Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
- (a)
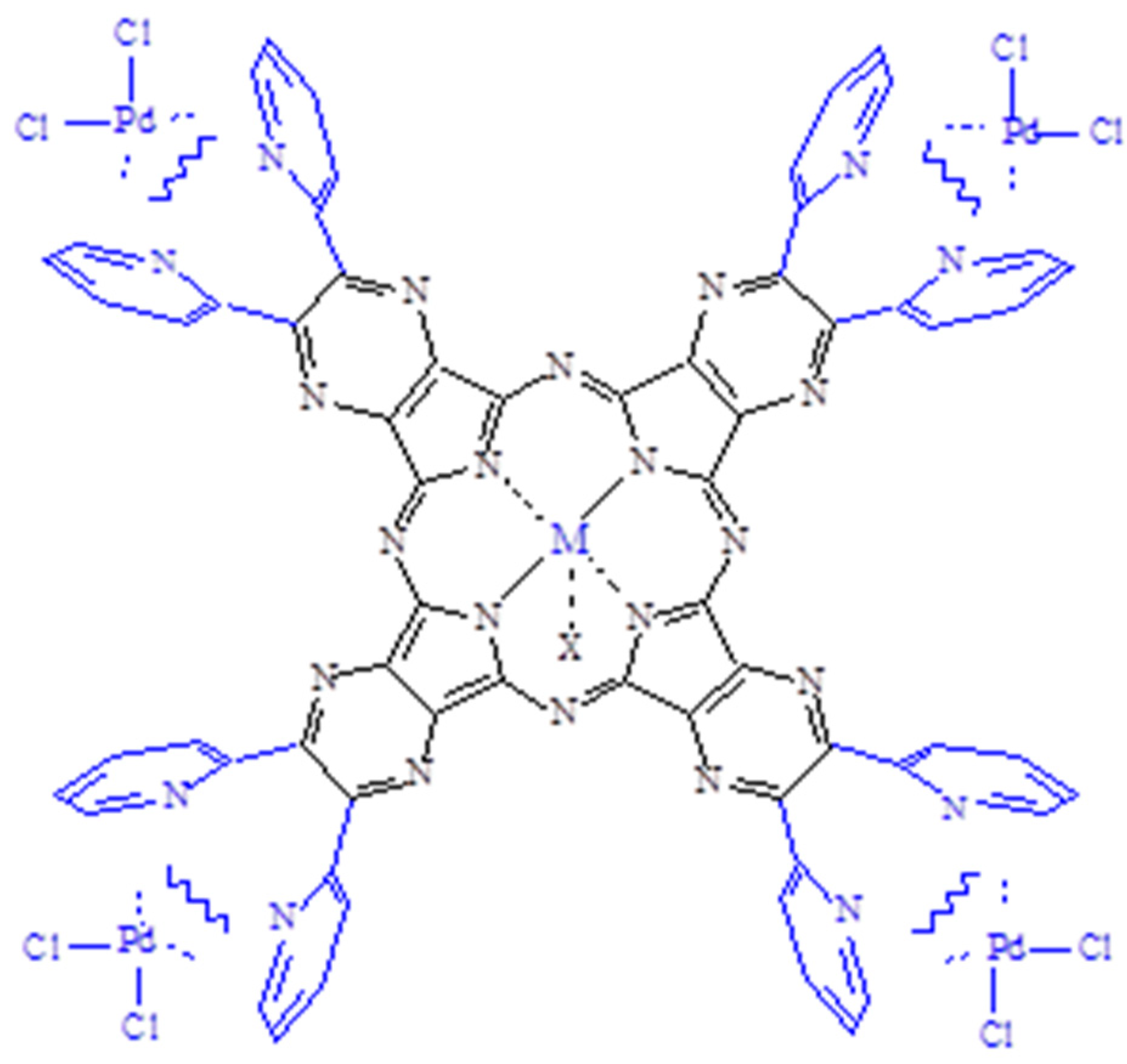
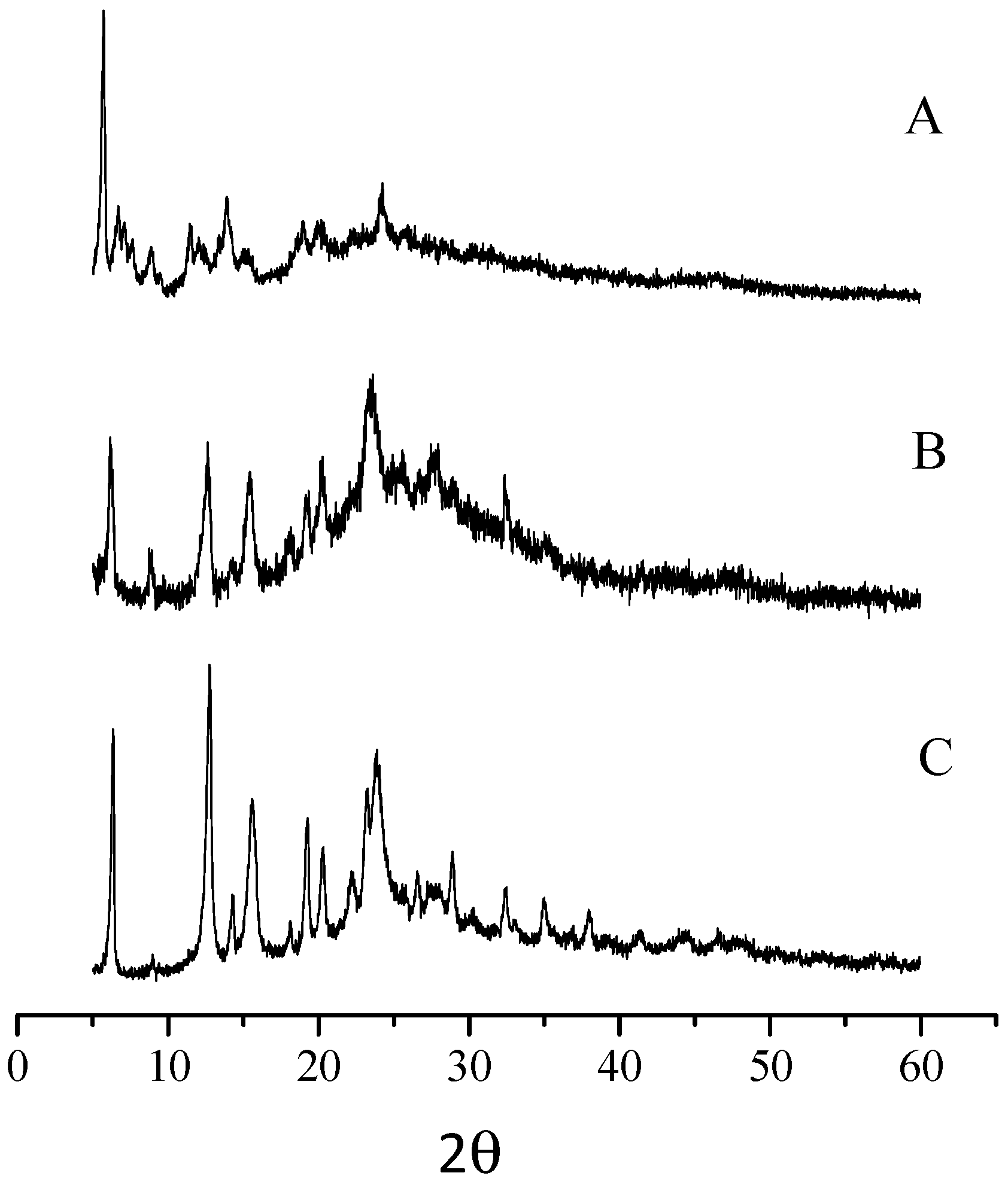
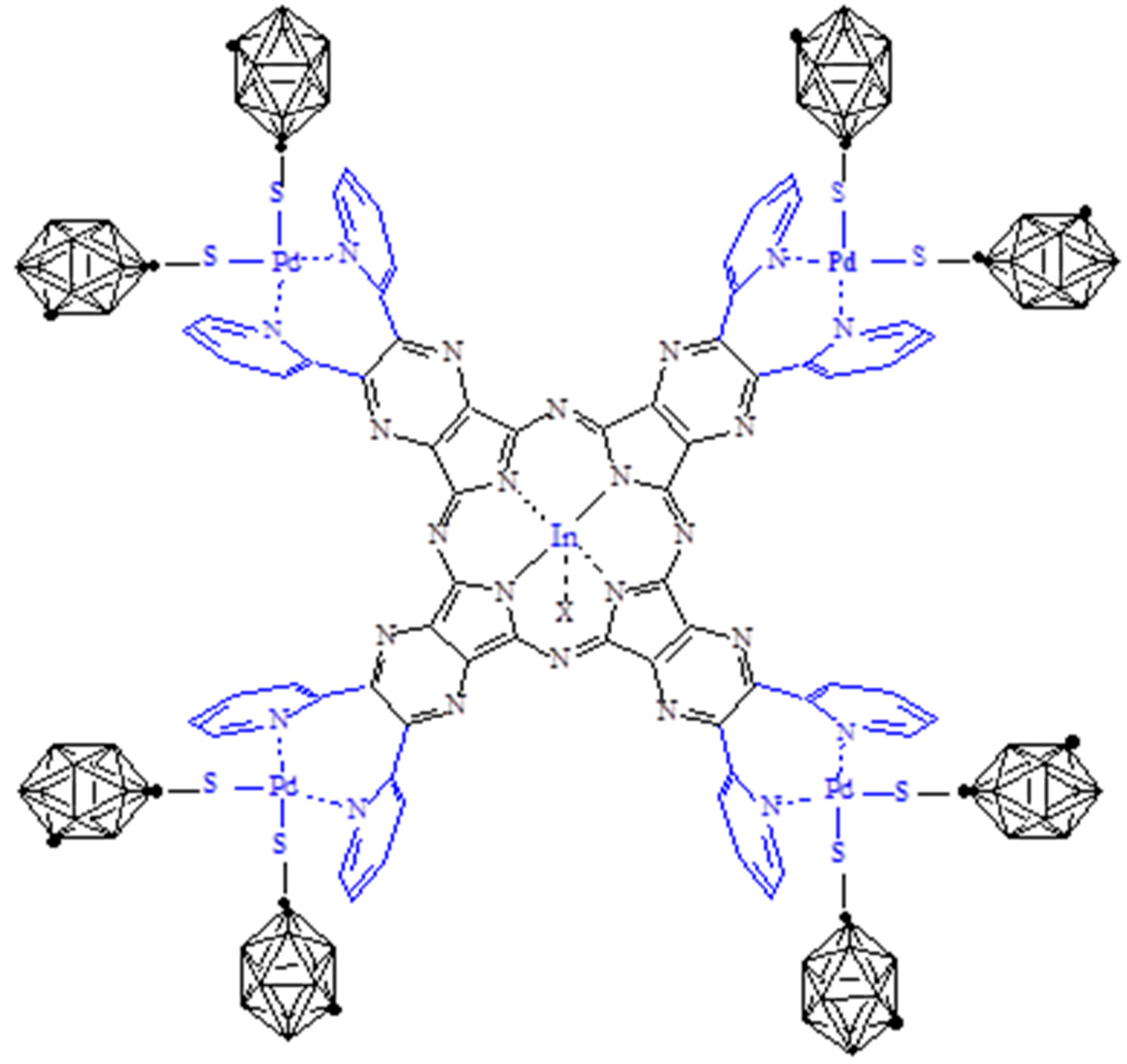
- General properties. The basic mononuclear InIII complex [Py8TPyzPzIn(OAc)]∙8H2O, prepared by heating in glacial CH3COOH the reactants [Py8TPyzPzH2]·2H2O and In(OAc)3 (Section 2), is generally obtained in good yield as a brilliant green solid. The X-ray powder spectrum of the complex (Figure 2A), indicative of a partial crystalline character, is closely approaching the X-ray powder of the spectra of the related macrocyclic analogs centrally carrying AlIIICl and GaIIICl units (Figure 2B,C) [7]. As established for the parallel series of tetrakis(thiadiazole)porphyrazines [TTDzPzMX] (M = AlIII, GaIII; X = Cl/M = InIII, X = OAc), [5] schematically shown in Figure 3, and supported by the molecular arrangement of the corresponding AlIII and GaIII species both elucidated by X-ray work [5] and by the findings for other similar tetrapyrrolic macrocycles given in ref. [7] (Table 1), providing for all of them (M = AlIII, GaIII) a distance M-Ct of 0.3–0.4 Å, the InIII center in the present basic macrocycle is given as axially positioned and residing out of the Ct of the inner N4 coordination site probably only very slightly exceeding the value of 0.4 Å. The water present in the complex, most likely involved in different forms of hydrogen bonds with the numerous N atoms of the macrocycle, could be eliminated by heating it under vacuum (100 °C, 10−2 mmHg); nevertheless, rehydration took place at least partially by exposition of samples to the air. Only traces of water were present in the corresponding macrocyclic salt-like species, most likely explained by the fact that external N atoms once positively charged are no longer available for water attraction.
