Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis
Abstract
1. Introduction
2. Results
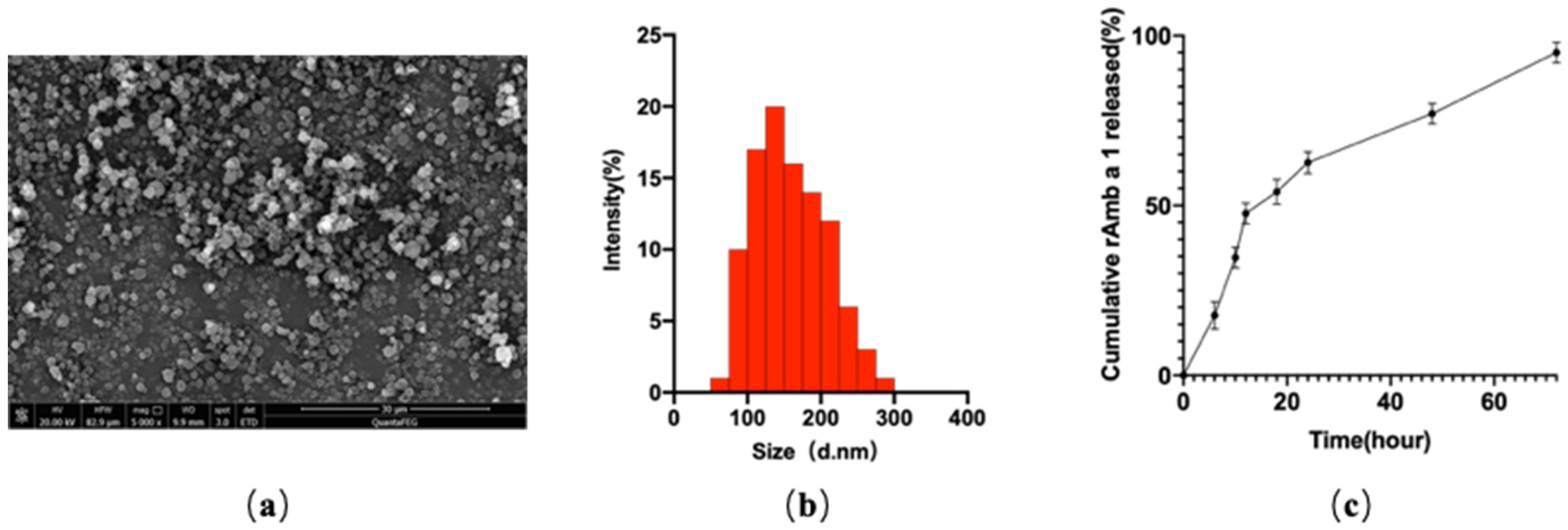
2.1. Characterization of rAmb a 1-Loaded PLGA-PEG Nanoparticles
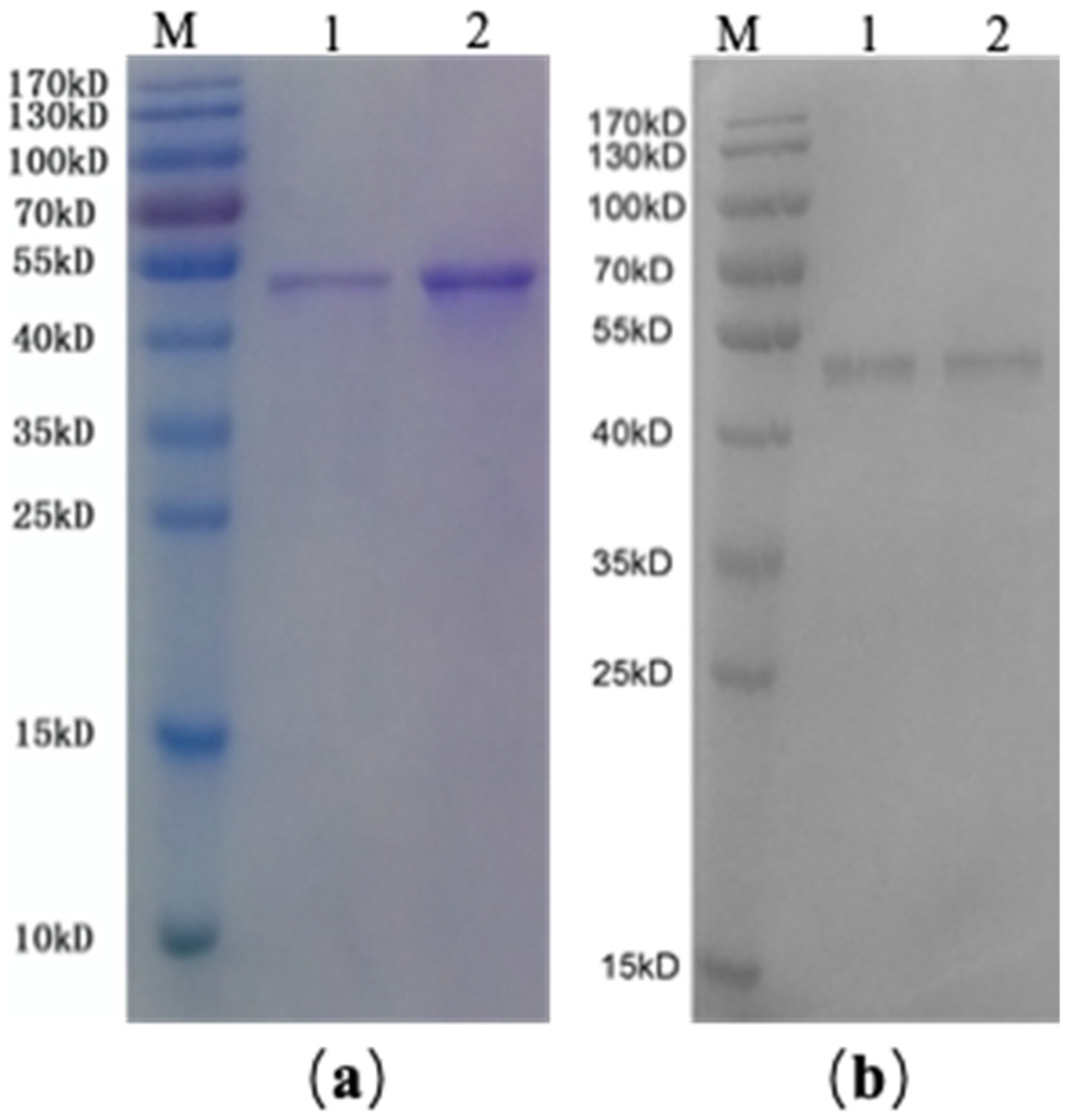
2.2. SDS-PAGE and Western Blotting of rAmb a 1-Loaded PLGA–PEG Nanoparticles
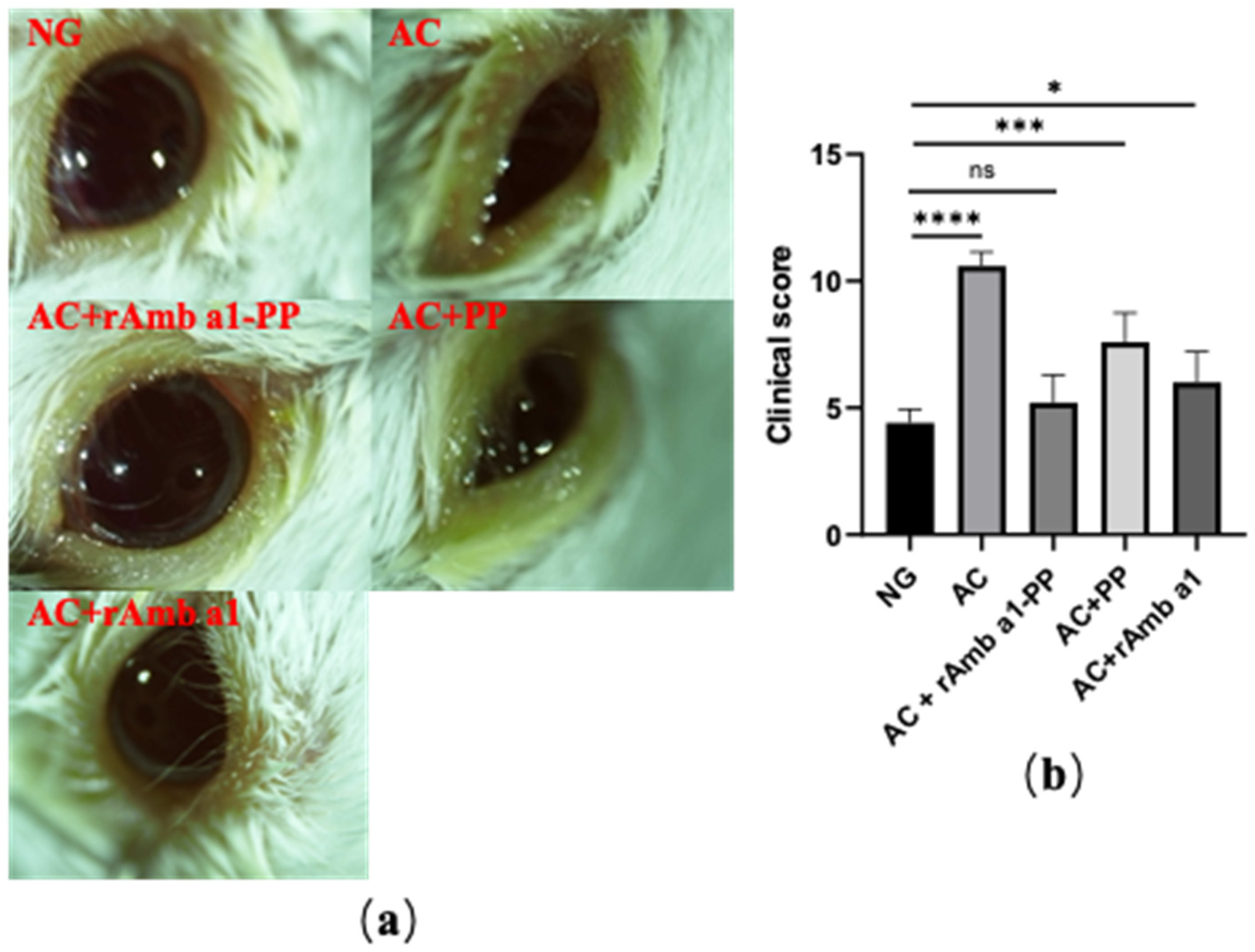
2.3. Evaluation of Ocular Signs
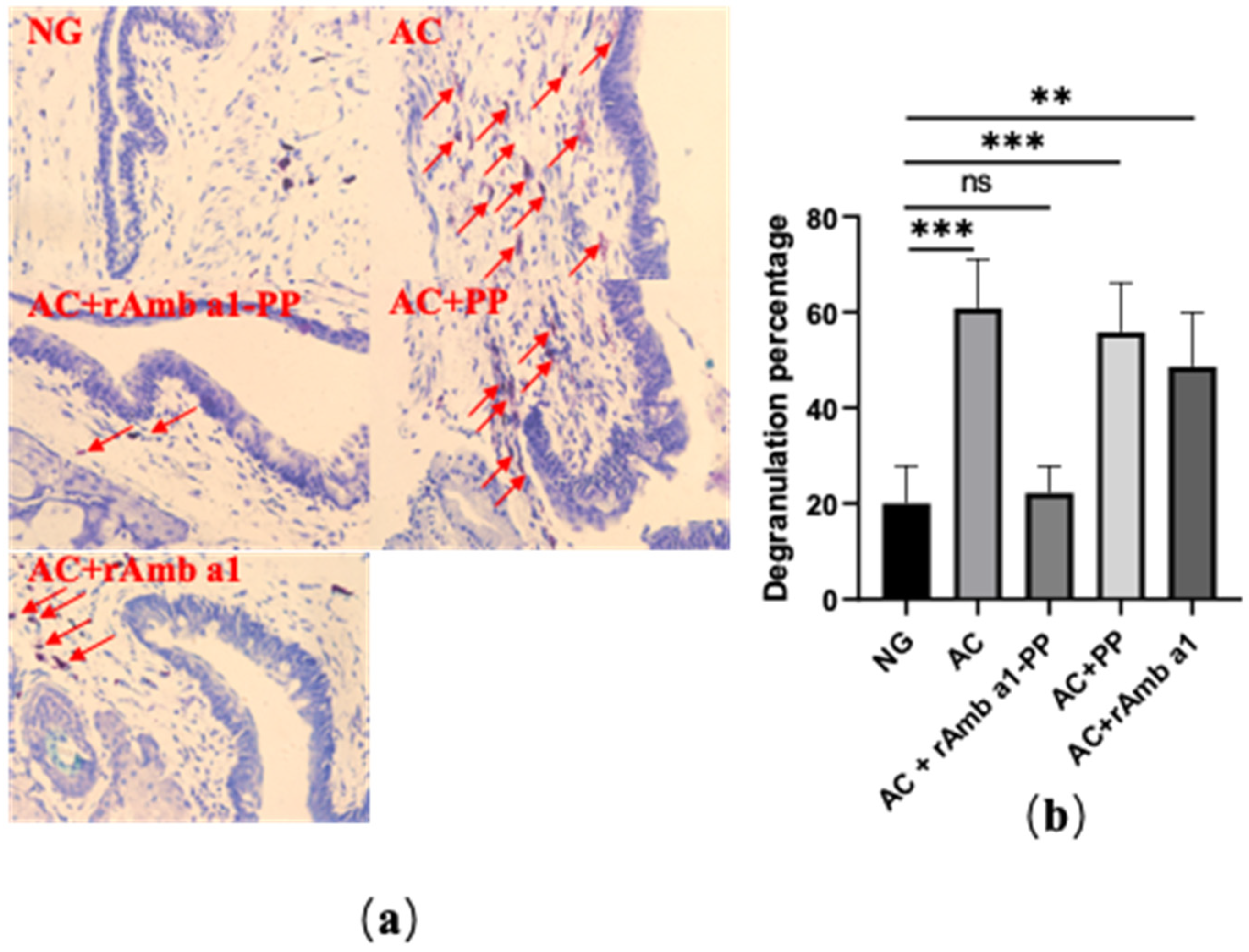
2.4. Analysis of Histological Examination
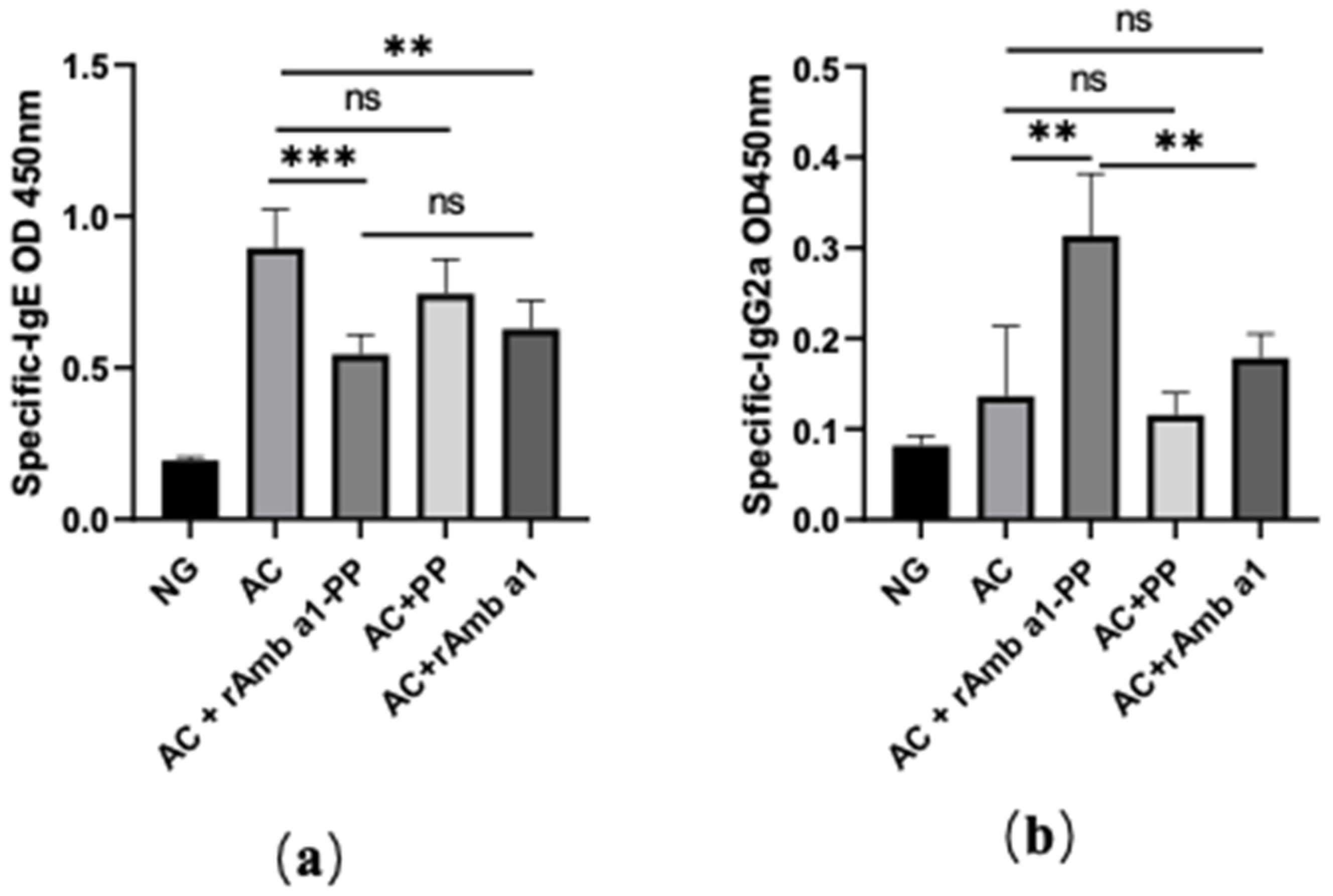
2.5. Specific IgE and IgG2a in Serum
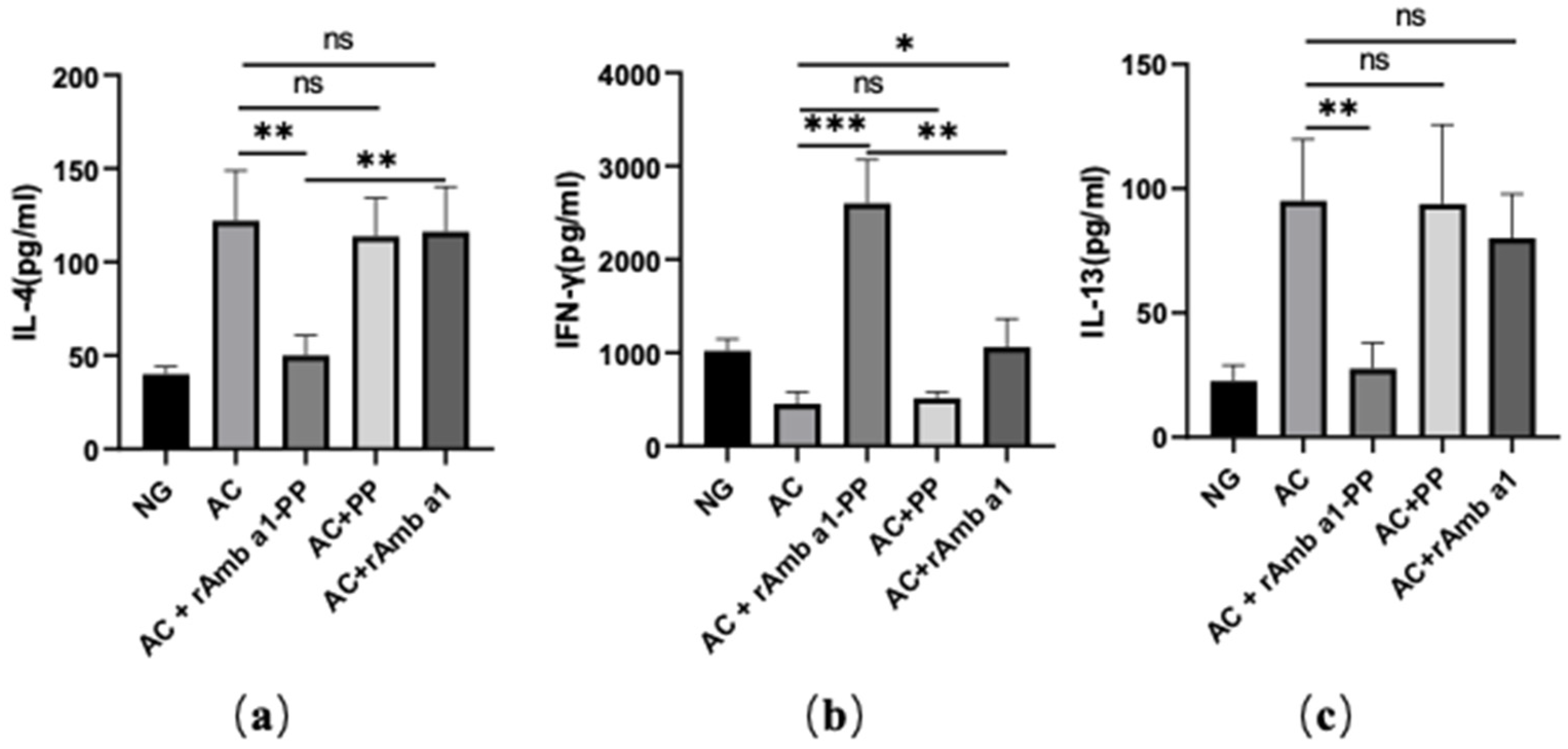
2.6. rAmb a 1-PLGA-PEG Nanoparticles Enhances the Th1/Th2 Ratio
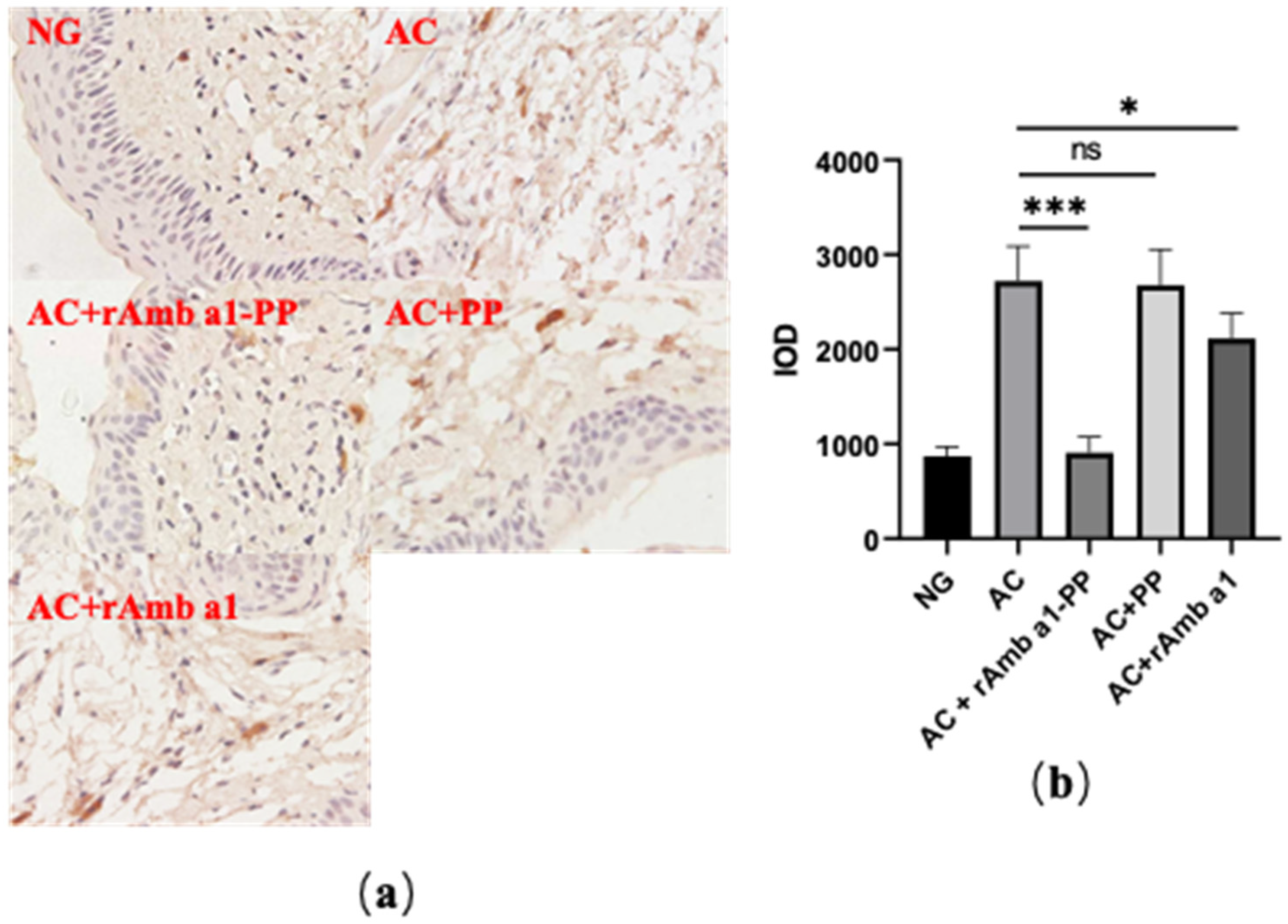
2.7. rAmb a 1-PLGA-PEG Nanoparticles Inhibits Mast Cell Degranulation
3. Discussion
4. Materials and Methods
4.1. Animals and Materials
4.2. Expression and Purification of rAmb a 1
4.3. Preparation of rAmb a 1-Loaded PLGA–PEG Nanoparticles
4.4. Characterization of rAmb a 1-Loaded PLGA-PEG Nanoparticles
