Natural Products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A Review on Their Structural Analysis, Quality Control, Pharmacology, and Pharmacokinetics
Abstract
1. Introduction
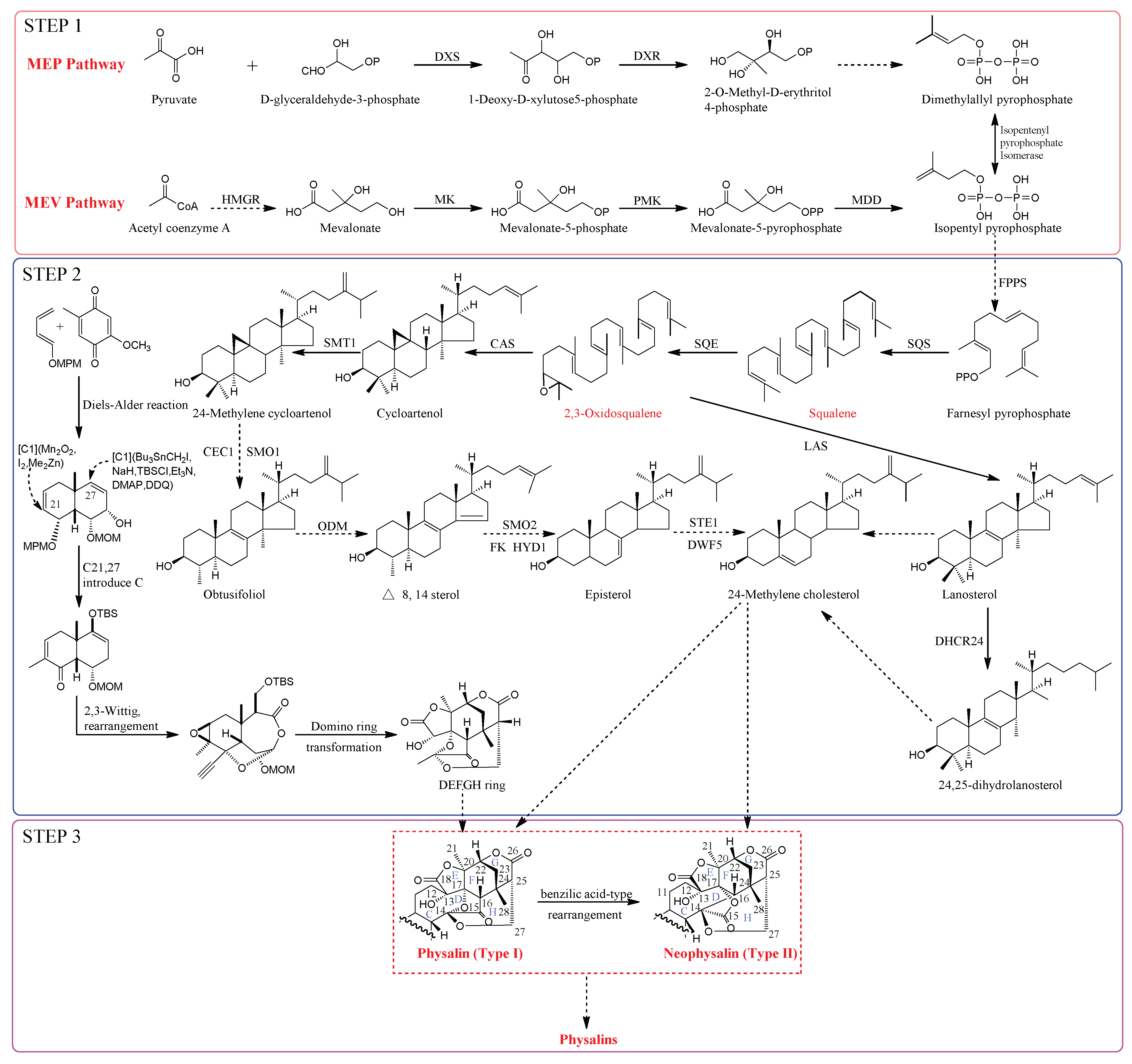
2. Structural Analysis
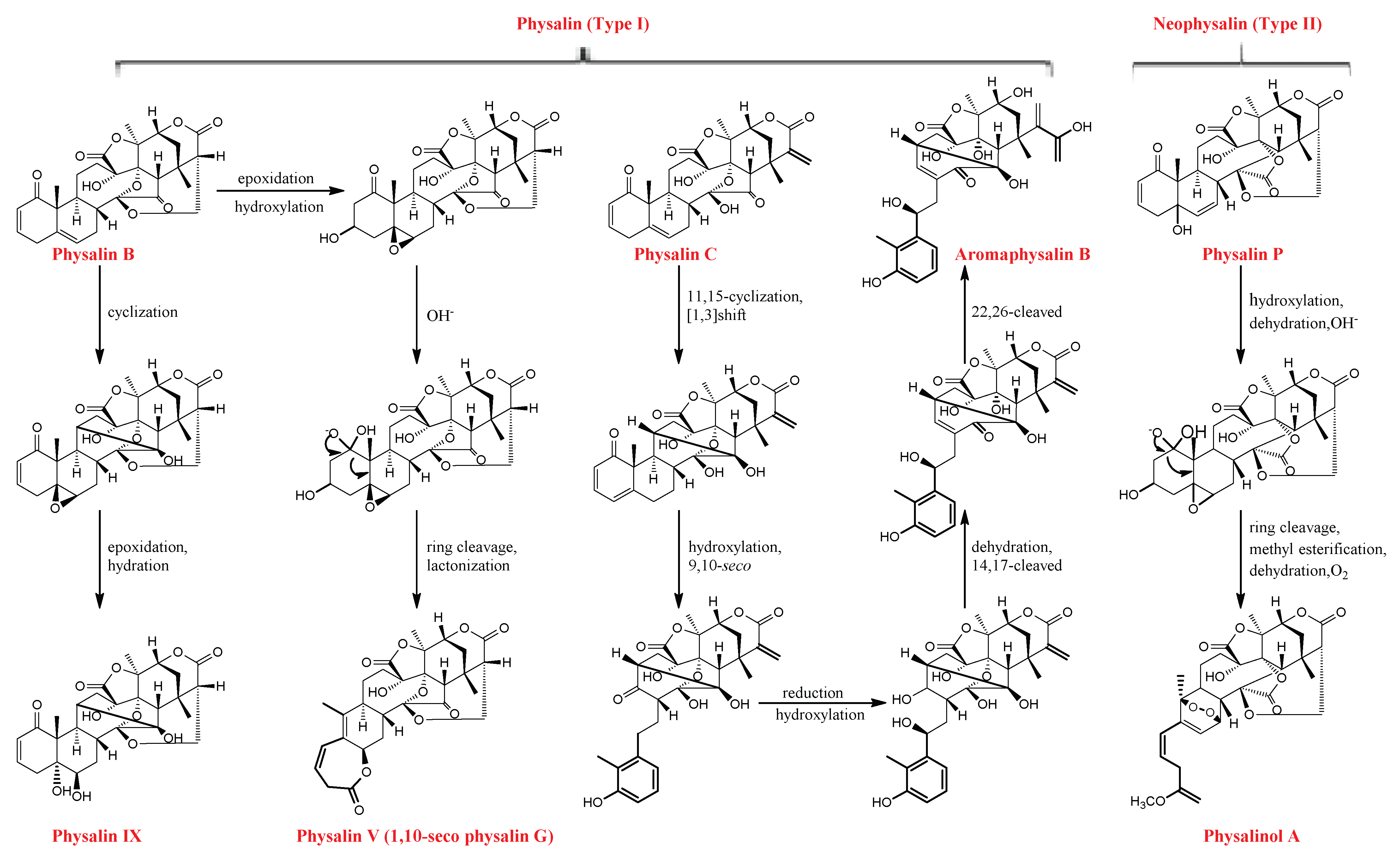
2.1. Physalins
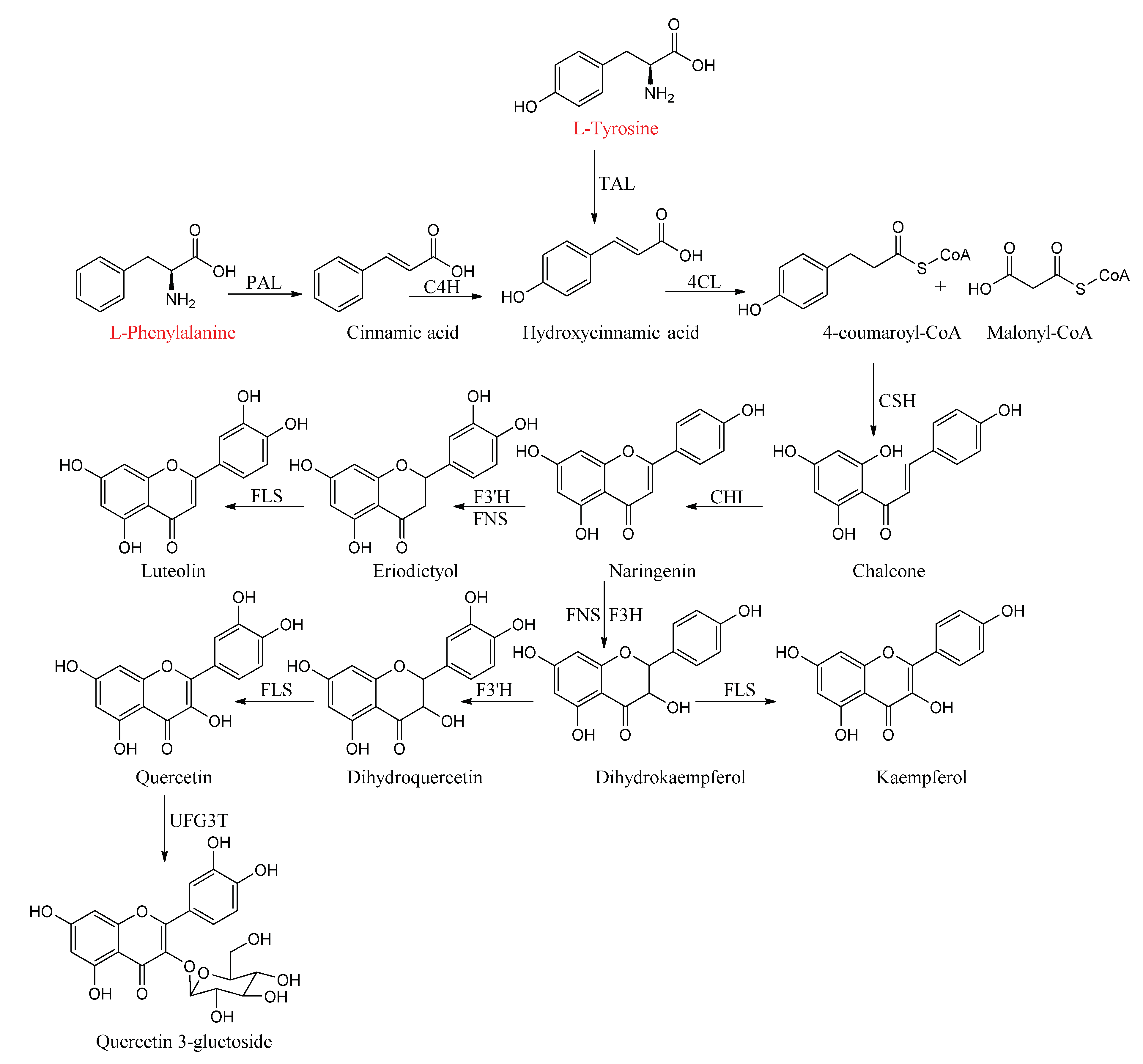
2.2. Flavonoids
3. Quality Control
4. Pharmacology
4.1. Anti-Inflammatory Activity
4.2. Anti-Tumor Activity
4.3. Immunosuppressive Activity
4.4. Antibacterial Activity
4.5. Antileishmanial Activity
4.6. Others
5. Pharmacology
5.1. Physalins
5.2. Flavonoids
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part I; People’s Medical Publishing House: Beijing, China, 2020; p. 360. (In Chinese) [Google Scholar]
- Zheng, W.J.; Fu, L.G. Flora of China; Editorial Committee of Flora of China, Chinese Academy of Sciences, Science Press: Beijing, China, 1978; p. 54. (In Chinese) [Google Scholar]
- Shu, Z.P.; Xu, B.Q.; Xing, N.; Li, X.L.; Wang, Q.H.; Yang, B.Y.; Kuang, H.X. Chemical constituents of Physalis Calyx seu Fructus. Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2014, 20, 99–102. [Google Scholar]
- Gao, P.Y.; Jin, M.; Du, C.L.; Liu, X.G. Research progress of Physalis alkekengi var. franchetii. Shenyang Yao Ke Da Xue Xue Bao 2014, 31, 732–737. [Google Scholar]
- Wen, X.; Erşan, S.; Li, M.; Wang, K.; Steingass, C.B.; Schweiggert, R.M.; Ni, Y.; Carle, R. Physicochemical characteristics and phytochemical profiles of yellow and red Physalis (Physalis alkekengi L. and P.pubescens L.) fruits cultivated in China. Food Res. Int. 2019, 120, 389–398. [Google Scholar] [CrossRef]
- Li, A.L.; Chen, B.J.; Li, G.H.; Zhou, M.X.; Li, Y.R.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Physalis alkekengi L. var. franchetii (Mast.) Makino: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2018, 210, 260–274. [Google Scholar] [CrossRef]
- Yang, L.J.; Wang, D.D.; Wu, H.J.; Chen, D.Z. Study on the action targets for anti-inflammatory bioactive components of Physalis alkekengi L. var. franchetii (Mast.) Makino based on network pharmacology. J. Tianjin Univ. Tradit. Chin. Med. 2018, 37, 399–403. [Google Scholar]
- Huang, M.; He, J.X.; Hu, H.X.; Zhang, K.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; Ren, D.M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2020, 72, 649–669. [Google Scholar] [CrossRef]
- Ozawa, M.; Morita, M.; Hirai, G.; Tamura, S.; Kawai, M.; Tsuchiya, A.; Oonuma, K.; Maruoka, K.; Sodeoka, M. Contribution of Cage-Shaped Structure of Physalins to Their Mode of Action in Inhibition of NF-κB Activation. ACS Med. Chem. Lett. 2013, 4, 730–735. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, J.; Zhao, J.; Zhang, T.; Gu, Y.; Khan, I.A.; Zou, Z.; Xu, Q. Naturally occurring physalins from the genus Physalis: A review. Phytochemistry 2021, 191, 112925. [Google Scholar] [CrossRef]
- Pandey, S.S.; Singh, S.; Pandey, H.; Srivastava, M.; Ray, T.; Soni, S.; Pandey, A.; Shanker, K.; Babu, C.S.V.; Banerjee, S.; et al. Endophytes of Withania somnifera modulate in planta content and the site of withanolide biosynthesis. Sci. Rep. 2018, 8, 5450. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef]
- Kushwaha, R.K.; Singh, S.; Pandey, S.S.; Kalra, A.; Babu, C.S.V. Fungal endophytes attune withanolide biosynthesis in Withania somnifera, prime to enhanced withanolide A content in leaves and roots. World J. Microbiol. Biotechnol. 2019, 35, 20. [Google Scholar] [CrossRef]
- Singh, S.; Pal, S.; Shanker, K.; Chanotiya, C.S.; Gupta, M.M.; Dwivedi, U.N.; Shasany, A.K. Sterol partitioning by HMGR and DXR for routing intermediates toward withanolide biosynthesis. Physiol. Plant. 2014, 152, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Goel, R.; Pathak, S.; Srivastava, A.; Singh, S.P.; Sangwan, R.S.; Asif, M.H.; Trivedi, P.K. De novo assembly, functional annotation and comparative analysis of Withania somnifera leaf and root transcriptomes to identify putative genes involved in the withanolides biosynthesis. PLoS ONE 2013, 8, e62714. [Google Scholar] [CrossRef]
- Singh, G.; Tiwari, M.; Singh, S.P.; Singh, S.; Trivedi, P.K.; Misra, P. Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci. Rep. 2016, 6, 25562. [Google Scholar] [CrossRef]
- Sharma, A.; Rather, G.A.; Misra, P.; Dhar, M.K.; Lattoo, S.K. Jasmonate responsive transcription factor WsMYC2 regulates the biosynthesis of triterpenoid withanolides and phytosterol via key pathway genes in Withania somnifera (L.) Dunal. Plant Mol. Biol. 2019, 100, 543–560. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Kojima, S.; Ohkubo, M.; Koshino, H.; Hashizume, D.; Hirai, G.; Maruoka, K.; Sodeoka, M. Synthesis of the right-side structure of type B physalins. Isr. J. Chem. 2017, 57, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, M.; Hirai, G.; Sodeoka, M. Synthesis of the DFGH ring system of type B physalins: Highly oxygenated, cage-shaped molecules. Angew. Chem. 2009, 48, 3862–3866. [Google Scholar] [CrossRef]
- Morita, M.; Hirai, G.; Ohkubo, M.; Koshino, H.; Hashizume, D.; Maruoka, K.; Sodeoka, M. Kinetically controlled one-pot formation of DEFGH-rings of type B physalins through domino-type transformations. Org. Lett. 2012, 14, 3434–3437. [Google Scholar] [CrossRef]
- Gupta, P.; Agarwal, A.V.; Akhtar, N.; Sangwan, R.S.; Singh, S.P.; Trivedi, P.K. Cloning and characterization of 2-C-methyl-d-erythritol-4-phosphate pathway genes for isoprenoid biosynthesis from Indian ginseng, Withania somnifera. Protoplasma 2013, 250, 285–295. [Google Scholar] [CrossRef]
- Kim, B.G.; Yang, S.M.; Kim, S.Y.; Cha, M.N.; Ahn, J.H. Biosynthesis and production of glycosylated flavonoids in Escherichia coli: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2979–2988. [Google Scholar] [CrossRef]