- (b)
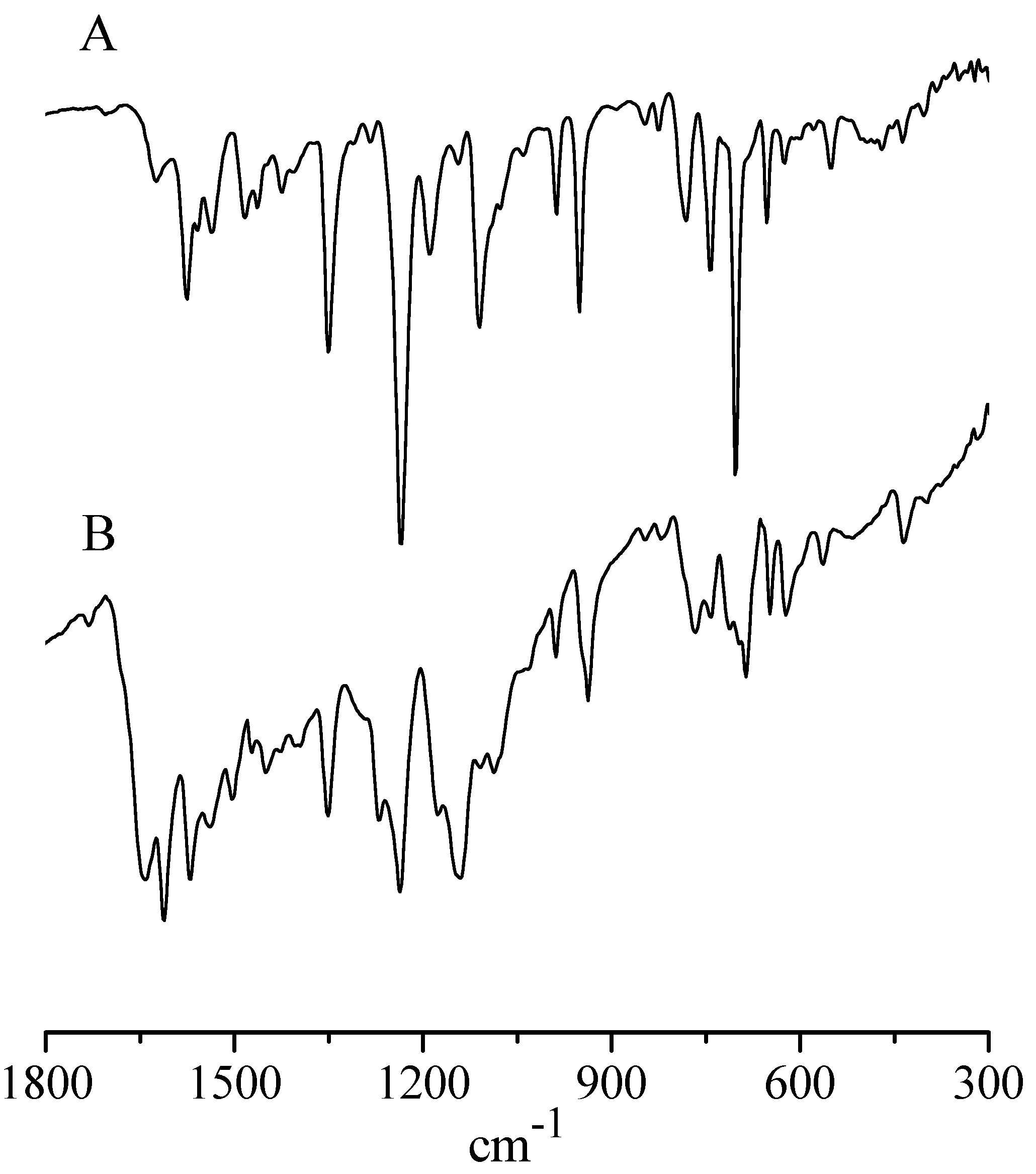
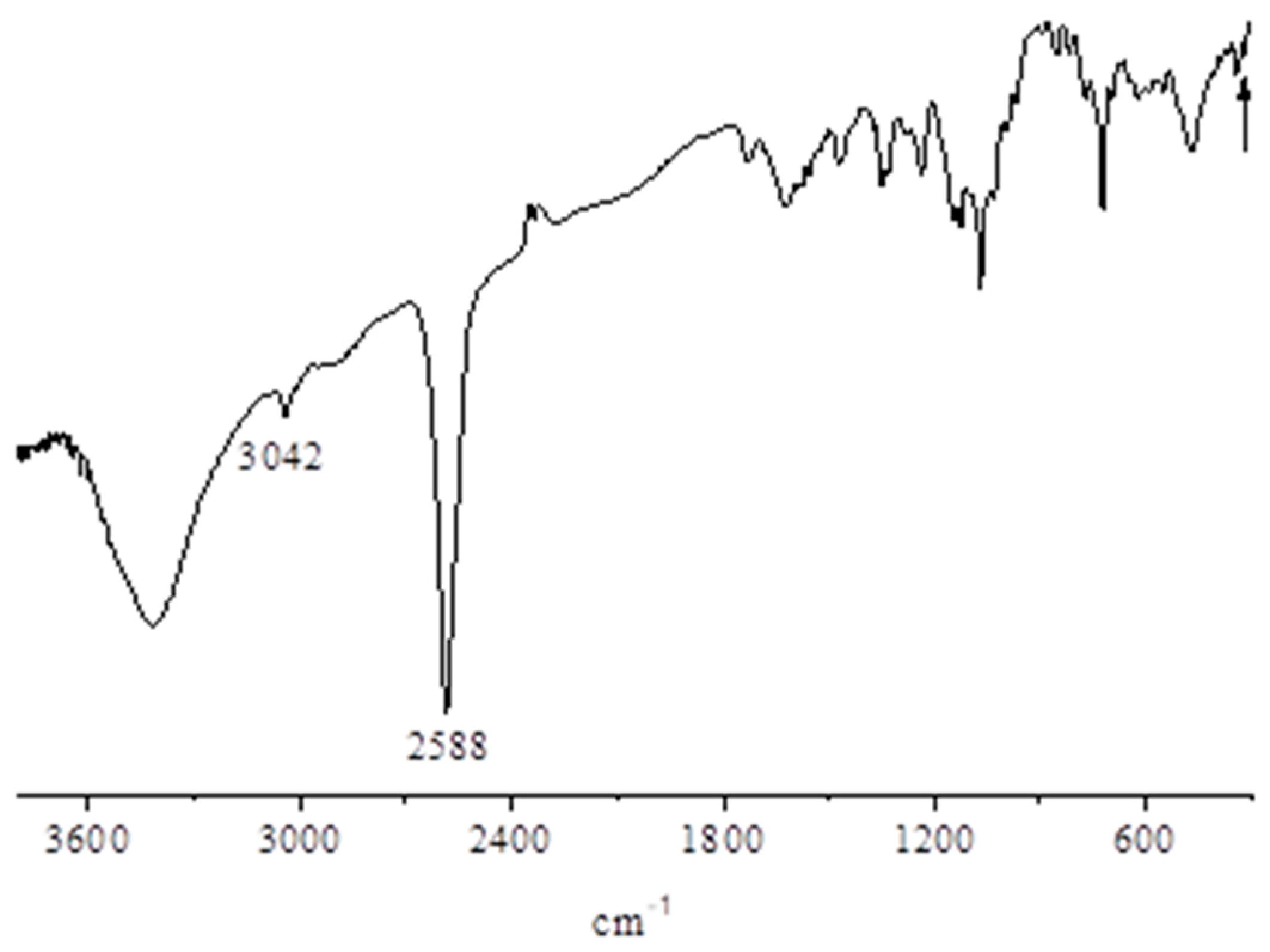
- IR and UV-visible spectral behavior. The IR spectrum of the present basic InIII complex shows, in addition to broad absorptions observed in the region just above 3000 cm−1 as due to the O-H stretching of the present water molecules, the typical absorptions of the Py8TPyzPz porphyrazine macrocycle present in the region 1800–280 cm−1 (Figure 6, top), including the doublet at 986 and 950 cm−1, closely recalling those observed previously for the AlIII and GaIII analogs and the complexes [Py8TPyzPzM] (M = bivalent metal center; ref. [7] (Table 1)). In the spectrum of the octacationic-related macrocycle (Figure 5, bottom), the essential skeletal absorptions of the macrocycle were kept in unchanged positions, with partly superimposed new peaks assigned to the presence of bending vibrations of the new CH3 groups and changes determined by the introduced N+ charged centers in the pyridine rings.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Szacilowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef] [PubMed]
- Donzello, M.P.; Agostinetto, R.; Ivanova, S.S.; Fujimori, M.; Suzuki, Y.; Hirofumi Yoshikawa, J.S.; Awaga, K.; Ercolani, C.; Kadish, K.M.; Stuzhin, P.A. Tetrakis(thiadiazole)porphyrazines. 4. Direct Template Synthesis, Structure, General Physicochemical Behavior, and Redox Properties of AlIII, GaIII, and InIII Complexes. Inorg. Chem. 2005, 44, 8539–8551. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Giustini, M.; Ercolani, C.; Monacelli, F. Tetrakis(thiadiazole)porphyrazines. 8. Singlet oxygen production, fluorescence response and liposomal incorporation of tetrakis(thiadiazole) porphyrazine macrocycles [TTDPzM] (M = MgII(H2O), ZnII, AlIIICl, GaIIICl, CdII, CuII, 2HI). Dalton Trans. 2012, 41, 6112–6121. [Google Scholar] [CrossRef]