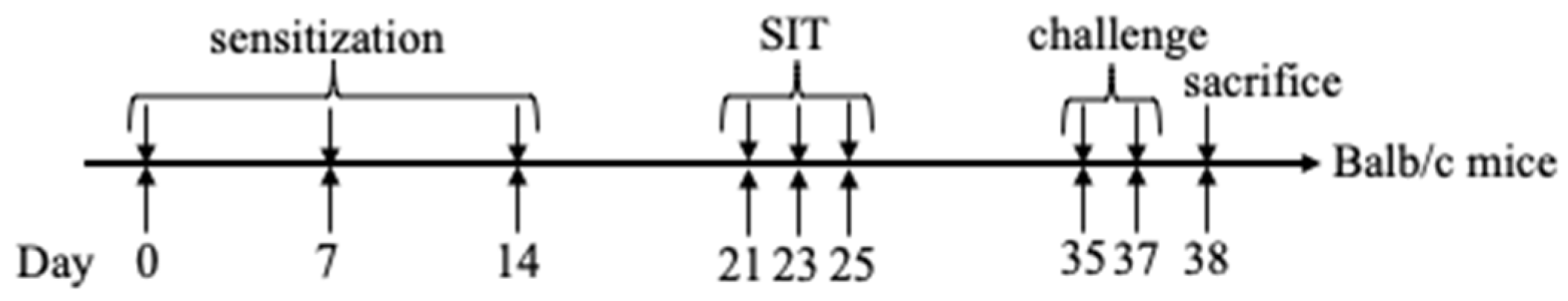
4.5. Animal Sensitization and Specific Immunotherapy
4.6. Histologic Evaluation
4.7. Detection of Allergen-Specific Antibodies in Serum
4.8. Cytokine Determination
4.9. Immunohistochemistry
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baab, S.; Le, P.H.; Kinzer, E.E. Allergic Conjunctivitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Baiula, M.; Bedini, A.; Carbonari, G.; Dattoli, S.D.; Spampinato, S. Therapeutic targeting of eosinophil adhesion and accumulation in allergic conjunctivitis. Front. Pharmacol. 2012, 3, 203. [Google Scholar] [CrossRef]
- La Rosa, M.; Lionetti, E.; Reibaldi, M.; Russo, A.; Longo, A.; Leonardi, S.; Tomarchio, S.; Avitabile, T.; Reibaldi, A. Allergic conjunctivitis: A comprehensive review of the literature. Ital. J. Pediatr. 2013, 39, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, F.; Feng, Z.; Li, W.; Li, N.; Du, X.; Zhao, X.; Li, S.; Yuan, J. Changes in Anterior and Posterior Corneal Elevation in Patients with Allergic Conjunctivitis. Front. Med. 2021, 8, 788302. [Google Scholar] [CrossRef]