- Qiu, L.; Jiang, Z.H.; Liu, H.X.; Chen, L.X.; Qu, G.X.; Qiu, F. Flavonoid glycosides of the calyx Physalis. J. Shenyang Pharm. Univ. 2007, 24, 744–747. [Google Scholar]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Zou, L.Q.; Wang, C.X.; Kuang, X.J.; Li, Y.; Sun, C. Advance in flavonoids biosynthetic pathway and synthetic biology. China J. Chin. Mater. Med. 2016, 22, 4124–4128. [Google Scholar]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant flavonoids—biosynthesis, transport and involvement in stress responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chen, X.R.; Wang, J.P.; Cui, W.Q.; Xing, X.X.; Chen, X.Y.; Ding, W.Y.; God’spower, B.O.; Eliphaz, N.; Sun, M.Q.; et al. Transcriptomic analysis reveals flavonoid biosynthesis of Syringa oblata Lindl. in response to different light intensity. BMC Plant Biol. 2019, 19, 487. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, X.T.; Feng, W.T.; Chen, S.S.; Xu, L.F.; Wang, Z.G.; Zhang, J.L.; Li, P.M.; Ma, F.W. Different biosynthesis patterns among flavonoid 3-glycosides with distinct effects on accumulation of other flavonoid metabolites in pears (Pyrus bretschneideri Rehd.). PLoS ONE 2014, 9, e91945. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, Y.; Fan, J.; Wang, D.; Gong, X.; Ouyang, Z. Accumulation of flavonoid glycosides and UFGT gene expression in mulberry leaves (Morus alba L.) before and after frost. Chem. Biodivers. 2017, 14, e1600496. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zou, M.; Zhao, S.; Sun, Y.J.; Xu, B.L. Investigated the germplasm resources of Physalis alkekengi L. Res. Pract. Chin. Med. 2019, 33, 16–19. [Google Scholar]
- Laczkó-Zöld, E.; Forgó, P.; Zupkó, I.; Sigrid, E.; Hohmann, J. Content determination of physalins in Physalis alkekengi by HPLC. J. China Pharm. 2011, 22, 1393–1395. [Google Scholar]
- Xu, B.L.; Li, X.K.; Wang, B. Simultaneous determination of five components in Physalis Calyx seu Fructus by HPLC. Chin. Tradit. Pat. Med. 2014, 36, 1700–1705. [Google Scholar]
- Yu, X.; Xu, B.L. ISSR analysis for genetic diversity of Physalis Calyx seu Fructus in different growing environment. Res. Pract. Chin. Med. 2017, 31, 15–17. [Google Scholar]
- Laczkó-Zöld, E.; Forgó, P.; Zupkó, I.; Sigrid, E.; Hohmann, J. Isolation and quantitative analysis of physalin D in the fruit and calyx of Physalis alkekengi L. Acta Biol. Hung. 2017, 68, 300–309. [Google Scholar] [CrossRef]
- Kranjc, E.; Albreht, A.; Vovk, I.; Glavnik, V. High performance thin-layer chromatography-mass spectrometry enables reliable analysis of physalins in different plant parts of Physalis alkekengi L. J. Chromatogr. A 2017, 1526, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luan, L.; Chen, Y.; Ren, Y.; Wu, Y. Characterization of physalins and fingerprint analysis for the quality evaluation of Physalis alkekengi L. var. franchetii by ultra-performance liquid chromatography combined with diode array detection and electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2012, 71, 54–62. [Google Scholar] [CrossRef]
- Huang, C.; Xu, Q.; Chen, C.; Song, C.; Xu, Y.; Xiang, Y.; Feng, Y.; Ouyang, H.; Zhang, Y.; Jiang, H. The rapid discovery and identification of physalins in the calyx of Physalis alkekengi L. var. franchetii (Mast.) Makino using ultra-high performance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J. Chromatogr. A 2014, 1361, 139–152. [Google Scholar] [PubMed]
- Zheng, Y.; Chen, Y.; Ren, Y.; Luan, L.; Wu, Y. Quantitative and transformation product analysis of major active physalins from Physalis alkekengi var. franchetii (Chinese Lantern) using ultraperformance liquid chromatography with electrospray ionisation tandem mass spectrometry and time-of-flight mass. Phytochem. Anal. 2012, 23, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, Y.Q.; Mu, C.X.; Feng, X.; Zhao, H.D.; Lin, C.X.; Cai, Q. Determination of 4,7-didehydro-neophysalin B in the fruits of Physalis alkekengi L. var. franchetii (mast.) Mskino by HPLC. Liaoning J. Tradit. Chin. Med. 2008, 35, 584–585. [Google Scholar]
- Zhao, H.D.; Lin, C.X.; Yin, C.X.; Yu, T.; Feng, X.; Cai, Q. Determination of 4,7-didehydro-neophysalinB in Physalis alkekengi L. var. franchetii (mast.) Mskino calyces by HPLC. J. Liaoning Univ. Tradit. Chin. Med. 2008, 10, 129–130. [Google Scholar]
- Cheng, X.M.; Zhang, C.H.; Chou, G.X.; Wang, Z.T. Investigation on quality standard of franchet groundcherry. China J. Chin. Mater. Medica 2010, 35, 2103–2105. [Google Scholar]
- Xu, B.L.; Li, X.K.; Wang, B. Change for sugar compositionin Physalis Calyx seu Fructus from different areas. Liaoning J. Tradit. Chin. Med. 2014, 41, 988–991. [Google Scholar]
- Wen, X.; Hempel, J.; Schweiggert, R.M.; Ni, Y.; Carle, R. Carotenoids and carotenoid esters of red and yellow physalis (Physalis alkekengi L. and P. pubescens L.) fruits and calyces. J. Agric. Food Chem. 2017, 65, 6140–6151. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhang, X.Y.; Song, X.B.; Li, Y.F.; Li, Q.H. Content determination of luteolin in Physalis Permviana Liquid by HPLC. Heilongjiang Med. 2004, 17, 10–11. [Google Scholar]
- Zhang, G.S.; Zhao, Y.L.; Yan, J.J.; Yu, Z.G. Simultaneous determination of 7 kinds of flavonoids in Jinhuang yanyan tablets by HPLC. J. Shenyang Pharm. Univ. 2012, 29, 693–696. [Google Scholar]
- Ji, L.; Yuan, Y.; Luo, L.; Chen, Z.; Ma, X.; Ma, Z.; Cheng, L. Physalins with anti-inflammatory activity are present in Physalis alkekengi var. franchetii and can function as michael reaction acceptors. Steroids 2012, 77, 441–447. [Google Scholar] [CrossRef]
- Soares, M.B.; Bellintani, M.C.; Ribeiro, I.M.; Tomassini, T.C.; dos Santos, R.R. Inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis angulata L. Eur. J. Pharmacol. 2003, 459, 107–112. [Google Scholar] [CrossRef]