- Saltini, G.; Cong, L.; Donzello, M.P.; Ercolani, C.; Viola, E.; Pettiti, I.; Stuzhin, P.A.; Kadish, K.M. Tetra-2,3-pyrazinoporphyrazines with Peripherally Appended Pyridine Rings. 20. Mono- and Pentanuclear AlIII and GaIII Complexes: Synthesis and Physicochemical and Photoactivity Studies. Inorg. Chem. 2019, 58, 15269–15282. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Seyler, R.C.; Mayo, F.R. Coordination Compounds of Palladous Chloride. J. Am. Chem. Soc. 1938, 60, 882–884. [Google Scholar] [CrossRef]
- Donzello, M.P.; Ou, Z.; Monacelli, F.; Ricciardi, G.; Rizzoli, C.; Ercolani, C.; Kadish, K.M. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. 1. Tetrakis-2,3-[5,6-di(2-pyridyl)pyrazino]porphyrazine: A New Macrocycle with Remarkable Electron-Deficient Properties. Inorg. Chem. 2004, 43, 8637–8648. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Cai, X.; Mannina, L.; Rizzoli, C.; Ricciardi, G.; Ercolani, C.; Kadish, K.M.; Rosa, A. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. 5. Synthesis, Physicochemical and Theoretical Studies of a Novel Pentanuclear Palladium(II) Complex and Related Mononuclear Species. Inorg. Chem. 2008, 47, 3903–3919. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Cai, X.; Mannina, L.; Ercolani, C.; Kadish, K.M. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. 8. Central (ZnII, CuII, MgII(H2O), CdII) and Exocyclic (PdII) Metal Ion Binding in Heteropentametallic Complexes from Tetrakis-2,3-[5,6-di(2-pyridyl)pyrazino]porphyrazine. Inorg. Chem. 2010, 49, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Donzello, M.P.; Viola, E.; Mannina, L.; Barteri, M.; Fu, Z.; Ercolani, C. Tetra-2,3-pyrazinoporphyrazines with externally appended pyridine rings. 