic activity. Colloids Surf. A 2019, 570, 293–305. [Google Scholar] [CrossRef]
- Worathanakul, P.; Jiang, J.; Biswas, P.; Kongkachuichay, P. Quench-ring assisted flame synthesis of SiO2-TiO2 nanostructured composite. J. Nanosci. Nanotechnol. 2008, 8, 6253–6259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Z.H.; Coomes, A.; Haghseresht, F.; Lu, G.Q. The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. J. Colloid Interface Sci. 2005, 284, 440–446. [Google Scholar] [CrossRef]
- Zhang, C.; Uchikoshi, T.; Liu, L.; Kikuchi, M.; Ichinose, I. Effect of surface modification with TiO2 coating on improving filtration efficiency of whisker-hydroxyapatite (HAp) membrane. Coatings 2020, 10, 670. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and protein adsorption: An overview of mechanisms and effects of surface features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef]
- Li, Z.; Hou, B.; Xu, Y.; Wu, D.; Sun, Y. Hydrothermal synthesis, characterization, and photocatalytic performance of silica-modified titanium dioxide nanoparticles. J. Colloid Interface Sci. 2005, 288, 149–154. [Google Scholar] [CrossRef]
- Bartošová, A.; Blinová, L.; Sirotiak, M.; Michalíková, M. Usage of FTIR-ATR as non-destructive analysis of selected toxic dyes. Res. Pap. Fac. Mater. Sci. Technol. Slovak. Univ. Technol. 2017, 25, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Ovchinnikov, O.V.; Evtukhova, A.V.; Kondratenko, T.S.; Smirnov, M.S.; Khokhlov, V.Y.; Erina, O.V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Cai, J.; Xin, W.; Liu, G.; Lin, D.; Zhu, D. Effect of calcination temperature on structural properties and photocatalytic activity of Mn-C-codoped TiO2. Mater. Res. 2016, 19, 401–407. [Google Scholar] [CrossRef] [Green Version]
- George, P.; Chowdhury, P. Complex dielectric transformation of UV-vis diffuse reflectance spectra for estimating optical band-gap energies and materials classification. Analyst 2019, 144, 3005–3012. [Google Scholar] [CrossRef] [PubMed]


| Sample Name | SBET [m2/g] | Vtotal [cm3/g] | Vmicro [cm3/g] | Vmeso [cm3/g] | Anatase in Crystallite Phase [%] | Anatase Crystallite Size [nm] | Rutile in Crystallite Phase [%] | Rutile Crystallite Size [nm] |
|---|---|---|---|---|---|---|---|---|
| starting TiO2 | 207 | 0.370 | 0.070 | 0.300 | 95 | 14 | 5 | 21 |
| TiO2-Ar-800 °C | 6 | 0.020 | 0.002 | 0.018 | 1 | >100 | 99 | >100 |
| TiO2-Ar-900 °C | 3 | 0.008 | 0.002 | 0.006 | - | - | 100 | >100 |
| TiO2-Ar-1000 °C | 4 | 0.009 | 0.001 | 0.008 | - | - | 100 | >100 |
| TiO2-4 h-180 °C-100 mM | 169 | 0.226 | 0.065 | 0.161 | 96 | 15 | 4 | 51 |
| TiO2-4 h-180 °C-100 mM-Ar-800 °C | 70 | 0.201 | 0.026 | 0.175 | 96 | 23 | 4 | 62 |
| TiO2-4 h-180 °C-100 mM-Ar-900 °C | 35 | 0.113 | 0.041 | 0.072 | 87 | 34 | 13 | >100 |
| TiO2-4 h-180 °C-100 mM-Ar-1000 °C | 8 | 0.044 | 0.003 | 0.041 | 6 | 48 | 94 | >100 |