- Vieira, A.T.; Pinho, V.; Lepsch, L.B.; Scavone, C.; Ribeiro, I.M.; Tomassini, T.; Ribeiro-dos-Santos, R.; Soares, M.B.; Teixeira, M.M.; Souza, D.G. Mechanisms of the anti-inflammatory effects of the natural secosteroids physalins in a model of intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 2005, 146, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, N.; Hu, X.; Zheng, Y. Anti-colitic effects of physalin B on dextran sodium sulfate-induced BALB/c mice by suppressing multiple inflammatory signaling pathways. J. Ethnopharmacol. 2020, 259, 112956. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wang, Y.; Dou, C.; Liu, F.; Guan, G.; Wei, K.; Yang, J.; Yang, M.; Tan, J.; Zeng, W.; et al. Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. J. Cell. Physiol. 2019, 234, 8788–8796. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Yi, L.; Wang, Q.; Xie, B.B.; Dong, Y.; Sha, C.W. Anti-inflammatory effects of physalin E from Physalis angulata on lipopolysaccharide-stimulated RAW 264.7 cells through inhibition of NF-κB pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.B.; Morais, T.C.; Carvalho, K.M.; Silva, C.R.; Andrade, G.M.; Brito, G.A.; Veras, M.L.; Pessoa, O.D.; Rao, V.S.; Santos, F.A. Topical anti-inflammatory potential of physalin E from Physalis angulata on experimental dermatitis in mice. Phytomedicine 2010, 17, 740–743. [Google Scholar] [CrossRef]
- Brustolim, D.; Vasconcelos, J.F.; Freitas, L.A.; Teixeira, M.M.; Farias, M.T.; Ribeiro, Y.M.; Tomassini, T.C.; Oliveira, G.G.; Pontes-de-Carvalho, L.C.; Ribeiro-dos-Santos, R.; et al. Activity of physalin F in a collagen-induced arthritis model. J. Nat. Prod. 2010, 73, 1323–1326. [Google Scholar] [CrossRef]
- Sun, C.P.; Oppong, M.B.; Zhao, F.; Chen, L.X.; Qiu, F. Unprecedented 22,26-seco physalins from Physalis angulata and their anti-inflammatory potential. Org. Biomol. Chem. 2017, 15, 8700–8704. [Google Scholar] [CrossRef]
- Sun, C.P.; Qiu, C.Y.; Zhao, F.; Kang, N.; Chen, L.X.; Qiu, F. Physalins V-IX, 16,24-cyclo-13,14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhao, F.; Jiang, Z.H.; Chen, L.X.; Zhao, Q.; Liu, H.X.; Yao, X.S.; Qiu, F. Steroids and flavonoids from Physalis alkekengi var. franchetii and their inhibitory effects on nitric oxide production. J. Nat. Prod. 2008, 71, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Medica 2007, 73, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, apigenin, luteolin and fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Xing, N.; Wang, Q.; Li, X.; Xu, B.; Li, Z.; Kuang, H.X. Antibacterial and anti-inflammatory activities of Physalis alkekengi var. franchetii and its main constituents. Evid.-Based Complement. Altern. Med. 2016, 2016, 4359394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.L.; Zhang, J.Y.; Song, X.N.; Zhang, Z.Y.; Li, J.F.; Li, S. Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent. J. Ethnopharmacol. 2018, 211, 197–206. [Google Scholar] [CrossRef]
- Qiu, L.; Zhao, F.; Liu, H.; Chen, L.; Jiang, Z.; Liu, H.; Wang, N.; Yao, X.; Qiu, F. Two New Megastigmane Glycosides, Physanosides A and B, from Physalisalkekengi L. var. franchetii, and Their Effect on NO Release in Macrophages. Chem. Biodivers. 2008, 5, 758–763. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, K.M.; Lee, H.J.; Yun, J.H.; Nho, C.W. Physalin A regulates the Nrf2 pathway through ERK and p38 for induction of detoxifying enzymes. BMC Complement. Altern. Med. 2019, 19, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Dai, C.; Fu, Y.; Loo, J.F.; Xia, D.; Gao, S.P.; Ma, Z.; Chen, Z. Physalin A exerts anti-tumor activity in non-small cell lung cancer cell lines by suppressing JAK/STAT3 signaling. Oncotarget 2016, 7, 9462–9476. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zang, L.H.; Feng, Y.S.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Qiu, F.; Ikejima, T. Physalin A induces apoptosis via p53-Noxa-mediated ROS generation, and autophagy plays a protective role against apoptosis through p38-NF-kappaB survival pathway in A375-S2 cells. J. Ethnopharmacol. 2013, 148, 544–555. [Google Scholar] [CrossRef]
- He, H.; Zang, L.H.; Feng, Y.S.; Wang, J.; Liu, W.W.; Chen, L.X.; Kang, N.; Tashiro, S.; Onodera, S.; Qiu, F.; et al. Physalin A induces apoptotic cell death and protective autophagy in HT1080 human fibrosarcoma cells. J. Nat. Prod. 2013, 76, 880–888. [Google Scholar] [CrossRef]
- He, H.; Feng, Y.S.; Zang, L.H.; Liu, W.W.; Ding, L.Q.; Chen, L.X.; Kang, N.; Hayashi, T.; Tashiro, S.; Onodera, S.; et al. Nitric oxide induces apoptosis and autophagy; autophagy down-regulates NO synthesis in physalin A-treated A375-S2 human melanoma cells. Food Chem. Toxicol. 2014, 71, 128–135. [Google Scholar] [CrossRef]
- Kang, N.; Jian, J.F.; Cao, S.J.; Zhang, Q.; Mao, Y.W.; Huang, Y.Y.; Peng, Y.F.; Qiu, F.; Gao, X.M. Physalin A induces G2/M phase cell cycle arrest in human non-small cell lung cancer cells: Involvement of the p38 MAPK/ROS pathway. Mol. Cell. Biochem. 2016, 415, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Qiu, L.; Wang, X.; Qiu, F.; Wong, Y.; Yao, X. Physalins A and B inhibit androgen-independent prostate cancer cell growth through activation of cell apoptosis and downregulation of androgen receptor expression. Biol. Pharm. Bull. 2011, 34, 1584–1588. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, L.; Chen, Y.; Wang, C.H.; ShenTu, J.Z.; Zheng, Y.L. Physalin B induces G2/M cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by altering mitochondrial function. Anti-Cancer Drugs 2019, 30, 128–137. [Google Scholar] [CrossRef]
- Hsu, C.C.; Wu, Y.C.; Farh, L.; Du, Y.C.; Tseng, W.K.; Wu, C.C.; Chang, F.R. Physalin B from Physalis angulata triggers the NOXA-related apoptosis pathway of human melanoma A375 cells. Food Chem. Toxicol. 2012, 50, 619–624. [Google Scholar] [CrossRef]
- Ma, Y.M.; Han, W.; Li, J.; Hu, L.H.; Zhou, Y.B. Physalin B not only inhibits the ubiquitin-proteasome pathway but also induces incomplete autophagic response in human colon cancer cells in vitro. Acta Pharmacol. Sin. 2015, 36, 517–527. [Google Scholar] [CrossRef]
- Vandenberghe, I.; Créancier, L.; Vispé, S.; Annereau, J.P.; Barret, J.M.; Pouny, I.; Samson, A.; Aussagues, Y.; Massiot, G.; Ausseil, F.; et al. Physalin B, a novel inhibitor of the ubiquitin-proteasome pathway, triggers NOXA-associated apoptosis. Biochem. Pharmacol. 2008, 76, 453–462. [Google Scholar] [CrossRef]