11. Photoactivity of a new Pt(II) pentanuclear macrocycle bearing four cisplatin-like functionalities and its related mono-platinated species. J. Porphyr. Phthalocyanines 2011, 15, 985–994. [Google Scholar] [CrossRef]
- Donzello, M.P.; Viola, E.; Ercolani, C.; Fu, Z.; Futur, D.; Kadish., K.M. Tetra-2,3-pyrazinoporphyrazines with Externally Appended Pyridine Rings. 12. New Heteropentanuclear Complexes Carrying Four Exocyclic Cis-platin-like Functionalities as Potential Bimodal (PDT/Cis-platin) Anticancer Agents. Inorg. Chem. 2012, 51, 12548–12559. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron Neutron Capture Therapy—A Literature Review. J. Clin. Diagn. Res. 2016, 10, ZEO1–ZEO4. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139–213158. [Google Scholar] [CrossRef]
- Bellucci, N.; Donzello, M.P.; Amati, M.; Viola, E.; Rizzoli, C.; Ercolani, C.; Ricciardi, G.; Rosa, A. Bis(CBT)palladium(II) Derivatives (CBT = m-carborane-1-thiolate): Synthesis, Molecular Structure and Physicochemical Properties of cis-[(bipy)Pd(CBT)2] and trans-[(py)2Pd(CBT)2]. Inorg. Chem. 2021, 60, 10478–10491. [Google Scholar] [CrossRef]
- Viola, E.; Donzello, M.P.; Testani, S.; Luccisano, G.; Astolfi, M.L.; Rizzoli, C.; Cong, L.; Man-nina, L.; Ercolani, C.; Kadish, K.M. Tetra-2,3-pyrazinoporphyrazines with Peripherally Appended Pyridine Rings. 19. Pentanuclear Octa(2-pyridyl)tetrapyrazinoporphyrazines Carrying Externally Carboranthiolate Groups: Physicochemical Properties and Potentialities as Anticancer Drugs. Inorg. Chem. 2019, 58, 1120–1133. [Google Scholar] [CrossRef]