- Leonardi, A.; Castegnaro, A.; La Gloria Valerio, A.; Lazzarini, D. Epidemiology of allergic conjunctivitis: Clinical appearance and treatment patterns in a population-based study. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 482–488. [Google Scholar] [CrossRef]
- Bielory, L.; Delgado, L.; Katelaris, C.H.; Leonardi, A.; Rosario, N.; Vichyanoud, P. ICON: Diagnosis and management of allergic conjunctivitis. Ann. Allergy Asthma Immunol. 2020, 124, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.; Norris, M.R.; Bielory, L. Seasonal ocular allergy and pollen counts. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Briza, P.; Thalhamer, J.; Ferreira, F. Specific immunotherapy in pollen allergy. Curr. Opin. Mol. Ther. 2007, 9, 160–167. [Google Scholar] [PubMed]
- Turkalj, M.; Banic, I.; Anzic, S.A. A review of clinical efficacy, safety, new developments and adherence to allergen-specific immunotherapy in patients with allergic rhinitis caused by allergy to ragweed pollen (Ambrosia artemisiifolia). Patient Prefer. Adherence 2017, 11, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Curin, M.; Khaitov, M.; Karaulov, A.; Namazova-Baranova, L.; Campana, R.; Garib, V.; Valenta, R. Next-Generation of Allergen-Specific Immunotherapies: Molecular Approaches. Curr. Allergy Asthma Rep. 2018, 18, 39. [Google Scholar] [CrossRef]
- Cao, H.; Liu, Z. Clinical significance of dust mite allergens. Mol. Biol. Rep. 2020, 47, 6239–6246. [Google Scholar] [CrossRef]
- Larsen, J.N.; Dreborg, S. Standardization of Allergen Extracts. Methods Mol. Biol. 2019, 2020, 63–76. [Google Scholar] [PubMed]
- Huang, H.J.; Curin, M.; Banerjee, S.; Chen, K.W.; Garmatiuk, T.; Resch-Marat, Y.; Carvalho-Queiroz, C.; Blatt, K.; Gafvelin, G.; Gronlund, H.; et al. A hypoallergenic peptide mix containing T cell epitopes of the clinically relevant house dust mite allergens. Allergy 2019, 74, 2461–2478. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, A.