- Wang, A.; Wang, S.; Zhou, F.; Li, P.; Wang, Y.; Gan, L.; Lin, L. Physalin B induces cell cycle arrest and triggers apoptosis in breast cancer cells through modulating p53-dependent apoptotic pathway. Biomed. Pharmacother. 2018, 101, 334–341. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, T.; Zhang, F.B.; Wang, Y.N.; Liu, Z.; Guo, S.; Li, L. Isolation and characterization of cytotoxic withanolides from the calyx of Physalis alkekengi L. var franchetii. Bioorg. Chem. 2020, 96, 103614. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, H.I.; Veras, M.L.; Torres, M.R.; Alves, A.P.; Pessoa, O.D.; Silveira, E.R.; Costa-Lotufo LV de Moraes, M.O.; Pessoa, C. In-vitro and in-vivo antitumour activity of physalins B and D from Physalis angulata. J. Pharm. Pharmacol. 2006, 58, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Jacobo-Herrera, N.J.; Bremner, P.; Marquez, N.; Gupta, M.P.; Gibbons, S.; Muñoz, E.; Heinrich, M. Physalins from Witheringia solanacea as modulators of the NF-kappaB cascade. J. Nat. Prod. 2006, 69, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, D.; Zhang, H.; Han, C.; Xue, G.; Zhu, T.; Luo, J.; Kong, L. YAP-dependent ubiquitination and degradation of beta-catenin mediates inhibition of Wnt signalling induced by physalin F in colorectal cancer. Cell Death Dis. 2018, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.L.; Muhammad, T.S.; Sulaiman, S.F. Physalin F from Physalis minima L. triggers apoptosis-based cytotoxic mechanism in T-47D cells through the activation caspase-3- and c-myc-dependent pathways. J. Ethnopharmacol. 2013, 150, 382–388. [Google Scholar] [CrossRef]
- Wu, S.Y.; Leu, Y.L.; Chang, Y.L.; Wu, T.S.; Kuo, P.C.; Liao, Y.R.; Teng, C.M.; Pan, S.L. Physalin F induces cell apoptosis in human renal carcinoma cells by targeting NF-kappaB and generating reactive oxygen species. PLoS ONE 2012, 7, e40727. [Google Scholar] [CrossRef]
- Sun, J.L.; Jiang, Y.J.; Cheng, L. Two new physalin derivatives from Physalis alkekengi L. var. franchetii (Mast.) Makino. Nat. Prod. Res. 2021, 35, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, C.; Zhang, H.; Xia, Y.Z.; Zhang, C.Y.; Luo, J.; Yang, L.; Kong, L.Y. Physakengose G induces apoptosis via EGFR/mTOR signaling and inhibits autophagic flux in human osteosarcoma cells. Phytomedicine 2018, 42, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.P.; Moraes, C.S.; Gonzalez, M.S.; Ribeiro, I.M.; Tomassini, T.C.; Azambuja, P.; Garcia, E.S. Physalin B inhibits Trypanosoma cruzi infection in the gut of Rhodnius prolixus by affecting the immune system and microbiota. J. Insect Physiol. 2012, 58, 1620–1625. [Google Scholar] [CrossRef]
- Garcia, E.S.; Castro, D.P.; Ribeiro, I.M.; Tomassini, T.C.; Azambuja, P. Trypanosoma rangeli: Effects of physalin B on the immune reactions of the infected larvae of Rhodnius prolixus. Exp. Parasitol. 2006, 112, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Meira, C.S.; Guimarães, E.T.; Bastos, T.M.; Moreira, D.R.; Tomassini, T.C.; Ribeiro, I.M.; Dos Santos, R.R.; Soares, M.B. Physalins B and F, seco-steroids isolated from Physalis angulata L., strongly inhibit proliferation, ultrastructure and infectivity of Trypanosoma cruzi. Parasitology 2013, 140, 1811–1821. [Google Scholar] [CrossRef]
- Soares, M.B.; Brustolim, D.; Santos, L.A.; Bellintani, M.C.; Paiva, F.P.; Ribeiro, Y.M.; Tomassini, T.C.; Dos Santos, R.R. Physalins B, F and G, seco-steroids purified from Physalis angulata L., inhibit lymphocyte function and allogeneic transplant rejection. Int. Immunopharmacol. 2006, 6, 408–414. [Google Scholar] [CrossRef]
- Pinto, L.A.; Meira, C.S.; Villarreal, C.F.; Vannier-Santos, M.A.; de Souza, C.V.; Ribeiro, I.M.; Tomassini, T.C.; Galvão-Castro, B.; Soares, M.B.; Grassi, M.F. Physalin F, a seco-steroid from Physalis angulata L., has immunosuppressive activity in peripheral blood mononuclear cells from patients with HTLV1-associated myelopathy. Biomed. Pharmacother. 2016, 79, 129–134. [Google Scholar] [CrossRef]
- Yu, Y.; Sun, L.; Ma, L.; Li, J.; Hu, L.; Liu, J. Investigation of the immunosuppressive activity of physalin H on T lymphocytes. Int. Immunopharmacol. 2010, 10, 290–297. [Google Scholar] [CrossRef]
- Yang, H.; Han, S.; Zhao, D.; Wang, G. Adjuvant effect of polysaccharide from fruits of Physalis alkekengi L. in DNA vaccine against systemic candidiasis. Carbohydr. Polym. 2014, 109, 77–84. [Google Scholar] [CrossRef]
- Helvacı, S.; Kökdil, G.; Kawai, M.; Duran, N.; Duran, G.; Güvenç, A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharm. Biol. 2010, 48, 142–150. [Google Scholar] [CrossRef]
- Yang, Y.K.; Xie, S.D.; Xu, W.X.; Nian, Y.; Liu, X.L.; Peng, X.R.; Ding, Z.T.; Qiu, M.H. Six new physalins from Physalis alkekengi var. franchetii and their cytotoxicity and antibacterial activity. Fitoterapia 2016, 112, 144–152. [Google Scholar] [CrossRef]
- Januário, A.H.; Filho, E.R.; Pietro, R.C.; Kashima, S.; Sato, D.N.; França, S.C. Antimycobacterial physalins from Physalis angulata L. (Solanaceae). Phytother. Res. 2002, 16, 445–448. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wu, D.; Tang, L.; Cao, X.; Xin, Y. In vitro effects on intestinal bacterium of physalins from Physalis alkekengi var. Francheti. Fitoterapia 2012, 83, 1460–1465. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Luo, J.G.; Liu, R.H.; Lin, R.; Yang, M.H.; Kong, L.Y. 1H NMR spectroscopy-guided isolation of new sucrose esters from Physalis alkekengi var. franchetii and their antibacterial activity. Fitoterapia 2016, 114, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Luo, J.G.; Liu, R.H.; Lin, R.; Yang, M.H.; Kong, L.Y. Physakengoses K-Q, seven new sucrose esters from Physalis alkekengi var. franchetii. Carbohydr. Res. 2017, 449, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, E.T.; Lima, M.S.; Santos, L.A.; Ribeiro, I.M.; Tomassini, T.B.; dos Santos, R.R.; dos Santos, W.L.; Soares, M.B. Activity of physalins purified from Physalis angulata in in vitro and in vivo models of cutaneous leishmaniasis. J. Antimicrob. Chemother. 2009, 64, 84–87. [Google Scholar] [CrossRef]
- Bao, C.L. Curative Effect of Chinese Physalis Alkekeng on Mice Allergic Asthuma; Yanbian University: Yanji, China, 2008. [Google Scholar]
- Hu, X.F.; Zhang, Q.; Zhang, P.P.; Sun, L.J.; Liang, J.C.; Morris-Natschke, S.L.; Chen, Y.; Lee, K.H. Evaluation of in vitro/in vivo anti-diabetic effects and identification of compounds from Physalis alkekengi. Fitoterapia 2018, 127, 129–137. [Google Scholar] [CrossRef]