| TiO2-4 h-180 °C-500 mM | 124 | 0.162 | 0.048 | 0.114 | 96 | 15 | 4 | 48 |
| TiO2-4 h-180 °C-500 mM-Ar-800 °C | 95 | 0.221 | 0.038 | 0.183 | 96 | 20 | 4 | 58 |
| TiO2-4 h-180 °C-500 mM-Ar-900 °C | 46 | 0.192 | 0.017 | 0.175 | 94 | 30 | 6 | 55 |
| TiO2-4 h-180 °C-500 mM-Ar-1000 °C | 16 | 0.069 | 0.006 | 0.063 | 12 | 47 | 88 | >100 |
| TiO2-4 h-180 °C-1000 mM | 121 | 0.174 | 0.049 | 0.125 | 96 | 15 | 4 | 51 |
| TiO2-4 h-180 °C-1000 mM-Ar-800 °C | 104 | 0.215 | 0.039 | 0.176 | 96 | 19 | 4 | 67 |
| TiO2-4 h-180 °C-1000 mM-Ar-900 °C | 55 | 0.166 | 0.021 | 0.145 | 94 | 30 | 6 | 54 |
| TiO2-4 h-180 °C-1000 mM-Ar-1000 °C | 12 | 0.046 | 0.005 | 0.041 | 16 | 45 | 84 | >100 |
| Sample Name | Zeta Potential δ [mV] | Carbon Content [wt.%] | Nitrogen Content [wt.%] | Eg [eV] |
|---|---|---|---|---|
| starting TiO2 | +12.8 | - | 0.18 | 3.29 |
| TiO2-Ar-800 °C | −35.9 | - | - | 3.03 |
| TiO2-Ar-900 °C | −36.7 | - | - | 3.03 |
| TiO2-Ar-1000 °C | −41.3 | - | - | 3.01 |
| TiO2-4 h-180 °C-100 mM | +13.6 | 2.10 | 0.79 | 3.27 |
| TiO2-4 h-180 °C-100 mM-Ar-800 °C | −38.5 | 0.17 | 0.08 | 3.27 |
| TiO2-4 h-180 °C-100 mM-Ar-900 °C | −45.8 | 0.08 | - | 3.21 |
| TiO2-4 h-180 °C-100 mM-Ar-1000 °C | −49.1 | 0.03 | - | 3.02 |
| TiO2-4 h-180 °C-500 mM | +22.8 | 3.82 | 1.41 | 3.27 |
| TiO2-4 h-180 °C-500 mM-Ar-800 °C | −47.4 | 0.26 | 0.08 | 3.24 |
| TiO2-4 h-180 °C-500 mM-Ar-900 °C | −51.0 | 0.22 | - | 3.24 |
| TiO2-4 h-180 °C-500 mM-Ar-1000 °C | −41.3 | 0.11 | - | 2.97 |
| TiO2-4 h-180 °C-1000 mM | +12.1 | 4.10 | 1.47 | 3.27 |
| TiO2-4 h-180 °C-1000 mM-Ar-800 °C | −51.6 | 0.27 | 0.11 | 3.23 |
| TiO2-4 h-180 °C-1000 mM-Ar-900 °C | −60.0 | 0.22 | - | 3.22 |
| TiO2-4 h-180 °C-1000 mM-Ar-1000 °C | −54.4 | 0.08 | - | 2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sienkiewicz, A.; Rokicka-Konieczna, P.; Wanag, A.; Kusiak-Nejman, E.; Morawski, A.W. Artificial Solar Light-Driven APTES/TiO2 Photocatalysts for Methylene Blue Removal from Water. Molecules 2022, 27, 947. https://doi.org/10.3390/molecules27030947
Sienkiewicz A, Rokicka-Konieczna P, Wanag A, Kusiak-Nejman E, Morawski AW. Artificial Solar Light-Driven APTES/TiO2 Photocatalysts for Methylene Blue Removal from Water. Molecules. 2022; 27(3):947. https://doi.org/10.3390/molecules27030947
Chicago/Turabian StyleSienkiewicz, Agnieszka, Paulina Rokicka-Konieczna, Agnieszka Wanag, Ewelina Kusiak-Nejman, and Antoni W. Morawski. 2022. "Artificial Solar Light-Driven APTES/TiO2 Photocatalysts for Methylene Blue Removal from Water" Molecules 27, no. 3: 947. https://doi.org/10.3390/molecules27030947
APA StyleSienkiewicz, A., Rokicka-Konieczna, P., Wanag, A., Kusiak-Nejman, E., & Morawski, A. W. (2022). Artificial Solar Light-Driven APTES/TiO2 Photocatalysts for Methylene Blue Removal from Water. Molecules, 27(3), 947. https://doi.org/10.3390/molecules27030947