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- D’Souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Huang, L.; Chen, H.; Zheng, Y.; Song, X.; Liu, R.; Liu, K.; Zeng, X.; Mei, L. Nanoformulation of D-α-tocopheryl polyethylene glycol 1000 succinate-b-poly(ε-caprolactone-ran-glycolide) diblock copolymer for breast cancer therapy. Integr. Biol. 2011, 3, 993–1002. [Google Scholar] [CrossRef]
- Iwaszko, M.; Bialy, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef]
- Tomic, J.; Jovanovic, M.; Tomic, D. Food allergy in children—Part I: Pathogenesis and diagnostic approach. Med. Pregl. 2003, 56, 54–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, H.R.; Pemberton, A.D. Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut. Immunology 2002, 105, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Revdekar, A.; Shende, P. Block copolymers in Alzheimer’s disease therapy: A perceptive to revolutionize biomaterials. J. Control. Release 2021, 340, 271–281. [Google Scholar] [CrossRef]
- Scholl, I.; Kopp, T.; Bohle, B.; Jensen-Jarolim, E. Biodegradable PLGA particles for improved systemic and mucosal treatment of Type I allergy. Immunol. Allergy Clin. N. Am. 2006, 26, 349–364. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Ming, L.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Coco, R.; Plapied, L.; Pourcelle, V.; Jerome, C.; Brayden, D.J.; Schneider, Y.J.; Preat, V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013, 440, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef]
- Shakya, A.K.; Lee, C.H.; Gill, H.S. Microneedle-Mediated Allergen-Specific Immunotherapy for the Treatment of Airway Allergy in Mice. Mol. Pharm. 2020, 17, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R. The future of antigen-specific immunotherapy of allergy. Nat. Rev. Immunol. 2002, 2, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 2015, 75, 14–24. [Google Scholar] [CrossRef]