- Pandey, R.K.; Zheng, G. Porphyrins as Photosensitizers in Photodynamic Therapy. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 6, pp. 157–230. [Google Scholar]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Kuznetsova, N.A.; Gretsova, N.S.; Derkacheva, V.M.; Mikhalenko, S.A.; Soloveva, L.I.; Yuzhakova, O.A.; Kaliya, O.L.; Lukyanets, E.A. Generation of singlet oxygen with anionic aluminum phthalocyanines in water. Russ. J. Gen. Chem. 2002, 72, 300–306. [Google Scholar] [CrossRef]
- Sekkat, N.; van den Bergh, H.; Nyokong, T.; Lange, N. Like a bolt from the blue: Phthalocyanines in biomedical optics. Molecules 2012, 17, 98–144. [Google Scholar] [CrossRef]
- Durmus, M.; Ahsen, V. Water-soluble cationic gallium(III) and indium(III) phthalocya-nines for photodynamic therapy. J. Inorg. Biochem. 2010, 104, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Fujishiroa, R.; Sonoya-maa, H.; Idea, Y.; Fujimuraa, T.; Sasaia, R.; Nagaib, A.; Moric, S.; Kaufmand, N.E.M.; Zhoud, Z.; Vicented, M.G.H.; et al. Synthesis, photodynamic activities, and cytotoxicity of new water-soluble cationic gallium(III) and zinc(II) phthalocyanines. J. Inorg. Biochem. 2019, 192, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Wang, J.; Chen, J.; Chidawanykia, W.; Nyokong, T.; Ishii, K.; Kobayashi, N. Gallium Phthalocyanine Photosensitizers: Carboxylation Enhances the Cellular Uptake and Improves the Photodynamic Therapy of Cancer, Anticancer Agents. Med. Chem. 2012, 12, 604–610. [Google Scholar]
- Ghazal, B.; Ewies, E.F.; Youssef, A.S.A.; Makhseed, S. Photo-physicochemical properties of water-soluble non-aggregated indium(III) phthalocyanines. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118244–118252. [Google Scholar] [CrossRef]
- Novakova, V.; Morkved, E.H.; Miletin, M.; Zimcik, P. Influence of protonation of peripheral substituents on photophysical and photochemical properties of tetrapyrazinoporphyrazines. J. Porphyrins Phthalocyanines 2010, 14, 582–591. [Google Scholar] [CrossRef]