- Guo, Y.; Li, S.; Li, J.; Ren, Z.; Chen, F.; Wang, X. Anti-hyperglycemic activity of polysaccharides from calyx of Physalis alkekengi var. franchetii Makino on alloxan-induced mice. Int. J. Biol. Macromol. 2017, 99, 249–257. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.F.; Xin, M.M.; Liu, H.B.; Sun, L.J.; Morris-Natschke, S.L.; Chen, Y.; Lee, K.H. Antidiabetic potential of the ethyl acetate extract of Physalis alkekengi and chemical constituents identified by HPLC-ESI-QTOF-MS. J. Ethnopharmacol. 2018, 225, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Z.; Wang, Y.; Zhong, R.; Feng, Y.; Xia, T.; Xie, Y.; Yang, B.; Sun, X.; Shu, Z. Systems pharmacology reveals the mechanism of activity of Physalis alkekengi L. var. franchetii against lipopolysaccharide-induced acute lung injury. J. Cell. Mol. Med. 2020, 24, 5039–5056. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, S.S.; Zhang, Q.; Shi, R.L.; Wang, H.C.; Liu, Y.C.; Ni, T.J.; Wu, Y.; Yao, Z.Y.; Sun, Y.; et al. Physalin B reduces Aβ secretion through down-regulation of BACE1 expression by activating FoxO1 and inhibiting STAT3 phosphorylation. Chin. J. Nat. Med. 2021, 19, 732–740. [Google Scholar] [CrossRef]
- Sá, M.S.; de Menezes, M.N.; Krettli, A.U.; Ribeiro, I.M.; Tomassini, T.C.; dos Santos, R.R.; de Azevedo, W.F.J.; Soares, M.B. Antimalarial activity of physalins B, D, F, and G. J. Nat. Prod. 2011, 74, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Piao, X.; Zhang, M.; Wang, X.; Xu, B.; Zhu, J.; Fang, Z.; Hou, Y.; Lu, Y.; Yang, B. Bioactivity-guided fractionation of the triglyceride-lowering component and in vivo and in vitro evaluation of hypolipidemic effects of Calyx seu Fructus Physalis. Lipids Health Dis. 2012, 11, 38. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Liang, K.L.; Su, J.; Chen, S.H.; Lv, G.Y. Relaxation effect of buddleoside combined with luteolin on isolated vessels in vivo and its mechanism. China J. Chin. Mater. Med. 2017, 42, 1370–1375. [Google Scholar]
- Yan, Q.; Li, Y.; Yan, J.; Zhao, Y.; Liu, Y.; Liu, S. Effects of luteolin on regulatory proteins and enzymes for myocyte calcium circulation in hypothermic preserved rat heart. Exp. Ther. Med. 2018, 15, 1433–1441. [Google Scholar] [CrossRef]
- Tariq, A.; Adnan, M.; Amber, R.; Pan, K.; Mussarat, S.; Shinwari, Z.K. Ethnomedicines and anti-parasitic activities of Pakistani medicinal plants against Plasmodia and Leishmania parasites. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 52. [Google Scholar] [CrossRef]
- Guimarães, E.T.; Lima, M.S.; Santos, L.A.; Ribeiro, I.M.; Tomassini, B.C.; Santos, R.R.; Santos, L.C.; Soares, B.P. Effects of seco-steroids purified from Physalis angulata L., Solanaceae, on the viability of Leishmania sp. Rev. Bras. Farmacogn. 2010, 20, 945–949. [Google Scholar] [CrossRef]
- Tan, X.; Jin, P.; Feng, L.; Song, J.; Sun, E.; Liu, W.; Shu, L.; Jia, X. Protective effect of luteolin on cigarette smoke extract-induced cellular toxicity and apoptosis in normal human bronchial epithelial cells via the Nrf2 pathway. Oncol. Rep. 2014, 31, 1855–1862. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, H.; Ding, L.; Oppong, M.; Pan, G.; Qiu, F. LC-MS/MS method for simultaneous determination of flavonoids and physalins in rat plasma: Application to pharmacokinetic study after oral administration of Physalis alkekengi var. franchetii (Chinese lantern) extract. Biomed. Chromatogr. 2017, 31, e3970. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Y.; Ren, Y.; Luan, L.; Wu, Y. An ultra-pressure liquid chromatography—Tandem mass spectrometry method for the simultaneous determination of three physalins in rat plasma and its application to pharmacokinetic study of Physalis alkekengi var. franchetii (Chinese lantern) in rats. J. Pharm. Biomed. Anal. 2012, 58, 94–101. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Y.L.; ShenTu, J.Z. Pharmacokinetic of physalin L in rat plasma. Chin. J. Mod. Appl. Pharm. 2017, 34, 1663–1667. [Google Scholar]
- Zheng, Y.; Lin, M.; Hu, X.; Zhai, Y.; Zhang, Q.; Lou, Y.; ShenTu, J.; Wu, L. Simultaneous pharmacokinetics and stability studies of physalins in rat plasma and intestinal bacteria culture media using liquid chromatography with mass spectrometry. J. Sep. Sci. 2018, 41, 1781–1790. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.; Liu, L.; Liang, X.; Hong, D. In vivo pharmacokinetics of and tissue distribution study of physalin B after intravenous administration in rats by liquid chromatography with tandem mass spectrometry. Biomed. Chromatogr. 2016, 30, 1278–1284. [Google Scholar] [CrossRef]
- Fang, L.; Chai, H.B.; Castillo, J.J.; Soejarto, D.D.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic constituents of Brachistus stramoniifolius. Phytother. Res. 2003, 17, 520–523. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.; Chen, N.; Luan, L.; Liu, X. Plasma pharmacokinetics and tissue distribution study of physalin D in rats by ultra-pressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2011, 879, 443–448. [Google Scholar] [CrossRef]
- Feng, X.; Huo, X.; Liu, H.; Chai, L.; Ding, L.; Qiu, F. Identification of absorbed constituents and in vivo metabolites in rats after oral administration of Physalis alkekengi var. franchetii by ultra-high-pressure liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2018, 32, e4121. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; Chai, L.; Ding, L.; Pan, G.; Qiu, F. Metabolic profiles of physalin A in rats using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. B 2017, 1046, 102–109. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, C.; Lin, M.; Zhai, Y.; Ge, Z.; ShenTu, J.; Wu, L.; Hu, X. Identification and quantitative analysis of physalin D and its metabolites in rat urine and feces by liquid chromatography with triple quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2017, 40, 2355–2365. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.P.; Figueiredo, M.B.; Ribeiro, I.M.; Tomassini, T.C.; Azambuja, P.; Garcia, E.S. Immune depression in Rhodnius prolixus by seco-steroids, physalins. J. Insect Physiol. 