- Nieto, J.E.; Casanova, I.; Serna-Ojeda, J.C.; Graue-Hernandez, E.O.; Quintana, G.; Salazar, A.; Jimenez-Martinez, M.C. Increased Expression of TLR4 in Circulating CD4+T Cells in Patients with Allergic Conjunctivitis and In Vitro Attenuation of Th2 Inflammatory Response by Alpha-MSH. Int. J. Mol. Sci. 2020, 21, 7861. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Alhamwe, B.A.; Miethe, S.; Garn, H. Epigenetic Mechanisms in Allergy Development and Prevention. Handb. Exp. Pharmacol. 2022, 268, 331–357. [Google Scholar] [PubMed]
- Albisa, A.; Piacentini, E.; Sebastian, V.; Arruebo, M.; Santamaria, J.; Giorno, L. Preparation of Drug-Loaded PLGA-PEG Nanoparticles by Membrane-Assisted Nanoprecipitation. Pharm. Res. 2017, 34, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Zeng, X.; Zhang, X.; Ma, L.; Liu, X.; Yu, H.; Mei, L.; Liu, Z. Effects of Caryota mitis profilin-loaded PLGA nanoparticles in a murine model of allergic asthma. Int. J. Nanomed. 2013, 8, 4553–4562. [Google Scholar]