- Zimcik, P.; Novakova, V.; Miletin, M.; Kopecky, K. Thianaphthene-Annulated Tetrapyrazinoporphyrazines. Macroheterocycles 2008, 1, 21–29. [Google Scholar] [CrossRef][Green Version]
- Zimcik, P.; Miletin, M.; Novakova, V.; Kopecky, K.; Nejedla, M.; Stara, V.; Sed-lackova, K. Effective Monofunctional Azaphthalocyanine Photosensitizers for Photodynamic Therapy. Aust. J. Chem. 2009, 62, 425–433. [Google Scholar] [CrossRef]
- Mitzel, F.; Fitzgerald, S.; Beeby, A.; Faust, R. The Synthesis of Arylalkyne-Substituted Tetrapyrazinoporphyrazines and an Evaluation of Their Potential as Photosensitisers for Photodynamic Therapy. Eur. J. Org. Chem. 2004, 1136–1142. [Google Scholar] [CrossRef]
- Machacek, M.; Cidlina, A.; Novakova, V.; Svec, J.; Rudolf, E.; Miletin, M.; Kučera, R.; Simunek, T.; Zimcik, P. Far-Red-Absorbing Cationic Phthalocyanine Photosensitizers: Synthesis and Evaluation of the Photodynamic Anticancer Activity and the Mode of Cell Death Induction. J. Med. Chem. 2015, 58, 1736–1749. [Google Scholar] [CrossRef]
- Wöhrle, D.; Tsaryova, O.; Semioshkin, A.; Gabel, D.; Suvorova, O. Synthesis and photochemi-cal properties of phthalocyanine zinc(II) complexes containing o-carborane units. J. Organomet. Chem. 2013, 747, 98–105. [Google Scholar] [CrossRef]
- Nyokong, T. Effects of substituents on the photochemical and photophysical properties of main group metal phthalocyanines. Coord. Chem. Rev. 2007, 251, 1707–1722. [Google Scholar] [CrossRef]
- Pietrangeli, D.; Rosa, A.; Pepe, A.; Altieri, S.; Bortolussi, S.; Postuma, I.; Protti, N.; Ferrari, C.; Cansolino, L.; Clerici, A.M.; et al. Water-soluble carboranyl-phthalocyanines for BNCT. Synthesis, characterization, and in vitro tests of the Zn(II)-nido-carboranylhexylthiophthalocyanine. Dalton Trans. 