2008, 54, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Otake, Y.; Hsieh, F.; Walle, T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab. Dispos. 2002, 30, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of flavonoids in human: A comprehensive review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; DuPont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver beta-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Shu, Z.; Tang, Y.; Yang, Y.; Ding, Z.; Zhong, R.; Xia, T.; Li, X.; Zheng, C.; Wen, Z.; Li, W.; et al. Two new 3-hexenol glycosides from the calyces of Physalis alkekengi var. franchetii. Nat. Prod. Res. 2021, 35, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; He, J.X.; Huang, M.; Hu, H.X.; Xu, L.T.; Fang, K.L.; Wang, X.N.; Shen, T. Two new physalins from Physalis alkekengi L. var. franchetii (Mast.) Makino. Nat. Prod. Res. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yoritate, M.; Morita, Y.; Gemander, M.; Morita, M.; Yamashita, T.; Sodeoka, M.; Hirai, G. Synthesis of DFGH-Ring Derivatives of Physalins via One-Pot Construction of GH-Ring and Evaluation of Their NF-κB-Inhibitory Activity. Org. Lett. 2020, 22, 8877–8881. [Google Scholar] [CrossRef]
| Analytes | Method | Part Used | Results | Reference |
|---|---|---|---|---|
| Physalins A, O, L, and B | HPLC | Fruits and calyxes | In 10 habitats: 1.04–3.12, 0.99–2.66, 0.59–0.91, and 0.54–1.31 mg/g, respectively. | [32] |
| Physalins B, D, G, and H, 4,7-didehydroneophysalin B | UPLC-MS/MS | Fruits and calyxes Calyxes | In 14 habitats: 30.75–749.13, 59.63–1046.63, 15.25–527.15, 1.00–254.05, 15.75–70.88 μg/g, respectively 467.84, 560.34, 352.06,156.69, 43.22 μg/g, respectively | [39] |
| Physalin D | RP-HPLC–UV | Calyxes Fruits | In mature and immature: 0.2028 ± 0.0160%, 0.7880 ± 0.0612%, respectively In mature and immature: 0.0259 ± 0.0021%, 0.0992 ± 0.0083%, respectively | [35] |
| 4,7-didehydroneophysalin B | HPLC | Fruits | 0.02% | [40] |
| 4,7-didehydroneophysalin B | HPLC | Fruits and calyxes | 50% and 70% ethanol extract: 2.18%, 0.42%, respectively | [41] |
| Physalins A, P and O, Luteoloside, luteolin | HPLC | Fruits | In 6 habitats: 0.048–0.24, 0.04–0.2, 0.36–1.8, 0. 052–0.26, 0.04–0.2 μg/mL, respectively | [33] |
| Luteoloside | TLC | Fruits and calyxes | In 11 habitats: 0.11–2.27 mg/g | [42] |
| Luteolin | HPLC | Physalis permviana liquid | 0.75 μg/mL | [45] |
| Polysaccharides Reducing sugar | UV | Calyxes Fruits | In 52 habitats: 0.34–9.67, 1.32–146.53 mg/g, respectively In 50 habitats: 2.47–11.82, 181.97–321.57 mg/g, respectively | [43] |
| Luteoloside Luteolin | HPLC | Jinhuang yanyan tablets | 0.14%–0.15%, 0.0066%–0.0070%, respectively | [46] |
| Lutein β-carotene | HPLC-DAD-APCI-MS | Fruits | 19.8–21.6 mg/100 g of total lutein and β-carotene contents | [44] |
| Citric acid Malic acid Tartaric acid Ascorbic acid | HPLC-UV | Fruits | 903–920 mg/100 g 396–554 mg/100 g 261–325 mg/100 g 26–32 mg/100 g | [5] |
| (hydroxy)cinnamoyl hexosides Sinapoyl Feruloyl hexosides | HPLC-DAD-ESI-MS | Fruits | 70.8–81.6 mg/kg 57.8–68.0 mg/kg 10.6–13.6 mg/kg | [5] |
| Aromatic amino acids and amino derivatives | HPLC-DAD-ESI-MS | Fruits | 50.9–63.5 mg/kg | [5] |
| Pharmacological Activity | Animal/Cell Models | Constituent/Extract | Detail | Dosage | Reference |
|---|---|---|---|---|---|
| Anti-inflammatory activity | LPS-induced 264.7 cells | Physalins A, O, L, G Isophysalin A | Induced NO production | 20 μM | [47] |
| IFN-γ-stimulated macrophages LPS-stimulated macrophages | Physalins B, F, G | Reduced NO production; inhibited TNF-α, IL-6, IL-12 | 2 μg/mL | [48] | |
| C57BL/6 mice | Physalins B, F | Suppressed the increase in TNF-α; increased vascular permeability; prevented neutrophil influx | 20 mg/kg | [49] | |
| LPS-induced 264.7 cells | Physalin B | Decreased the levels of TNF-α, IL-6, IL-1β | 0.25, 0.5, 1.0 μM | [50] | |
| LPS/IFN-γ-induced macrophages IL-4/IL-13-induced macrophages LPS-induced C57BL/6 mice | Physalin D | In vitro: activated signal transducer and activator of STAT6 pathway; suppressed STAT1 activation; blocked STAT1 nuclear translocation In vivo: reduced inducible iNOS cell number; increased CD206+ cell number | 5 μM | [51] | |
| LPS-stimulated RAW 264.7 cells | Physalin E | Inhibited the generation of TNF-α, IL-6, NF-κB p65; reduced the degradation of I-kappa B protein | 12.5, 25, 50 μM | [52] | |
| TPA-induced acute ear edema in mice Oxazolone-induced chronic dermatitis in mice | Physalin E | Reduced ear edema response and myeloperoxidase activity; suppressed increase in ear thickness and levels of TNF-α and IFN-γ | 0.125, 0.25, 0.5 mg/ear | [53] | |
| DBA/1 mice | Physalin F | Decreased paw edema and joint inflammation | 60 mg/kg | [54] | |
| LPS-induced macrophages | Physalin X Aromaphysalin B | Inhibited NO production | IC50 = 68.50, 29.69 μM, respectively | [55] | |
| LPS-induced macrophages | Physalins B, F, H, V, D1, VII, I Isophysalin B | Inhibited NO production | IC50 = 0.32–4.03, 12.83–34.19 μM, respectively. | [56] | |
| LPS-induced macrophages | Physalins A, B, F Ombuine Luteolin | Inhibited NO production | IC50 = 2.57 ± 1.18, 0.84 ± 0.64, 0.33 ± 0.17, 2.23 ± 0.19, 7.39 ± 2.18 µM, respectively. | [57] | |
| LPS/IFN-γ-stimulated macrophages ICR mice | Luteolin | In vitro: suppressed the production of IL-6, IL-12, and TNF-α In vivo: inhibited paw edema | 20 μM 20 mg/kg | [58] | |
| KF-8 cells | Apigenin Lutelin | Inhibited NF-κB activation and the expression of CCL2/MCP-1 and CXCL1/KC | 20 μM | [59] | |
| LPS-induced macrophages | Kaempferol Quercetin | Inhibited STAT-1 and NF-κB activation, iNOS protein and mRNA expression, and NO production | 100 μM | [60,61] | |
| LPS-stimulated THP-1 cells ICR mice | 70% ethanol extract | In vitro: reduced the production of NO, PGE2, TNF-α, IL-1, iNOS, and COX-2 In vivo: reduced ear edema; induced granulomatous tissue formation | 500 μg/mL | [62] | |
| Wistar rats | Methanol extract | Reduced the paw volume | 500 mg/kg | [63] | |
| LPS-induced macrophages | Physanosides B | Inhibited NO production | IC50 = 9.93 μM | [64] | |
| LPS-induced macrophages | (6S,9R)-roseoside | Inhibited NO production | IC50 = 7.31 μM | [65] | |