- Hong, J.; Gao, Q.; Xiao, X.; Cao, H.; Yuan, R.; Liu, Z.; Chen, T. T cell epitope of arginine kinase with CpG co-encapsulated nanoparticles attenuates a shrimp allergen-induced Th2-bias food allergy. Biosci. Biotechnol. Biochem. 2020, 84, 804–814. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Liu, L.; Wang, J.; Gong, M.; Yuan, R.; Lu, J.; Xiao, X.; Liu, X. Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules 2022, 27, 598. https://doi.org/10.3390/molecules27030598
Cao H, Liu L, Wang J, Gong M, Yuan R, Lu J, Xiao X, Liu X. Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules. 2022; 27(3):598. https://doi.org/10.3390/molecules27030598
Chicago/Turabian StyleCao, Hui, Ling Liu, Junyi Wang, Miao Gong, Ruyi Yuan, Jiahua Lu, Xiaojun Xiao, and Xiaoyu Liu. 2022. "Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis" Molecules 27, no. 3: 598. https://doi.org/10.3390/molecules27030598
APA StyleCao, H., Liu, L., Wang, J., Gong, M., Yuan, R., Lu, J., Xiao, X., & Liu, X. (2022). Effects of rAmb a 1-Loaded PLGA-PEG Nanoparticles in a Murine Model of Allergic Conjunctivitis. Molecules, 27(3), 598. https://doi.org/10.3390/molecules27030598