2015, 44, 11021–11028. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, D.; Rosa, A.; Ristori, S.; Salvati, A.; Altieri, S.; Ricciardi, G. Carboranyl-porphyrazines and derivatives for boron neutron capture therapy: From synthesis to in vitro tests. Coord. Chem. Rev. 2013, 257, 2213–2231. [Google Scholar] [CrossRef]
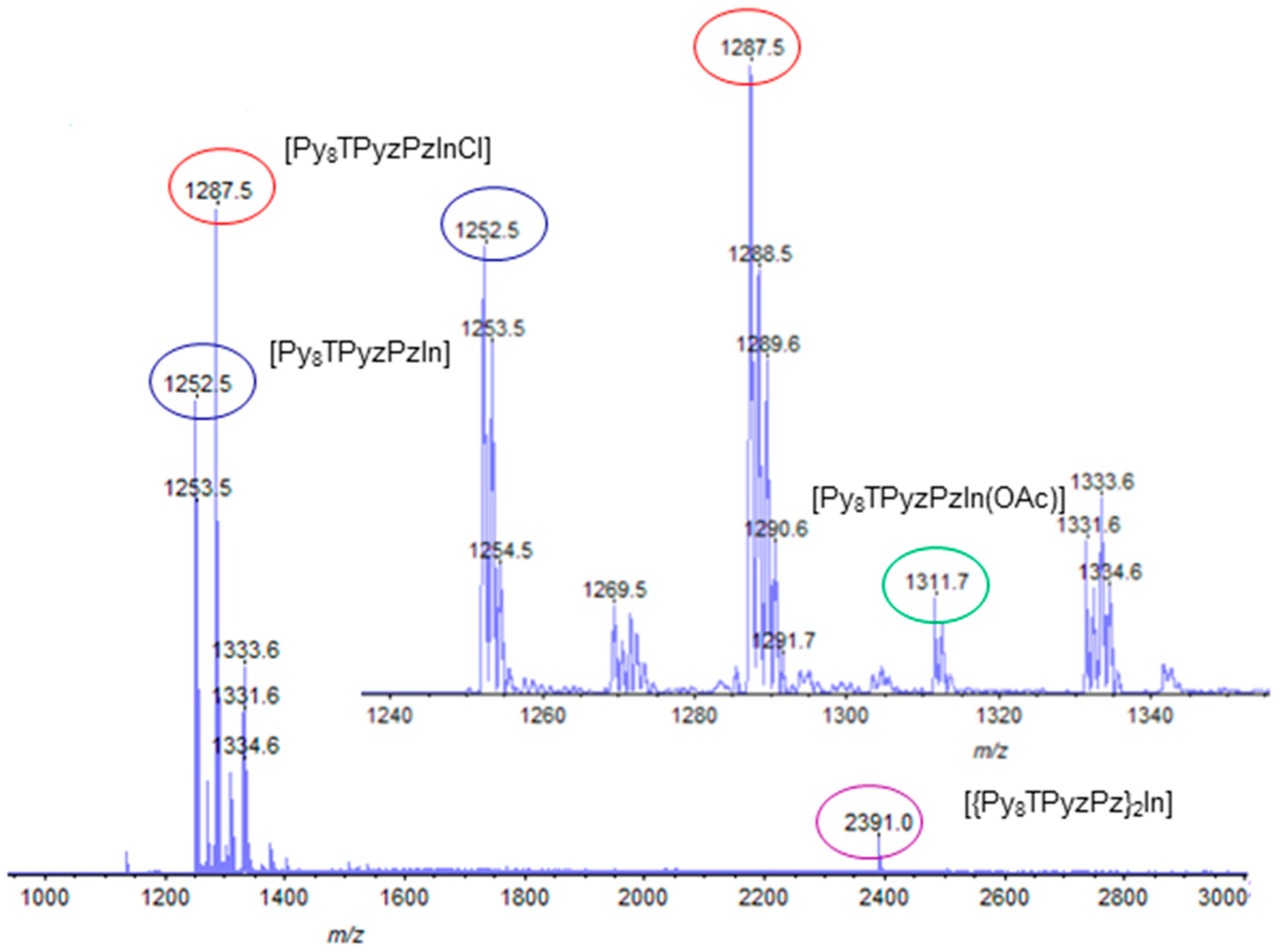
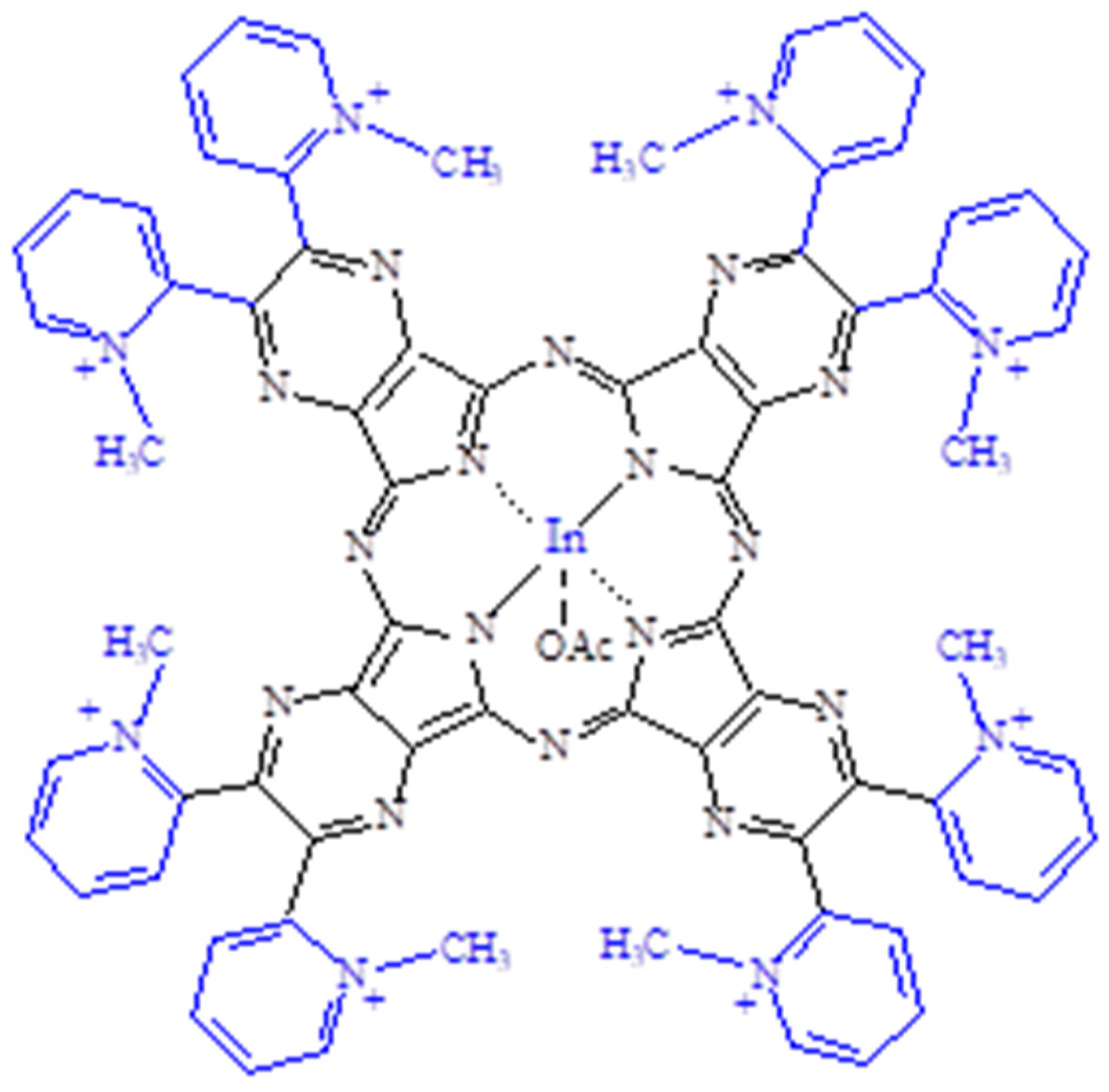
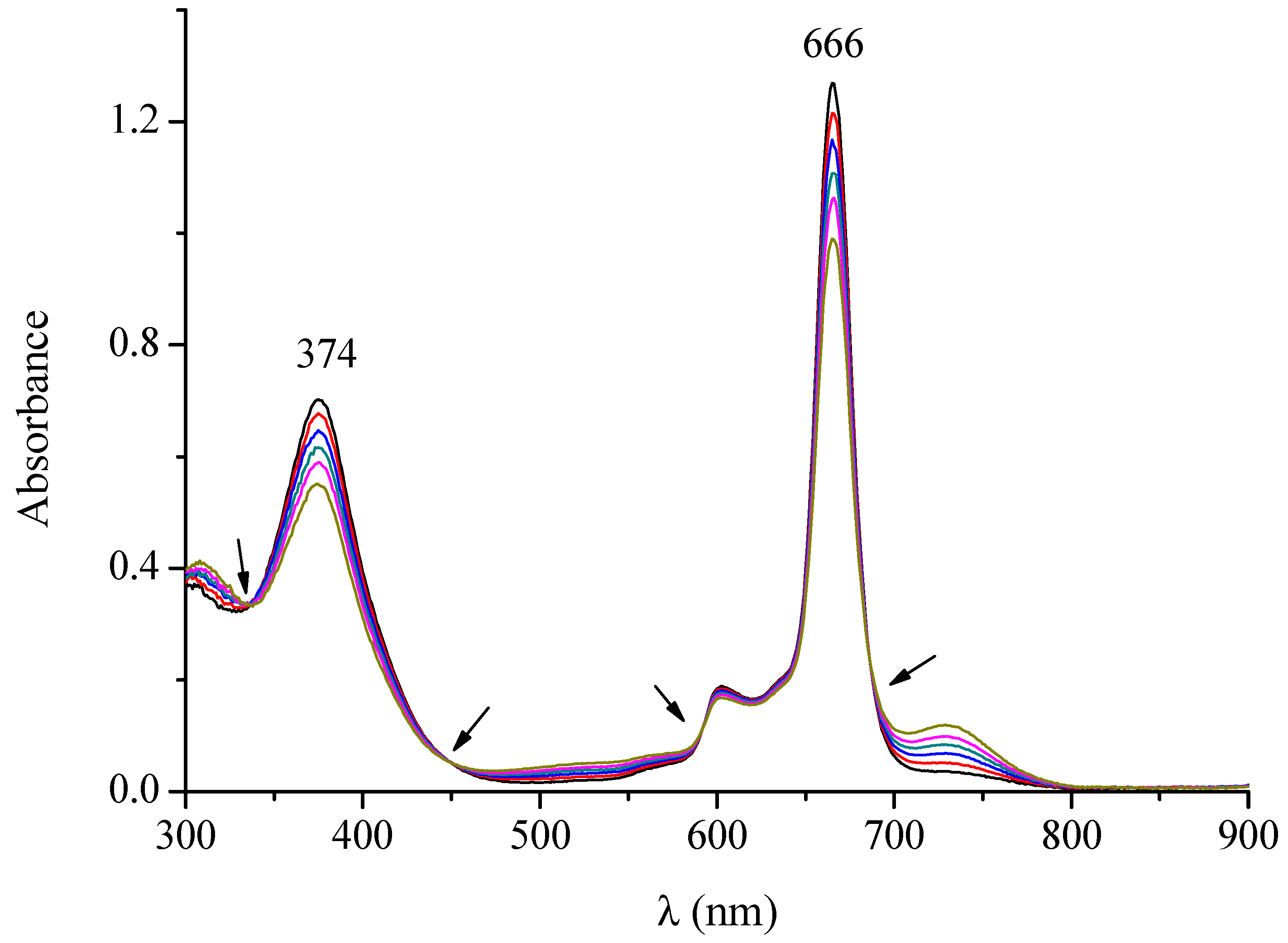

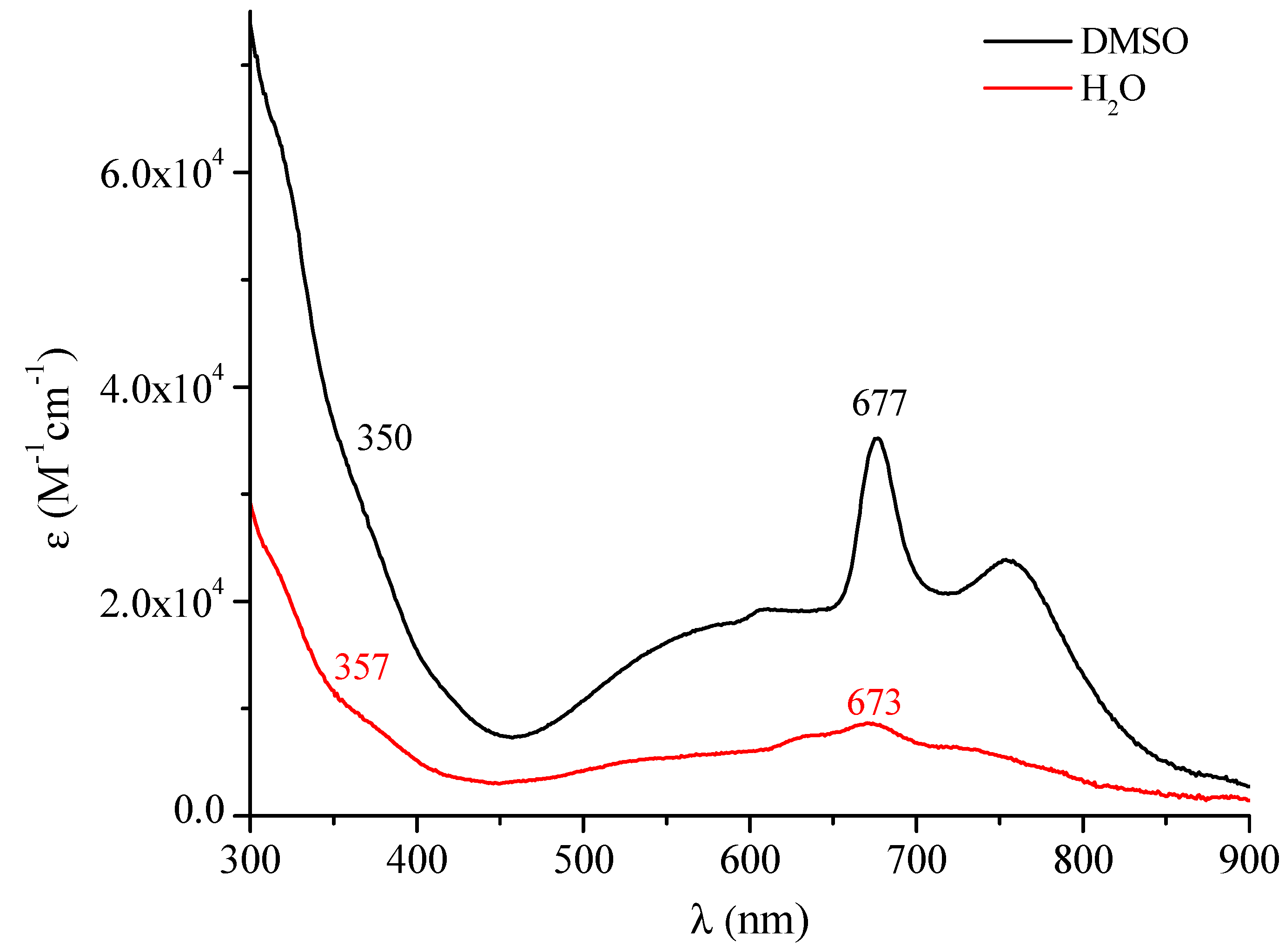

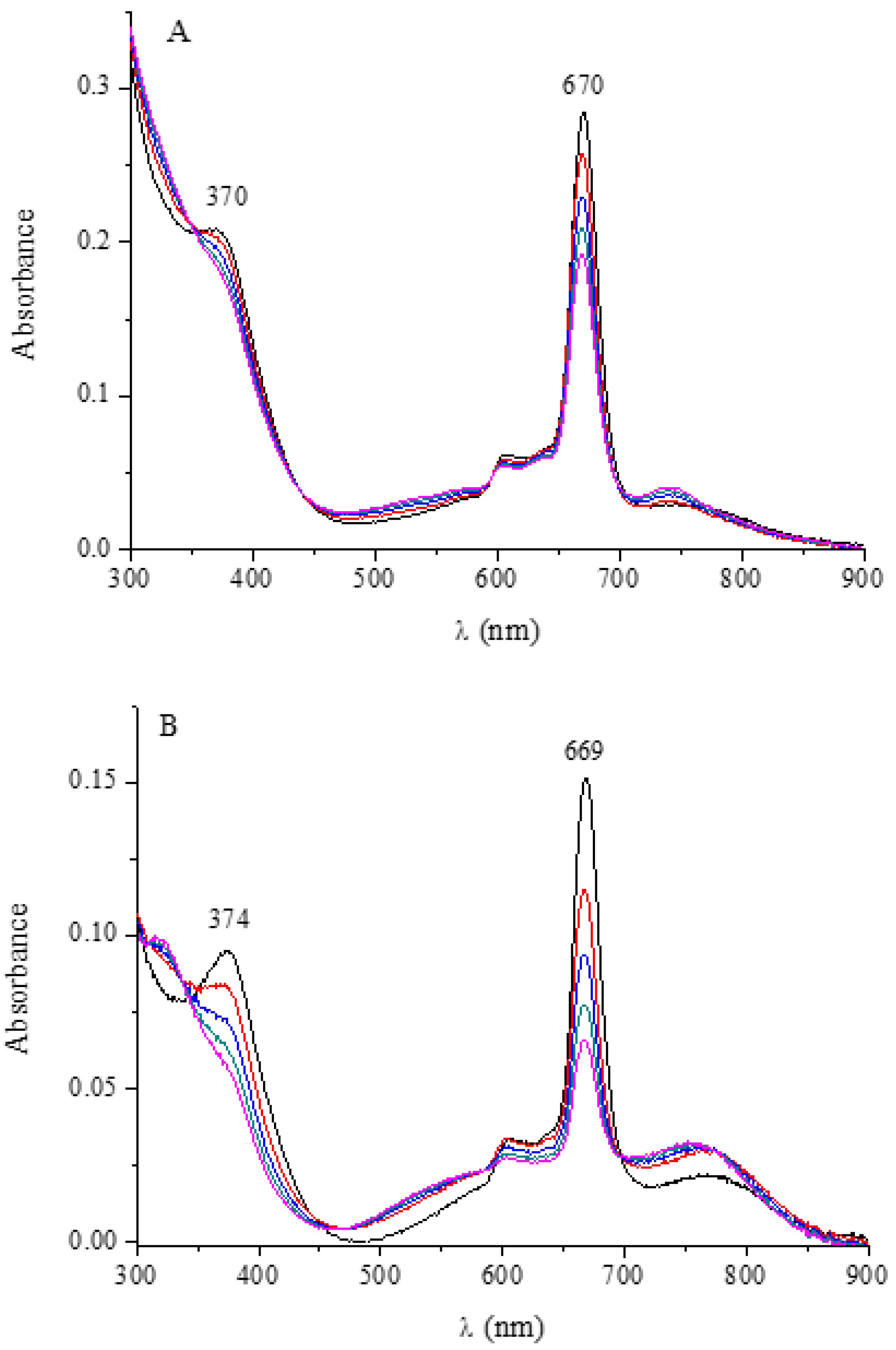
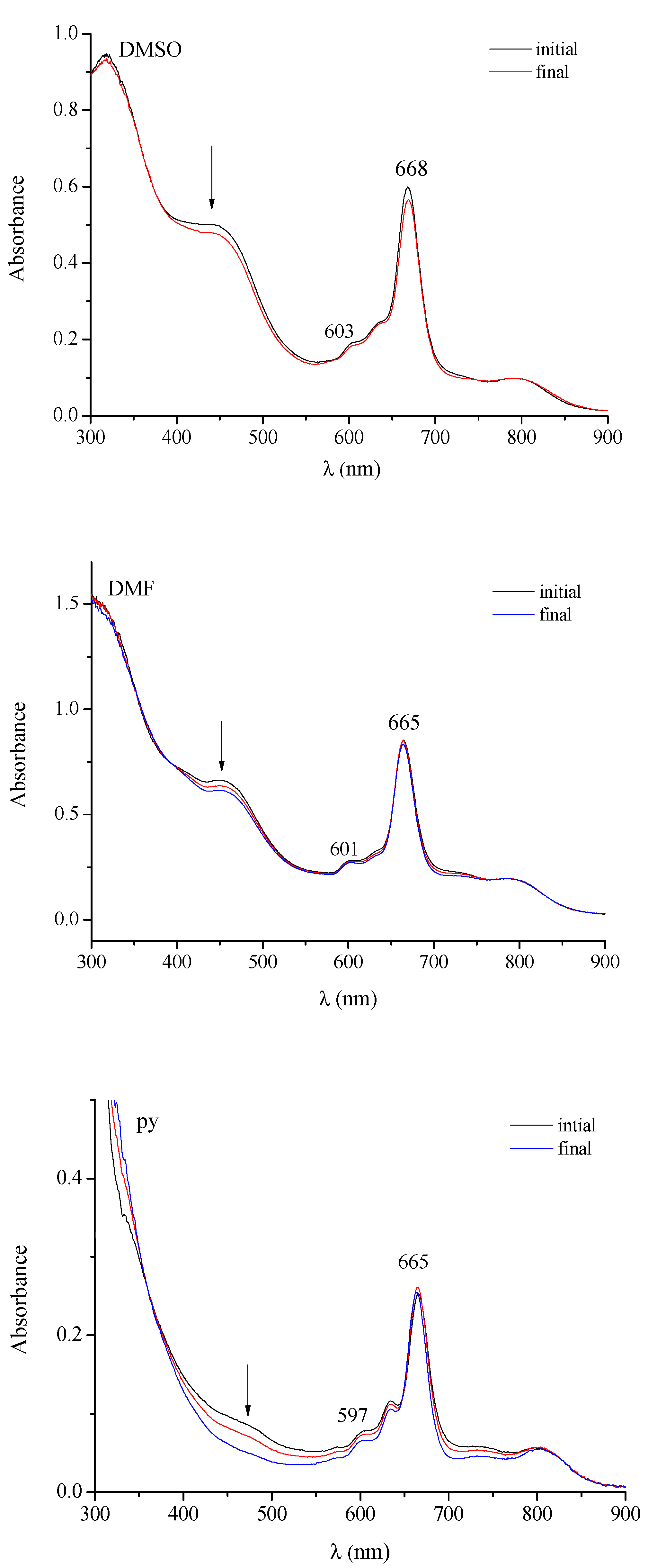
| Macrocyclic Species | Solvent | Soret Region | Q-Band Region | |||
|---|---|---|---|---|---|---|
| [Py8TPyzPzIn(OAc)]·8H2O | CH3CN | 371 (4.97) | 599 (4.36) | 662 (5.21) | ||
| CHCl3 | 371 (4.99) | 600 (4.42) | 662 (5.29) | |||
| DMF | 374 (4.97) | 603 (4.19) | 666 (5.22) | |||
| DMSO | 376 (4.97) | 603 (4.40) | 667 (5.26) | |||
| Py | 378 (5.01) | 606 (4.41) | 669 (5.26) | |||
| [(2-Mepy)8TPyzPzIn(OAc)](I)8·H2O | CH3CN | 356 (4.77) | 560 (4.28) | 603 (4.32) | 671 (4.74) | 745 (4.50) |
| DMF | 314 (4.80) 358(sh) (4.55) | 575 (4.27) | 609 (4.33) | 676 (4.61) | 763 (4.45) | |
| DMSO | 315(sh) (4.81) 350 (sh) (4.56) | 577 (4.25) | 608 (4.28) | 677 (4.55) | 755 (4.38) | |
| H2O | 357(sh) (4.02) | 570 (3.74) | 636 (3.87) 673 (3.93) | 719 (2.81) | ||
| Py | 357(sh) (4.32) | 573 (3.93) | 611 (4.00) | 681 (4.27) | 771 (4.09) | |
| [(PdCl2)4Py8TPyzPzIn(OAc)]·8H2O | DMSO | 377 (4.37) | 603 (4.07) | 672 (4.74) | ||
| DMF | 366 sh (4.52) | 608 (4.08) | 672 (4.61) | |||
| Py | 377 (4.59) | 605 (4.10) | 668 (4.79) | |||
| CH3CN | 673 | |||||
| [(PtCl2)4Py8TPyzPzIn(OAc)]·H2O | DMSO | 374 (4.48) | 604 (4.03) | 669 (4.69) | ||
| DMF | 373 (4.65) | 603 (4.20) | 667 (4.83) | |||
| Py | 378 (4.57) | 607 (4.12) | 668 (4.77) | |||