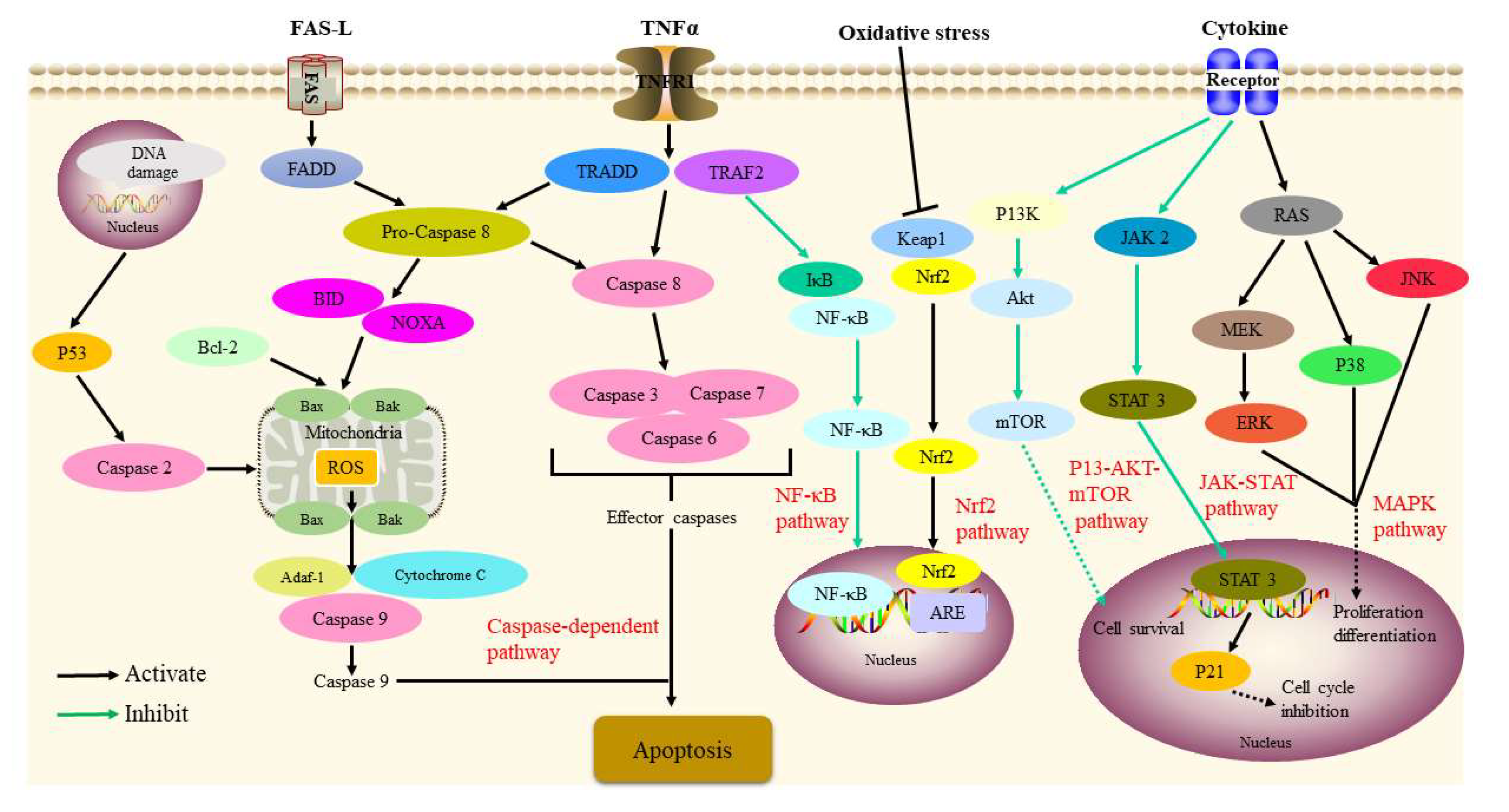
| Anti-tumor activity | HepG2 cells | Physalin A | Activated the Nrf2–ARE pathway and its target genes | 20 μM | [65] |
| Non-small cell lung cancer BALB /c mice | Physalin A | In vitro: suppressed both constitutive and induced STAT3 activity In vivo: suppressed tumor xenograft growth | 5,10, 15 μM 40, 80 mg/kg | [66] | |
| Human melanoma A375-S2 cells | Physalin A | Activated transmembrane death receptor; Induced poptosis via apoptotic (intrinsic and extrinsic) pathway; up-regulated p53-NOXA-mediated ROS generation | 15 μM | [67] | |
| Human HT1080 fibrosarcoma cells | Physalin A | Upregulated CASP3, CASP8 expression | IC50 = 10.7 ± 0.91 μM | [68] | |
| Human melanoma A375-S2 cells | Physalin A | Repressed the production of RNS and ROS; triggered the expression of iNOS and NO | 15 μM | [69] | |
| Non-small cell lung cancer | Physalin A | Induced G2/M cell cycle arrest; increased the amount of intracellular ROS | IC50 = 28.4 μM | [70] | |
| Prostate cancer cells (CWR22Rv1, C42B) | Physalins A, B | Inhibited the growth of two cells; activated the JNK and ERK pathway | IC50 = 14.2, 9.6 μM, respectively | [71] | |
| Non-small cell lung cancer | Physalin B | Exhibited anti-proliferative and apoptotic activity; downregulated the CDK1/CCNB1 complex; upregulated p21 | 5, 10, 20 μmol/L | [72] | |
| Human melanoma A375 cells | Physalin B | Activated the expression of the NOXA, BCL2 associated X (Bax), and CASP3 | 3 μg/mL | [73] | |
| Human HCT116 colon cancer cells | Physalin B | Activated the ERK, JNK, and p38 MAPK pathways; increased ROS generation | IC50 = 1.35 μmol/L | [74] | |
| Human DLD-1 colon cancer cells | Physalin B | Inhibited TNFα-induced NF-κB activation; induced the proapoptotic protein NOXA generation | 5 μM | [75] | |
| Breast cancer cells (MCF-7, MDA-MB-231, T-47D) | Physalin B | Induced cell cycle arrest at G2/M phase; promoted the cleavage of PARP, CASP3, CASP7, and CASP9; inactivated Akt and P13K phosphorylation | 2.5, 5, 10 μM | [76] | |
| TNF-α-stimulated HeLa cells | Physalins B, C, F | Inhibited the phosphorylation and degradation of IκBα and NF-κB activation | IC50 = 6.07, 6.54, 2.53 μM, respectively | [9] | |
| Tumor cells (A549, K562) | (17S,20R,22R)-5β,6β-epoxy-18,20-dihydroxy-1-ox- owitha-2,24-dienolide withaphysalin B | Suppressed the PI3K/Akt/mTOR signaling pathway | IC50 = 1.9–4.3 μM | [77] | |
| Tumor cells (B-16, HCT-8, PC3, MDA-MB-435, MDA-MB-231, MCF-7, K562, CEM, HL-60) Swiss mice | Physalins B, D | In vitro: displayed activity against several cancer cell lines In vivo: inhibited the proliferation of cells; reduced Ki67 staining | 0.58–15.18, 0.28–2.43 μg/mL, respectively 10, 25 mg/kg | [78] | |
| Human cancer cells (C4-2B, 22Rv1, 786-O, A-498, ACHN, A375-S2) | Physalins B, F | Showed anti-proliferative activities | IC50 = 0.24–3.17 μM | [56] | |
| Human T cell leukemia Jurkat cells | Physalins B, F | Inhibited PMA-induced NF-κB and TNF-α-induced NF-κB activation | 8, 16 µM, respectively | [79] | |
| HEK293T cells BALB/c-nu/nu mice | Physalin F | In vitro: decreased TOPFlash reporter activity; promoted the proteasomal degradation of β-catenin In vivo: downregulated β-catenin | 4 μM 10, 20 mg/kg | [80] | |
| T-47D cells | Physalin F | Activated the CASP3 and c-myc pathways | IC50 = 3.60 μg/mL | [81] | |
| Human renal, carcinoma cells (A498, ACHN, UO-31) | Physalin F | Induced cell apoptosis through the ROS-mediated mitochondrial pathway; suppressed NF-κB activation | 1, 3, 10 μg/mL | [82] | |
| PC-3 cancer cell lines | 7β-ethoxyl-isophysalin C | Showed apparent moderate activities | IC50 = 8.26 µM | [83] | |
| Human osteosarcoma cells | Physakengose G | Inhibited the epidermal growth factor receptor/mTOR (EGFR/mTOR) pathway; blocked autophagic flux through lysosome dysfunction | 5, 10, 20 μM | [84] | |
| Immunosuppressive activity | Trypanosoma cruzi (T. cruzi)-infected insects | Physalin B | Decreased number of T. cruzi Dm28c and T. cruzi transmission; inhibited the development of parasites | 1 mg/mL 20 ng 57 ng/cm2 | [85] |
| H14 Trypanosoma rangeli-infected Rhodnius prolixus larvae | Physalin B | Reduced the production of hemocyte microaggregation and NO | 0.1, 1 μg/mL | [86] | |
| T. cruzi trypomastigotes BALB/c mice macrophages | Physalin B Physalin F | Displayed strongest effects against epimastigote forms of T. cruzi | IC50 = 5.3 ± 1.9, 5.8 ± 1.5 μM, respectively IC50 = 0.68 ± 0.01, 0.84 ± 0.04 μM, respectively | [87] | |
| Con A-induced spleen cells CBA mice | Physalins B, F, G | In vitro: inhibited MLR and IL-2 production In vivo: prevented the rejection of allogeneic heterotopic heart transplant | 2 μg/mL 1 mg/mouse/day | [88] | |
| Human T-cell lymphotropic virus type 1 (HTLV-1)-infected subjects | Physalin F | Inhibited spontaneous proliferation; reduced the levels of IL-2, IL-6, IL-10, TNF-α, and IFN-γ | 10 μM | [89] | |
| T cells BALB/c mice | Physalin H | In vitro: suppressed proliferation and MLR In vivo: inhibited delayed-type hypersensitivity reactions and T-cell response | IC50 = 0.69, 0.39 μg/mL, respectively IC50 = 2.75 or 3.61 μg/mL | [90] | |
| ICR mice | Polysaccharides | Enhanced specific antibody titers immunoglobulin G (IgG), IgG1, and IgG2b, as well as the concentration of IL-2 and IL-4 | 40 µg/mice | [91] | |
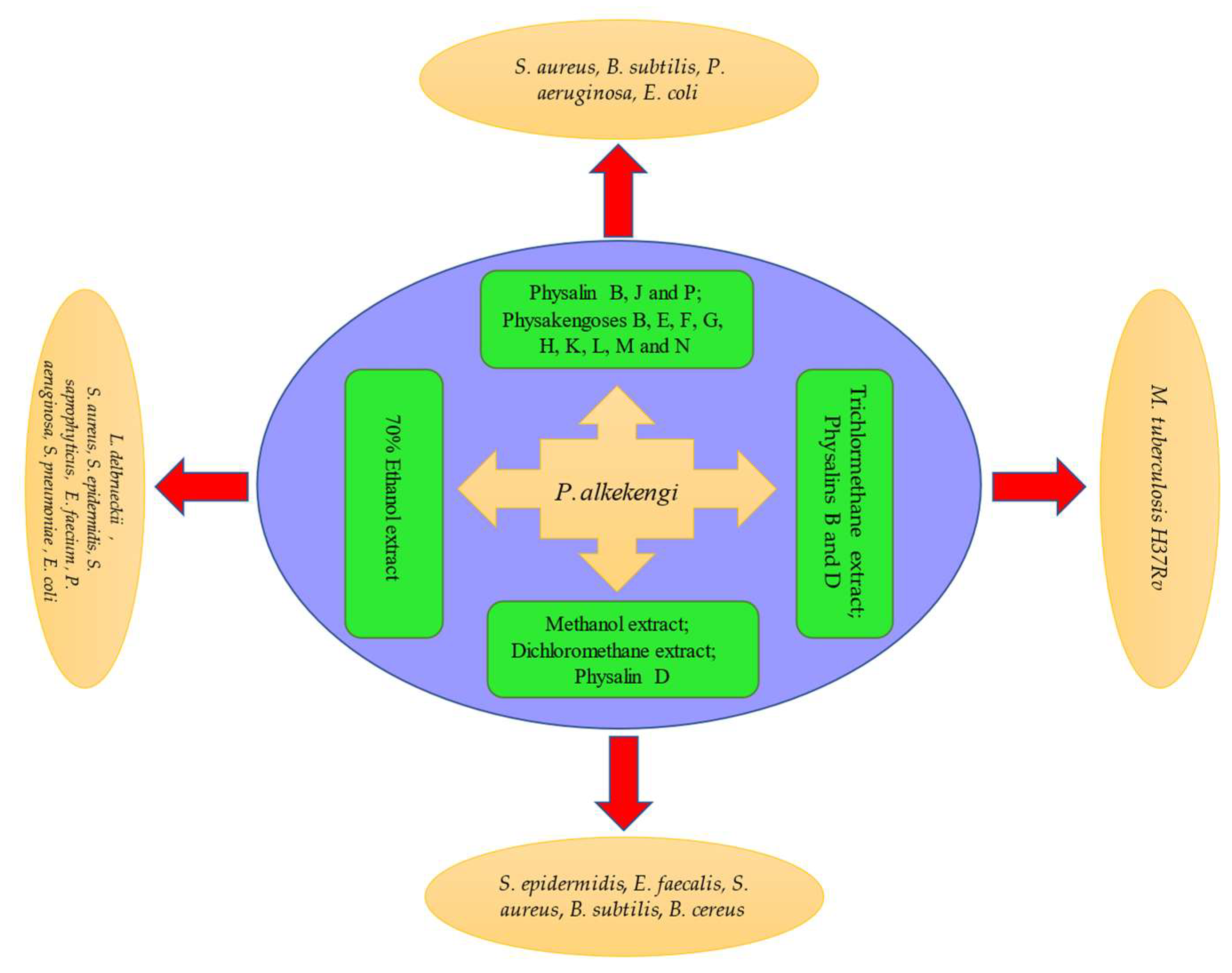
| Anti-microbial activity | Gram-positive bacteria: Staphylococcus epidermidis (S. epidermidis), Enterococcus faecalis (E. faecalis), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), Bacillus cereus (B. cereus) | Methanol extract Dichloromethane extract Physalin D | Displayed moderate antibacterial activity | MIC = 32–128 µg/mL | [92] |
| Escherichia coli (E. coli), B. subtilis | Physalins B, J, P | Showed high antibacterial activity | MIC = 12.5–23.7, 23.23–24.34, 22.8–27.98 µg/mL, respectively | [93] | |
| Mycobacterium tuberculosis H37Rv | Trichlormethane extract Physalins B, D | Showed antibacterial activity | MIC = 32, >128, 32 µg/mL, respectively | [94] | |
| Lactobacillus delbrueckii (L. delbrueckii), E. coli | 70% ethanol extract | Promoted the growth of L. delbrueckii; inhibited the growth of E. coli | 0.78–1.56 mg/mL | [95] | |