| [{Pd(CBT)2}4Py8TPyzPzIn(OAc)]∙19H2O | DMSO | 320 (4.84) | 454 (4.52) | 603 (3.98) | 668 (4.42) | |
| DMF | 318 (4.91) | 454 (4.60) | 603 (4.15) | 666 (4.50) | ||
| Py | 320 (4.94) | 468 (4.43) | 601 (4.27) | 666 (4.56) | ||
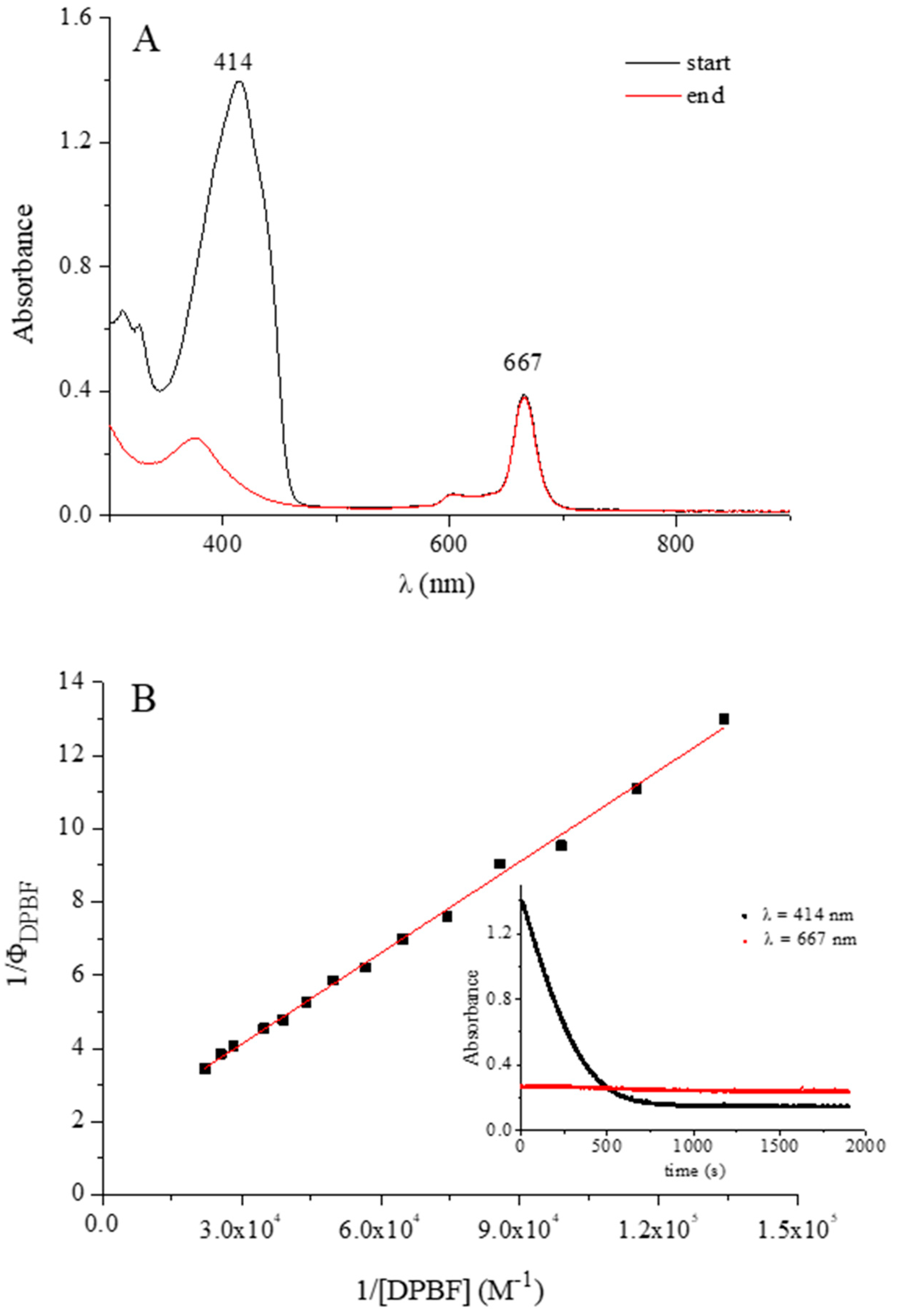
| Compound | HCl [M] | λmax [nm] | λirr [nm] | ΦΔ a | Ref. |
|---|---|---|---|---|---|
| [Py8TPyzPzIn(OAc)] | 0 | 667 | 670 | 0.55 | tw |
| [(PdCl2)4Py8TPyzPzIn(OAc)] | 0 | 672 | 670 | 0.36 | tw |
| [(PtCl2)4Py8TPyzPzIn(OAc)] | 0 | 667 | 670 | 0.46 | tw |
| [Py8TPyzPzAlCl] | 1 × 10−4 | 656 | 660 | 0.24 | 7 |
| [Py8TPyzPzGaCl] | 1 × 10−4 | 652 | 650 | 0.68 | 7 |
| [(PdCl2)4Py8TPyzPzAlCl] | 1 × 10−4 | 662 | 660 | 0.21 | 7 |
| [(PdCl2)4Py8TPyzPzGaCl] | 1 × 10−4 | 656 | 660 | 0.42 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donzello, M.P.; Capobianco, G.; Pettiti, I.; Ercolani, C.; Stuzhin, P.A. Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes. Molecules 2022, 27, 849. https://doi.org/10.3390/molecules27030849
Donzello MP, Capobianco G, Pettiti I, Ercolani C, Stuzhin PA. Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes. Molecules. 2022; 27(3):849. https://doi.org/10.3390/molecules27030849
Chicago/Turabian StyleDonzello, Maria Pia, Giulia Capobianco, Ida Pettiti, Claudio Ercolani, and Pavel A. Stuzhin. 2022. "Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes" Molecules 27, no. 3: 849. https://doi.org/10.3390/molecules27030849
APA StyleDonzello, M. P., Capobianco, G., Pettiti, I., Ercolani, C., & Stuzhin, P. A. (2022). Tetra-2,3-Pyrazinoporphyrazines with Externally Appended Pyridine Rings 22 Synthesis, Physicochemical and Photoactivity Studies on In(III) Mono- and Heteropentanuclear Complexes. Molecules, 27(3), 849. https://doi.org/10.3390/molecules27030849