| Gram-positive bacteria: S. aureus, S. epidermidis, Staphylococcus saprophyticus (S. saprophyticus), Enterococcus faecium (E. faecium) Gram-negative bacteria: Pseudomonas aeruginosa (P. aeruginosa), Streptococcus pneumoniae (S. pneumoniae), E. coli | 70% ethanol extract | Showed antibacterial activity | MIC = 0.825–1.65 mg/mL | [62] | |
| S. aureus, B. subtilis, P. aeruginosa, E. coli | Physakengoses B, E, F, G, H, K, L, M, N, O | Showed potent inhibitory effects | MIC = 2.16–14.9 μg/mL | [96,97] | |
| Anti-leishmanial | Leishmania-infected macrophages Leishmania amazonensis -infected BALB/c mice | Physalins B, F | In vitro: reduced the percentage of macrophages In vivo: reduced the lesion size, the parasite load, and histopathological alterations | IC50 = 0.21 and 0.18 μM, respectively | [98] |
| Others | Kunming mice | Water extract | Decreased the expression of white blood cells and eosinophils, IL-5, IFN-γ, Th1, and Th2 | 0.25, 5, 1 g/mL | [99] |
| 3T3-L1 pre-adipocyte cells HepG2 cells Male Sprague–Dawley (SD) rats | Ethyl acetate extract | In vitro: relieved oxidative stress; inhibited α-glucosidase activity. In vivo: decreased FBG, TC, and TG | 300 mg/kg | [100] | |
| Alloxan-induced mice | Polysaccharides | Decreased FBG and GSP; increased FINS; upregulated the PI3K, Akt, and GLUT4 mRNA | 200, 400, 800 mg/kg | [101] | |
| High-fat diet-fed and streptozotocin-induced diabetic SD rats | Ethyl acetate extract | Reduced the FBG, TC, TG, and GSP; increased FINS | 300, 600 mg/kg | [102] | |
| Wistar rats Albino mice | Aqueous methanolic extract | Reduced the intensity of gastric mucosal damage; inhibited pain sensation | 500 μg/mL 500 mg/kg | [63] | |
| LPS-induced acute lung injury in BALB/c mice | 70% ethanol extract | Reduced the release of TNF-α and the accumulation of oxidation products; decreased the levels of NF-κB, phosphorylated-p38, ERK, JNK, p53, CASP3, and COX-2 | 500 mg/kg | [103] | |
| 4% dextran sulfate sodium--induced colitis in BALB/c mice | Physalin B | Reduced MPO activity; suppressed the activation of NF-κB, STAT3, arrestin beta 1 (ARRB1), and NLR family pyrin domain containing 3 (NLRP3) | 10, 20 mg/kg | [50] | |
| N2a/APPsw cells | Physalin B | Downregulated β-amyloid (Aβ) secretion and the expression of beta-secretase 1 (BACE1) | 3 μmol/L | [104] | |
| DPPH TBA | Physalin D | Exhibited antioxidant activity | IC50 ≥ 10 ± 2.1 µg/mL | [92] | |
| Plasmodium berghei-infected mice | Physalins B, D, F, G | Caused parasitemia reduction and delay | 50, 100 mg/kg | [105] | |
| High glucose-induced primary mouse hepatocytes Oleic acid-induced HepG2 cells Kunming mice | 75% ethanol extract Luteolin-7-O-β-d-glucopyranoside | In vitro: decreased the levels of TG in HepG2 cells In vivo: decreased the levels of TC and TG | 50, 100 μg/mL, respectively 1 or 2 g/kg, 0.54 g/kg, respectively | [106] | |
| SD mice | Luteolin | Increased NO; activated PI3K/Akt/NO signaling pathway; enhanced the activity of endothelial NOS | 7.5 µg/mL | [107] | |
| SD rats | Luteolin | Conferred a cardioprotective effect; ameliorated Ca2+ overload | 7.5, 15, 30 μmol/L | [108] |
| Methods | Compounds | Dose/ mg/kg | t1/2/h | Cmax/ ng/mL | Tmax/h | CL/L/min/kg | MRT0-t/ h | MRT0-∞/h | AUC0-t/ ng·h/mL | AUC0-∞/ ng·h/mL | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LC-MS/MS | Physalin A Physalin D Physalin L | 2 16 3 | 2.52 ± 0.40 3.36 ± 0.26 2.82 ± 0.25 | 5.30 ± 1.76 11.5 ± 3.57 56.4 ± 15.4 | 1.29 ± 2.31 1.67 ± 1.46 1.28 ± 1.33 | - - - | 3.63 ± 0.57 4.85 ± 0.37 4.07 ± 0.37 | - - | 21.0 ± 3.14 70.5 ± 10.10 200 ± 31.30 | 113 ± 103 103 ± 30.2 266 ± 53.0 | [112] |
| UPLC–MS/MS | Physalin D Physalin G 4,7-Didehydroneophysalin B | 35.6 13.9 32.6 | 3.67 ± 1.04 8.04 ± 3.42 6.15 ± 1.20 | 47.6 ± 4.10 20.9 ±4.40 23.6 ± 4.90 | 1.17 ± 0.00 1.17 ± 0.00 1.17 ± 0.00 | 4.4 ± 0.60 3.2 ± 0.70 8.7 ± 1.90 | 3.42 ± 0.33 4.69 ± 1.41 4.89 ± 0.43 | - - | 60.82 ± 14.32 61.24 ± 11.53 60.82 ± 12.85 | 136.94 ± 17.18 74.56 ± 17.46 64.82 ± 14.80 | [113] |
| LC-MS/MS | Physalin L | 18.52 | 2.89 ± 1.14 | 77.48 ± 28.30 | 0.69 ± 0.26 | 50.26 ± 11.50 | 3.13 ± 0.63 | 4.33 ± 1.50 | 280.78 ± 86.48 | 313.10 ± 101.24 | [114] |
| HPLC-MS/MS | Physalin B | 5 | 5.35 ± 0.49 | 395.0 ± 35.4 | 0.08 ± 0.0 | 0.18 ± 0.03 | - | - | 382.25 ± 24.87 | 449.92 ± 27.46 | [116] |
| HPLC-MS/MS | Physalin D | 2 | 0.09 ± 0.07 | 941.3 ± 272.1 | 0.08 ± 0.0 | 0.12 ± 0.01 | 0.30 ± 0.12 | - | 28.30 ± 29.02 | 283.89± 28.37 | [118] |
| SPE-LC-MS/MS | Physalin A Physalin D Physalin G 4,7-Didehydroneophysalin B | 29 38.8 18.3 31.6 | 1.83 ± 0.61 3.11 ± 1.37 2.24 ± 1.47 2.32 ± 1.01 | 12.73 ± 2.08 64.58 ± 21.30 89.93 ± 26.05 19.63 ± 7.21 | 0.67 ± 0.15 1.29 ± 0.78 0.67 ± 0.00 1.13 ± 0.32 | - | - | - | 65.21 ± 10.52 615.39 ± 97.86 159.12 ± 34.76 105.5 ± 28.21 | 96.31 ± 30.50 885.18 ± 230.68 205.07 ± 49.8 173.58 ± 17.90 | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Sun, Y.; Cao, F.; Yang, B.; Kuang, H. Natural Products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A Review on Their Structural Analysis, Quality Control, Pharmacology, and Pharmacokinetics. Molecules 2022, 27, 695. https://doi.org/10.3390/molecules27030695
Yang J, Sun Y, Cao F, Yang B, Kuang H. Natural Products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A Review on Their Structural Analysis, Quality Control, Pharmacology, and Pharmacokinetics. Molecules. 2022; 27(3):695. https://doi.org/10.3390/molecules27030695
Chicago/Turabian StyleYang, Jing, Yanping Sun, Feng Cao, Bingyou Yang, and Haixue Kuang. 2022. "Natural Products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A Review on Their Structural Analysis, Quality Control, Pharmacology, and Pharmacokinetics" Molecules 27, no. 3: 695. https://doi.org/10.3390/molecules27030695
APA StyleYang, J., Sun, Y., Cao, F., Yang, B., & Kuang, H. (2022). Natural Products from Physalis alkekengi L. var. franchetii (Mast.) Makino: A Review on Their Structural Analysis, Quality Control, Pharmacology, and Pharmacokinetics. Molecules, 27(3), 695. https://doi.org/10.3390/molecules27030695





